Pyrometallurgical Scheme Intended to Process Arsenic-Containing Concentrates with Recovery of Precious Metals
Abstract
1. Introduction
| Concentrate | Contents | References | |||||
|---|---|---|---|---|---|---|---|
| Au, g/t | Ag, g/t | As, wt. % | Cu, wt. % | Fe, wt. % | S, wt. % | ||
| Concentrate of gold mine in Liangshan District (Sichuan Province, China) | 63.76 | 69.30 | 8.90 | 0.09 | 21.03 | 18.48 | [10] |
| Serrenti-Furtei gold-bearing deposit in Southern Sardinia (Italy) | 90.25 | no data | 12.55 | 33.15 | 9.16 | no data | [7] |
| Arsenic-containing gold-bearing concentrate from Hunan Province (China) | 60.16 | 50.27 | 13.84 | 0.097 | 32.33 | 23.62 | [3] |
| Gold concentrate from Golden Sun Co. Ltd. (Jeollanam-do, Korea) | 130.20 | 986.6 | 0.25 | 0.19 | 8.68 | 0.25 | [4] |
| Concentrate from Uderey deposit (Krasnoyarsk Region, Russia) | 30.00 | no data | 12.20 | no data | 21.60 | 20.00 | [11] |
| Arsenopyrite concentrate from New England Antimony Mine (New South Wales, Australia) | no data | no data | 31.60 | no data | 36.80 | 28.0 | [12] |
| Gravioconcentrate from the Vasilkovskoye deposit (Kazakhstan) | 5–50 | 16–20 | 1.60–5.10 | 0.29–4.00 | 4.00–9.00 | 2.05 | [13] |
| Concentrate from a deposit of Yenisei ridge (Yakutia, Russia) | 15–80 | no data | 3.00–13.20 | no data | 7.26–22.44 | 8.22–22.44 | [14] |
| Concentrate from pyrite-arsenopyrite mineralization in northern Finland | no data | no data | 6.00 | no data | 21.60 | 19.50 | [15] |
| Concentrate from Olympias deposit (Chalkidiki, Greece) | 21.00 | no data | 7.50 | no data | 43.70 | no data | [16] |
| Concentrate from GENMIN’sFairview Mine (Barberton, South Africa) | 131.50 | no data | 6.71 | no data | no data | no data | [17] |
| Concentrate from Hunan (China) | 30.00 | 66.90 | 8.87 | no data | 19.94 | 30.71 | [18] |
2. Materials and Methods
2.1. Equipment
2.2. Materials
2.3. Technological Experiment Procedure
2.4. Characterizations
3. Results and Discussion
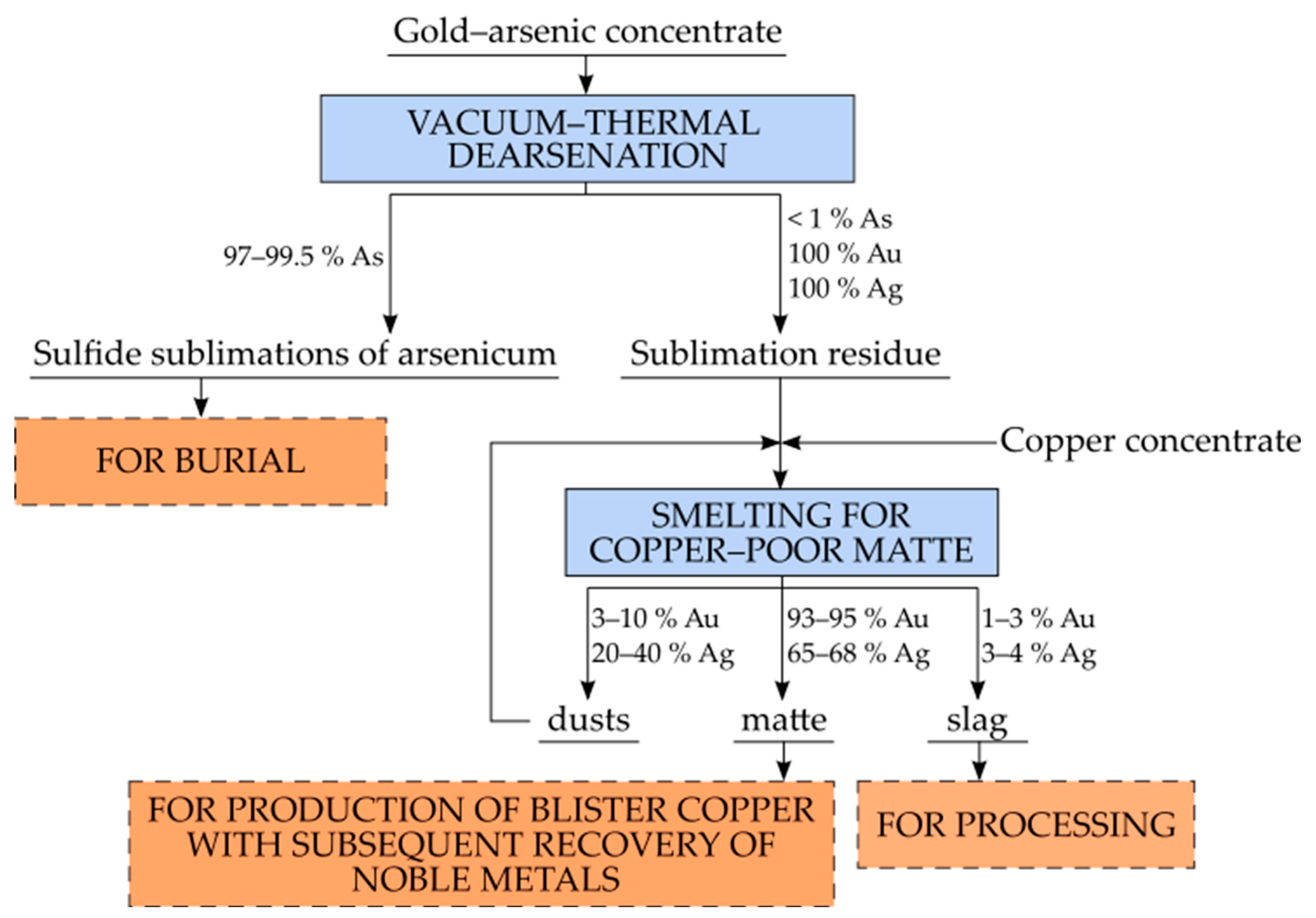
3.1. Vacuum-Thermal Dearsenation of Gold-Arsenic Concentrate
3.2. Pyrometallurgical Processing of Arsenic Sublimation Residues from Gold-Containing Concentrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isakova, R.A.; Chelokhsaev, L.S.; Khrapunov, V.E.; Spivak, M.M.; Fedulov, J.O. Vacuum-thermal arsenic recovery from gold-containing sulfide concentrate. J. Kunming Inst. Technol. 1993, 18, 28–34. [Google Scholar]
- Luganov, V.A.; Khrapunov, V.E.; Isakova, R.A.; Chnyrenkova, T. Processing of Arsenic-Bearing Gold Ores. In Proceedings of the SME Annual Meeting & Exhibit, Phoenix, AZ, USA, 25–27 February 2002; p. 63. [Google Scholar]
