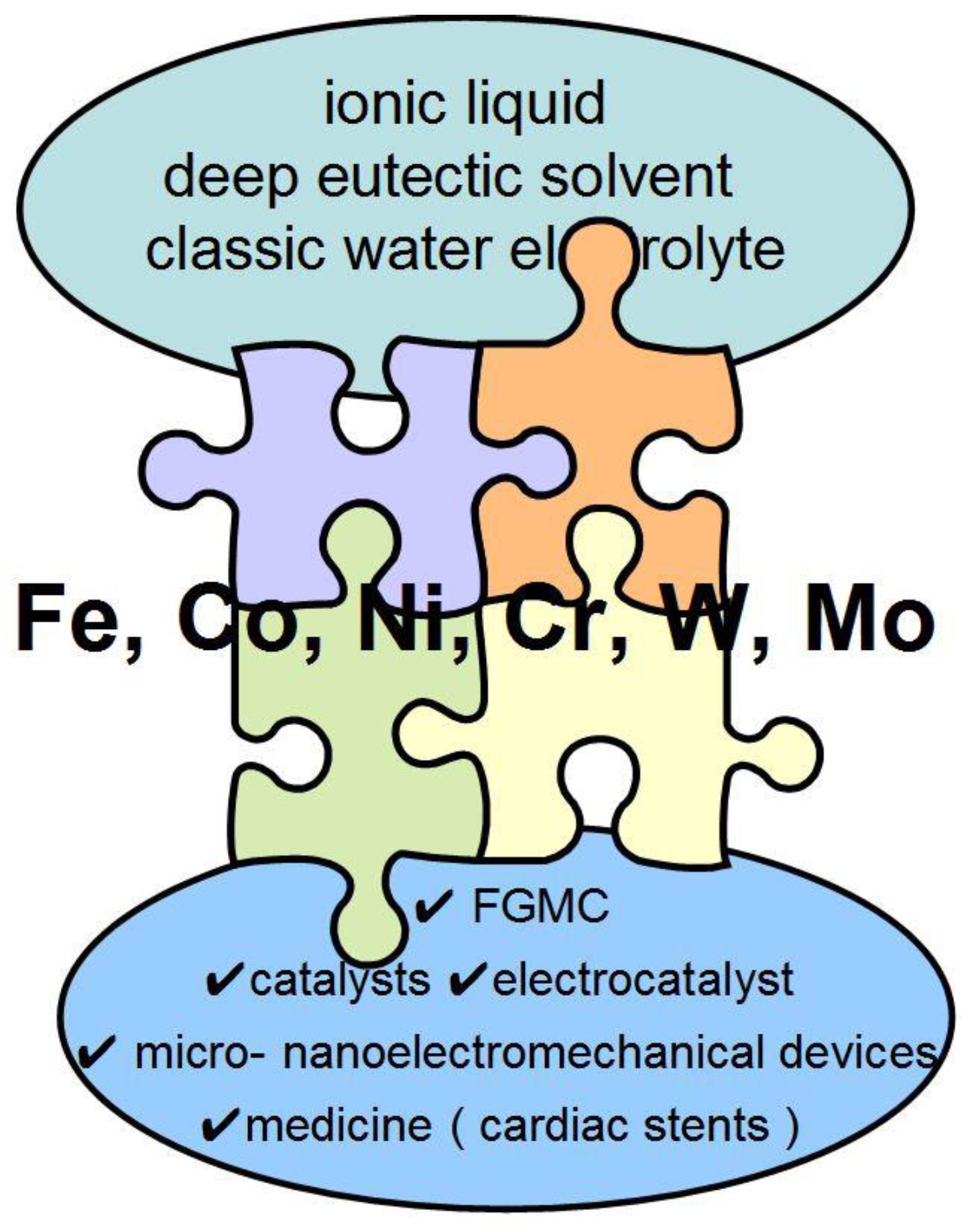
Electrodeposition of Iron Triad Metal Coatings: Miles to Go
Abstract
1. Introduction
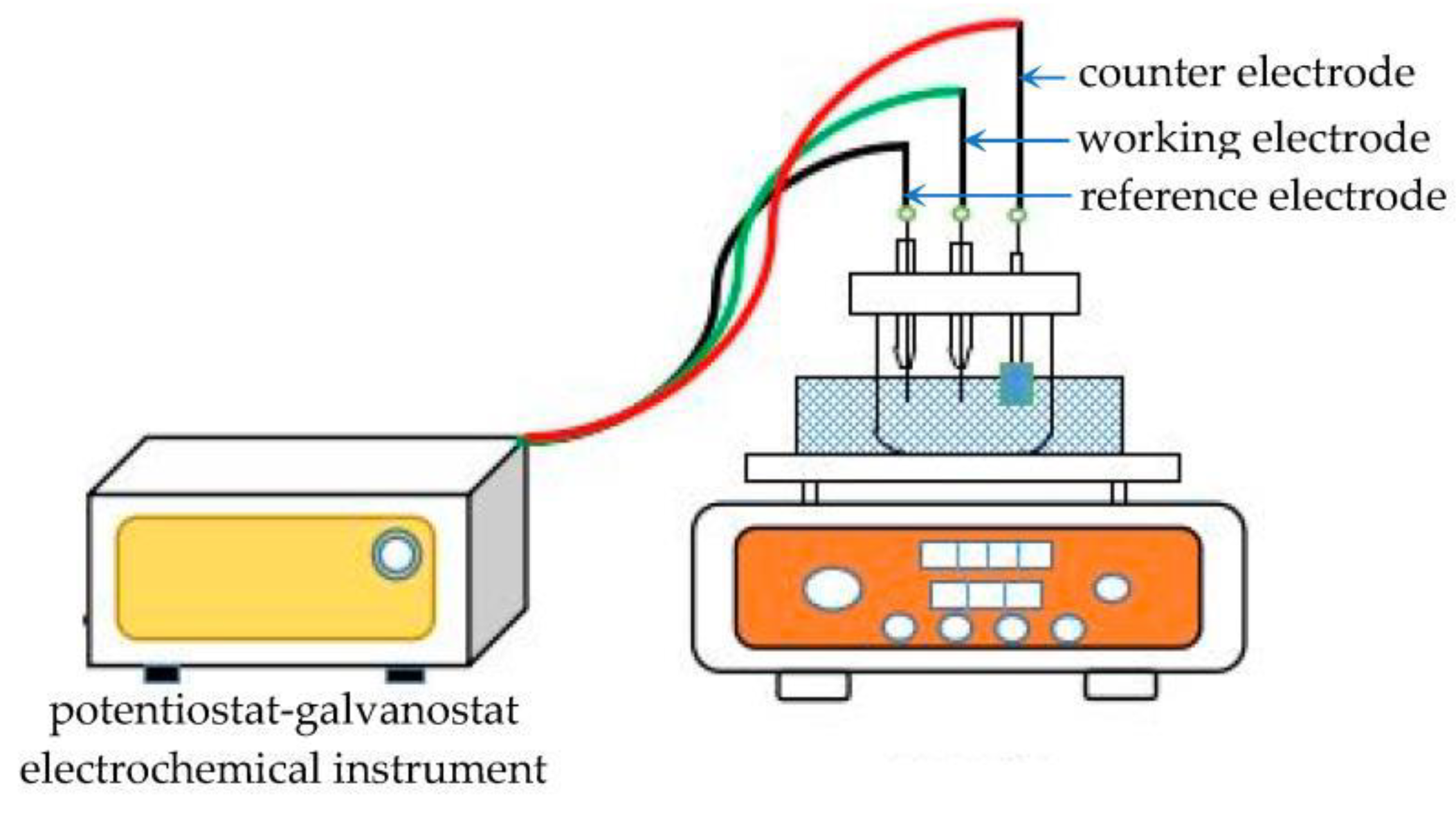
2. Electrodeposition from Water Solutions
2.1. Bi- and Ternary Alloys of Metals of the Iron Triad with Chromium and Metalloids
2.2. Iron Triad Metal Alloys with Molybdenum and Tungsten
- Electrochemical generation of reactive forms of nickel and tungsten. The intermediate forms of these metals with an unpaired electron, i.e., particles of the radical type, exhibit a particularly high reactivity;
- Formation of refractory metals from reactive particles of heteropoly compounds due to electrode initiation of the polymerization process. The film formed in this case has a low electron conductivity;
- Electrochemical reduction of metal ions in the non-metallic system at the point of contact of parts of the film with ions in different oxidized states;
- Final electrochemical reduction of metal ions at the film-alloy interface.
