Electrooxidation Performance of a Cotton-Cloth-Derived, Ni-Based, Hollow Microtubular Weave Catalytic Electrode for Methanol and Urea
Abstract
:1. Introduction
2. Materials and Methods
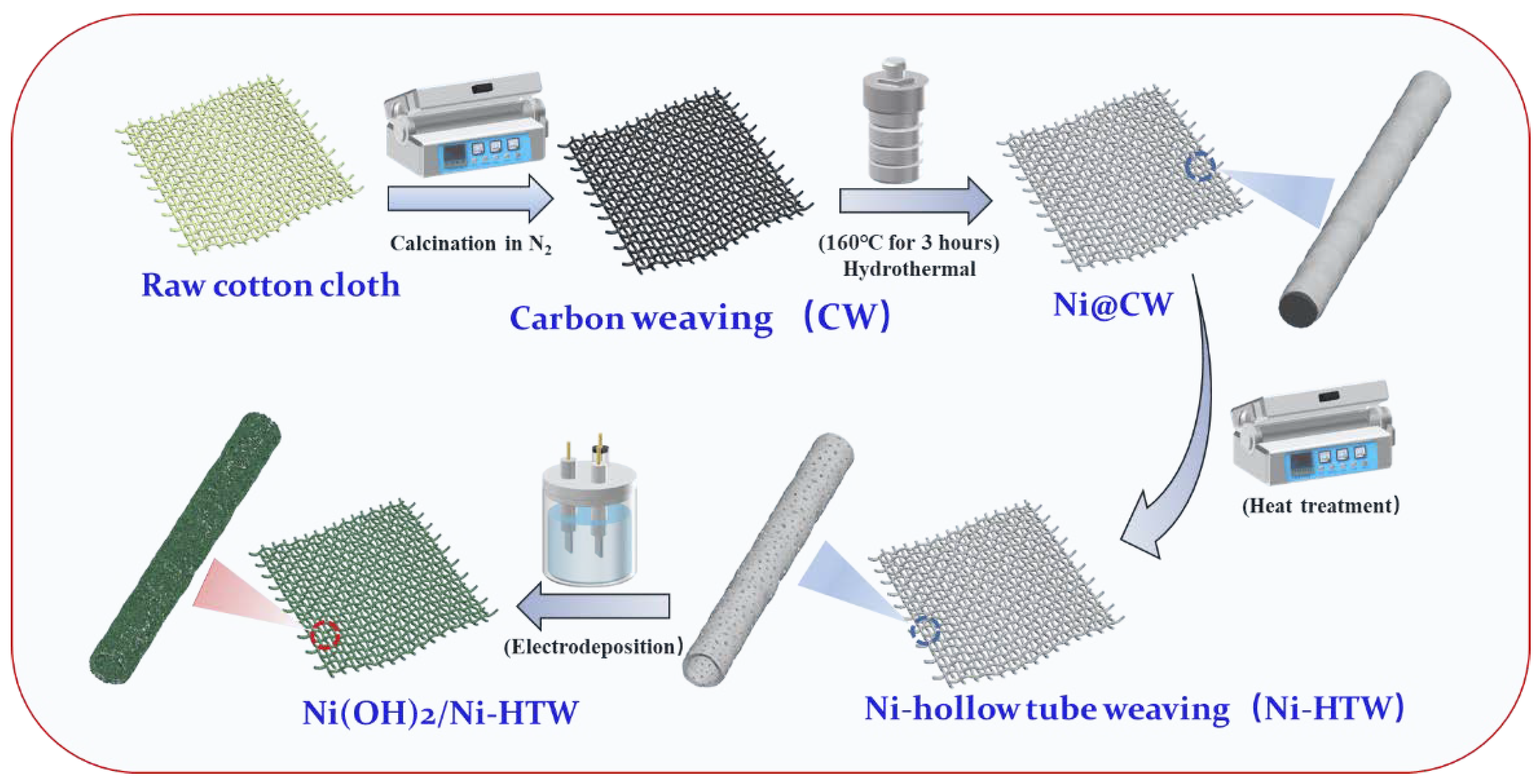
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Test
3. Results and Discussion
3.1. XRD and XPS
3.2. Microstructure
3.3. Electrochemical Performance
3.4. Electrochemical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zan, G.; Wu, Q. An ultra-high-performance anode material for supercapacitors: Self-assembled long CO3O4 hollow tube network with multiple heteroatom (C-, N- and S-) doping. J. Mater. Chem. A 2016, 4, 9097–9105. [Google Scholar] [CrossRef]
- Li, L.; Han, L.; Han, Y.; Yang, Z.; Su, B.; Lei, Z. Preparation and enhanced photocatalytic properties of 3D nanoarchitectural ZnO hollow spheres with porous shells. Nanomaterials 2018, 8, 687. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zhu, X.; Lou, G.; Wu, Y.; Xu, K.; Zhang, Y.; Zhang, L.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable hierarchical porous biomass carbons enriched with pyridinic and pyrrolic nitrogen for asymmetric supercapacitor. Mater. Des. 2018, 149, 184–193. [Google Scholar] [CrossRef]
- Su, B.; Zhong, M.; Han, L.; Wei, M.; Liu, Y.; Yang, H.; Lei, Z. Eco-friendly preparation of hierarchically self-assembly porous ZnO nanosheets for enhanced photocatalytic performance. Mater. Res. Bull. 2020, 124, 110777. [Google Scholar] [CrossRef]
- Jiang, S.; Shao, H.; Cao, G.; Li, H.; Xu, W.; Li, J.; Fang, J.; Wang, X. Waste cotton fabric derived porous carbon containing Fe3O4/NiS nanoparticles for electrocatalytic oxygen evolution. J. Mater. Sci. Technol. 2020, 59, 92–99. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Luo, H.; Zhong, M.; Lei, Z. Biomass-templated preparation of hierarchically multi-functional Ni/NiO@C hollow-fibers. Part. Part. Syst. Charact. 2020, 38, 2000268. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Prasankumar, T.; Jose, S.; Ajayan, P.M.; Ashokkumar, M. Functional carbons for energy applications. Mater. Res. Bull. 2021, 142, 111425. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Li, W.; Zhu, J.; Huang, W. Structure Engineering in Biomass-Derived Carbon Materials for Electrochemical Energy Storage. Research 2020, 2020, 8685436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-H.; Yuan, S.; Bao, D.; Yin, Y.-B.; Zhong, H.-X.; Zhang, X.-B.; Yan, J.-M.; Jiang, Q. Decorating Waste Cloth via Industrial Wastewater for Tube-Type Flexible and Wearable Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1603719. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, B.; Chen, M.; Wang, L.; Fu, X.-Z.; Luo, J.-L. Electrodeposited porous spherical Ni(OH)2@Ni on carbon paper for high-efficiency hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 1540–1547. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Wu, Z.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, e2006292. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system–A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Song, X.; Gao, L.; Li, Y.; Chen, W.; Mao, L.; Yang, J.-H. Nickel phosphate-based materials with excellent durability for urea electro-oxidation. Electrochim. Acta 2017, 251, 284–292. [Google Scholar] [CrossRef]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 12465–12469. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, Y.; Jin, Z.; Duan, Y.; Xu, M.; Yu, J. Facile Synthesis of PtPd Network Structure Nanochains Supported on Multi-Walled Carbon Nanotubes for Methanol Oxidation. Metals 2022, 12, 1911. [Google Scholar] [CrossRef]
