Spherical CdS Nanoparticles Precipitated from a Cadmium Thiosulfate Complex Using Ultraviolet Light for Photocatalytic Dye Degradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Procedure
2.3. Analytical Methods
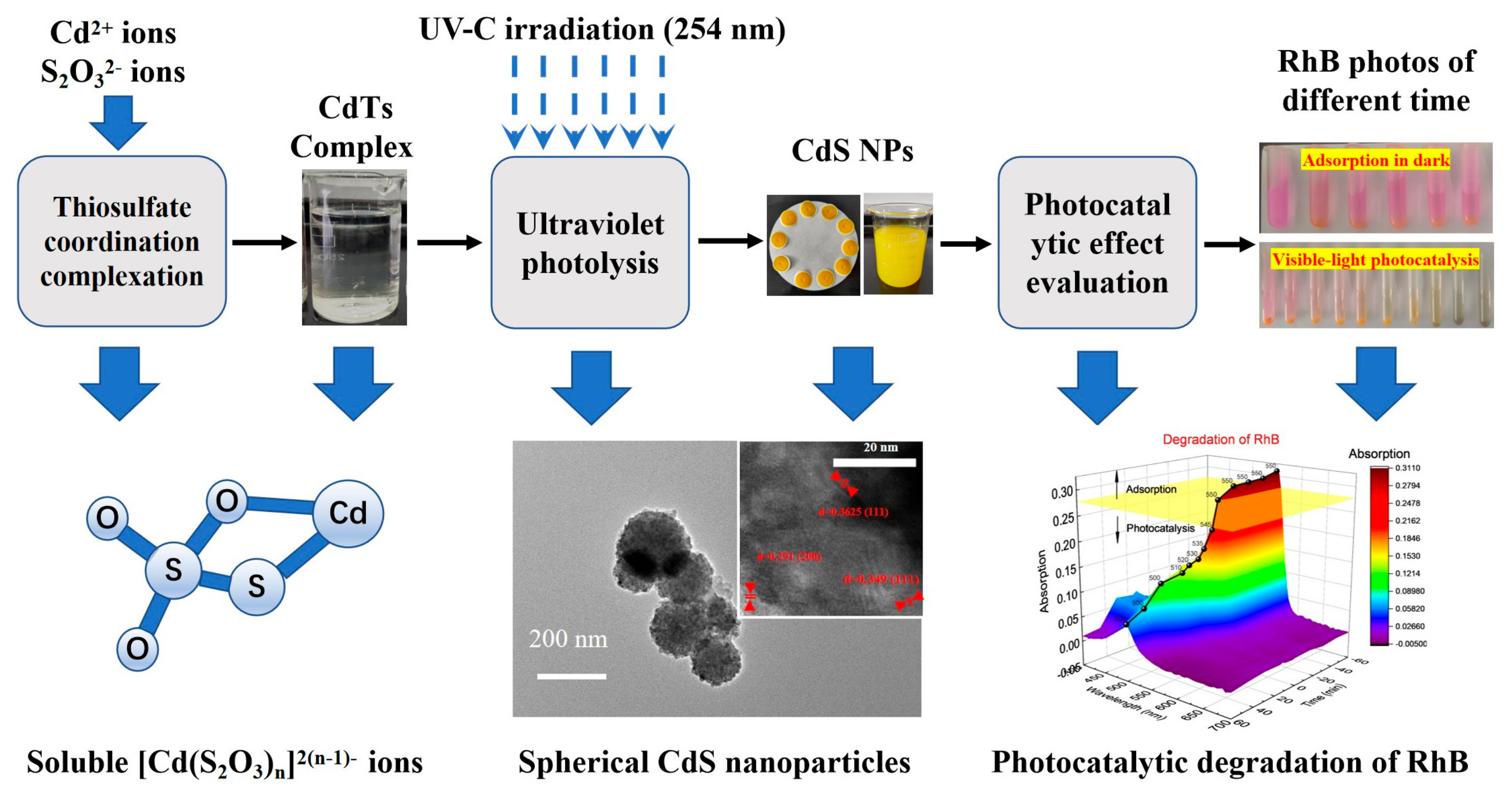
3. Results
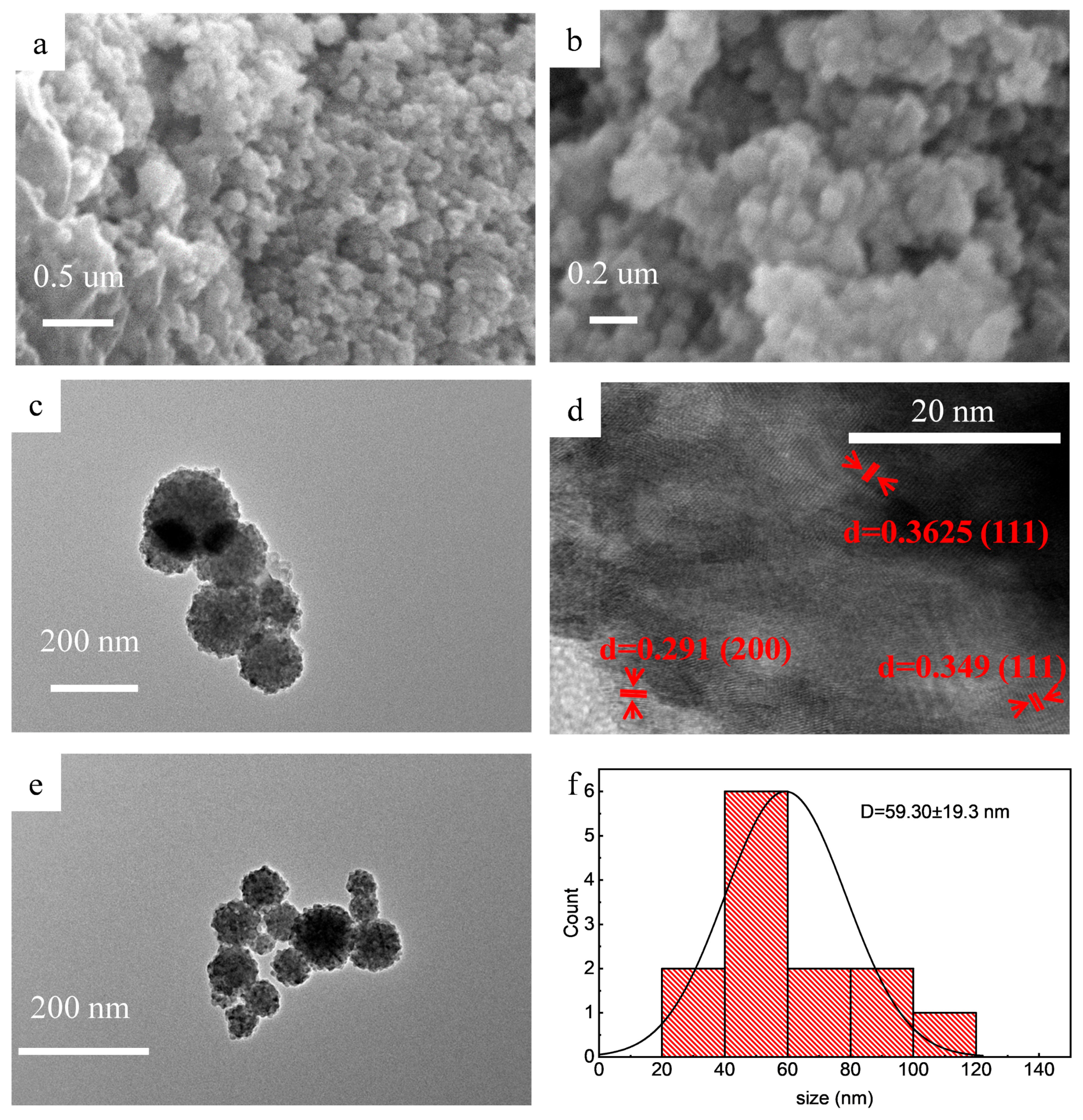
3.1. Characterization of UV-Decomposition Products
3.2. Photocatalytic Activity of the Spherical CdS NPs
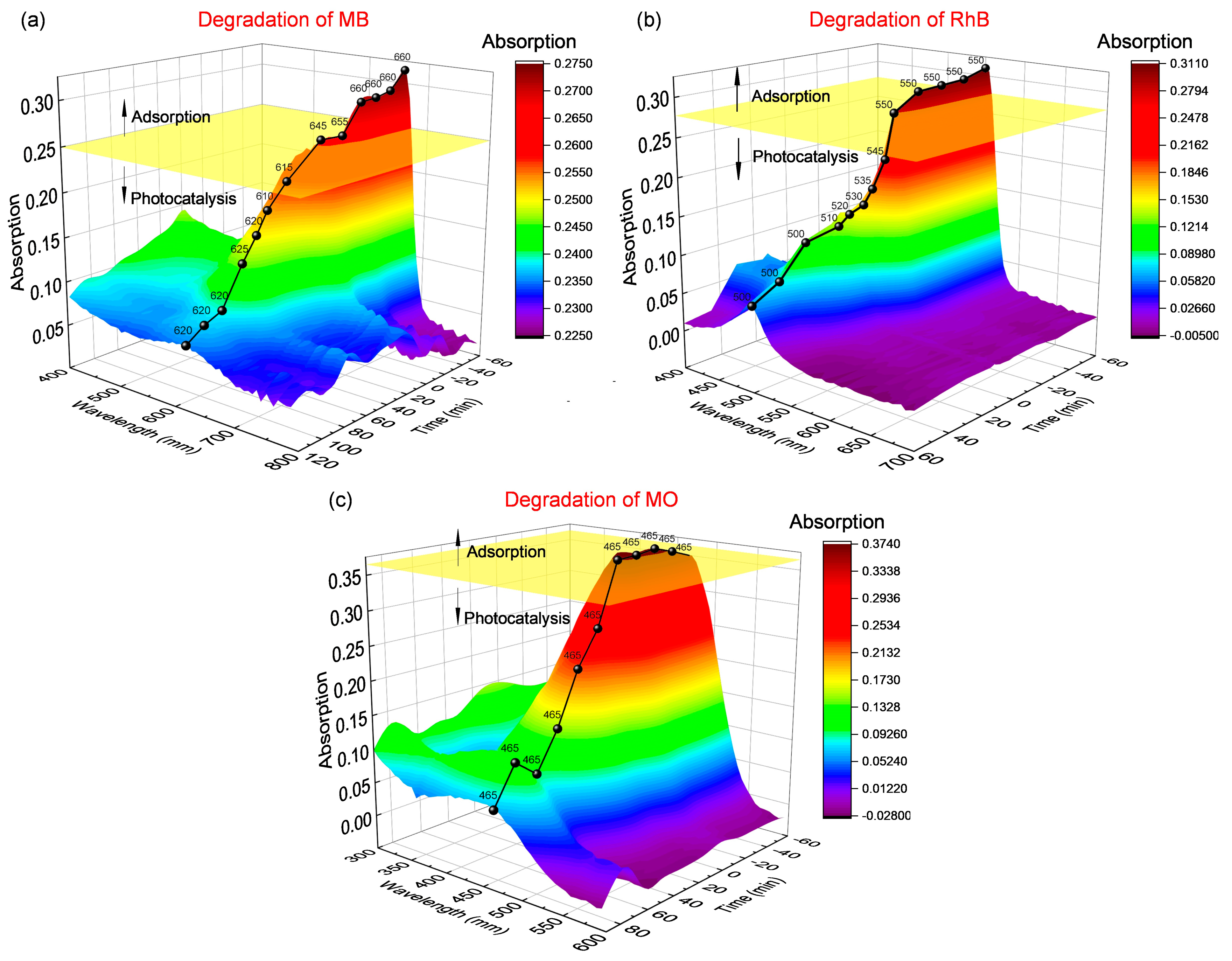
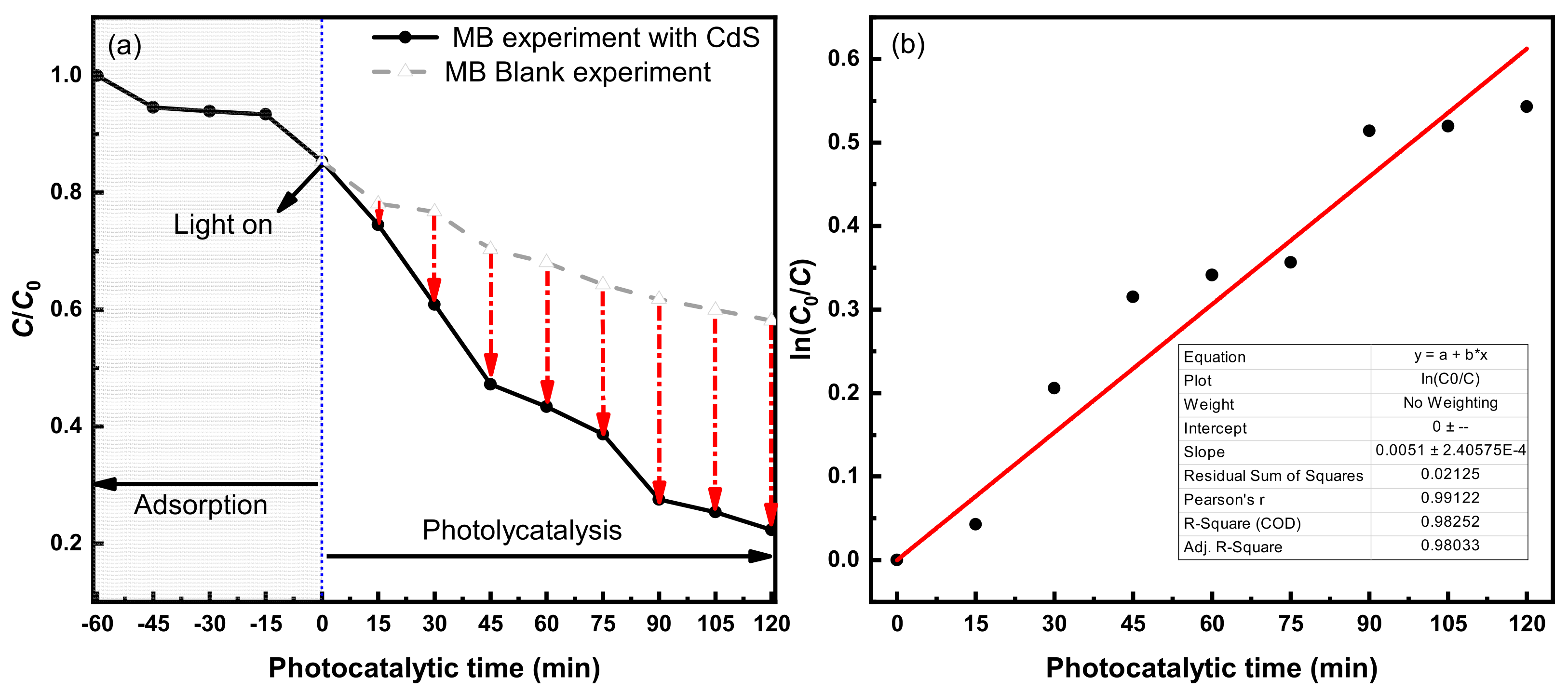
3.2.1. Photocatalytic Degradation Effect of MB
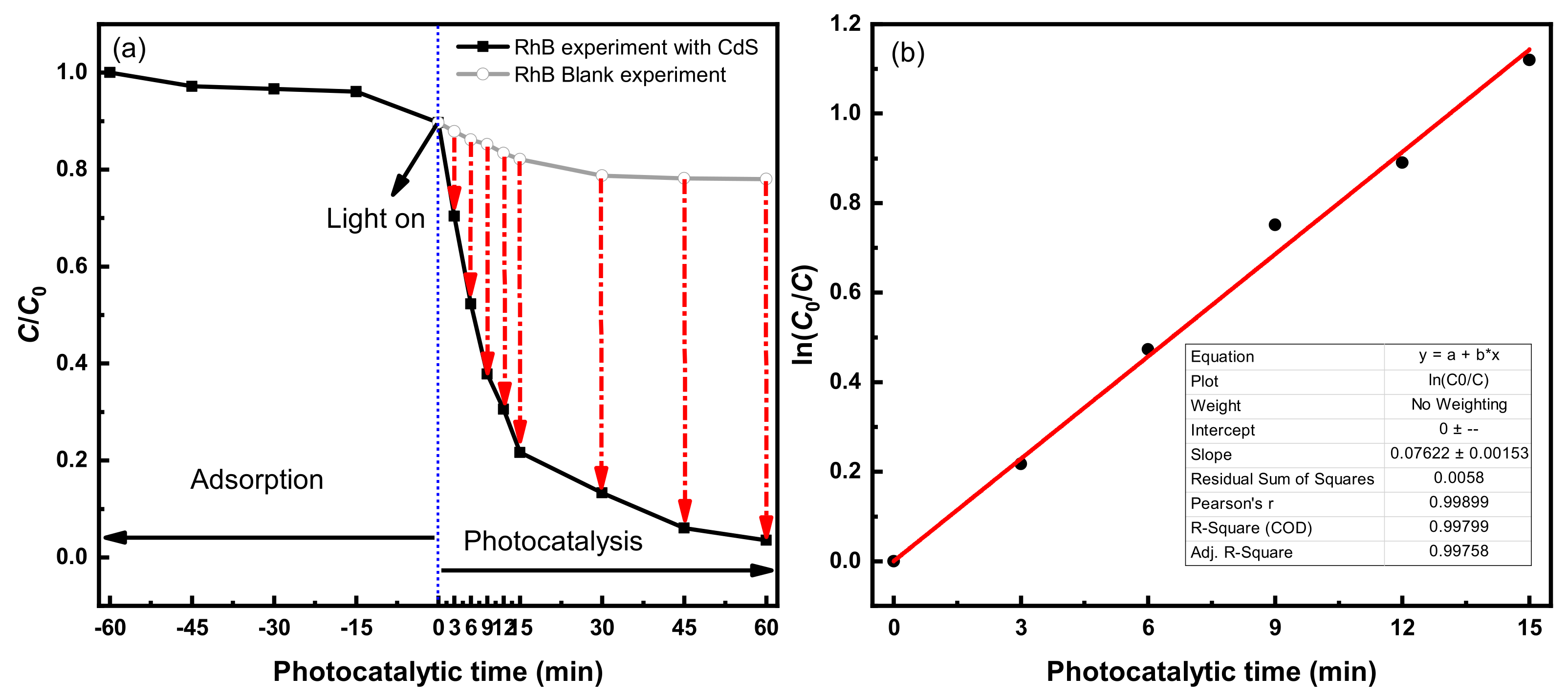
3.2.2. Photocatalytic Degradation Effect of RhB
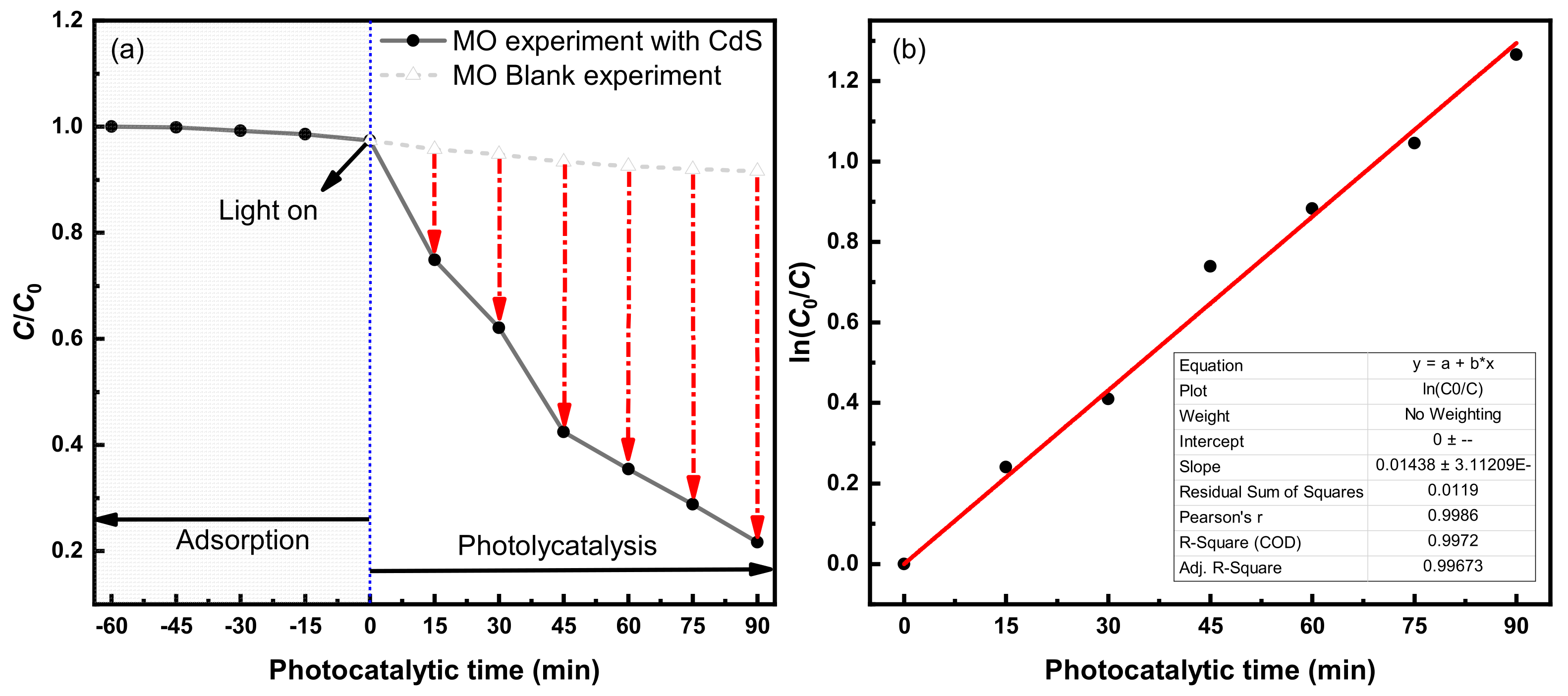
3.2.3. Photocatalytic Degradation Effect of MO
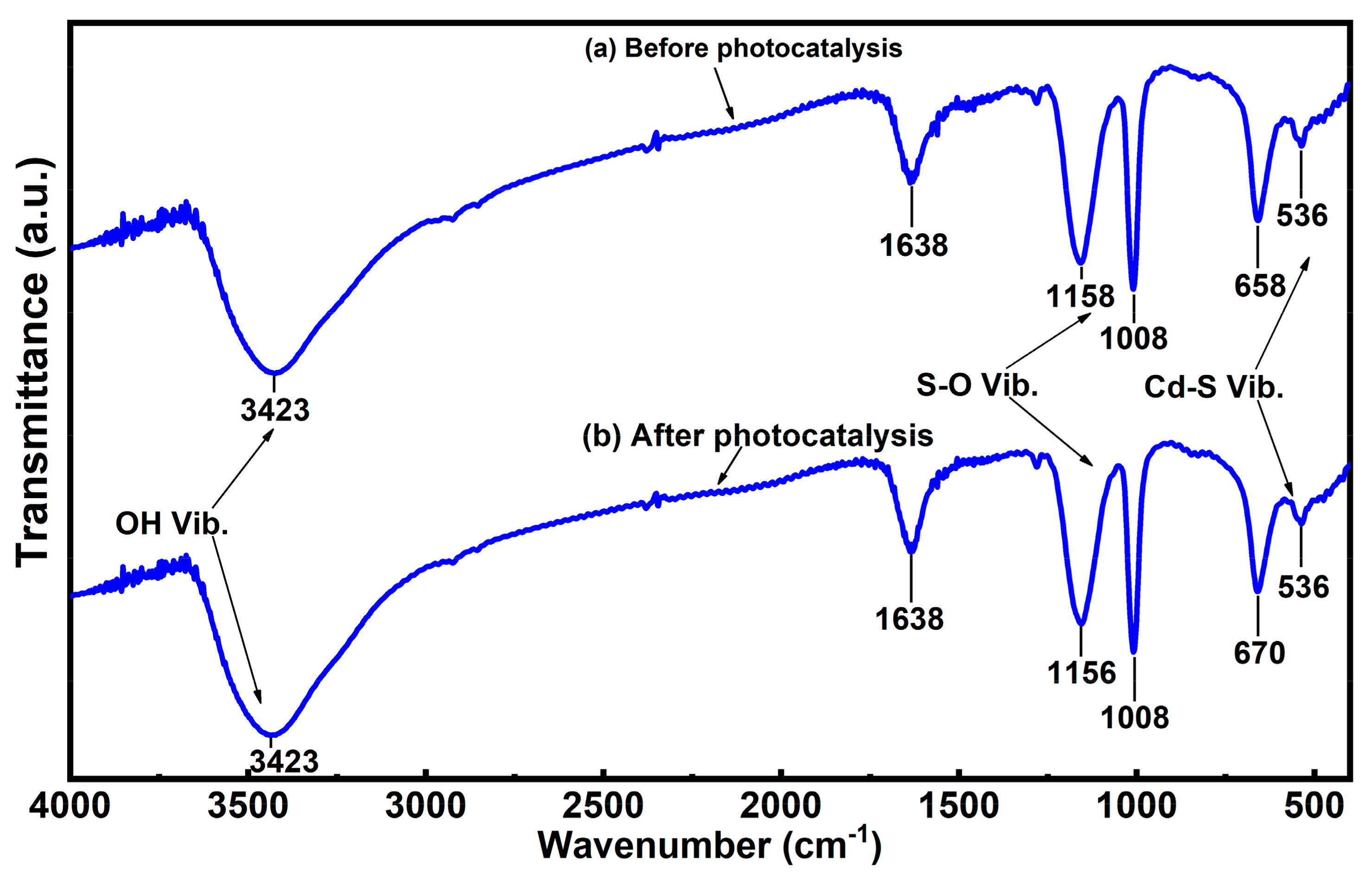
3.3. Photocatalyst Stability Experiments
4. Discussion
4.1. Photocatalytic Degradation Kinetics of the Spherical CdS NPs
4.1.1. Photocatalytic Degradation Rate of MB
4.1.2. Photocatalytic Degradation Rate of RhB
4.1.3. Photocatalytic Degradation Rate of MO