3. Electrodeposition of Two- and Three-Component Alloys in Ionic Liquids and Deep Eutectic Solvents
3.1. Electrodeposition of Alloys in Ionic Liquids
- The relationship between the precipitate structure and the composition of the IL has not been studied in detail yet;
- Coatings must achieve quality standards, and process development is required to a large extent;
- Some applications are at the fundamental research stage with associated higher risk, that is, electroless, semiconductor, anodizing, and nanocomposite coatings;
- Process economics has been determined for a limited number of processes.
3.2. Electrodeposition in Deep Eutectic Solvents (DESs)
4. Conclusions
- The relevance of electrodeposited alloys with a wide range of useful properties, such as corrosive, electrocatalytic, and magnetic properties, is shown. Due to these properties, the scope of applications for these alloys is very wide. The ability to deposit alloys on parts of a complex shape increases the wear and corrosion resistance of the latter.
- It is very important to study the correlation between the concentration of metal ions in the electrolytic bath and the metal content in the reduced form to describe the technique of manufacturing the coatings.
- Also promising, in our opinion, will be the establishment of a correlation between the magnetic and catalytic properties of electrodeposited alloys based on iron triad metals. Another promising direction of research on the properties of electrodeposited nickel- and cobalt-based alloys with modifying additives is the use of these materials in medicine, for example, as a replacement for platinum cardiac stents [61,62,63,64].
- The potentiostatic, ultrasonic, and plasma generation techniques in liquid are promising approaches in the electrodeposition process. Solution plasma sputtering is a simple and facile technique for the preparation of highly dispersed nanomaterials (metals and alloys) on a variety of carriers, such as metal oxides and carbon materials.
- The electrochemical deposition of alloys based on Fe-triad with aqueous (classical) solvents due to their advantages (Table 3) is today the most commonly used technology for industry. Due to their specific physicochemical and electrochemical properties, ionic liquids and DES are promising for obtaining coatings with new compositions and unique properties that cannot be obtained from classical solvents.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zangari, G. Electrodeposition of Alloys and Compounds in the Era of Microelectronics and Energy Conversion Technology. Coatings 2015, 5, 195–218. [Google Scholar] [CrossRef]
- Ma, L.; Xiaoli, X.; Nie, Z.; Dong, T.; Mao, Y. Electrodeposition and Characterization of Co-W Alloy from Regenerated Tungsten Salt. Int. J. Electrochem. Sci. 2017, 12, 1034–1051. [Google Scholar] [CrossRef]
- Li, Y.; Cai, X.; Zhang, G.; Xu, C.; Guo, W.; An, M. Optimization of Electrodeposition Nanocrytalline Ni-Fe Alloy Coatings for the Replacement of Ni Coatings. J. Alloys Compd. 2022, 903, 163761. [Google Scholar] [CrossRef]
- Li, A.; Zhu, Z.; Xue, Z.; Liu, Y. Periodic Ultrasound-Assisted Electrodeposition of Fe–Ni Alloy Foil. Mater. Res. Bull. 2022, 150, 111778. [Google Scholar] [CrossRef]