- Zheng, Y.; Wei, D.; Liu, W.; Duan, H.; Zhor, S. One Novel two-step Bio-Oxidation Pretreatment of Arsenic-Containing Gold-Bearing Concentrate. Int. J. Electrochem. Sci. 2018, 13, 5983–5994. [Google Scholar] [CrossRef]
- Kim, B.; Park, C.; Cho, K.; Kim, J.; Choi, N.; Lee, S. Sulfuric Acid Baking-Water Leaching for Gold Enrichment and Arsenic Removal from Gold Concentrate. Minerals 2021, 11, 1332. [Google Scholar] [CrossRef]
- Gurman, M.A.; Shcherbak, L.I.; Rasskazov, A.V. Gold and arsenic recovery from calcinates of rebellious pyrite-arsenopyrite concentrates. J. Min. Sci. 2015, 51, 586–590. [Google Scholar] [CrossRef]
- Mesa Espitia, S.L.; Lapidus, G.T. Arsenic removal strategy in the processing of an arsenopyritic refractory gold ore. Hydrometallurgy 2021, 203, 105628. [Google Scholar] [CrossRef]
- Curreli, L.; Garbarino, C.; Ghiani, M.; Orrù, G. Arsenic leaching from a gold bearing enargite flotation concentrate. Hydrometallurgy 2009, 96, 258–263. [Google Scholar] [CrossRef]
- Rakhmanov, O.B.; Aksenov, A.V.; Mineyev, G.G.; Nazarov, K.M.; Karimov, M.I. Processing of refractory gold-bearing arsenic flotation concentrate of the Ikkizhelon deposit using autoclave oxidation. Bull. INRTU 2018, 22, 163–172. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Myung, E.; Purev, O.; Choi, N.; Park, C. Recovery of Gold from the Refractory Gold Concentrate Using Microwave Assisted Leaching. Metals 2020, 10, 571. [Google Scholar] [CrossRef]
- Yaozhong, L.; Smith, R.W. Arsenic removal from high arsenic bearing gold sulphide concentrate. Miner. Process. Extr. Metall. 2004, 113, 189–191. [Google Scholar] [CrossRef]
- Karimov, K.; Rogozhnikov, D.; Kuzas, E.; Dizer, O.; Golovkin, D.; Tretiak, M. Deposition of Arsenic from Nitric Acid Leaching Solutions of Gold-Arsenic Sulphide Concentrates. Metals 2021, 11, 889. [Google Scholar] [CrossRef]
- Dunn, J.G.; Chamberlain, A.C. The Recovery of Gold from Refractory Arsenopyrite Concentrates by Pyrolysis-Oxidation. Miner. Eng. 1997, 10, 919–928. [Google Scholar] [CrossRef]
- Varentsov, V.K.; Varentsova, V.I. Gold and Silver Recovery from Persistent Arsenopyrite Gravioconcentrate. Chem. Sustain. Dev. 2019, 27, 257–261. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching Kinetics of Sulfides from Refractory Gold Concentrates by Nitric Acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef]
- Rusanen, L.; Aromaa, J.; Forsen, O. Pressure Oxidation of Pyrite-Arsenopyrite Refractory Gold Concentrate. Physicochem. Probl. Miner. Process. 2013, 49, 101–109. [Google Scholar] [CrossRef]
- Kydros, K.A.; Matis, K.A.; Papadoyannis, I.N.; Mavros, P. Selective Separation of Arsenopyrite from an Auriferous Pyrite Concentrate by Sulphonate Flotation. Int. J. Miner. Process. 1993, 38, 141–151. [Google Scholar] [CrossRef]