4.2. The Degradation Mechanism Study of CdS NPs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Yamagami, T.; Ezaki, T.; Moriguchi, J.; Fukui, Y.; Okamoto, S.; Ukai, H.; Sakurai, H.; Aoshima, K.; Ikeda, M. Low-level cadmium exposure in Toyama City and its surroundings in Toyama prefecture, Japan, with references to possible contribution of shellfish intake to increase urinary cadmium levels. Sci. Total Environ. 2006, 362, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, F.; Wang, W.; Bai, Y.L.; Fu, Y.; Dreisinger, D. Eco-friendly leaching of gold from a carbonaceous gold concentrate in copper-citrate-thiosulfate solutions. Hydrometallurgy 2020, 191, 105204. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Dong, Z.l.; Yang, Y.b.; Li, Q.; Liu, X.l.; Jiang, T. Eco-friendly and economical gold extraction by nickel catalyzed ammoniacal thiosulfate leaching-resin adsorption recovery. J. Clean. Prod. 2019, 233, 1475–1485. [Google Scholar] [CrossRef]
- Deutsch, J.L.; Dreisinger, D.B. Silver sulfide leaching with thiosulfate in the presence of additives Part I: Copper–ammonia leaching. Hydrometallurgy 2013, 137, 156–164. [Google Scholar] [CrossRef]
