Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Scheme
2.3. Detection Method
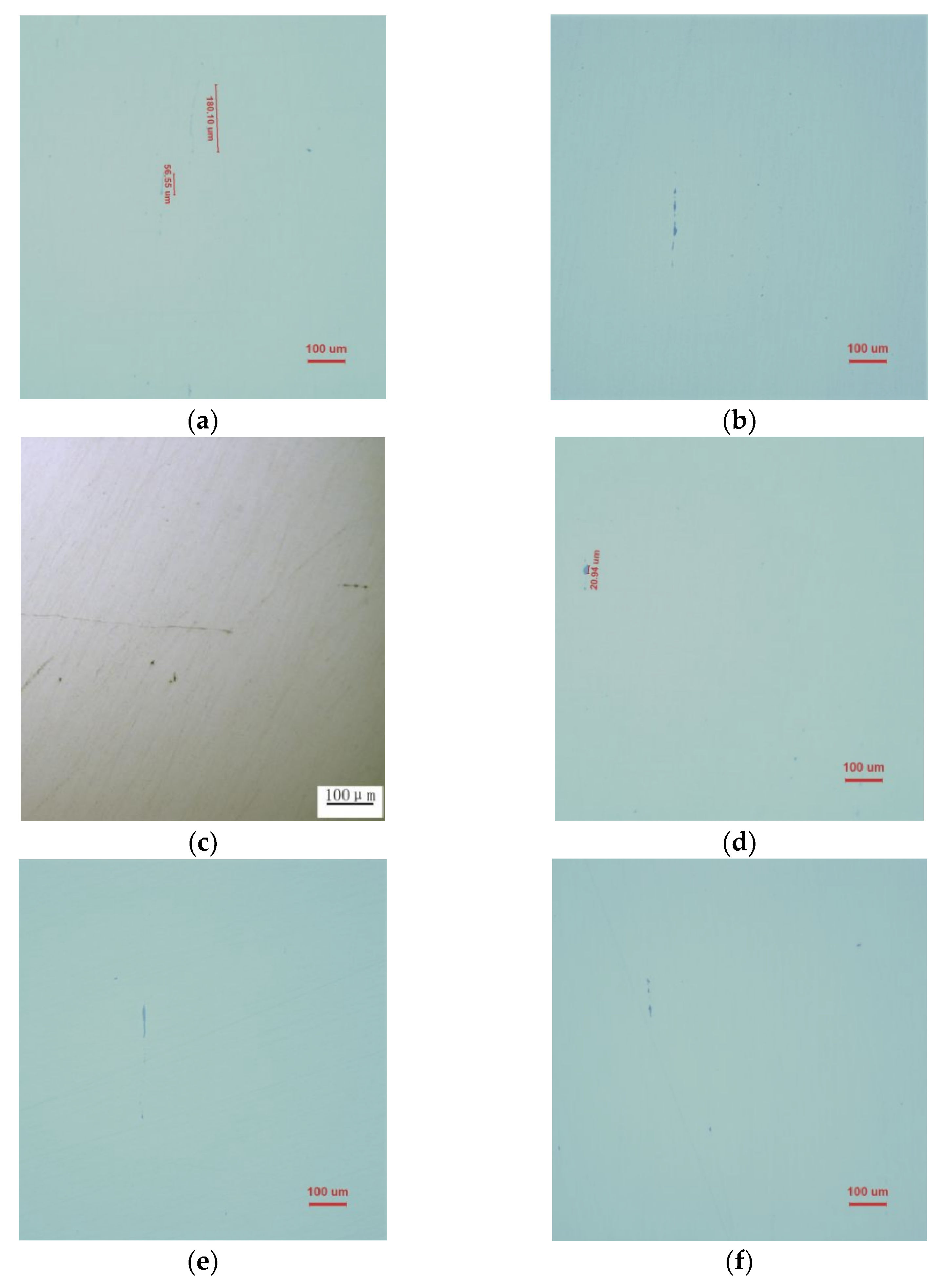
2.3.1. Low-Multiplier Detection of Inclusions in Steel
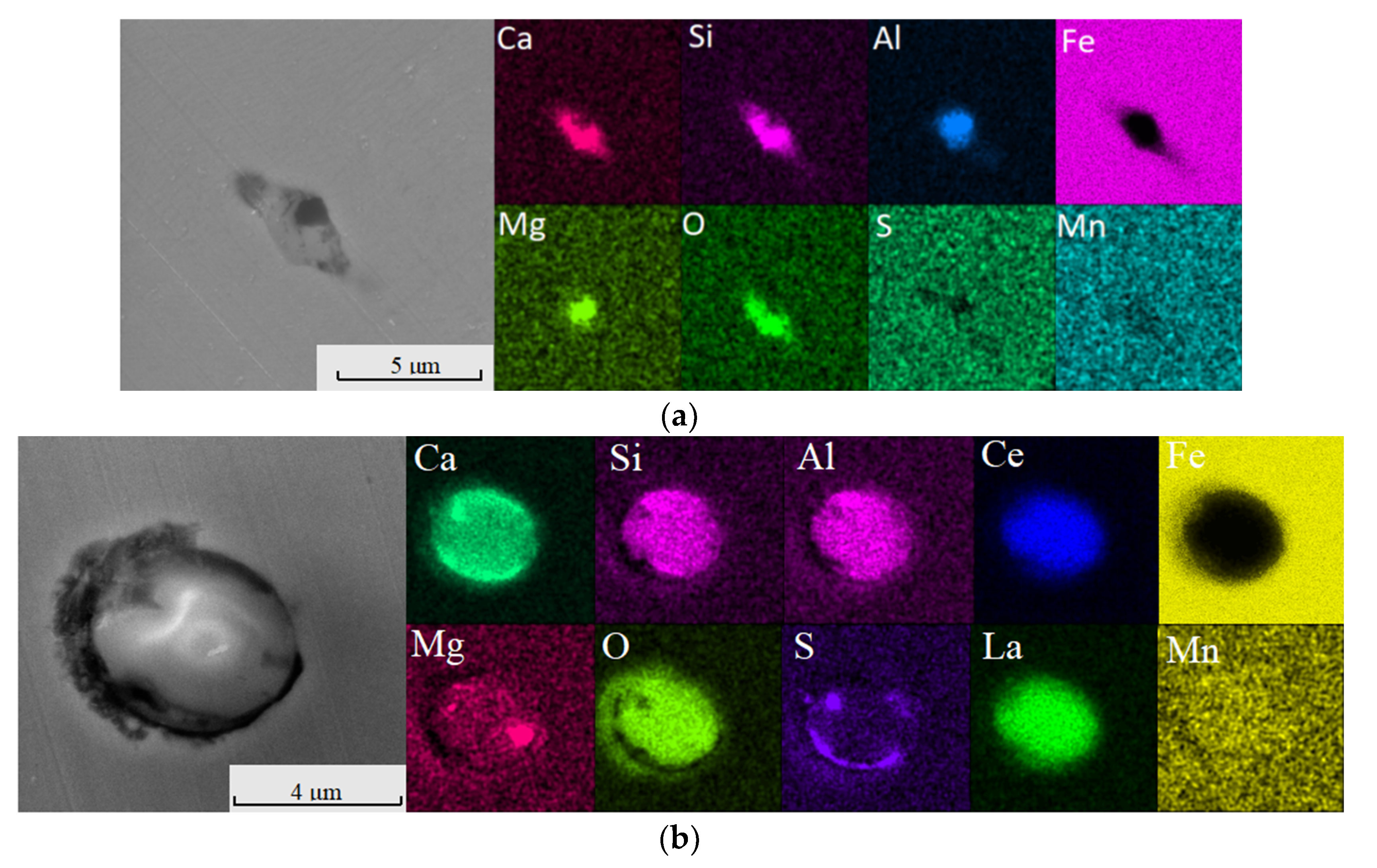
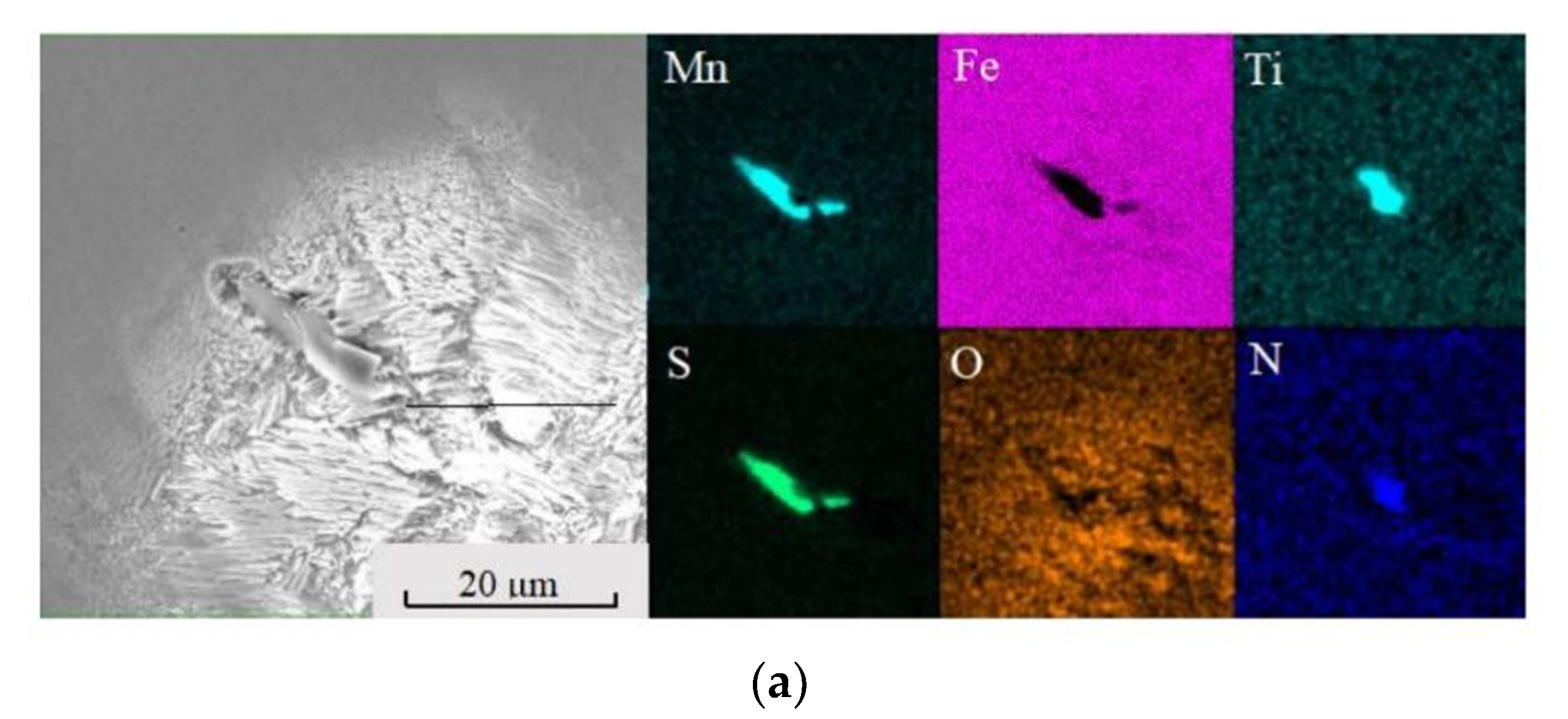
