Influence of the Alloying Elements on the Corrosion Behavior of As-Cast Magnesium–Gallium–Zinc Alloys in Simulated Body Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloys Manufacturing
2.2. Evaluation of Corrosion and Bioactivity
3. Results and Discussion
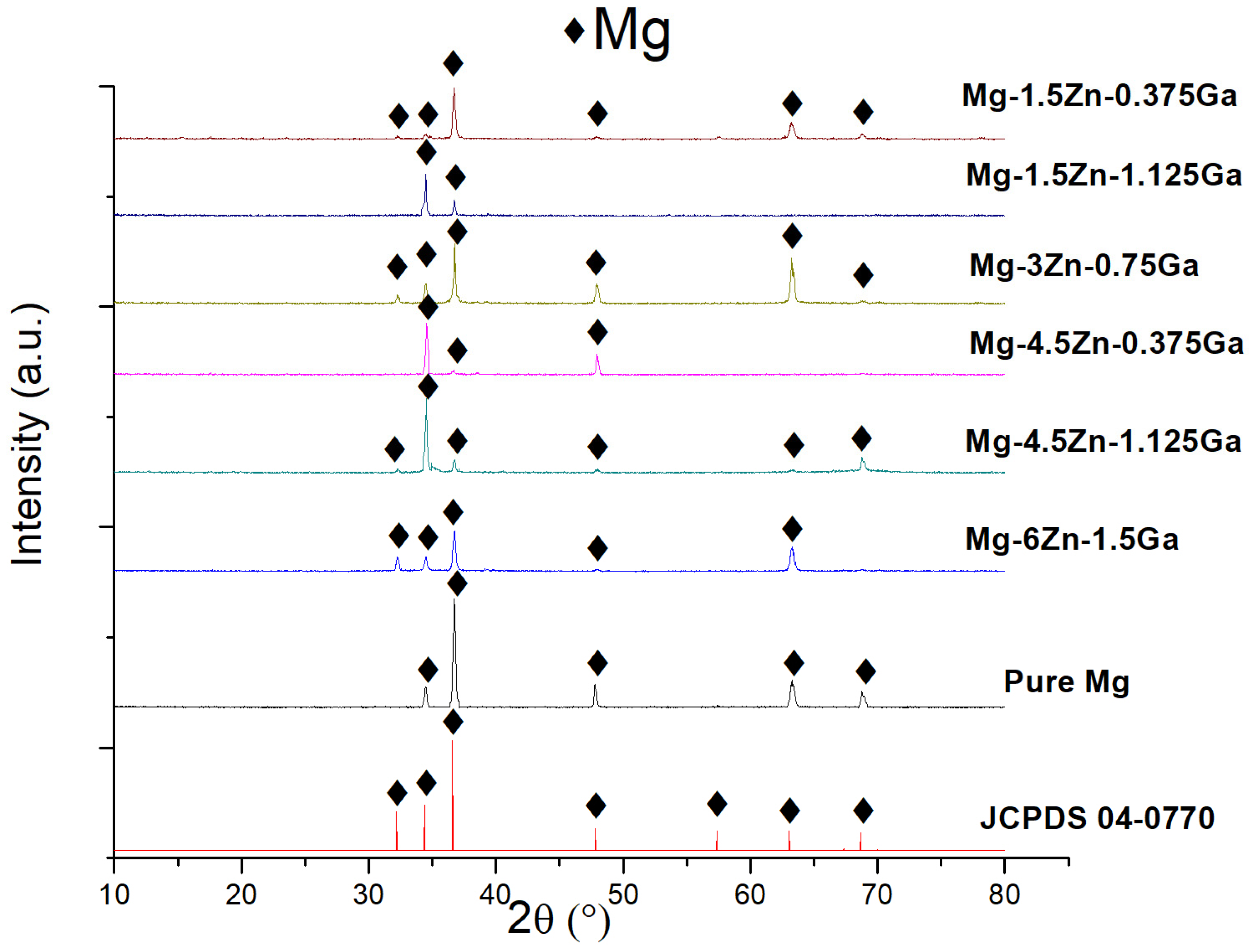
3.1. Microstructural Analysis
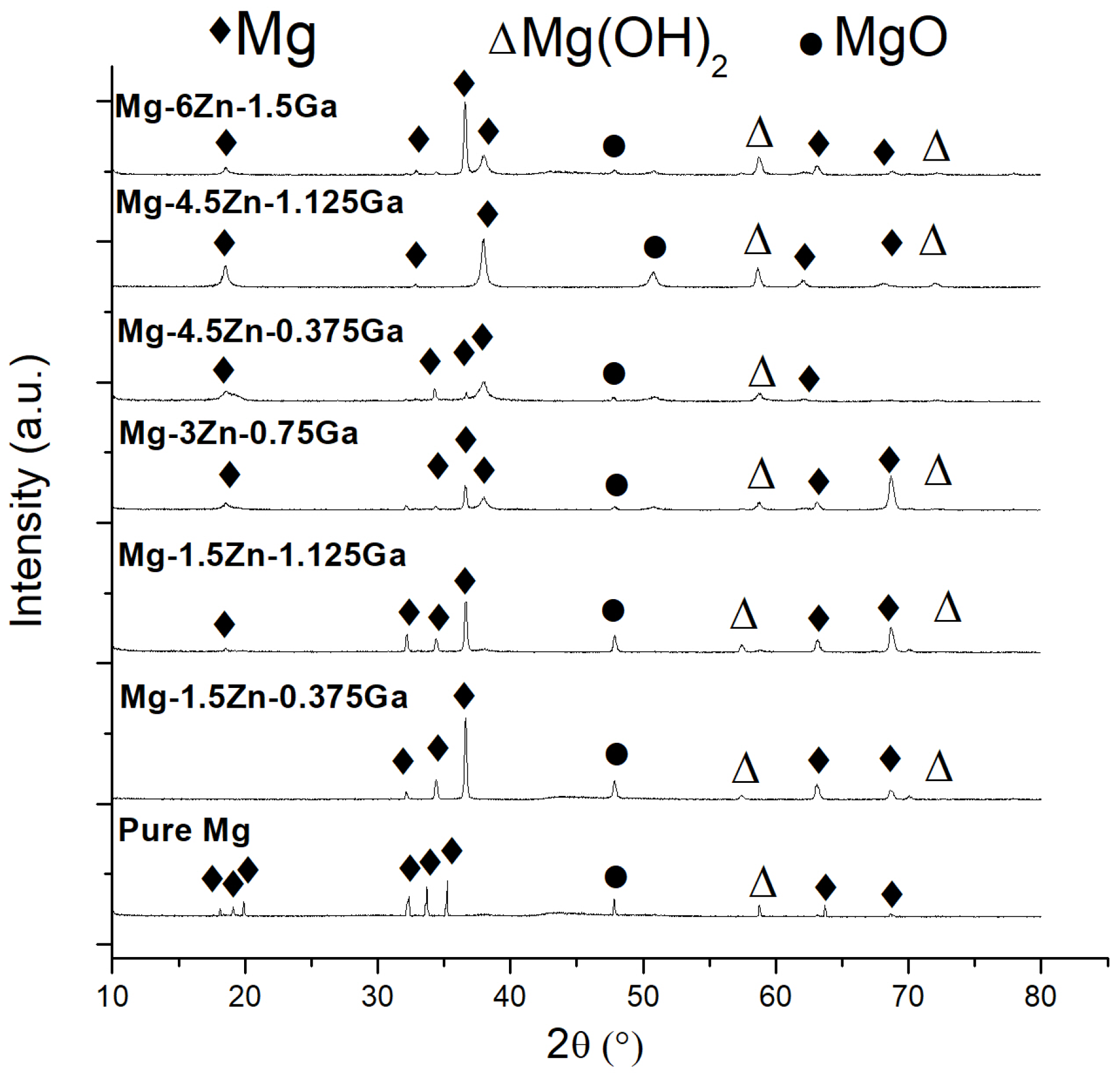
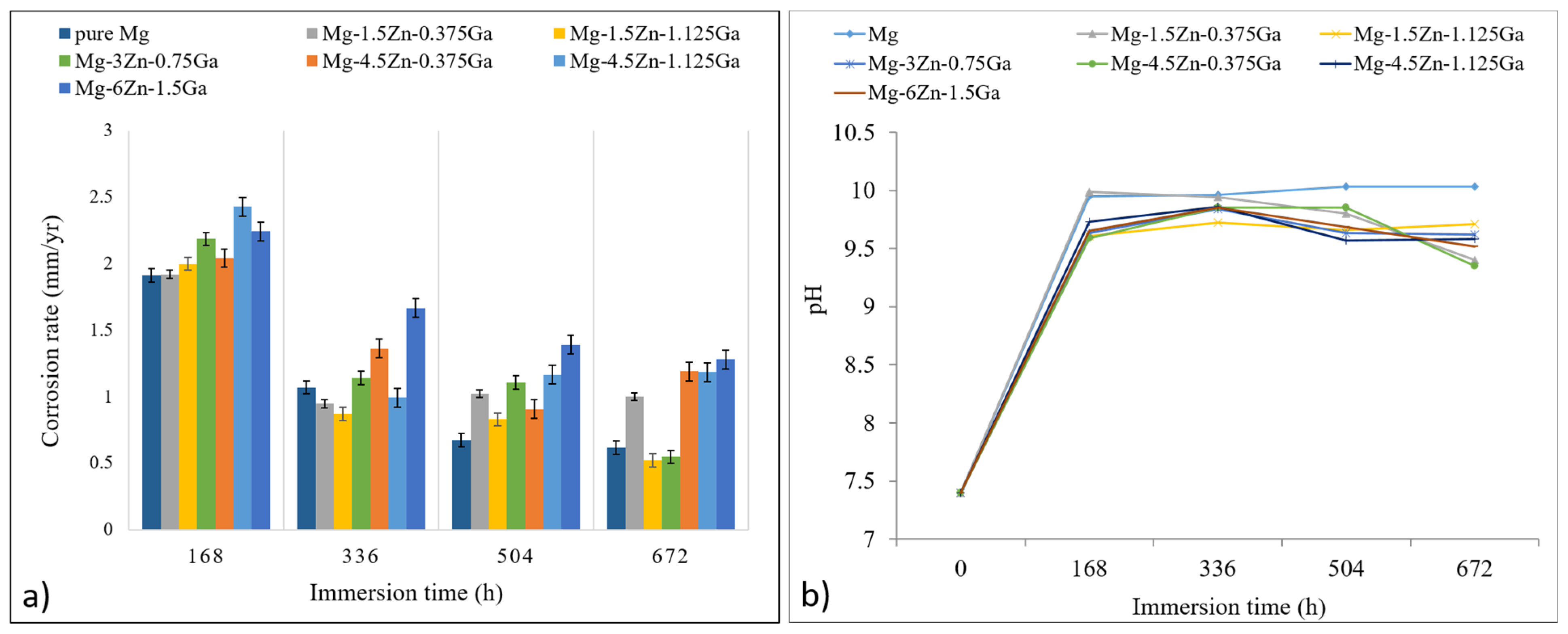
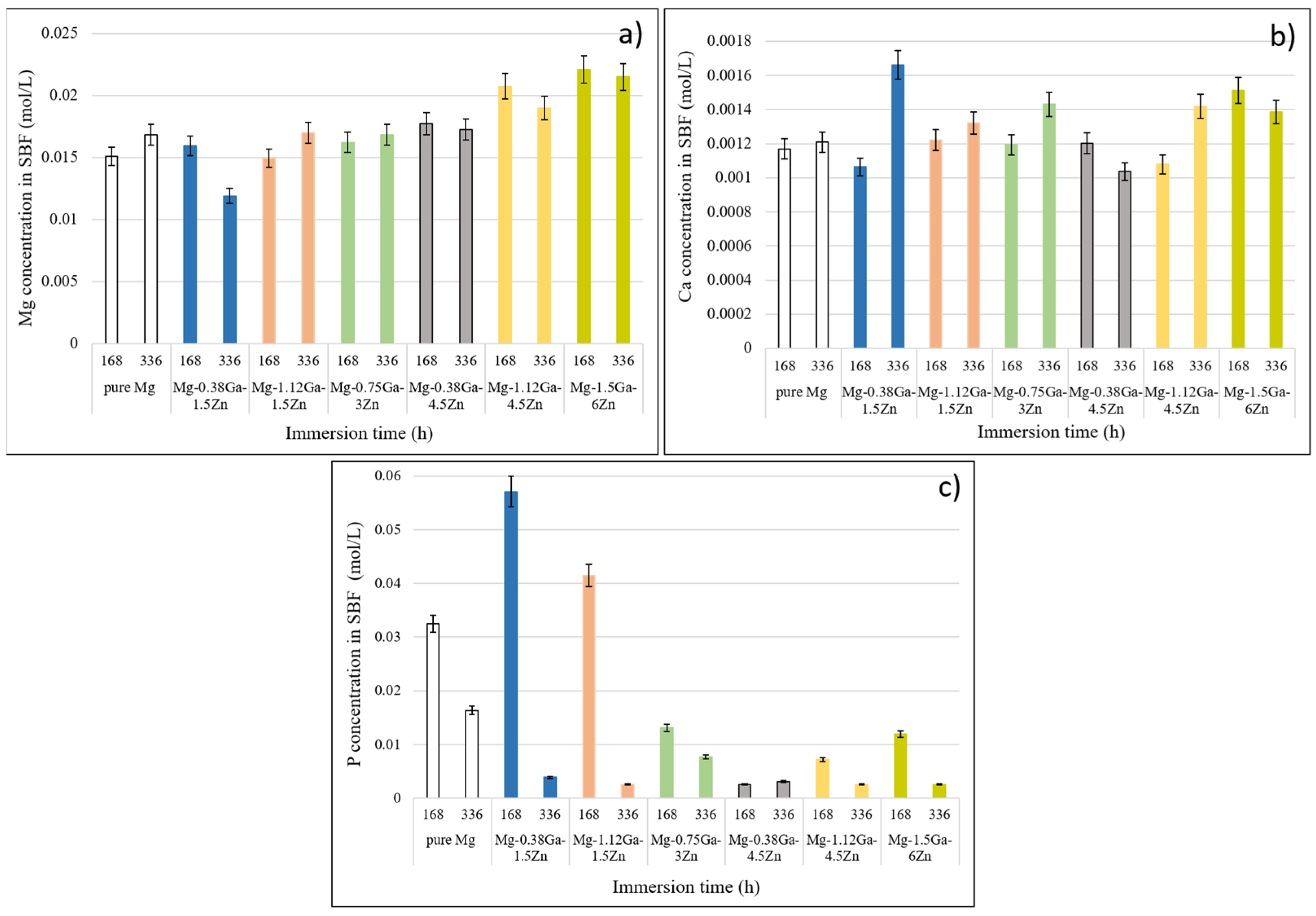
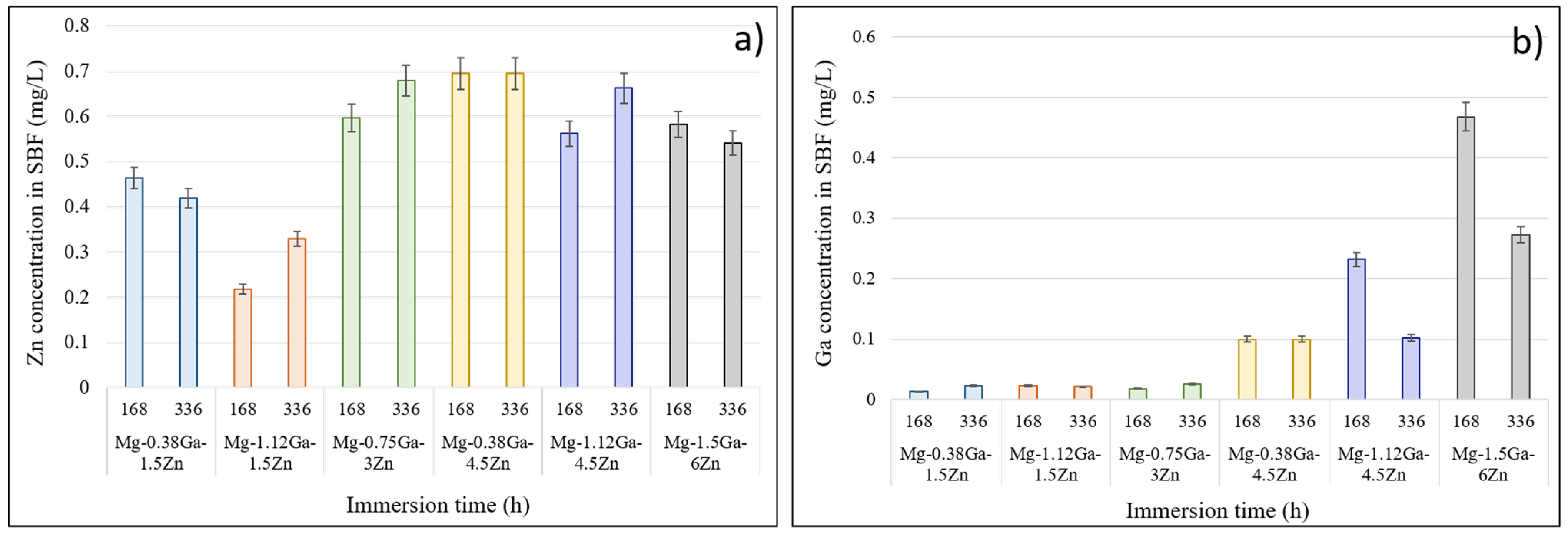
3.2. Degradation of the Mg-Ga-Zn Alloys in SBF
3.3. Degradation Rate Measurement
3.4. Formation of Ca−P Coatings on the Alloys Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra, G.; Pandey, A. Preparation Strategies for Mg-Alloys for Biodegradable Orthopaedic Implants and Other Biomedical Applications: A Review. IRBM 2022, 43, 229–249. [Google Scholar] [CrossRef]
- Stanciu, L.; Diaz-Amaya, S. Biodegradable materials for medical applications. Introd. Biomater. 2022, 307–346. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Ibrahim, H.; Esfahani, S.N.S.