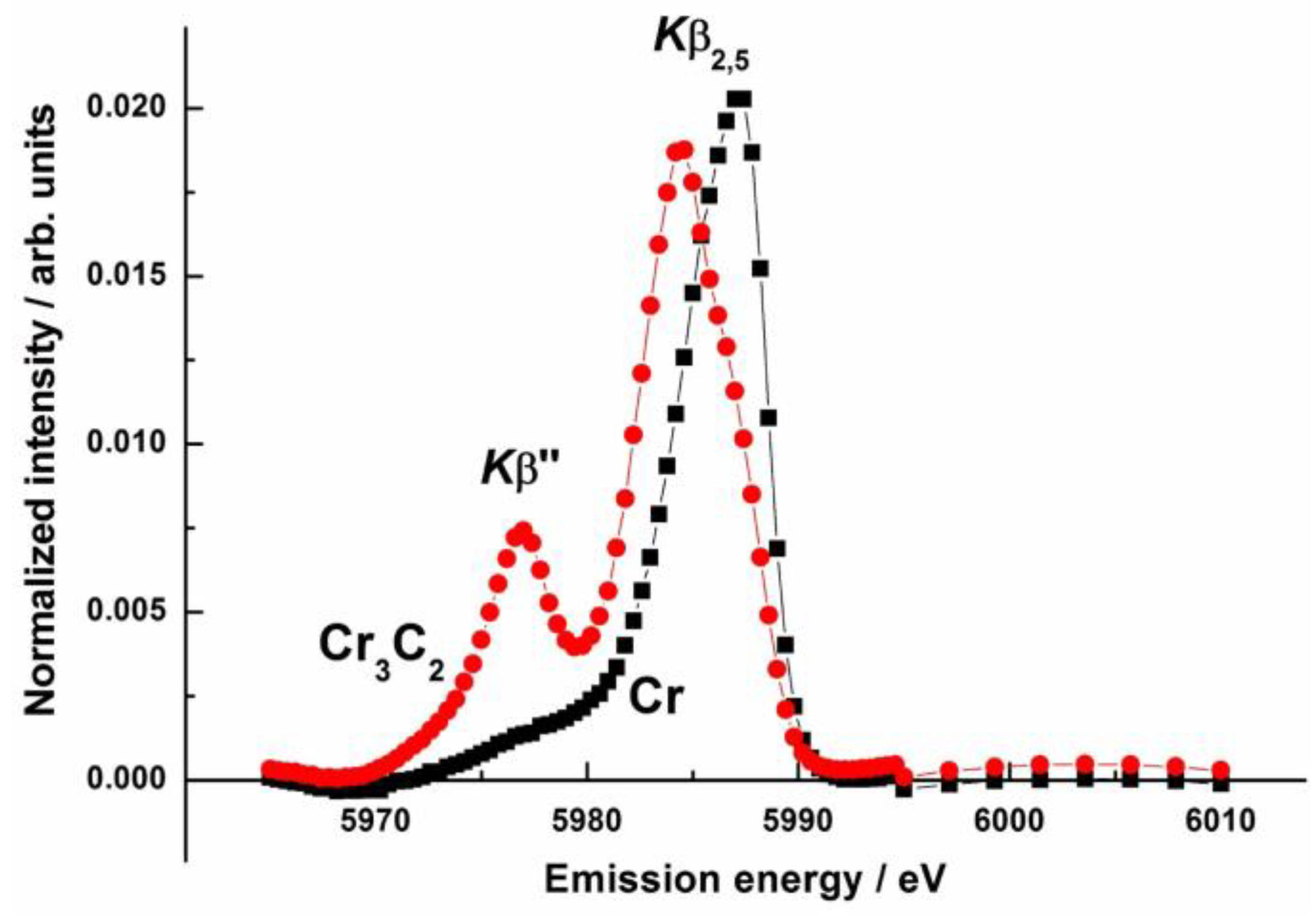
- Safonov, V.A.; Habazaki, H.; Glatzel, P.; Fishgoit, L.A.; Drozhzhin, O.A.; Lafuerza, S.; Safonova, O.V. Application of Valence-to-Core X-Ray Emission Spectroscopy for Identification and Estimation of Amount of Carbon Covalently Bonded to Chromium in Amorphous Cr-C Coatings Prepared by Magnetron Sputtering. Appl. Surf. Sci. 2018, 427, 566–572. [Google Scholar] [CrossRef]
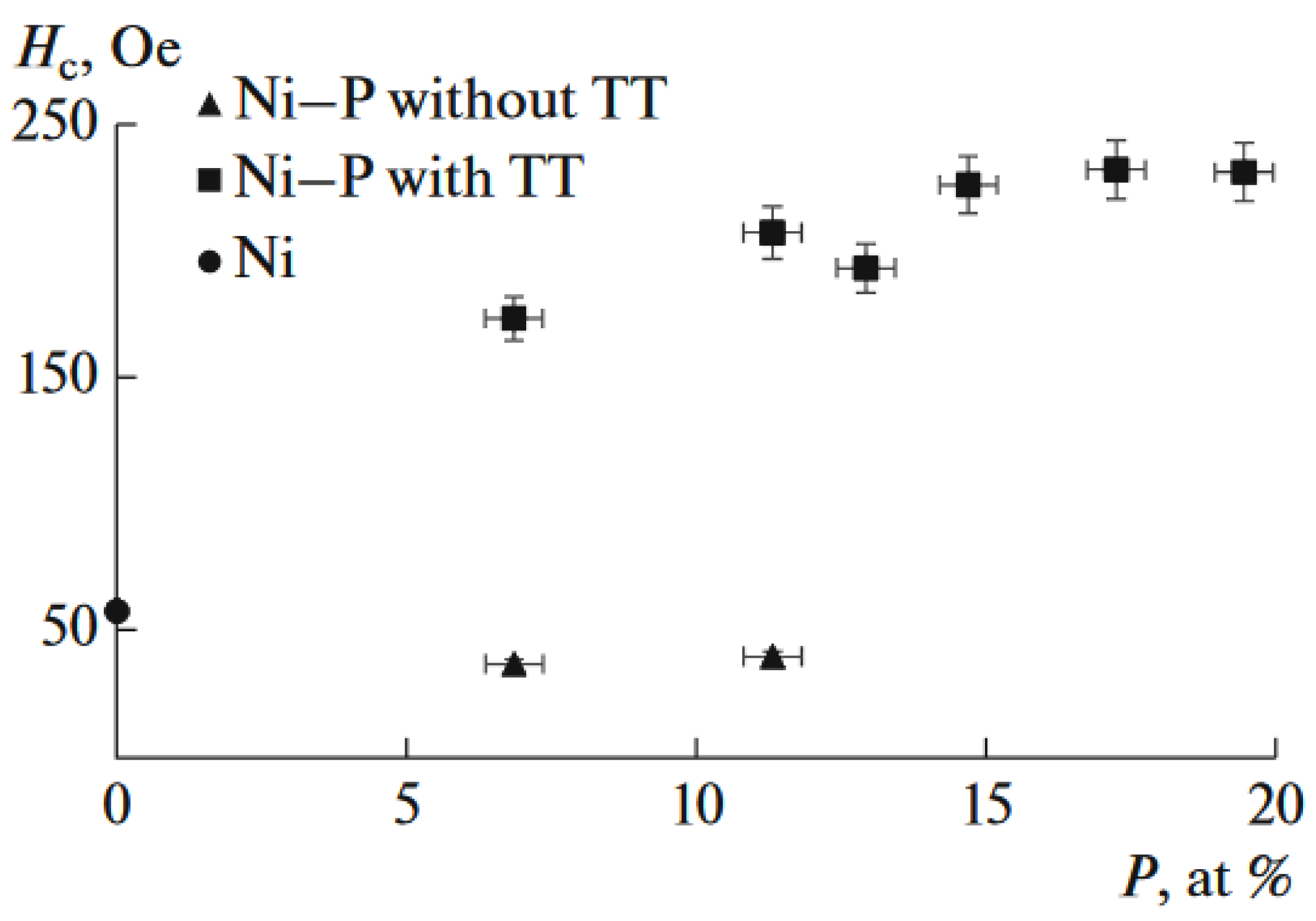
- Knyazev, A.V.; Fishgoit, L.A.; Chernavskii, P.A.; Safonov, V.A.; Filippova, S.E. Magnetic Properties of Electrodeposited Ni–P Alloys with Varying Phosphorus Content. Russ. J. Phys. Chem. 2017, 91, 260–263. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Fishgoit, L.A.; Chernavskii, P.A.; Safonov, V.A.; Filippova, S.E. Magnetic Properties of Electrodeposited Amorphous Nickel–Phosphorus Alloys. Russ. J. Electrochem. 2017, 53, 270–274. [Google Scholar] [CrossRef]
- Liu, S.; Shohji, I.; Kobayashi, T.; Hirohashi, J.; Wake, T.; Yamamoto, H.; Kamakoshi, Y. Mechanistic Study of Ni–Cr–P Alloy Electrodeposition and Characterization of Deposits. J. Electroanal. Chem. 2021, 897, 115582. [Google Scholar] [CrossRef]
- Jiang, W.; Li, H.; Lao, Y.; Li, X.; Fang, M.; Chen, Y. Synthesis and Characterization of Amorphous NiCoP Alloy Films by Magnetic Assisted Jet Electrodeposition. J. Alloys Compd. 2022, 910, 164848. [Google Scholar] [CrossRef]
- Safonov, V.A.; Safonova, O.V.; Fishgoit, L.A.; Kvashnina, K.; Glatzel, P. Chemical State of Phosphorus in Amorphous Ni–Fe–P Electroplates. Surf. Coat. Technol. 2015, 275, 239–244. [Google Scholar] [CrossRef]
- Safonov, V.A.; Fishgoit, L.A.; Safonova, O.V.; Glatzel, P. On the Presence of Covalently Bound Phosphorus in Amorphous Ni–Co–P and Fe–Co–P Electroplates. Mater. Chem. Phys. 2021, 272, 124987. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumar, R.; Walia, R.S. Functionally Graded Material Coatings (FGMC)—A Review. J. Phys. Conf. Ser. 2021, 2007, 012068. [Google Scholar] [CrossRef]
- Moiseev, I.I.; Loktev, A.S.; Shlyakhtin, O.A.; Mazo, G.N.; Dedov, A.G. New Approaches to the Design of Nickel, Cobalt, and Nickel–Cobalt Catalysts for Partial Oxidation and Dry Reforming of Methane to Synthesis Gas. Pet. Chem. 2019, 59, S1–S20. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kalmykov, K.; Snytko, V.; Kuznetsova, I.; Orekhov, A.; Zakharov, A.; Kustov, L. Nanorolls Decorated with Nanotubes as a Novel Type of Nanostructures: Fast Anodic Oxidation of Amorphous Fe–Cr–B Alloy in Hydrophobic Ionic Liquid. ACS Appl. Mater. Interfaces 2021, 13, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, R.; Panzeri, G.; Accogli, A.; Liberale, F.; Nobili, L.; Magagnin, L. Electrodeposition from Deep Eutectic Solvents. In Progress and Developments in Ionic Liquids; Handy, S., Ed.; InTechOpen: London, UK, 2017; ISBN 978-953-51-2901-1. [Google Scholar]
- Yamasaki, T. High-Strength Nanocrystalline Ni-W Alloys Produced by Electrodeposition and Their Embrittlement Behaviors during Grain Growth. Scr. Mater. 2001, 44, 1497–1502. [Google Scholar] [CrossRef]