- Mozaffari, S.; Najafi, S.; Norouzi, Z. Hierarchical NiO@Ni(OH)2 nanoarrays as high-performance supercapacitor electrode material. Electrochim. Acta 2021, 368, 137633. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Chen, Y.; Xu, Z.; Guan, H. Modulation electronic structure of NiS nanoarray induced by Fe, V doping for high efficiency water and urea electrolysis. J. Ind. Eng. Chem. 2022, 113, 170–180. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Chen, G.; Kim, H.; Kim, S.; Hu, Z.; Chen, J.; Haw, S.; Ciucci, F.; Jung, W. Hierarchical Structure of CuO Nanowires Decorated with Ni(OH)2 Supported on Cu Foam for Hydrogen Production via Urea Electrocatalysis. Small Methods 2022, 6, 2101017. [Google Scholar] [CrossRef]
- Xia, L.; Liao, Y.; Qing, Y.; Xu, H.; Gao, Z.; Li, W.; Wu, Y. In Situ Growth of Porous Ultrathin Ni(OH)2 Nanostructures on Nickel Foam: An Efficient and Durable Catalysts for Urea Electrolysis. ACS Appl. Energy Mater. 2020, 3, 2996–3004. [Google Scholar] [CrossRef]
- Wang, X.; Pan, F.; Sun, X.; Li, Y.; Zhou, J.; Wang, Z.; Qin, C. Performance and Mechanism of Nanoporous Ni@NiO composites for RhB ultrahigh electro-catalytic degradation. Metals 2022, 13, 38. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, J.; Chen, W.; Wu, Y.; Li, S.; Du, X.; Zhang, M. Nickel-cobalt derived nanowires/nanosheets as electrocatalyst for efficient H2 generation via urea oxidation reaction. J. Alloys Compd. 2022, 891, 161790. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, H.; Humayun, M.; Fu, Y.; Xu, X.; Feng, C.; Wang, C. Constructing nanoporous crystalline/amorphous NiFe2O4/NiO electrocatalyst for high efficiency OER/UOR. J. Alloys Compd. 2023, 936, 168206. [Google Scholar] [CrossRef]
- Lv, S.; Yang, F.; Chu, X.; Wang, H.; Yang, J.; Chi, Y.; Yang, X. In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition. Metals 2019, 9, 826. [Google Scholar] [CrossRef] [Green Version]
- Alkan, G.; Košević, M.; Mihailović, M.; Stopic, S.; Friedrich, B.; Stevanović, J.; Panić, V. Characterization of defined Pt particles prepared by ultrasonic spray pyrolysis for one-step synthesis of supported ORR composite catalysts. Metals 2022, 12, 290. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Kim, H.; Song, S.; Fei, L.; Hu, Z.; Lin, H.; Chen, C.; Ciucci, F.; Jung, W. Ni-Doped CuO Nanoarrays Activate Urea Adsorption and Stabilizes Reaction Intermediates to Achieve High-Performance Urea Oxidation Catalysts. Adv. Sci. 2022, 9, 2204800. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Wu, D.; Chen, M.; Liang, Y.; Wang, Q.; Wang, L.; Fu, X.-Z.; Luo, J.-L. In situ facile fabrication of Ni(OH)2 nanosheet arrays for electrocatalytic co-production of formate and hydrogen from methanol in alkaline solution. Appl. Catal. B Environ. 2021, 281, 119510. [Google Scholar] [CrossRef]
- Qin, H.; Ye, Y.; Li, J.; Jia, W.; Zheng, S.; Cao, X.; Lin, G.; Jiao, L. Synergistic Engineering of Doping and Vacancy in Ni(OH)2 to Boost Urea Electrooxidation. Adv. Funct. Mater. 2022, 33, 2209698. [Google Scholar] [CrossRef]