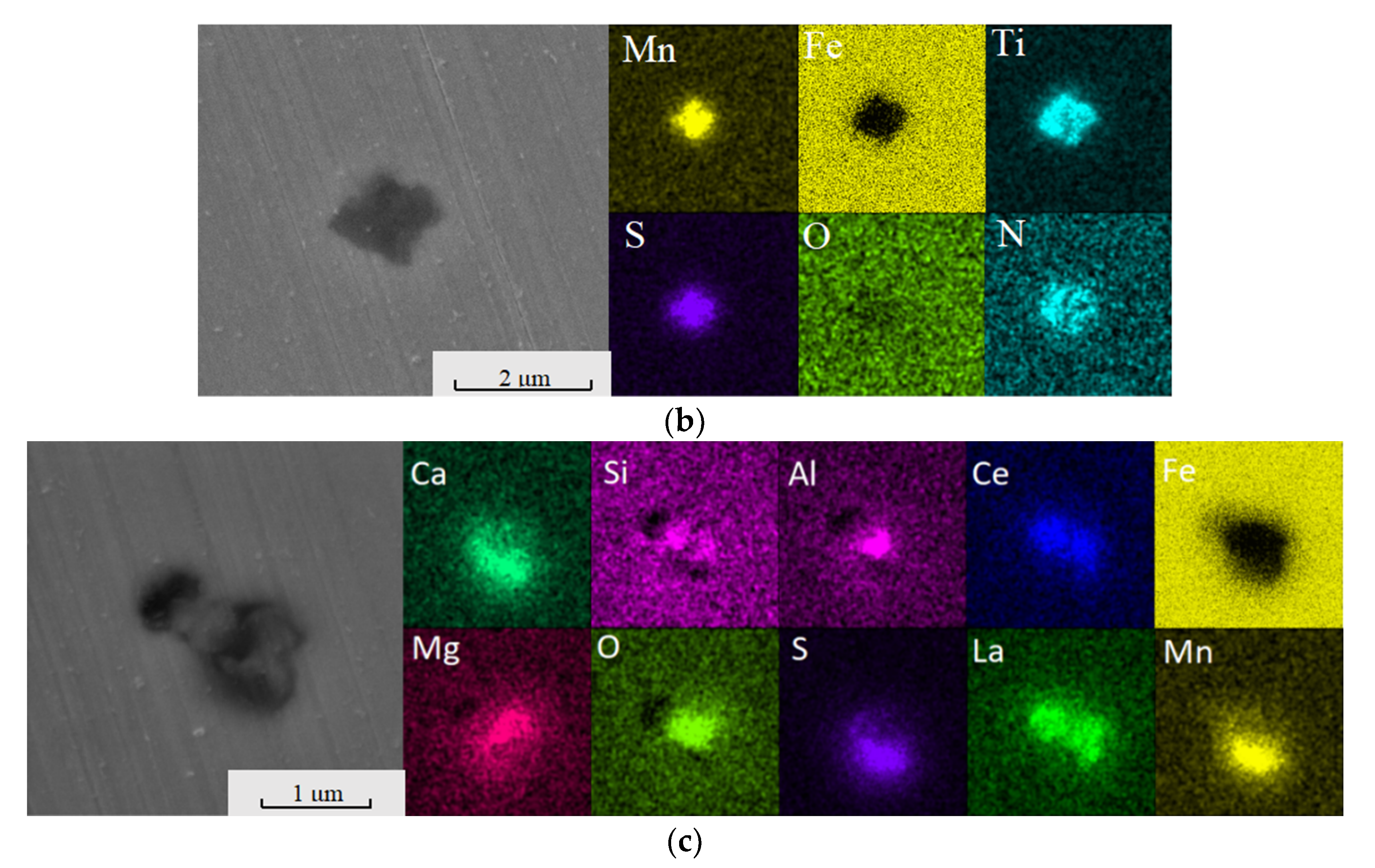
2.3.2. Scanning Electron Microscopy–Energy-Dispersive X-ray Spectroscopy (SEM-EDS) Analysis
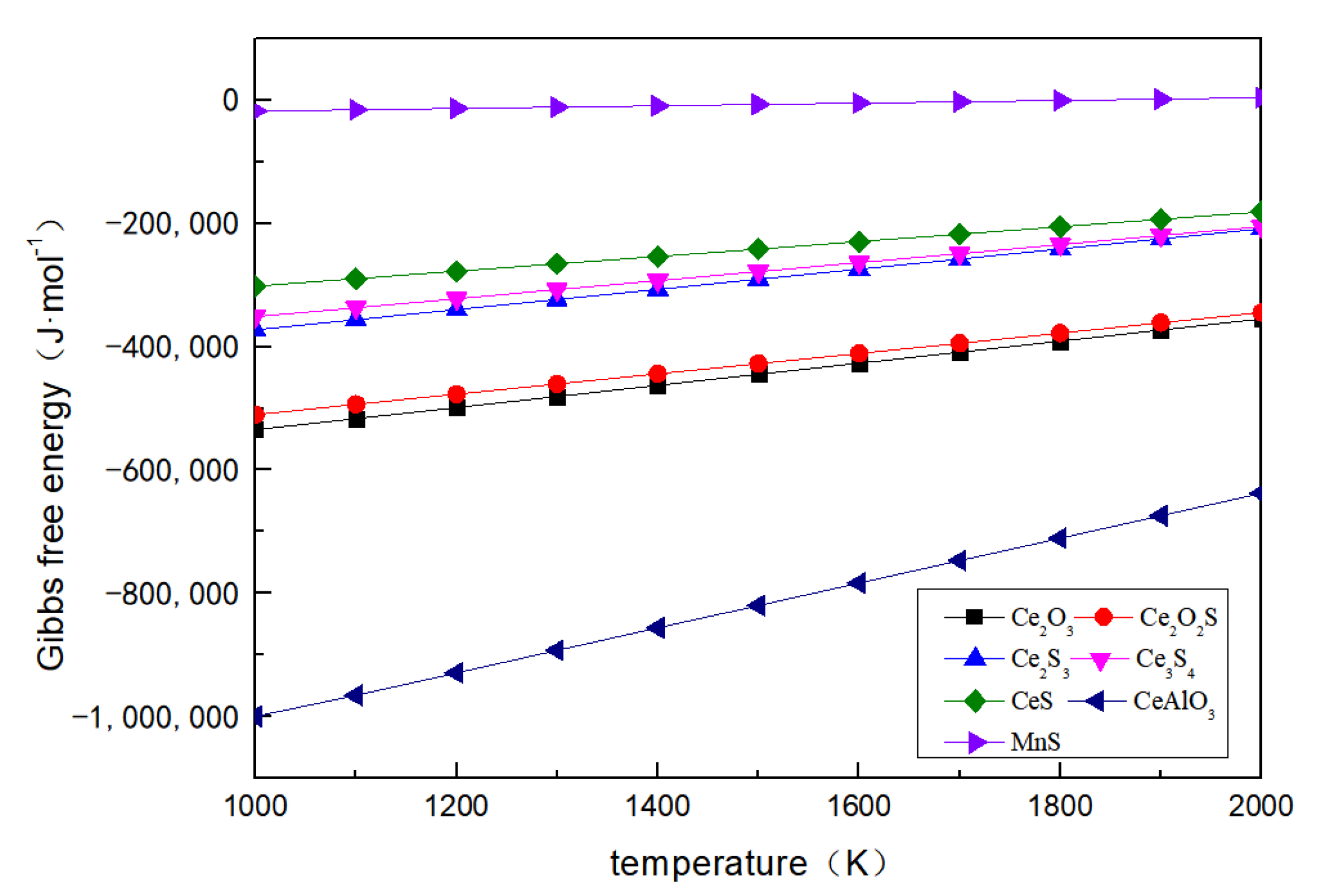
3. Thermodynamic Calculations
4. Results
4.1. Effect of Ce on Inclusions in Heavy Rail Steel
4.2. Effect of Ce in Heavy Rail Steel on Aluminium Inclusion