- Quiroga Argañaraz, M.P.; Ribotta, S.B.; Folquer, M.E.; Benítez, G.; Rubert, A.; Gassa, L.M.; Vela, M.E.; Salvarezza, R.C. The Electrochemistry of Nanostructured Ni–W Alloys. J. Solid State Electrochem. 2013, 17, 307–313. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Geng, X.; Ru, J.; Hua, Y.; Bu, J.; Xue, Y.; Wang, D. The Role of Electrolyte Ratio in Electrodeposition of Nanoscale Fe Cr Alloy from Choline Chloride-Ethylene Glycol Ionic Liquid: A Suitable Layer for Corrosion Resistance. J. Mol. Liq. 2022, 346, 117059. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Al-Fakih, A.M.; Aziz, M.; Emran, K.M. Part II: Impact of Ionic Liquids as Anticorrosives and Additives on Ni-Co Alloy Electrodeposition: Experimental and DFT Study. Arab. J. Chem. 2021, 14, 102909. [Google Scholar] [CrossRef]
- Maizi, R. Electrodeposition of Ni, Fe and Ni-Fe Alloys in Two Ionic Liquids: (Tri (n-Butyl) [2-Methoxy-2-Oxoethyl] Ammonium Bis (Trifluoromethylsulfonyl) [BuGBOEt] [Tf2N] and (1-Butyl-1- Methylpyrrolidinium Bis Trifluoromethylsulfonyl) Imide ([P1,4] [Tf2N]). Int. J. Electrochem. Sci. 2016, 11, 7111–7124. [Google Scholar] [CrossRef]
- Wallace, A.G.; Symes, M.D. Water-Splitting Electrocatalysts Synthesized Using Ionic Liquids. Trends Chem. 2019, 1, 247–258. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Ouyang, P.; Zhang, R.; Liu, H.; Xu, C.; Liu, Z. Electrochemical Behavior and Electrodeposition of Fe-Co-Ni Thin Films in Choline Chloride/Urea Deep Eutectic Solvent. J. Electroanal. Chem. 2022, 919, 116516. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials 2021, 11, 3270. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advances in Application of Ionic Liquids: Fabrication of Surface Nanoscale Oxide Structures by Anodization of Metals and Alloys. Surf. Interfaces 2022, 34, 102345. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of Metals and Alloys from Ionic Liquids. J. Alloys Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Costa, J.G. dos R. da; Costa, J.M.; Almeida Neto, A.F. de Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals 2022, 12, 2095. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys; Principles and Practice; Academic Press: New York, NY, USA; London, UK, 1963; Volume 1. [Google Scholar]
- Landolt, D. Fundamental aspects of alloy plating. Plat. Surf. Finish. 2001, 88, 70–79. [Google Scholar]
- Cao, X.; Wang, H.; Liu, T.; Shi, Y.; Xue, X. Electrodeposition of Bi from Choline Chloride-Malonic Acid Deep Eutectic Solvent. Materials 2023, 16, 415. [Google Scholar] [CrossRef]
- Lv, Q.; Yao, B.; Zhang, W.; She, L.; Ren, W.; Hou, L.; Fautrelle, Y.; Lu, X.; Yu, X.; Li, X. Controlled Direct Electrodeposition of Crystalline NiFe/Amorphous NiFe-(Oxy)Hydroxide on NiMo Alloy as a Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Eng. J. 2022, 446, 137420. [Google Scholar] [CrossRef]
- Saeki, R.; Yakita, T.; Ohgai, T. Magnetization and Microhardness of Iron–Chromium Alloy Films Electrodeposited from an Aqueous Solution Containing N, N-Dimethylformamide. J. Mater. Res. Technol. 2022, 18, 2735–2744. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe Alloys, Composites, and Nano Coatings—A Review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Shetty, A.R.; Hegde, A.C. Effect of Magnetic Field on Corrosion Performance of Ni–Co Alloy Coatings. J. Bio- Tribo-Corros. 2023, 9, 16. [Google Scholar] [CrossRef]