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Reports 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloy. 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 116–134. [Google Scholar]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Grillo, C.A.; Alvarez, F.; Fernández Lorenzo de Mele, M.A. Degradation of bioabsorbable Mg-based alloys: Assessment of the effects of insoluble corrosion products and joint effects of alloying components on mammalian cells. Mater. Sci. Eng. C 2016, 58, 372–380. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, R.; Chen, R.; Liu, C.; Gao, J. Preparation of calcium phosphate coatings on Mg-1.0Ca alloy. Trans. Nonferr. Met. Soc. China 2010, 20, s655–s659. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloy. 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Keim, S.; Brunner, J.G.; Fabry, B.; Virtanen, S. Control of magnesium corrosion and biocompatibility with biomimetic coatings. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2011, 96 B, 84–90. [Google Scholar] [CrossRef]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng.—Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, M.; Gao, J.; Hu, J.; Zhang, Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008, 62, 2181–2184. [Google Scholar] [CrossRef]
- Kuwahara, H.; Al-Abdullat, Y.; Mazaki, N.; Tsutsumi, S.; Aizawa, T. Precipitation of Magnesium Apatite on Pure Magnesium Surface during Immersing in Hank´s Solution. Mater. Trans. 2001, 42, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of Corrosion-Resistant Conversion Coatings for Magnesium and Its Alloys. Corrosion 2011, 67, 35005–35016. [Google Scholar] [CrossRef]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Almanza-Robles, J.M. Effect of gallium content and heat treatment on the microstructure and corrosion rate of magnesium binary alloys. Metals 2019, 9, 990. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, L.; Tanner, T.; Godfrey, C.; Noll, B. Chemistry and Pharmacokinetics of Gallium Maltolate, a Compound With High Oral Gallium Bioavailability. Met. Based. Drugs 2000, 7, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.Y.; Lian, J.S.; Niu, L.Y.; Jiang, Z.H.; Jiang, Q. Growth of zinc phosphate coatings on AZ91D magnesium alloy. Surf. Coatings Technol. 