4.3. Effect of Ce in Sulphide Inclusion in Heavy Rail Steel
5. Conclusions
- (1)
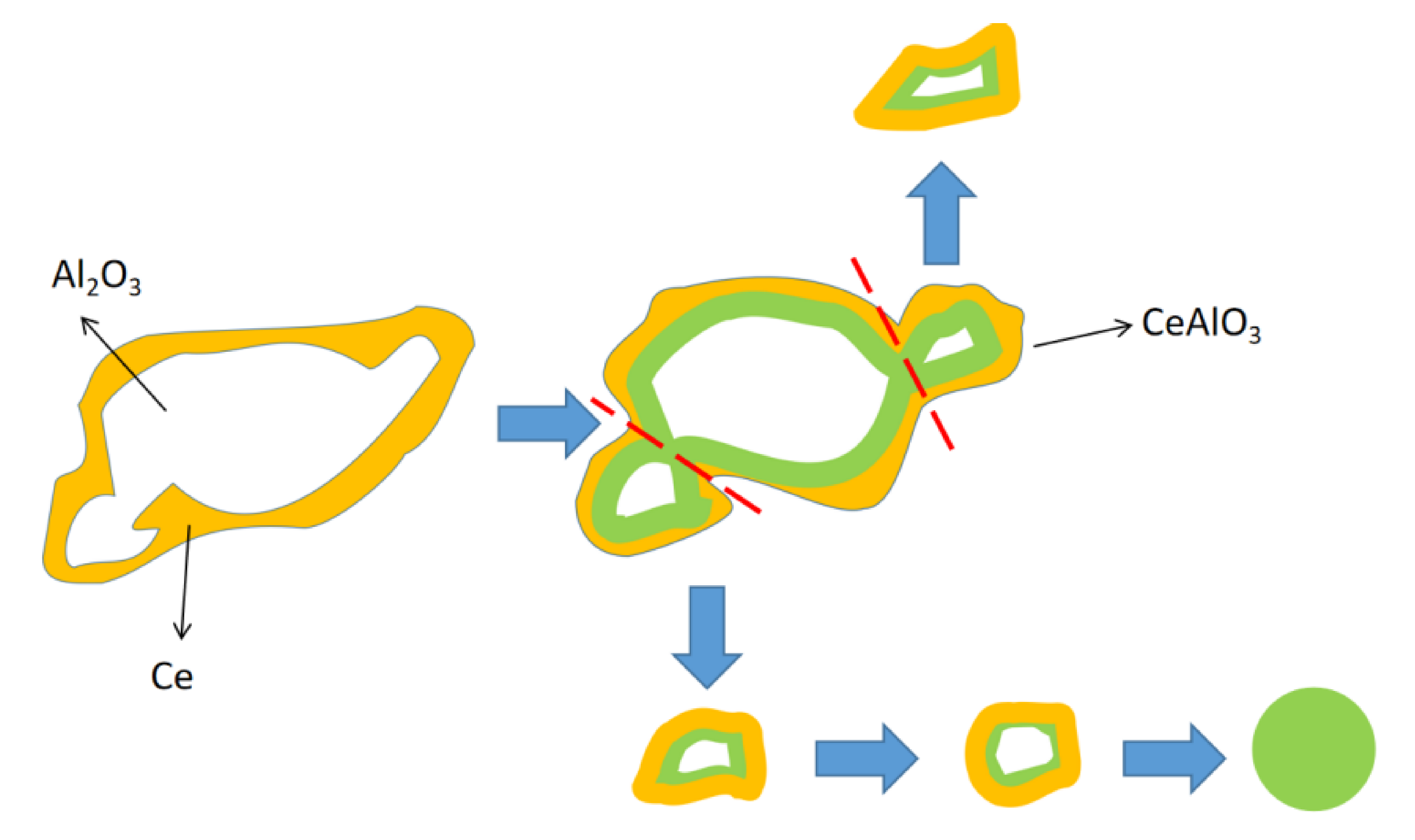
- Under the test conditions, the addition of Ce can effectively reduce the number and size of class A and B inclusions in the cast billet samples and make class D and Ds inclusions completely disappear in heavy rail steel. Meanwhile, the CaO–MgO–Al2O3–SiO2 inclusions change from irregularly shaped to round, and the MnS inclusions change in shape from long strips to squares with similar lengths and widths.