- Miller, D.M.; Hansford, G.S. Batch Biooxidation of a Gold-Bearing Pyrite-Arsenopyrite Concentrate. Miner. Eng. 1992, 5, 613–629. [Google Scholar] [CrossRef]
- Bai, T.; Lei, H.; Lei, Z.; Li, S. Dearsenification of gold concentrates via microwave roasting. Can. J. Metall. Mater. Sci. 2020, 59, 60–66. [Google Scholar] [CrossRef]
- Isabayev, S.M.; Kuzgibekova, K.M.; Zhinova, E.V.; Zikanova, T.A. Development of technology for oxidative roasting of gold-arsenic concentrates of double resistance. Kompleks. Ispolz. Mineral. Syra. 2015, 3, 27–31. [Google Scholar]
- Qin, H.; Guo, X.; Tian, Q.; Uu, D.; Zhang, L. Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold recovery. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; He, Y.; Xu, S.; Hu, B.; Gao, H.; Zheng, G. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Miner. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Khrapunov, V.Y.; Chelokhsayev, L.S.; Takezhanov, S.T.; Isakova, R.A. Processing of gold-arsenic concentrates by the blooming method. Kompleks. Ispolz. Mineral. Syra. 1991, 6, 42–45. [Google Scholar]
- Paleyev, P.L.; Gulyashinov, A.N.; Antropova, I.G.; Gulyashinov, P.A. Gold recovery from refractory arsenopyrite ores and concentrates. Gold Technol. 2013, 2, 36–38. [Google Scholar]
- Paleyev, P.L.; Gulyashinov, A.N.; Antropova, I.G. Dearsenization of gold-bearing arsenopyrite ore in an atmosphere of superheated water vapor. Sib. Branch Rus. Ac. Scien. Phys. Technol. Prob. Min. 2013, 6, 175–179. [Google Scholar]
- Paleyev, P.L.; Gulyashinov, P.A.; Gulyashinov, A.N.; Antropova, I.G. Roasting of gold-bearing arsenopyrite ore in an atmosphere of superheated steam. Inter. J. App. Fund. Res. 2017, 12, 233–237. [Google Scholar]
- Lissina, N.N.; Kharitidi, G.P.; Ovchinnikova, L.A.; Skopov, G.V. Suppression of high-temperature sublimation of arsenic sulfides. Non-Ferr. Met. 1985, 5, 31–35. [Google Scholar]
- Shustrov, A.Y.; Matsenko, Y.A.; Maslov, V.I. Development of technology to process the flotation gold concentrate by soda smelting. Tsvet. Met. 1999, 10, 29–31. [Google Scholar]
- Kozhakhmetov, S.M.; Kvyatkovsky, S.A.; Ospanov, E.A.; Semenova, A.S. Extraction of gold into collector iron-copper alloys under conditions of reduction smelting of gold ores resistant to opening. Tsvet. Met. 2017, 8, 39–42. [Google Scholar] [CrossRef]
- Kvyatkovskiy, S.A.; Kozhakhmetov, S.M.; Ospanov, Y.A.; Semenova, A.S. Pyrometallurgical opening of refractory carbonaceous and arsenic gold ledge ores with noble metal recovery to matte. Tsvet. Met. 2017, 9, 53–58. [Google Scholar] [CrossRef]