- Haché, M.J.R.; Tam, J.; Erb, U.; Zou, Y. Electrodeposited Nanocrystalline Medium-Entropy Alloys—An Effective Strategy of Producing Stronger and More Stable Nanomaterials. J. Alloys Compd. 2022, 899, 163233. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Rouhaghdam, A.S. Facile Electrodeposition of Ternary Ni-Fe-Co Alloy Nanostructure as a Binder Free, Cost-Effective and Durable Electrocatalyst for High-Performance Overall Water Splitting. J. Colloid Interface Sci. 2019, 547, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Bachvarov, V.; Lefterova, E.; Rashkov, R. Electrodeposited NiFeCo and NiFeCoP Alloy Cathodes for Hydrogen Evolution Reaction in Alkaline Medium. Int. J. Hydrogen Energy 2016, 41, 12762–12771. [Google Scholar] [CrossRef]
- Kothanam, N.; Harachai, K.; Qin, J.; Boonyongmaneerat, Y.; Triroj, N.; Jaroenapibal, P. Hardness and Tribological Properties of Electrodeposited Ni–P Multilayer Coatings Fabricated through a Stirring Time-Controlled Technique. J. Mater. Res. Technol. 2022, 19, 1884–1896. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Köçkar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of Electrodeposited Multilayer Coatings: A Review of Fabrication, Microstructure, Properties and Applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Ma, C.; Wang, S.; Walsh, F.C. The Electrodeposition of Nanocrystalline Cobalt–Nickel–Phosphorus Alloy Coatings: A Review. Trans. Inst. Met. Finish. 2015, 93, 275–280. [Google Scholar] [CrossRef]
- Filgueira de Almeida, A.; Venceslau de Souto, J.I.; Lima dos Santos, M.; Costa de Santana, R.A.; Alves, J.J.N.; Nascimento Campos, A.R.; Prasad, S. Establishing Relationships between Bath Composition and the Properties of Amorphous Ni–Mo Alloys Obtained by Electrodeposition. J. Alloys Compd. 2021, 888, 161595. [Google Scholar] [CrossRef]
- Benavente Llorente, V.; Diaz, L.A.; Lacconi, G.I.; Abuin, G.C.; Franceschini, E.A. Effect of Duty Cycle on NiMo Alloys Prepared by Pulsed Electrodeposition for Hydrogen Evolution Reaction. J. Alloys Compd. 2022, 897, 163161. [Google Scholar] [CrossRef]
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of Electrodeposition Methods for the Preparation of High-Entropy Alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683–1696. [Google Scholar] [CrossRef]
- Yue, Z.; Muig, M.; Xiao-Ming, X.; Ze-Lin, L.; Shi-Xun, L.; Sbao-Min, Z. Kinetic Model of Induced Codeposition of Ni-Mo Alloys. Chin. J. Chem. 2010, 18, 29–34. [Google Scholar] [CrossRef]
- Jović, B.M.; Jović, V.D.; Maksimović, V.M.; Pavlović, M.G. Characterization of Electrodeposited Powders of the System Ni–Mo–O. Electrochim. Acta 2008, 53, 4796–4804. [Google Scholar] [CrossRef]
- Podlaha, E.J.; Landolt, D. Induced Codeposition: II. A Mathematical Model Describing the Electrodeposition of Ni-Mo Alloys. J. Electrochem. Soc. 1996, 143, 893–899. [Google Scholar] [CrossRef]