- (2)
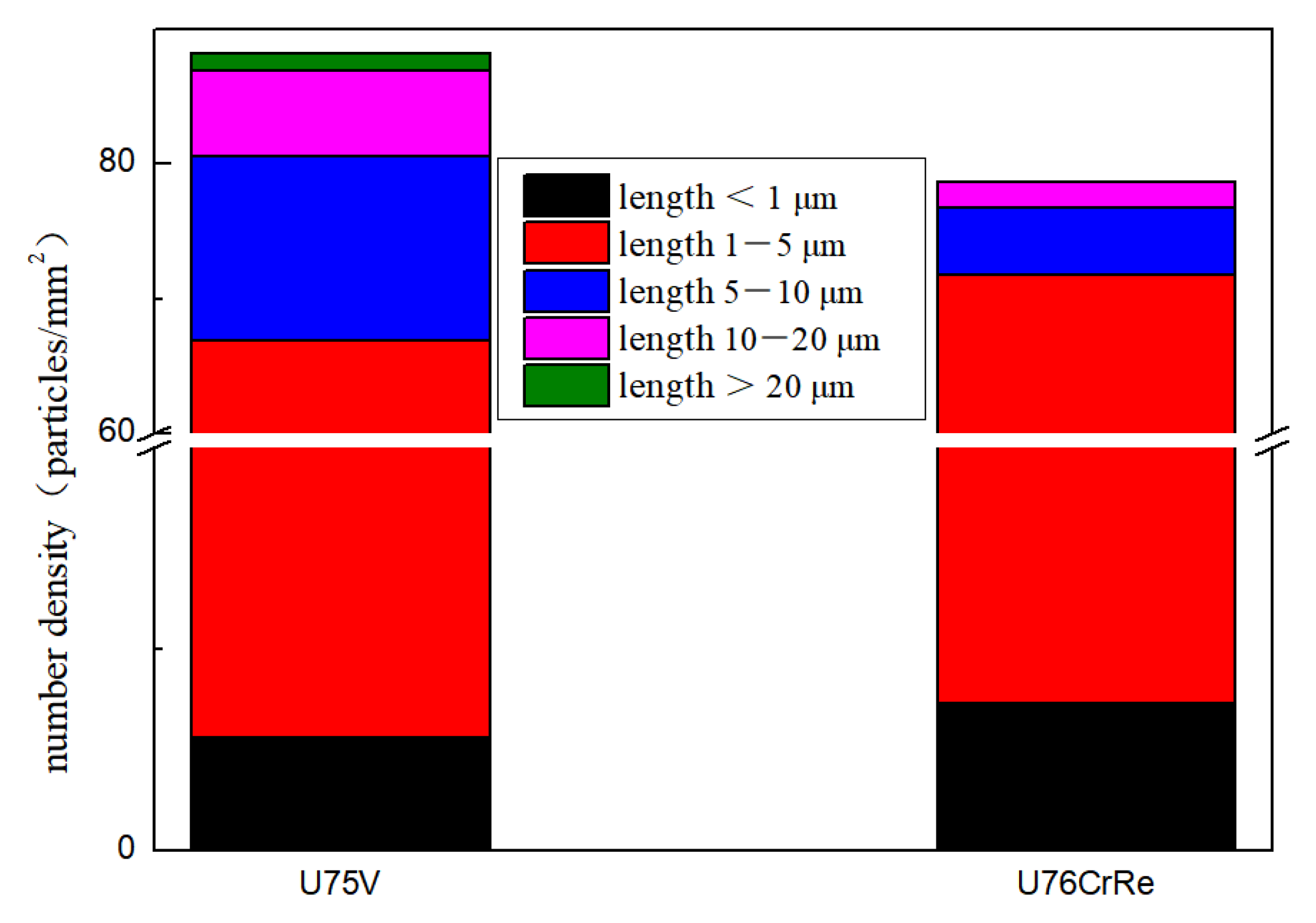
- Multi-field analysis shows that the addition of Ce can effectively reduce the number density and size of inclusions in heavy rail steel; in particular, inclusions longer than 5 μm decreased significantly, while those shorter than 5 μm increased, indicating that Ce can promote the dispersion distribution of inclusions in heavy rail steel.