- Han, C.; Wang, W.; Xie, F.; Zhang, T.A. Mechanism and kinetics of mercuric sulfide leaching with cuprous-thiosulfate solutions. Sep. Purif. Technol. 2017, 177, 223–232. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.N.; Wang, J.; Wang, W. Review of gold leaching in thiosulfate-based solutions. T. Nonferr. Metal. Soc. 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Petter, P.M.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Murali, A.; Zhang, Z.; Shine, A.E.; Free, M.L.; Sarswat, P.K. E-wastes derived sustainable Cu recovery using solvent extraction and electrowinning followed by thiosulfate-based gold and silver extraction. J. Hazard. Mater. 2022, 8, 100196. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, Y.B.; Li, Q. Recovery of Gold from Pregnant Thiosulfate Solutions by the Resin Adsorption Technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, Y.B.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Han, C.; Wang, G.X.; Zou, M.J.; Shi, C.Y. Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals 2019, 9, 1178. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Han, C.; Xie, F. Efficient mercury recovery from mercuric-thiosulfate solutions by ultraviolet photolysis. Environ. Chem. Lett. 2018, 16, 1049–1054. [Google Scholar] [CrossRef]
- Egorov, N.B.; Eremin, L.P.; Usov, V.F.; Larionov, A.M. Preparation of lead sulfide nanoparticles in the photolysis of aqueous solutions of lead thiosulfate complex. High Energ. Chem. 2007, 41, 251–254. [Google Scholar] [CrossRef]
- Han, C.; Wang, G.X.; Cheng, C.; Shi, C.Y.; Yang, Y.C.; Zou, M.J. A kinetic and mechanism study of silver-thiosulfate complex photolysis by UV-C irradiation. Hydrometallurgy 2020, 191, 105212. [Google Scholar] [CrossRef]
- Egorov, N.B.; Eremin, L.P.; Larionov, A.M.; Usov, V.F.; Tsepenko, E.A.; D’yachenko, A.S. Products of photolysis of cadmium thiosulfate aqueous solutions. High Energ. Chem. 2011, 42, 119–122. [Google Scholar] [CrossRef]
- Iqbal, T.; Ali, F.; Khalid, N.R.; Tahir, M.B.; Ijaz, M. Facile synthesis and antimicrobial activity of CdS-Ag2S nanocomposites. Bioorg. Chem. 2019, 90, 103064. [Google Scholar] [CrossRef]