- Khrapunov, V.Y.; Chelokhsayev, L.S.; Issakova, R.A. Compaction of sulfide arsenic-containing wastes. Kompleks. Ispolz. Mineral. Syra. 2000, 3, 84–88. [Google Scholar]
- Tkachenko, O.B.; Ganzha, T.I.; Morozova, T.P. Processing of gold-arsenic-coal concentrates. Tsvet. Metall. 1972, 10, 20–23. [Google Scholar]
- Isakova, R.A.; Lodeyshchikov, V.V.; Chelokhsayev, L.S. Gold recovery from cinders of evacuation of sulfide arsenic-containing concentrates. Kompleks. Ispolz. Mineral. Syra. 1989, 7, 39–42. [Google Scholar]
- Khrapunov, V.Y.; Kenzhaliyev, B.K.; Bolotova, L.S.; Isakova, R.A. Gold recovery from refractory arsenic-carbon-antimony-containing concentrates. Tsvet. Met. 2001, 11, 33–36. [Google Scholar]
- Chelokhsayev, L.S.; Kozhakhmetov, S.M.; Lebedev, N.I.; Isakova, R.A.; Khrapunov, V.E. Pyrometallurgical method to recover gold from cinders from vacuum removal of arsenic from concentrate. Kompleks. Ispolz. Mineral. Syra. 1991, 7, 60–62. [Google Scholar]
- Khrapunov, V.Y.; Spivak, M.M.; Spitsyn, V.A.; Isakova, R.A. On the thermal behavior of arsenopyrite. J. Inorg. Chem. 1991, 36, 2786–2790. [Google Scholar]
- Khrapunov, V.Y.; Chelokhsayev, L.S.; Isakova, R.A. Composition and purification of exhaust gases of the process of vacuum-thermal processing of gold-arsenic concentrates. Tsvet. Met. 1992, 12, 53–55. [Google Scholar]
- Khrapunov, V.Y.; Isakova, R.A.; Fudulov, I.O. Laboratory study of vacuum-thermal processing of gold-arsenic concentrate of the People’s Republic of China. Kompleks. Ispolz. Mineral. Syra. 1991, 11, 55–59. [Google Scholar]
- Khrapunov, V.Y. Vacuum thermal sublimation of arsenic from gold arsenic-, antimony-, copper-containing concentrates. Ind. Kazakhstan 2001, 6, 82–83. [Google Scholar]
- Khrapunov, V.Y.; Isakova, R.A.; Chelokhsayev, L.S. About vacuum processing of gold-arsenic concentrates of Yakutia. Tsvet. Met. 1993, 4, 9–12. [Google Scholar]
- Khrapunov, V.Y.; Isakova, R.A.; Chelokhsayev, L.S. Isolation of arsenic from gold-bearing concentrates of Central Asia by vacuum thermal sublimation. Kompleks. Ispolz. Mineral. Syra. 1992, 12, 26–28. [Google Scholar]
- Volodin, V.N.; Trebukhov, S.A.; Nitsenko, A.V.; Trebukhov, S.A.; Tuleutay, F.K. Some problems of processing dispersed arsenic-coating raw materials by sublimation in vacuum. J. Phys. Conf. Ser. 2021, 2059, 012026. [Google Scholar] [CrossRef]
- Miroshnikova, A.; Frolova, O.; Mataibayeva, I.; Rafailovich, M. Geochemical and Mineralogical Characteristics of the Giant Bakyrchik Gold Deposit, East Kazakhstan. In Proceedings of the 12th Biennial SGA Meeting on Mineral Deposit Research for a High-Tech World, Uppsala, Sweden, 12–15 August 2013; Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000337983900055 (accessed on 24 November 2022).