- (3)
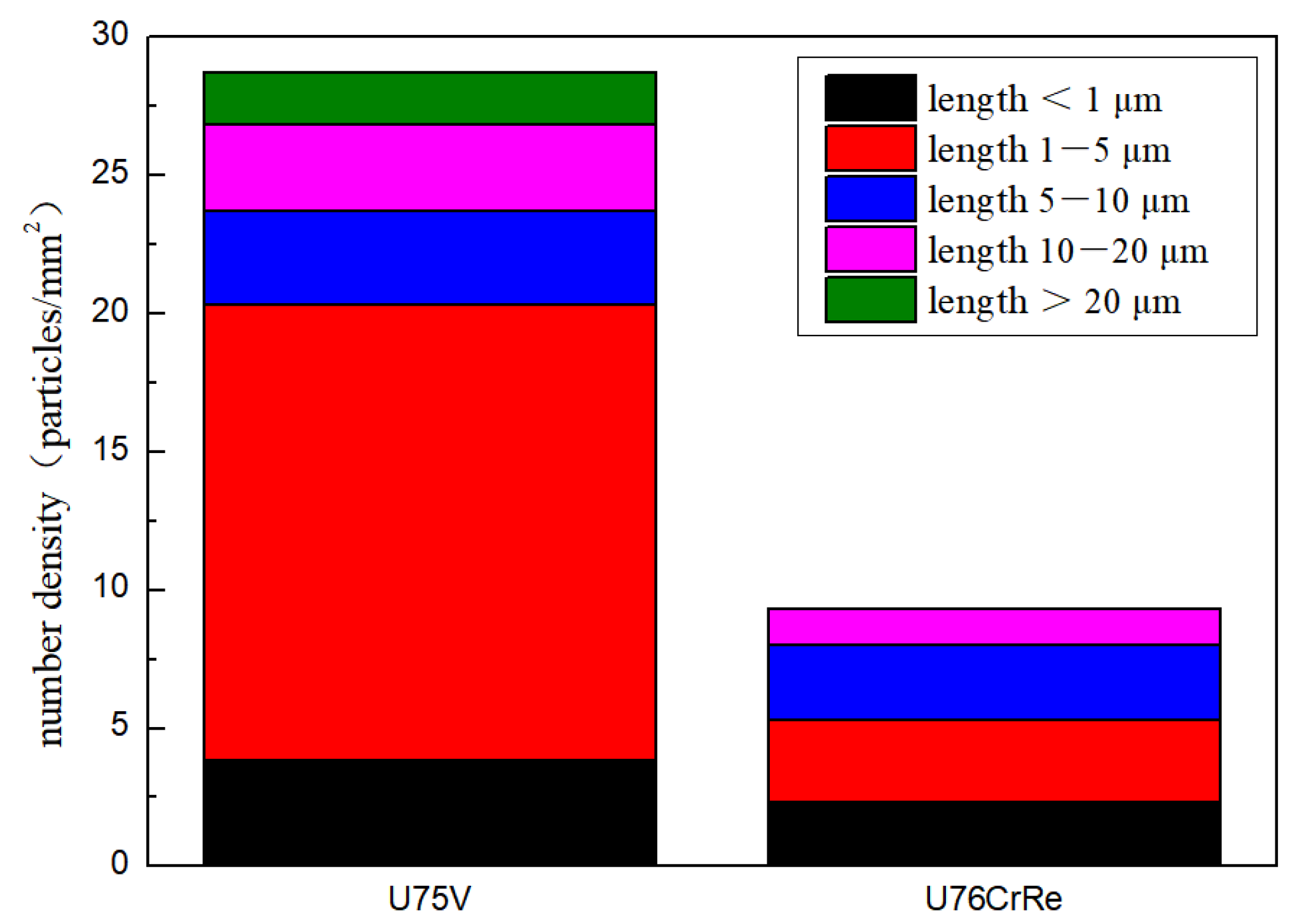
- Under these test conditions, the aluminium-containing inclusions were converted into smaller spherical CeAlO3 inclusions after the addition of Ce, which can reduce the collision growth probability of such inclusions and simplify their floating and removal. The number density of aluminium inclusions in steel decreased from 28.72 particles/mm2 to 9.33 particles/mm2. The size distribution of aluminium inclusions changed: the number density of aluminium inclusions with the length of 1–5 μm decreased from 16.43 particles/mm2 to 3 particles/mm2, while those of other length inclusions also decreased to different degrees.
