Research Regarding Molybdenum Flakes’ Improvement on the Hydrogen Storage Efficiency of MgH2
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of MgH2
2.2. Synthesis of Flake-like Mo
2.3. Preparation of MgH2-MoCl5 and MgH2-Mo Composites
2.4. Characterization
3. Results and Discussion
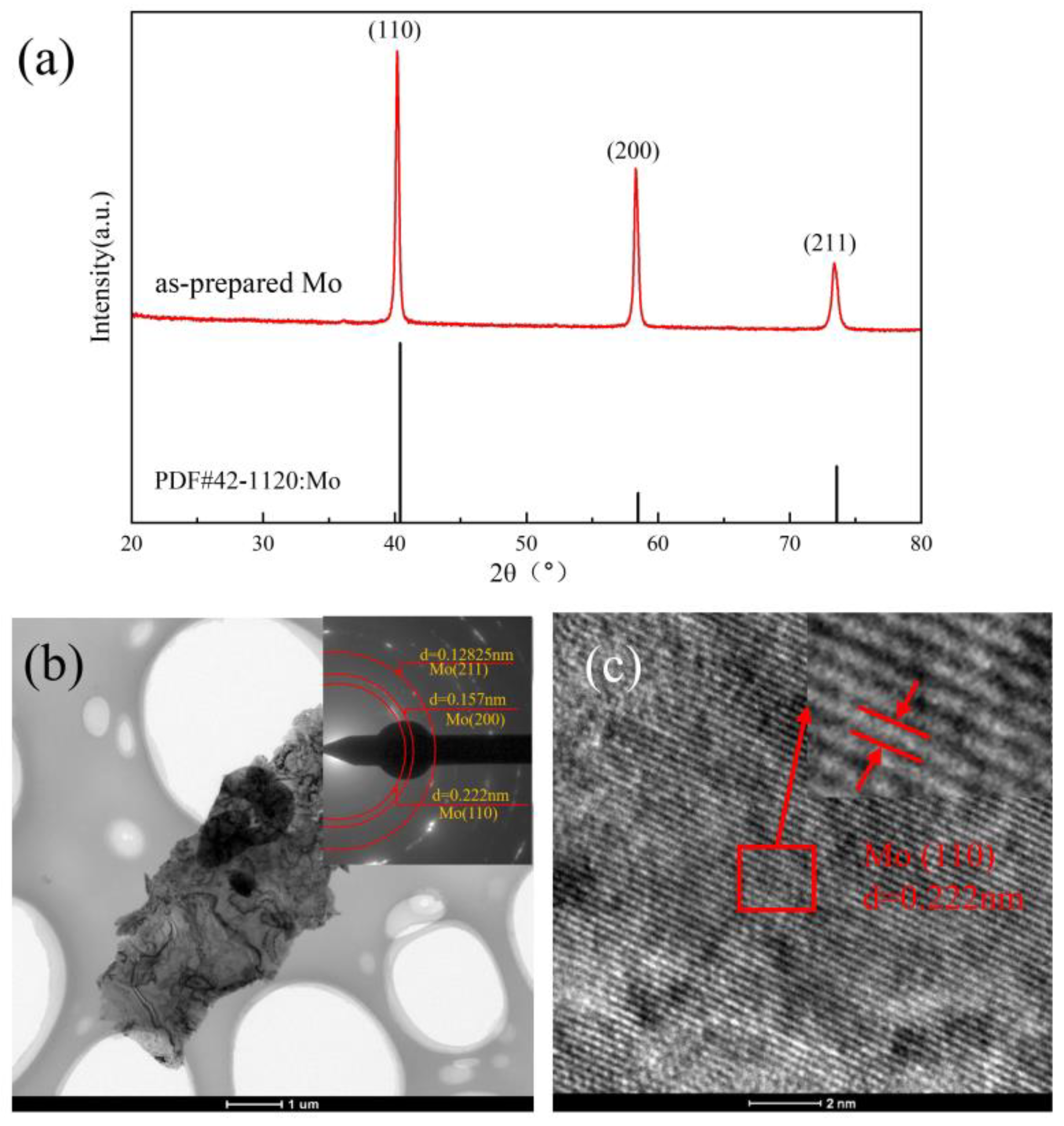
3.1. Characterization of As-Prepared Mo
3.2. Catalytic Effect of Mo on the Hydrogen Storage Properties of MgH2
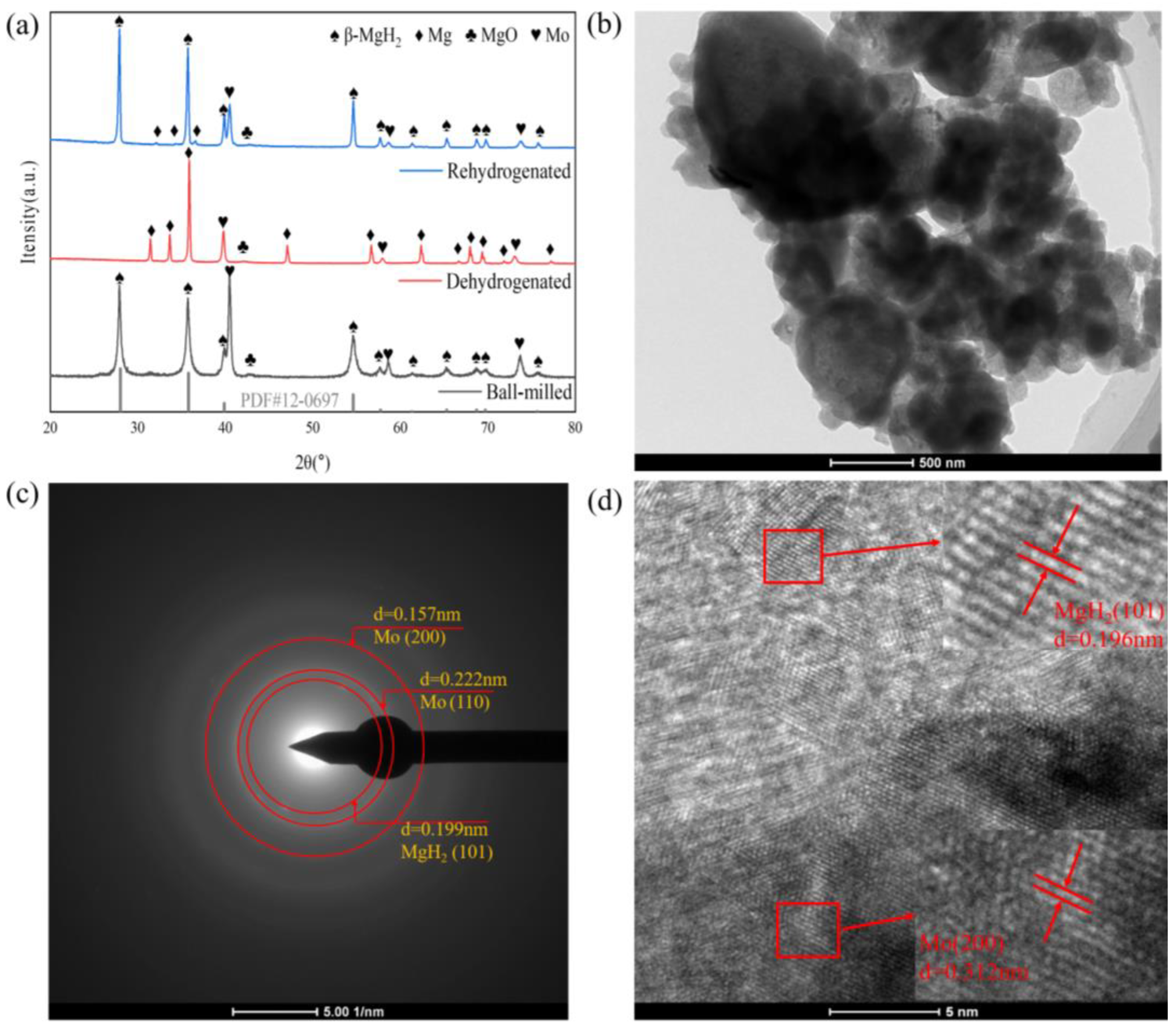
3.3. Evolution of Flake-like Molybdenum in Cyclic Processes and Its Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, H.; He, L.; Lin, H. Progress and Trends in Mg-based Materials for Energy Storage Research: A Review. Energy Technol. 2018, 6, 445–448. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int. J. Hydrogen Energy 2018, 43, 1545–1565. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, X. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 10064. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H. Stress/strain effects on thermodynamic properties of magnesium hydride: A brief review. Int. J. Hydrogen Energy 2017, 42, 16603–16610. [Google Scholar] [CrossRef]
- Kim, C.K. A review on design strategies for metal hydrides with enhanced reaction thermodynamics for hydrogen storage applications. Int. J. Energy Res. 2018, 42, 1455–1468. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sust. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019, 9, 9907–9930. [Google Scholar] [CrossRef]
- Tian, M.; Shang, C.X. Nano-structured MgH2 catalyzed by TiC nanoparticles for hydrogen storage. J. Chem. Technol. Biotechnol. 2011, 8, 69–74. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Johnson, J.K.; Shaz, M.A.; Srivastava, O.N. H2 as a dynamic additive for improving the de/rehydrogenation properties of MgH2: A combined experimental and theoretical mechanistic investigation. J. Phys. Chem. C 2018, 122, 21248–21261. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Kumar, S.; Ankur, J.; Yamaguchi, J.S. Surface modification of MgH2 by ZrCl4 to tailor the reversible hydrogen storage performance. Int. J. Hydrogen Energy 2017, 42, 6152–6159. [Google Scholar] [CrossRef]