- Manazoğlu, M.; Hapçı, G.; Orhan, G. Electrochemical Deposition and Characterization of Ni-Mo Alloys as Cathode for Alkaline Water Electrolysis. J. Mater. Eng. Perform. 2016, 25, 130–137. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Cesiulis, H.; Donten, M.; Sort, J.; Pellicer, E.; Podlaha-Murphy, E.J. Modern Trends in Tungsten Alloys Electrodeposition with Iron Group Metals. Surf. Eng. Appl. Electrochem. 2012, 48, 491–520. [Google Scholar] [CrossRef]
- Fishgoit, L.A.; Fedorayev, I.I.; Knyazev, A.V.; Kasyanov, F.V.; Perkovskii, E.A. Electrodeposition of Nanocoatings Involving Iron Triad Metals. Gal’vanotekh. Obrab. Poverkhn. 2022, 30, 13–28. [Google Scholar] [CrossRef]
- Zhu, Z.; Meng, H.; Ren, P. CoNiWReP High Entropy Alloy Coatings Prepared by Pulse Current Electrodeposition from Aqueous Solution. Colloids Surf. A 2022, 648, 129404. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Li, B.; Li, M.; Hong, M.; Zhang, Z. Influences of Co and Process Parameters on Structure and Corrosion Properties of Nanocrystalline Ni-W-Co Ternary Alloy Film Fabricated by Electrodeposition at Low Current Density. Surf. Coat. Technol. 2022, 439, 128457. [Google Scholar] [CrossRef]
- Crousier, J.; Eyraud, M.; Crousier, J.-P.; Roman, J.-M. Influence of Substrate on the Electrodeposition of Nickel-Molybdenum Alloys. J. Appl. Electrochem. 1992, 22, 749–755. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Chassaing, E. Electrochemical Investigation of the Ni±Cu±Mo Electrodeposition System. J. Appl. Electrochem. 1997, 27, 568–572. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W Electrodeposited Coatings: Characterization, Properties and Applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Proverbio, E. Highly Hydrophobic Ni-W Electrodeposited Film with Hierarchical Structure. Surf. Coat. Technol. 2018, 344, 626–635. [Google Scholar] [CrossRef]
- Lee, S.; Choi, M.; Park, S.; Jung, H.; Yoo, B. Mechanical Properties of Electrodeposited Ni-W ThinFilms with Alternate W-Rich and W-Poor Multilayers. Electrochim. Acta 2015, 153, 225–231. [Google Scholar] [CrossRef]
- Slavcheva, E.; Mokwa, W.; Schnakenberg, U. Electrodeposition and Properties of NiW Films for MEMS Application. Electrochim. Acta 2005, 50, 5573–5580. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Liao, Z.; Chen, C.; Wei, G. A Novel Synthesis Method for Functionally Graded Alloy Coatings by Induced Electrodeposition. Mater. Lett. 2022, 312, 131681. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L.; Yin, Y.; Xu, Z.; Lv, Y.; Liao, Z.; Wei, G.; Zhong, F.; Yuan, M. Electrodeposition and Wear Behavior of NiCoW Ternary Alloy Coatings Reinforced by Al2O3 Nanoparticles: Influence of Current Density and Electrolyte Composition. Surf. Coat. Technol. 2022, 431, 128030. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Budreika, A.; Skridaila, N. Electrodeposition of CoMo and CoMoP Alloys from the Weakly Acidic Solutions. Surf. Eng. Appl. Electrochem. 2010, 46, 406–415. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile Fabrication of Platinum-Cobalt Alloy Nanoparticles with Enhanced Electrocatalytic Activity for a Methanol Oxidation Reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef]