2006, 201, 1814–1820. [Google Scholar] [CrossRef]
- Dong, J.; Tümer, N.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zadpoor, A.A.; Zhou, J. Extrusion-based additive manufacturing of Mg-Zn alloy scaffolds. J. Magnes. Alloy. 2022, 10, 2491–2509. [Google Scholar] [CrossRef]
- Pham, D.N.; Hiromoto, S.; Yamazaki, T.O.M.; Kobayashi, E. Enhanced Corrosion Resistance and In Vitro Biocompatibility of Mg-Zn Alloys by Carbonate Apatite Coating. Appl. Bio Mater. 2021, 4, 6881–6892. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Qiao, Y.; Wang, X.; Lin, Q. Preparation of medical Mg–Zn alloys and the effect of different zinc contents on the alloy. J. Mater. Sci. Mater. Med. 2022, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg-X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 2421–2432. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Vazquez-Montiel, R.; Peralta-Montes, J.S.; Almanza-Robles, J.M. Microstructure, corrosion rate, and mechanical properties of unidirectionally and cross-rolled Mg-0.375Ga and Mg-0.750Ga alloys. J. Biomed. Mater. Res. B. Appl. Biomater. 2022, 110, 646–659. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- G31-12a; ASTM. Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–10. [CrossRef]
- ASTM_G-1; ASTM. G1-17 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Ga-Mg (Gallium-Magnesium) system. Bull. Alloy Phase Diagr. 1985, 6, 434–439. [Google Scholar] [CrossRef]
- Kevorkov, D.; Pekguleryuz, M. Experimental study of the Ce-Mg-Zn phase diagram at 350 °C via diffusion couple techniques. J. Alloy. Compd. 2009, 478, 427–436. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion. A framework for improved alloy performance. Adv. Eng. Mater. 2009, 5, 837–858. [Google Scholar] [CrossRef]
- Chen, J.; Ai, M.; Wang, J.; Han, E.H.; Ke, W. Formation of hydrogen blister on AZ91 magnesium alloy during cathodic charging. Corros. Sci. 2009, 51, 1197–1200. [Google Scholar] [CrossRef]