- (4)
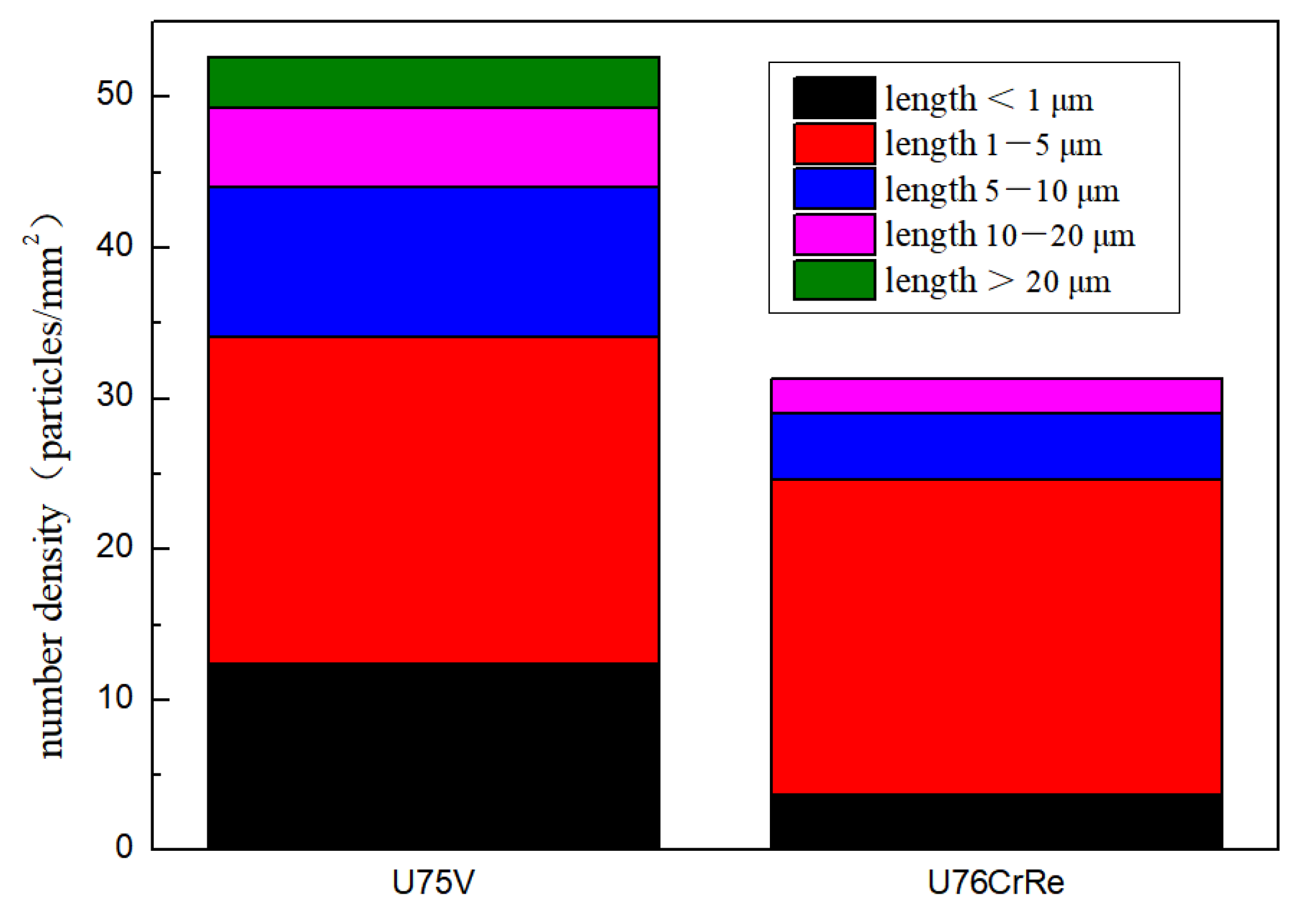
- Under the experimental conditions, Ce reacted with the solid solution [S] in the liquid steel to reduce the [S] concentration in the liquid steel, which aided the reduction in the number and size of the inclusion core required for the growth of MnS inclusions in steel; meanwhile, CaO–MgO–Al2O3–SiO2–CeO and MnS composite inclusions were formed more. Thus, the number density of MnS inclusions in steel decreased from 52.7 particles/mm2 to 31.33 particles/mm2, and the size distribution of MnS inclusions changed. The number density of inclusions with lengths between 5 and 10, lengths between 10 and 20, and larger than 20 μm, which showed the most obvious change, decreased from 12.4, 21.7, 10, and 5.2 particles/mm2 to 4.33, 2.33, and 0 particles/mm2, respectively.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belalia, A.; Kamla, Y.; Amara, M.; Hadj Meliani, M.; El Azizi, A.; Azari, Z. Experimental and numerical investigation of UIC 54 rail degradation. Eng. Fail. Anal. 2020, 111, 104163. [Google Scholar] [CrossRef]
- Kormyshev, V.E.; Gromov, V.E.; Ivanov, Y.F.; Glezer, A.; Yuriev, A.A.; Semin, A.P.; Sundeev, R.V. Structural phase states and properties of rails after long-term operation. Mater. Lett. 2020, 268, 127499. [Google Scholar] [CrossRef]
- Jing, J.L.; Ya, F.G.; Yue, S.W.; Chang, H.Y. Experimental Research on Crack Propagation in U71Mn and U75V Rail Steels. Key Eng. Mater. 2006, 43, 807–810. [Google Scholar]
- Wang, H.; Bao, Y.; Duan, C.; Lu, L.; Liu, Y.; Zhang, Q. Effect of Rare Earth Ce on Deep Stamping Properties of High-Strength Interstitial-Free Steel Containing Phosphorus. Materials 2020, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Chen, X.-Q.; Fu, P.; Luan, Y.; Hu, X.; Liu, H.; Sun, M.; Chen, Y.; Cao, Y.; et al. Low-oxygen rare earth steels. Nat. Mat. 2022, 21, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.R.; Wang, J.A.; Wang, X.D.; Guo, F.; Liu, Y. Effects of lanthanum on hot deformation behaviour of Mn-Cr-Mo bainitic rail steel. J. Rare Earths 2018, 36, 772–780. [Google Scholar] [CrossRef]
- Xu, Y.W.; Song, S.H.; Wang, J.W. Effect of rare earth cerium on the creep properties of modified 9Cr-1Mo heat-resistant steel. Mater. Lett. 2015, 161, 616–619. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.P.; Zhi, J.G.; Duan, C.-y.; Gao, S.; Wang, M. Effect of Rare Earth Ce on the Morphology and Distribution of Al2O3 Inclusions in High Strength IF Steel Containing Phosphorus during Continuous Casting and Rolling Process: Fundamentals of High Temperature Processes. ISIJ Int. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Cheng, W.S.; Song, B.; Yang, Z.B.; Mao, J. Effect of Rare Earth Ce on Modifying Inclusions in Al-killed X80 Pipeline Steel. Trans. Indian Inst. Met. 2022, 75, 2837–2846. [Google Scholar] [CrossRef]
- Lan, F.-J.; Zhuang, C.-L.; Li, C.-R.; Yang, H.; Yang, G.-K.; Yao, H.-J.; Zhang, Z.-Z. Effect of Rare-Earth Cerium on Nonmetallic Inclusions in Fe–Mn–C–Al Twinning-Induced Plasticity. Steel. Steel Res. Int. 2022, 94, 2200421. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, C.J. Evolution and Deformability of Inclusions in Steel Containing Rare-Earth Element Under Different Deoxidation Conditions. Steel. Steel Res. Int. 2022, 93, 2200027. [Google Scholar] [CrossRef]