- Saito, G.; Akiyama, T. Nanomaterial Synthesis Using Plasma Generation in Liquid. J. Nanomater. 2015, 16, 299. [Google Scholar] [CrossRef]
- Endres, F.; Abbott, A.; MacFarlane, D. (Eds.) Electrodeposition from Ionic Liquids, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; ISBN 978-3-527-68270-6. [Google Scholar]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Ispas, A.; Bund, A. Electrodeposition in Ionic Liquids. Electrochem. Soc. Interface 2014, 23, 47–51. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Part I: Ni-Co Alloy Foils Electrodeposited Using Ionic Liquids. Arab. J. Chem. 2020, 13, 7707–7719. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Tripathy, B.C.; Singh, P.; Keshavarz, A.; Iglauer, S. Roles of Organic and Inorganic Additives on the Surface Quality, Morphology, and Polarization Behavior during Nickel Electrodeposition from Various Baths: A Review. J. Appl. Electrochem. 2019, 49, 847–870. [Google Scholar] [CrossRef]
- Danilov, F.I.; Bogdanov, D.A.; Smyrnova, O.V.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–Fe Alloy from a Choline Chloride-Containing Ionic Liquid. J. Solid State Electrochem. 2022, 26, 939–957. [Google Scholar] [CrossRef]
- Tułodziecki, M.; Tarascon, J.-M.; Taberna, P.L.; Guéry, C. Importance of the Double Layer Structure in the Electrochemical Deposition of Co from Soluble Co2+—Based Precursors in Ionic Liquid Media. Electrochim. Acta 2014, 134, 55–66. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys. 2: Practical and Specific Information; Academic Press: New York, NY, USA, 1963; ISBN 978-1-4832-0967-8. [Google Scholar]
- Abbott, A.P. Deep Eutectic Solvents and Their Application in Electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Smith, E.L. Deep Eutectic Solvents (DESs) and the Metal Finishing Industry: Where Are They Now? Trans. Inst. Met. Finish. 2013, 91, 241–248. [Google Scholar] [CrossRef]
- Gómez, E.; Fons, A.; Cestaro, R.; Serrà, A. Electrodeposition of CoNi Alloys in a Biocompatible DES and Its Suitability for Activating the Formation of Sulfate Radicals. Electrochim. Acta 2022, 435, 141428. [Google Scholar] [CrossRef]
- Landa-Castro, M.; Sebastián, P.; Giannotti, M.I.; Serrà, A.; Gómez, E. Electrodeposition of Nanostructured Cobalt Films from a Deep Eutectic Solvent: Influence of the Substrate and Deposition Potential Range. Electrochim. Acta 2020, 359, 136928. [Google Scholar] [CrossRef]
- Oliveira, F.G.S.; Santos, L.P.M.; da Silva, R.B.; Correa, M.A.; Bohn, F.; Correia, A.N.; Vieira, L.; Vasconcelos, I.F.; de Lima-Neto, P. FexNi(1-x) Coatings Electrodeposited from Choline Chloride-Urea Mixture: Magnetic and Electrocatalytic Properties for Water Electrolysis. Mater. Chem. Phys. 2022, 279, 125738. [Google Scholar] [CrossRef]
- Higashino, S.; Abbott, A.P.; Miyake, M.; Hirato, T. Iron(III) Chloride and Acetamide Eutectic for the Electrodeposition of Iron and Iron Based Alloys. Electrochim. Acta 2020, 351, 136414. [Google Scholar] [CrossRef]
- Niciejewska, A.; Ajmal, A.; Pawlyta, M.; Marczewski, M.; Winiarski, J. Electrodeposition of Ni–Mo Alloy Coatings from Choline Chloride and Propylene Glycol Deep Eutectic Solvent Plating Bath. Sci. Rep. 2022, 12, 18531. [Google Scholar] [CrossRef] [PubMed]