- Jafari, L.; Pourahmad, A.; Asadpour, L. Rice husk based MCM-41 nanoparticles loaded with Ag2S nanostructures by a green and room temperature method and its antimicrobial property. Inorg. Nano-Met. Chem. 2017, 47, 1552–1559. [Google Scholar] [CrossRef]
- Devi, R.A.; Latha, M.; Velumani, S.; Oza, G.; Reyes-Figueroa, P.; Rohini, M.; Becerril-Juarez, I.G.; Lee, J.H.; Yi, J. Synthesis and Characterization of Cadmium Sulfide Nanoparticles by Chemical Precipitation Method. J. Nanosci. Nanotechnol. 2015, 15, 8434–8439. [Google Scholar] [CrossRef]
- Bie, C.; Fu, J.; Cheng, B.; Zhang, L. Ultrathin CdS nanosheets with tunable thickness and efficient photocatalytic hydrogen generation. Appl. Surf. Sci. 2018, 462, 606–614. [Google Scholar] [CrossRef]
- Xia, Z.L.; Yu, R.; Yang, H.; Luo, B.F.; Huang, Y.Y.; Li, D.; Shi, J.Y.; Xu, D.B. Novel 2D Zn-porphyrin metal organic frameworks revived CdS for photocatalysis of hydrogen production. Int. J. Hydrogen Energy 2022, 47, 13340–13350. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Guo, F.Q.; Guo, R.Q.; Bai, H.N.; Zhang, B.H.; Li, X.; Qian, Y.; Liu, X.B. Facile Synthesis of Hexagonal CdS Nanofilm on FTO Glass Substrate via Hydrothermal Method. Int. J. Electrochem. Sci. 2020, 15, 10129–10140. [Google Scholar] [CrossRef]