- Junussov, M.; Umarbekova, Z. Mineralogical and Morphological Studies of Gold-Bearing Arsenopyrite and Pyrite Minerals of Bakyrchik and Bolshevik Gold Black Shale Deposits (Eastern Kazakhstan). Contemp. Trends Geosci. 2018, 7, 153–165. [Google Scholar] [CrossRef]
- Kanayeva, Z.K.; Kanayeva, A.T.; Semenchenko, G.V. Geological structure of the gold-arsenic deposit Bakyrchik of East Kazakhstan. Fund. Res. 2014, 11, 2405–2410. [Google Scholar]



| Concentrates | Composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au, g/t | Ag, g/t | wt. % | ||||||||||
| As | Fe | S | C | Cu | Pb | Zn | SiO2 | Al2O3 | CaO | |||
| Gravitational I | 230 | 20 | 21.4 | 35.8 | 32.0 | 0.6 | 0.05 | 0.24 | 0.06 | 4.80 | 2.38 | 1.12 |
| Gravitational II | 230 | 21 | 26.3 | 37.0 | 28.2 | 0,7 | 0,04 | 0.20 | 0.06 | 1.35 | 0.77 | 0.82 |
| Flotation | 81 | 59 | 14.1 | 22.3 | 20.1 | 9.2 | 0.19 | 1.0 | 0.80 | 16.8 | 4.89 | 1.65 |
| Products | Quantity | As | S | Fe | ||||
|---|---|---|---|---|---|---|---|---|
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Gravity concentrate I | ||||||||
| Loaded: | ||||||||
| Gravitational concentrate I | 26.13 | 100 | 21.40 | 100 | 32.00 | 100 | 35.80 | 100 |
| Residue | 15.46 | 59.17 | 0.26 | 0.72 | 32.81 | 60.66 | 57.68 | 95.33 |
| Condensate | 9.55 | 36.55 | 57.90 | 98.88 | 29.78 | 34.01 | 0.04 | 0.04 |
| Loss + discrepancy | −1.12 | −4.28 | – | −0.40 | – | −5.33 | – | −4.63 |
| Gravity concentrate II | ||||||||
| Loaded: | ||||||||
| Gravitational concentrate II | 24.65 | 100 | 26.30 | 100 | 28.20 | 100 | 37.00 | 100 |
| Residue | 14.85 | 60.24 | 0.21 | 0.48 | 33.17 | 70.86 | 57.79 | 94.10 |
| Condensate | 8.41 | 34.12 | 76.59 | 99.36 | 19.96 | 24.15 | 0.03 | 0.03 |
| Loss + discrepancy | −1.39 | −5.64 | – | −0.16 | – | −4.99 | – | −5.87 |
| Flotation concentrate | ||||||||
| Loaded: | ||||||||
| Flotation concentrate | 22.41 | 100 | 14.10 | 100 | 20.10 | 100 | 22.30 | 100 |
| Received: | ||||||||
| Residue | 16.23 | 72.42 | 0.12 | 0.62 | 14.66 | 52.82 | 29.32 | 95.22 |
| Condensate | 5.02 | 22.40 | 61.30 | 97.39 | 37.71 | 42.03 | 0.02 | 0.02 |
| Loss + discrepancy | −1.16 | −5.18 | – | −1.99 | – | −5.15 | – | −4.76 |
| Products | Quantity | Cu | Au | Ag | ||||
|---|---|---|---|---|---|---|---|---|
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Residue | 51.40 | 71.38 | 0.07 | 1.48 | 3.90 × 10−2 | 99.85 | 3.40 × 10−3 | 45.90 |
| Copper concentrate | 10.30 | 14.31 | 23.30 | 98.52 | 3.00 × 10−4 | 0.15 | 2.00 × 10−2 | 54.10 |
| Limestone | 10.30 | 14.31 | – | – | – | – | – | – |
| Total | 72.00 | 100.00 | – | 100.00 | – | 100.00 | – | 100.00 |
| Received: | ||||||||
| Matte | 16.8 | 23.33 | 13.01 | 89.73 | 1.12 × 10−1 | 93.72 | 1.49 × 10−2 | 65.74 |
| Slag | 34.9 | 48.47 | 0.17 | 2.44 | 7.36 × 10−4 | 1.28 | 3.20 × 10−4 | 2.93 |