- Schmutz, P.; Guillaumin, V.; Lillard, R.S.; Lillard, J.A.; Frankel, G.S. Influence of Dichromate Ions on Corrosion Processes on Pure Magnesium. J. Electrochem. Soc. 2003, 150, B99. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.D.; Covino, B.S.; ASM International Handbook Committee. ASM Handbook. Volume 13C, Corrosion: Environments and Industries; ASM International: Materials Park, OH, USA, 2006; Available online: https://ansto.on.worldcat.org/oclc/1013920715 (accessed on 6 April 2020).
- Kutz, M. Handbook of Environmental Degradation of Materials: Second Edition; Elsevier: London, UK, 2013; pp. 1–910. [Google Scholar] [CrossRef]
- López-Buisán Natta, M.G. Evidence of two anodic processes in the polarization curves of magnesium in aqueous media. Corrosion 2001, 57, 712–720. [Google Scholar] [CrossRef]
- Mayyas, M.; Mousavi, M.; Ghasemian, M.B.; Abbasi, R.; Li, H.; Christoe, M.J.; Han, J.; Wang, Y.; Zhang, C.; Rahim, M.A.; et al. Pulsing liquid alloys for nanomaterials synthesis. ACS Nano 2020, 14, 14070–14079. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Hofstetter, J.; Uggowitzer, P.J.; Song, G.; Liu, M.; Atrens, A. Corrosion of ultra-high-purity Mg in 3.5% NaCl solution saturated with Mg(OH)2. Corros. Sci. 2013, 75, 78–99. [Google Scholar] [CrossRef]