- Shevlin, S.A.; Guo, Z.X. MgH2 dehydrogenation thermodynamics: Nanostructuring and transition metal doping. J. Phys. Chem. C 2013, 117, 10883–10891. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Cao, Z.J.; Wang, H. Enhanced dehydriding thermodynamics and kinetics in Mg(In)–MgF2 composite directly synthesized by plasma milling. J. Alloys Compd. 2014, 586, 113–117. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M.; Ishikawa, T.T.; Huot, J.; Fruchart, D.; Miraglia, S.; Kiminami, C.S.; Botta, W.J. Nanoscale Grain Refinement and H-Sorption Properties of MgH2 Processed by High-Pressure Torsion and Other Mechanical Routes. Adv. Eng. Mater. 2010, 12, 786–792. [Google Scholar] [CrossRef]
- Alsabawi, K.; Gray, E.M.; Webb, C.J. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Wang, X.; Chen, M.; Lu, Y.; Liu, M.; Chen, L. Excellent catalysis of TiO2 nanosheets with high-surface-energy {001} facets on the hydrogen storage properties of MgH2. Nanoscale 2019, 11, 7465–7473. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Yu, H.J.; Lu, X.; Song, M.C.; Wu, F.Y.; Zheng, J.G.; Yuan, Z.F.; Zhang, L.T. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Yan, N.Y.; Lu, X.; Lu, Z.Y.; Yu, H.J.; Wu, F.Y.; Zheng, J.G.; Wang, X.Z.; Zhang, L.T. Enhanced hydrogen storage properties of Mg by the synergistic effect of grain refinement and NiTiO3 nanoparticles. J. Magnes. Alloys 2022, 10, 3542–3552. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Song, M.C.; Zhang, L.T.; Zheng, J.G.; Yu, Z.D.; Wang, S.N. Constructing graphene nanosheets supported FeOOH nanodots for hydrogen storage of MgH2. Int. J. Min. Met. Mater. 2022, 29, 1464–1473. [Google Scholar] [CrossRef]
- Soni, P.K.; Bhatnagar, A.; Shaz, M.A.; Srivastava, O.N. Effect of graphene templated fluorides of Ce and La on the de/rehydrogenation behavior of MgH2. Int. J. Hydrogen Energy 2017, 42, 20026–20035. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Chen, J. Enhancing hydrogen storage performances of MgH2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 2018, 29, 265705. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kim, H.; Sakaki, K.; Hayashi, S.; Jimura, K.; Asano, K. Destabilizing the Dehydrogenation Thermodynamics of Magnesium Hydride by Utilizing the Immiscibility of Mn with Mg. Inorg. Chem. 2019, 58, 14600–14607. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Khan, D.; Zhu, W.; Hu, C.; Zeng, X.; Ding, W. Preparation and hydrogen storage properties of MgH2-trimesic acid-TM MOF (TM = Co, Fe) composites. J. Mater. Sci. Technol. 2019, 35, 2132–2143. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.; Li, X.; Ren, S.; Zhou, S.; Zhang, H.; Han, S. Improved hydrogen storage properties of MgH2 by nickel@nitrogen-doped carbon spheres. Dalton Trans. 2020, 20, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Ding, Z.; Fu, Y. Enhanced H2 sorption performance of magnesium hydride with hard-carbon-sphere-wrapped nickel. RSC Adv. 2018, 8, 28787–28796. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.T.; Yu, H.J.; Lu, Z.Y.; He, J.H.; Zheng, J.G.; Wu, F.Y.; Chen, L.X. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Mozhzhukhin, S.A.; Arbuzov, A.A.; Volodin, A.A.; Fokina, E.E.; Fursikov, P.V.; Lototskyy, M.V.; Yartys, V.A. Features of the Hydrogenation of Magnesium with a Ni-Graphene Coating. Russ. J. Phys. Chem. A 2020, 94, 996–1001. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Lu, Z.; Zhao, C.; Zheng, J.; Wei, T.; Wu, F.; Xiao, B. The effect of different Co phase structure (FCC/HCP) on the catalytic action towards the hydrogen storage performance of MgH2. Chin. J. Chem. Eng. 2022, 43, 343–352. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Q. Constructing Core-Shell Co@N-Rich Carbon Additives Toward Enhanced Hydrogen Storage Performance of Magnesium Hydride. Front. Chem. 2020, 8, 233. [Google Scholar] [CrossRef]
- Ding, X.; Ding, H.; Song, Y. Activity-Tuning of Supported Co–Ni Nanocatalysts via Composition and Morphology for Hydrogen Storage in MgH2. Front. Chem. 2020, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, L.; Yang, X.; Zhu, X.; Chen, L. Remarkably improved hydrogen storage performance of MgH2 by the synergetic effect of FeNi/rGO nanocomposite. Dalton Trans. 2020, 49, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, H.G.; Huot, J.; Chapon, L.C.; Tichelaar, F.D.; Mulder, F.M. Hydrogen Cycling of Niobium and Vanadium Catalyzed Nanostructured Magnesium. J. Am. Chem. Soc. 2005, 127, 14348–14354. [Google Scholar] [CrossRef]