- Ysea, N.B.; Benavente Llorente, V.; Loiácono, A.; Lagucik Marquez, L.; Diaz, L.; Lacconi, G.I.; Franceschini, E.A. Critical Insights from Alloys and Composites of Ni-Based Electrocatalysts for HER on NaCl Electrolyte. J. Alloys Compd. 2022, 915, 165352. [Google Scholar] [CrossRef]
- You, Y.; Gu, C.; Wang, X.; Tu, J. Electrochemical Synthesis and Characterization of Ni–P Alloy Coatings from Eutectic–Based Ionic Liquid. J. Electrochem. Soc. 2012, 159, D642–D648. [Google Scholar] [CrossRef]
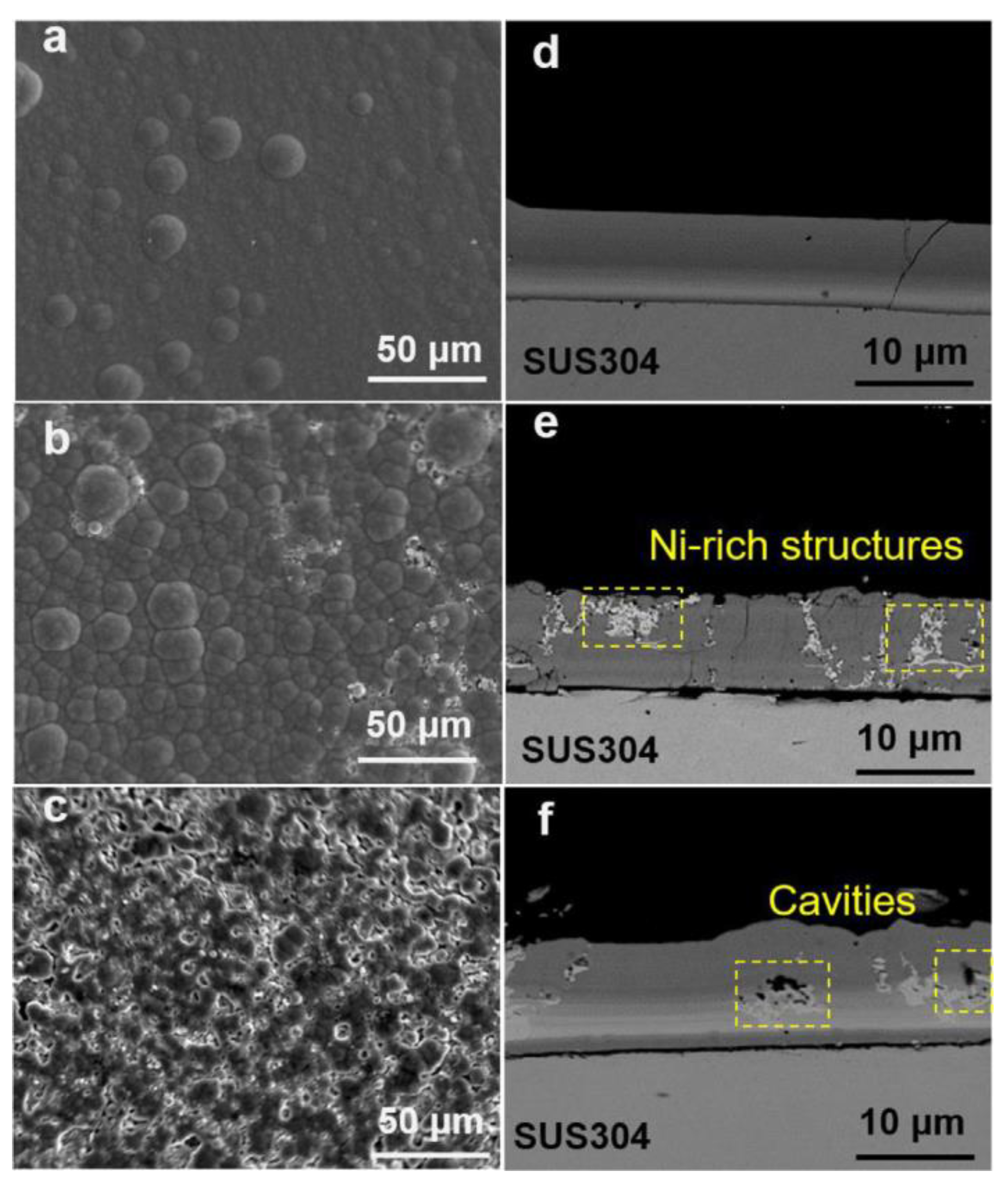
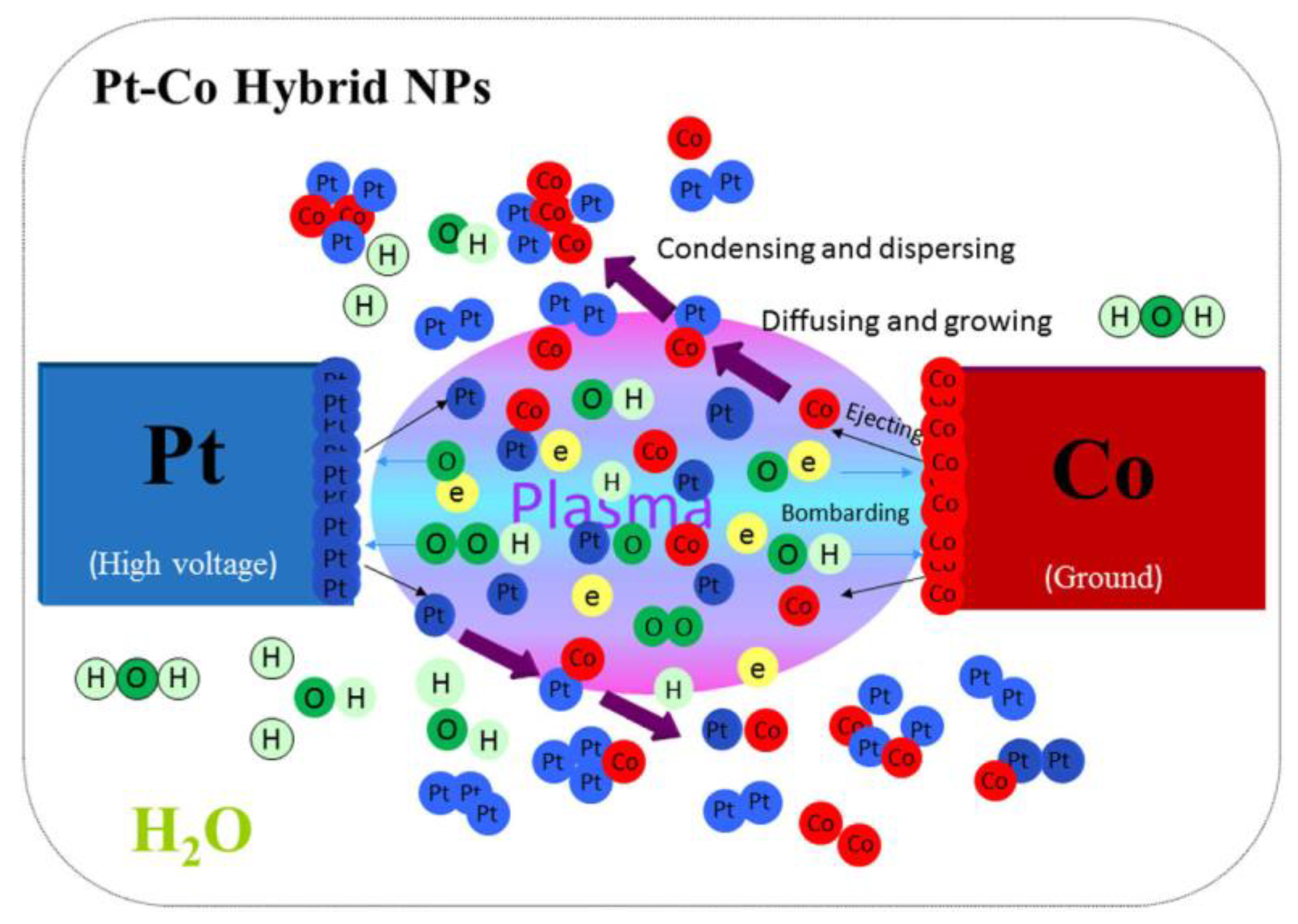
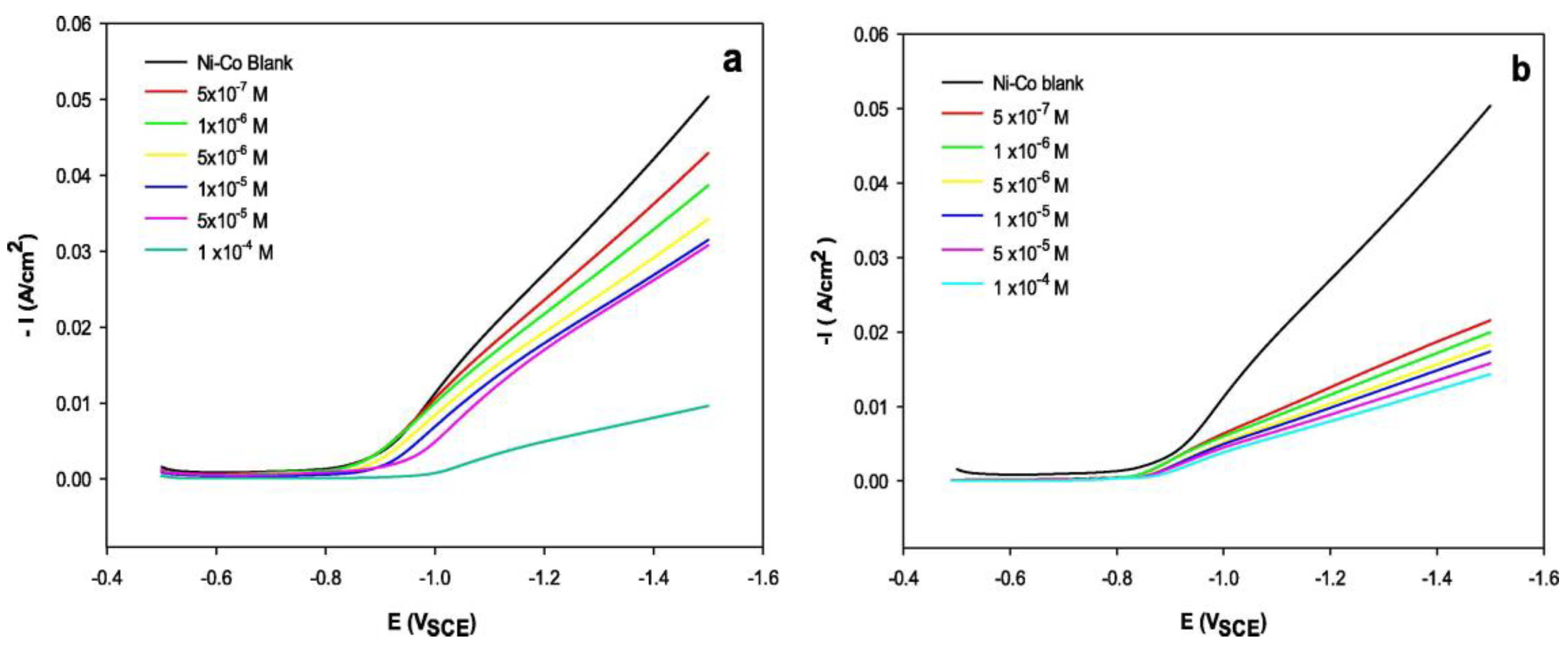
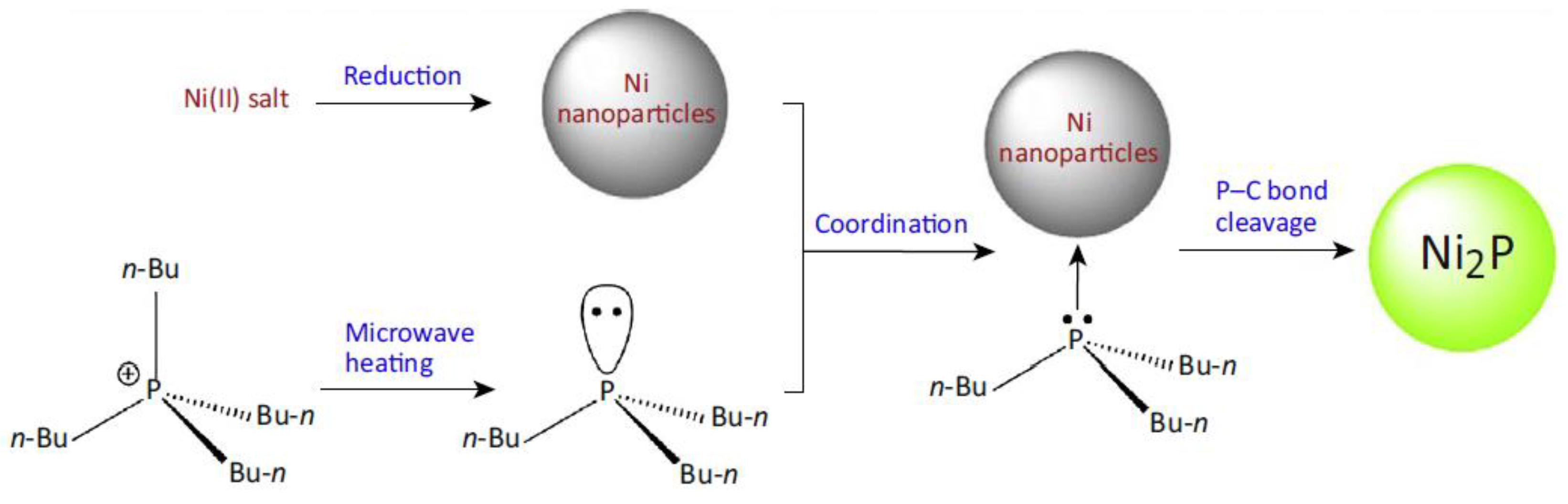

| Parameter | Ref. [3] | Ref. [4] |
|---|---|---|
| Electroplating process parameters | ||
| Substrate | steel | steel |
| Temperature (°C) | 50 | 55 |
| Cathode current density (A/dm2) | 3 | 1 |
| Periodic ultrasound application | no | yes |
| Characteristics of deposits | ||
| Roughness (nm) | 5 | 95 |
| Iron content (wt.%) | 34.55 | 63.00 |
| Grain size (nm) | 4.8 | 11–12 |
| Composition | Cobalt Content | ||
|---|---|---|---|
| Composition of the electrolytic solution | 30 | 50 | 70 |
| Composition of deposited alloy | |||
| 1. without ILs | 38.03 | 59.8 | 79.0 |
| 2. with [MOFIM]I | 35.9 | 65.65. | 85.33 |
| 3. with ([FPIM]Br | 48.96 | 60.76 | 83.62 |
| Electrolyte | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Classic water solutions | low temperature, low cost, fast, good adhesion of deposit, easy to control thickness of the deposit, wide range of metal salt concentration | pH dependent, low current efficiency and porosity due to hydrogen evolution. High concentration of toxic component, the anion formation in the solvent decomposition process can lead to the occurrence of a precipitation with the metal ion | [26,70] |
| ILs | pH independent, deposition metals that are not accessible with conventional aqueous solution, good solvent for organic and inorganic substances | High cost, toxic, different impurities (water et al.) | [3,4,25] |
| DES | Deposition metals that are not accessible with conventional aqueous solution, the anion formation in the solvent decomposition process can not lead to the occurrence of a precipitation with the metal ion, low cost, non toxic, easy preparation, high purity | There are few systematic studies | [15,23,20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, O.; Fishgoit, L.; Knyazev, A.; Kultin, D.; Kustov, L. Electrodeposition of Iron Triad Metal Coatings: Miles to Go. Metals 2023, 13, 657. https://doi.org/10.3390/met13040657
Lebedeva O, Fishgoit L, Knyazev A, Kultin D, Kustov L. Electrodeposition of Iron Triad Metal Coatings: Miles to Go. Metals. 2023; 13(4):657. https://doi.org/10.3390/met13040657
Chicago/Turabian StyleLebedeva, Olga, Larisa Fishgoit, Andrey Knyazev, Dmitry Kultin, and Leonid Kustov. 2023. "Electrodeposition of Iron Triad Metal Coatings: Miles to Go" Metals 13, no. 4: 657. https://doi.org/10.3390/met13040657
APA StyleLebedeva, O., Fishgoit, L., Knyazev, A., Kultin, D., & Kustov, L. (2023). Electrodeposition of Iron Triad Metal Coatings: Miles to Go. Metals, 13(4), 657. https://doi.org/10.3390/met13040657