| Chamber dust | 4.30 | 5.97 | 1.82 | 3.21 | 1.97 × 10−2 | 4.22 | 1.46 × 10−2 | 16.49 |
| Cyclone dust | 1.00 | 1.40 | 2.66 | 1.09 | 1.54 × 10−2 | 0.77 | 1.34 × 10−2 | 3.52 |
| Bag filter dust | 8.10 | 11.25 | 1.13 | 3.76 | 1.13 × 10−2 | 4.56 | 1.17 × 10−2 | 24.89 |
| Total | 65.10 | 90.42 | – | 100.23 | – | 104.55 | – | 113.57 |
| Loss + discrepancy | −6.90 | −9.58 | – | +0.23 | – | +4.55 | – | +13.57 |
| Products | Quantity | Cu | Au | Ag | ||||
|---|---|---|---|---|---|---|---|---|
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Residue | 62.70 | 66.57 | 0.26 | 3.58 | 1.12 × 10−2 | 100.00 | 8.14 × 10−3 | 50.51 |
| Copper concentrate | 12.50 | 13.33 | 35.10 | 96.42 | – | – | 4.00 × 10−2 | 49.49 |
| Limestone | 18.80 | 20.00 | – | – | – | – | – | – |
| Total | 94.00 | 100.00 | – | 100.00 | – | 100.00 | – | 100.00 |
| Received: | ||||||||
| Matte | 14.40 | 15.32 | 29.64 | 93.79 | 4.58 × 10−2 | 93.92 | 4.77 × 10−2 | 67.98 |
| Slag | 56.10 | 59.68 | 0.24 | 2.96 | 3.00 × 10−4 | 2.40 | 6.14 × 10−4 | 3.41 |
| Chamber dust | 1.00 | 1.06 | 2.10 | 0.46 | 2.38 × 10−3 | 0.34 | 1.47 × 10−2 | 1.45 |
| Cyclone dust | 1.10 | 1.17 | 4.10 | 0.99 | 4.08 × 10−3 | 0.64 | 1.85 × 10−2 | 2.01 |
| Bag filter dust | 7.30 | 7.77 | 2.37 | 3.80 | 2.32 × 10−3 | 2.41 | 2.38 × 10−2 | 17.20 |
| Total | 79.90 | 85.00 | – | 102.00 | – | 99.71 | – | 92.05 |
| Loss + discrepancy | −14.10 | −15.00 | – | +2.00 | – | −0.29 | – | −7.95 |
| Elements and Compounds | Cu | Pb | Zn | Fe | S | SiO2 | CaO | Al2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content, wt. % | 33.12 | 2.41 | 1.75 | 6.75 | 15.6 | 23.0 | 1.76 | 4.71 | 10.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volodin, V.; Trebukhov, S.; Nitsenko, A.; Linnik, X.; Tuleutay, F.; Trebukhov, A.; Ruzakhunova, G. Pyrometallurgical Scheme Intended to Process Arsenic-Containing Concentrates with Recovery of Precious Metals. Metals 2023, 13, 540. https://doi.org/10.3390/met13030540
Volodin V, Trebukhov S, Nitsenko A, Linnik X, Tuleutay F, Trebukhov A, Ruzakhunova G. Pyrometallurgical Scheme Intended to Process Arsenic-Containing Concentrates with Recovery of Precious Metals. Metals. 2023; 13(3):540. https://doi.org/10.3390/met13030540
Chicago/Turabian StyleVolodin, Valeriy, Sergey Trebukhov, Alina Nitsenko, Xeniya Linnik, Farkhat Tuleutay, Alexey Trebukhov, and Galiya Ruzakhunova. 2023. "Pyrometallurgical Scheme Intended to Process Arsenic-Containing Concentrates with Recovery of Precious Metals" Metals 13, no. 3: 540. https://doi.org/10.3390/met13030540
APA StyleVolodin, V., Trebukhov, S., Nitsenko, A., Linnik, X., Tuleutay, F., Trebukhov, A., & Ruzakhunova, G. (2023). Pyrometallurgical Scheme Intended to Process Arsenic-Containing Concentrates with Recovery of Precious Metals. Metals, 13(3), 540. https://doi.org/10.3390/met13030540