- Hernandez-Gordillo, A.; Oros-Ruiz, S.; Gomez, R. Preparation of efficient cadmium sulfide nanofibers for hydrogen production using ethylenediamine (NH2CH2CH2NH2) as template. J. Colloid Interface Sci. 2015, 451, 40–45. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, B.; Xu, F.; Zhang, L.; Yu, J. In Situ Grown Monolayer N-Doped Graphene on CdS Hollow Spheres with Seamless Contact for Photocatalytic CO(2) Reduction. Adv. Mater. 2019, 31, e1902868. [Google Scholar] [CrossRef]
- He, B.W.; Bie, C.B.; Fei, X.G.; Cheng, B.; Yu, J.G.; Ho, W.K.; Al-Ghamdi, A.A.; Wageh, S. Enhancement in the photocatalytic H2 production activity of CdS NRs by Ag2S and NiS dual cocatalysts. Appl. Catal. B-Environ. 2021, 288, 119994. [Google Scholar]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.H.; Zhang, P.; Li, X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, J.l.; Jiang, H.; Liu, D.c.; Zhang, L. CdS/Ag2S nanocomposites photocatalyst with enhanced visible light photocatalysis activity. Solid State Sci. 2019, 98, 106020. [Google Scholar] [CrossRef]
- Oghabi, H.; Haghshenas, D.F.; Firoozi, S. Selective separation of Cd from spent Ni-Cd battery using glycine as an eco-friendly leachant and its recovery as CdS nanoparticles. Sep. Purif. Technol. 2020, 242, 116832. [Google Scholar] [CrossRef]
- Maleki, M.; Haghighi, M. Sono-dispersion of CuS-CdS over TiO 2 in one-pot hydrothermal reactor as visible-light-driven nanostructured photocatalyst. J. Mol. Catal. A-Chem. 2016, 424, 283–296. [Google Scholar] [CrossRef]
- Lu, X.; Yu, Y.; Chen, L.; Mao, H.; Zhang, W.; Wei, Y. Preparation and characterization of polyaniline microwires containing CdS nanoparticles. Chem. Commun. 2004, 13, 1522–1523. [Google Scholar] [CrossRef]
- Banizi, Z.T.; Seifi, M.; Askari, M.B.; Dehaghi, S.B.; Zadeh, M.H.R. Photoluminescence and photocatalytic studies of cadmium sulfide/multiwall carbon nanotube (CdS/MWCNT) nanocomposites. Optik 2018, 158, 882–892. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. ZnO/CdS/CuS heterostructure: A suitable candidate for applications in visible-light photocatalysis. J. Phys. Chem. Solids 2022, 160, 110344. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Free, M.L. Adsorption-coupled reduction mechanism in ZnO-Functionalized MWCNTs nanocomposite for Cr (VI) removal and improved anti-photocorrosion for photocatalytic reduction. J. Alloy. Compd. 2020, 843, 155835. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Han, C.; Sun, P.; Wang, G.; Li, J.; Chang, Q. Spherical CdS Nanoparticles Precipitated from a Cadmium Thiosulfate Complex Using Ultraviolet Light for Photocatalytic Dye Degradation. Metals 2023, 13, 554. https://doi.org/10.3390/met13030554
Liu F, Han C, Sun P, Wang G, Li J, Chang Q. Spherical CdS Nanoparticles Precipitated from a Cadmium Thiosulfate Complex Using Ultraviolet Light for Photocatalytic Dye Degradation. Metals. 2023; 13(3):554. https://doi.org/10.3390/met13030554
Chicago/Turabian StyleLiu, Fengling, Chao Han, Panbo Sun, Guangxin Wang, Jiwen Li, and Qinghua Chang. 2023. "Spherical CdS Nanoparticles Precipitated from a Cadmium Thiosulfate Complex Using Ultraviolet Light for Photocatalytic Dye Degradation" Metals 13, no. 3: 554. https://doi.org/10.3390/met13030554