- Xiong, K.; Yu, L.; Xiang, Y.; Zhang, H.; Chen, J.; Gao, Y. Cerium-incorporated Ni2P nanosheets for enhancing hydrogen production from overall water splitting and urea electrolysis. J. Alloys Compd. 2022, 912, 165234. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Hou, C.; Li, B.; Gao, H.; Lin, J.; Luo, X. Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation. Appl. Catal. B Environ. 2019, 259, 118020. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Hu, R.; Zheng, Z.; Zhang, W.; Lu, C.; Deng, Y. Flexible and Conductive Carbonized Cotton Fabrics Coupled with a Nanostructured Ni(OH)2 Coating for High Performance Aqueous Symmetric Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 5231–5239. [Google Scholar] [CrossRef]
- Ko, Y.; Park, J.; Mo, J.; Lee, S.; Song, Y.; Ko, Y.; Lee, H.; Kim, Y.; Huh, J.; Lee, S.; et al. Layer-by-layer assembly-based electrocatalytic fibril electrodes enabling extremely low overpotentials and stable operation at 1 A cm-2 in water-splitting reaction. Adv. Funct. Mater. 2021, 31, 2102530. [Google Scholar] [CrossRef]
- Han, X.; Yu, Y.; Huang, Y.; Liu, D.; Zhang, B. Photogenerated Carriers Boost Water Splitting Activity over Transition-Metal/Semiconducting Metal Oxide Bifunctional Electrocatalysts. ACS Catal. 2017, 7, 6464–6470. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894–1897. [Google Scholar] [CrossRef] [Green Version]
- Gultom, N.S.; Abdullah, H.; Hsu, C.-N.; Kuo, D.-H. Activating nickel iron layer double hydroxide for alkaline hydrogen evolution reaction and overall water splitting by ele哎ctrodepositing nickel hydroxide. Chem. Eng. J. 2021, 419, 129608. [Google Scholar] [CrossRef]
- Ming, L.; Wu, X.-Y.; Wang, S.-S.; Wu, W.; Lu, C.-Z. Facile growth of transition metal hydroxide nanosheets on porous nickel foam for efficient electrooxidation of benzyl alcohol. Green Chem. 2021, 23, 7825–7830. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Zhang, P.; Ma, H.; Gao, Z.; Wang, H.; Lu, Y.; He, J.; Zhao, Y. Transition metal ions regulated oxygen evolution reaction performance of Ni-based hydroxides hierarchical nanoarrays. Sci. Rep. 2017, 7, 46154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Li, W.; Liu, L. Vertically Aligned Porous Nickel(II) Hydroxide Nanosheets Supported on Carbon Paper with Long-Term Oxygen Evolution Performance. Chem.—Asian J. 2017, 12, 543–551. [Google Scholar] [CrossRef]
- Tao, S.; Yang, F.; Schuch, J.; Jaegermann, W.; Kaiser, B. Electrodeposition of Nickel Nanoparticles for the Alkaline Hydrogen Evolution Reaction: Correlating Electrocatalytic Behavior and Chemical Composition. Chemsuschem 2018, 11, 948–958. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Tian, Q.; Liu, M.; Wang, X.; Li, P.; Li, W.; Cai, N.; Chen, W.; Yu, F. Papillae-like morphology of Ni/Ni(OH)2 hybrid crystals by stepwise electrodeposition for synergistically improved HER. Crystengcomm 2019, 21, 3431–3438. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Li, Y.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Sun, J.; Xu, P. A crystalline–amorphous Ni–Ni(OH)2 core–shell catalyst for the alkaline hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 23323–23329. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Z.; Zhang, F.; Duan, D.; Li, Y.; Wei, G.; Liu, S.; Yuan, Q.; Wang, E.; Hao, X. New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media. Catalysts 2019, 9, 749. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Kang, L.; Zhao, T.; Feng, J.; Celorrio, V.; Zhang, G.; Cibin, G.; Kucernak, A.; Brett, D.J.L.; Corà, F.; et al. Identification and manipulation of dynamic active site deficiency-induced competing reactions in electrocatalytic oxidation processes. Energy Environ. Sci. 2022, 15, 2386–2396. [Google Scholar] [CrossRef]