- Yan, C.H.; Zhang, H.Y.; Wu, F.Y.; Ze, S.U.; Zheng, J.G.; Zhang, L.T.; Chen, L.X. Mn nanoparticles enhanced dehydrogenation and hydrogenation kinetics of MgH2 for hydrogen storage. Trans. Nonferrous Met. Soc. China 2021, 31, 3469–3477. [Google Scholar]
- Jia, Y.; Han, S.; Zhang, W.; Zhao, X.; Sun, P.; Liu, Y.; Shi, H.; Wang, J. Hydrogen absorption and desorption kinetics of MgH2 catalyzed by MoS2 and MoO2. Int. J. Hydrogen Energy 2013, 38, 2352–2356. [Google Scholar] [CrossRef]
- Chen, M.; Pu, Y.; Li, Z.; Huang, G.; Liu, X.; Lu, Y.; Tang, W.; Xu, L.; Liu, S.; Yu, R.; et al. Synergy between metallic components of MoNi alloy for catalyzing highly efficient hydrogen storage of MgH2. Nano Res. 2020, 13, 2063–2071. [Google Scholar] [CrossRef]
- Xia, G.; Leng, H.; Xu, N.; Li, Z.; Wu, Z.; Du, J.; Yu, X. Enhanced hydrogen storage properties of LiBH4-MgH2 composite by the catalytic effect of MoCl3. Int. J. Hydrogen Energy 2011, 36, 7128–7135. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Sun, L.; Li, X. Effects of F and Cl on the stability of MgH2. Int. J. Hydrogen Energy 2014, 39, 877–883. [Google Scholar] [CrossRef]
- Song, M.; Zhang, L.; Yao, Z.; Zheng, J.; Shang, D.; Chen, L.; Li, H. Unraveling the degradation mechanism for the hydrogen storage property of Fe nanocatalyst-modified MgH2. Inorg. Chem. Front. 2022, 9, 3874. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zhang, H.; Song, M.; Wu, F.; Zhang, L. Research Regarding Molybdenum Flakes’ Improvement on the Hydrogen Storage Efficiency of MgH2. Metals 2023, 13, 631. https://doi.org/10.3390/met13030631
Cheng C, Zhang H, Song M, Wu F, Zhang L. Research Regarding Molybdenum Flakes’ Improvement on the Hydrogen Storage Efficiency of MgH2. Metals. 2023; 13(3):631. https://doi.org/10.3390/met13030631
Chicago/Turabian StyleCheng, Changshan, Haoyu Zhang, Mengchen Song, Fuying Wu, and Liuting Zhang. 2023. "Research Regarding Molybdenum Flakes’ Improvement on the Hydrogen Storage Efficiency of MgH2" Metals 13, no. 3: 631. https://doi.org/10.3390/met13030631
APA StyleCheng, C., Zhang, H., Song, M., Wu, F., & Zhang, L. (2023). Research Regarding Molybdenum Flakes’ Improvement on the Hydrogen Storage Efficiency of MgH2. Metals, 13(3), 631. https://doi.org/10.3390/met13030631