- Zhuo, C.; Liu, R.; Zhao, Z.; Zhang, Y.; Hao, X.; Wu, H.; Sun, Y. Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel. Metals 2022, 12, 1012. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, F.; Zhu, X.Y. Behaviors of Lanthanum and Cerium on Grain Boundaries in Carbon Manganese Clean Steel. J. Rare Earths 2007, 25, 485–489. [Google Scholar] [CrossRef]
- Yu, Y.C.; Chen, W.Q.; Zhen, H.G. Effect of Cerium and Mischmetal on Soildfication Structure of Fe-36Ni Low Expansionalloy. J. Chin. Soc. Rare Earths 2012, 30, 175–180. [Google Scholar]
- Huang, Y.; Cheng, G.G.; Xie, Y. Modification Mechanism of Cerium on the inclusionsin Drill Steel. Acta Metall. Sin. 2018, 54, 1253–12661. [Google Scholar]
| Steel Grade | wB/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | V | Cr | Al | Re | O | Fe | |
| U75V | 0.75 | 0.55 | 0.79 | 0.014 | 0.008 | 0.005 | 0 | 0.0035 | 0 | 0.0014 | balance |
| U75V | 0.72 | 0.60 | 0.81 | 0.010 | 0.012 | 0.005 | 0 | 0.0031 | 0 | 0.0018 | balance |
| U75V | 0.71 | 0.59 | 0.77 | 0.011 | 0.011 | 0.004 | 0 | 0.0045 | 0 | 0.0017 | balance |
| U75V | 0.74 | 0.56 | 0.79 | 0.010 | 0.005 | 0.006 | 0 | 0.0041 | 0 | 0.0018 | balance |
| U76CrRe | 0.79 | 0.69 | 0.94 | 0.016 | 0.003 | 0.048 | 0.0724 | 0.0028 | 0.0085 | 0.0015 | balance |
| U76CrRe | 0.79 | 0.73 | 0.97 | 0.017 | 0.001 | 0.042 | 0.0765 | 0.0034 | 0.0091 | 0.0016 | balance |
| U76CrRe | 0.79 | 0.67 | 0.93 | 0.015 | 0.002 | 0.043 | 0.0772 | 0.0028 | 0.0083 | 0.0012 | balance |
| U76CrRe | 0.80 | 0.68 | 0.94 | 0.014 | 0.001 | 0.051 | 0.0786 | 0.0029 | 0.0084 | 0.0018 | balance |
| Reaction Formula | ∆G0 | ∆G (1873 K) |
|---|---|---|
| [Ce] + 3/2[O] = 1/2Ce2O3 | −714,380 + 179.74T | −377,726.98 |
| [Ce] + [O] + 1/2[S] = 1/2Ce2O2S | −675,700 + 165.50T | −365,718.50 |
| [Ce] + 3/2[S] = 1/2Ce2S3 | −536,420 + 163.86T | −229,510.22 |
| [Ce] + 4/3[S] = 1/3Ce3S4 | −497,670 + 146.30T | −223,650.10 |
| [Ce] + [S] = CeS | −422,100 + 120.38T | −196,628.26 |
| [Ce] + 3[O] + [Al] = CeAlO3 | −1,366,460 + 364.30T | −684,126.10 |
| [Mn] + [S] = MnS | −39,469 + 21.71T | 1193.83 |
| C | Si | Mn | P | Al | O | S | Ce | |
|---|---|---|---|---|---|---|---|---|
| O | −0.45 | −0.131 | −0.021 | 0.07 | −3.9 | −0.02 | −0.133 | −0.57 |
| S | 0.122 | 0.063 | −0.026 | 0.029 | 0.035 | −0.27 | −0.026 | - |
| Ce | - | - | - | - | - | −5.03 | - | - |
| Al | 0.091 | 0.0056 | 0.035 | 0.033 | 0.045 | −6.6 | 0.03 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Yang, J.; Liang, W. Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel. Metals 2023, 13, 614. https://doi.org/10.3390/met13030614
Bai G, Yang J, Liang W. Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel. Metals. 2023; 13(3):614. https://doi.org/10.3390/met13030614
Chicago/Turabian StyleBai, Guojun, Jichun Yang, and Wenjing Liang. 2023. "Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel" Metals 13, no. 3: 614. https://doi.org/10.3390/met13030614
APA StyleBai, G., Yang, J., & Liang, W. (2023). Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel. Metals, 13(3), 614. https://doi.org/10.3390/met13030614