- Chen, F.; Ho, Y.; Chang, H.; Tsai, Y. Nanocomposite integrating tube-like NiCo2S4 and carbon nanotubes for electrooxidation of methanol. Electrochem. Commun. 2020, 117, 106783. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, X.; Yang, J.; Liu, J.; Zhang, L.; Zhang, L. Three-dimensional Ni-MoN nanorod array as active and non-precious metal electrocatalyst for methanol oxidation reaction. Electroanal. Chem. 2022, 906, 116001. [Google Scholar] [CrossRef]
- Sreekanth, T.; Tamilselvan, M.; Yoo, K.; Kim, J. Microwave-assisted in situ growth of VO2 nanoribbons on Ni foam as inexpensive bifunctional electrocatalysts for the methanol oxidation and oxygen evolution reactions. Appl. Surf. Sci. 2021, 570, 151119. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.; Cho, M.; Seo, J. Electrochemical deposition of self-supported bifunctional copper oxide electrocatalyst for methanol oxidation and oxygen evolution reaction. J. Ind. Eng. Chem. 2019, 76, 515–523. [Google Scholar] [CrossRef]
- Wu, N.; Guo, R.; Zhang, X.; Gao, N.; Chi, X.; Cao, D.; Hu, T. Nickel/nickel oxide nanocrystal nitrogen-doped carbon composites as efficient electrocatalysts for urea oxidation. J. Alloy. Compd. 2021, 870, 159408. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Xue, Y.; Miao, B.; Li, S.; Jiang, Y.; Liu, X.; Chen, Y. Atomically thick Ni(OH)2 nanomeshes for urea electrooxidation. Nanoscale 2019, 11, 1058–1064. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, W.; Li, M.; Yang, C.; Zhang, N. Ultrafast formation of an FeOOH electrocatalyst on Ni for efficient alkaline water and urea oxidation. Chem. Commun. 2020, 56, 14713–14716. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Shen, Z.; Tang, Y.; Chen, Q.; Cao, H.; Zhang, H.; Zheng, G.; Zhang, J. Ni-WC nanoparticles/carbon aerogel electrocatalytic electrode for methanol and urea electrooxidation. Int. J. Hydrog. Energy 2023, 48, 991–1000. [Google Scholar] [CrossRef]
- Zhan, J.; Cai, M.; Zhang, C.; Wang, C. Synthesis of mesoporous NiCO2O4 fibers and their electrocatalytic activity on direct oxidation of ethanol in alkaline media. Electrochim. Acta 2015, 154, 70–76. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, G.; Wei, J.; Chen, Q.; Zhang, J.; Tang, Y. Electrooxidation Performance of a Cotton-Cloth-Derived, Ni-Based, Hollow Microtubular Weave Catalytic Electrode for Methanol and Urea. Metals 2023, 13, 659. https://doi.org/10.3390/met13040659
Hou G, Wei J, Chen Q, Zhang J, Tang Y. Electrooxidation Performance of a Cotton-Cloth-Derived, Ni-Based, Hollow Microtubular Weave Catalytic Electrode for Methanol and Urea. Metals. 2023; 13(4):659. https://doi.org/10.3390/met13040659
Chicago/Turabian StyleHou, Guangya, Jiaxuan Wei, Qiang Chen, Jianli Zhang, and Yiping Tang. 2023. "Electrooxidation Performance of a Cotton-Cloth-Derived, Ni-Based, Hollow Microtubular Weave Catalytic Electrode for Methanol and Urea" Metals 13, no. 4: 659. https://doi.org/10.3390/met13040659