- Chou, Y.F.; Chiou, W.A.; Xu, Y.; Dunn, J.C.Y.; Wu, B.M. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials 2004, 25, 5323–5331. [Google Scholar] [CrossRef]
- Kibalczyc, W.; Christoffersen, J.; Christoffersen, M.R.; Zielenkiewicz, A.; Zielenkiewicz, W. The effect of magnesium ions on the precipitation of calcium phosphates. J. Cryst. Growth 1990, 106, 355–366. [Google Scholar] [CrossRef]
- Donnelly, R.; Boskey, A. The effect of gallium on seeded hydroxyapatite growth. Calcif. Tissue Int. 1989, 44, 138–142. [Google Scholar] [CrossRef]
- Roohani, I.; Cheong, S.; Wang, A. How to build a bone?—Hydroxyapatite or Posner’s clusters as bone minerals. Open Ceram. 2020, 6, 100092. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H.; Barroug, A. Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. C 2007, 27, 198–205. [Google Scholar] [CrossRef] [Green Version]
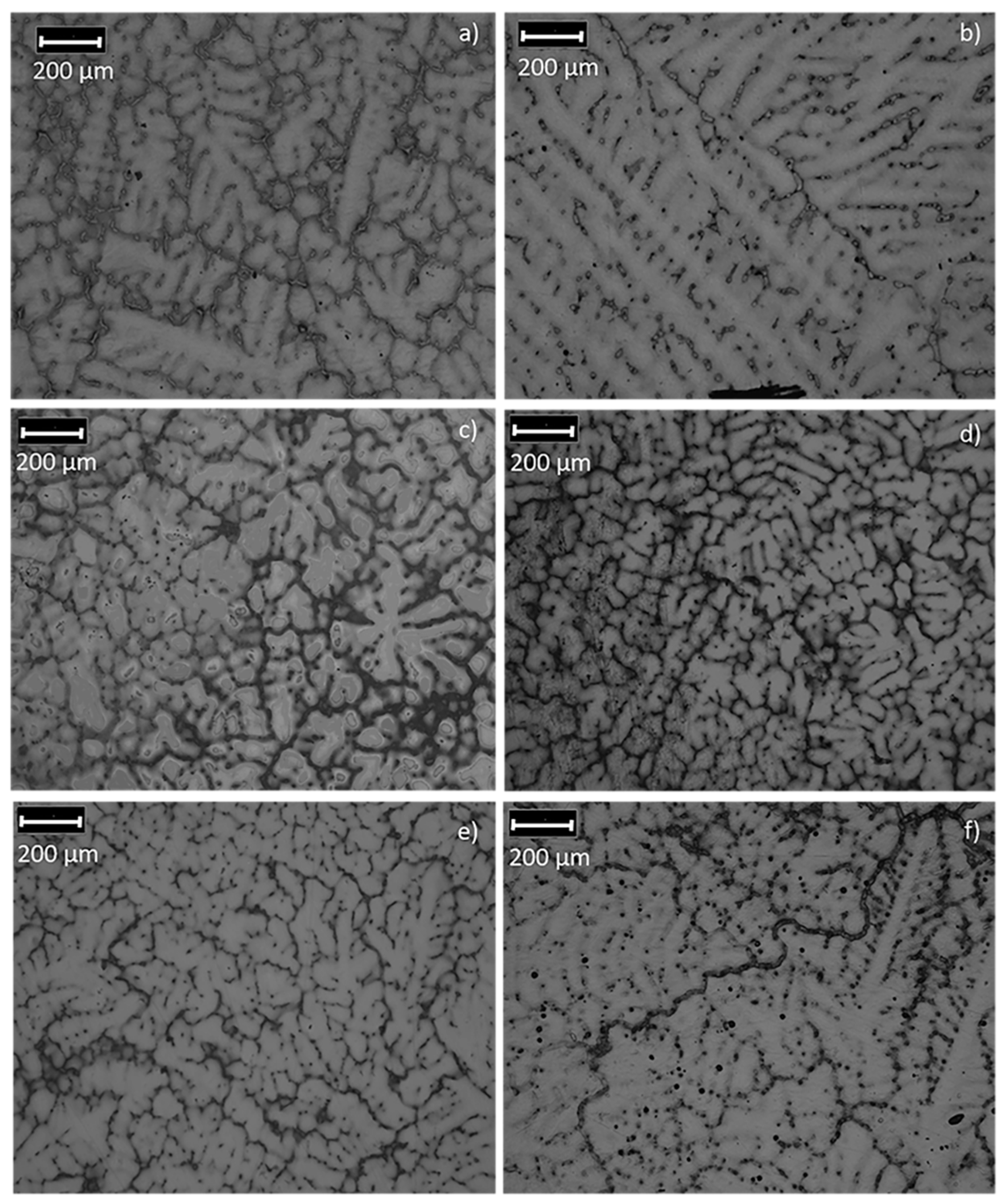
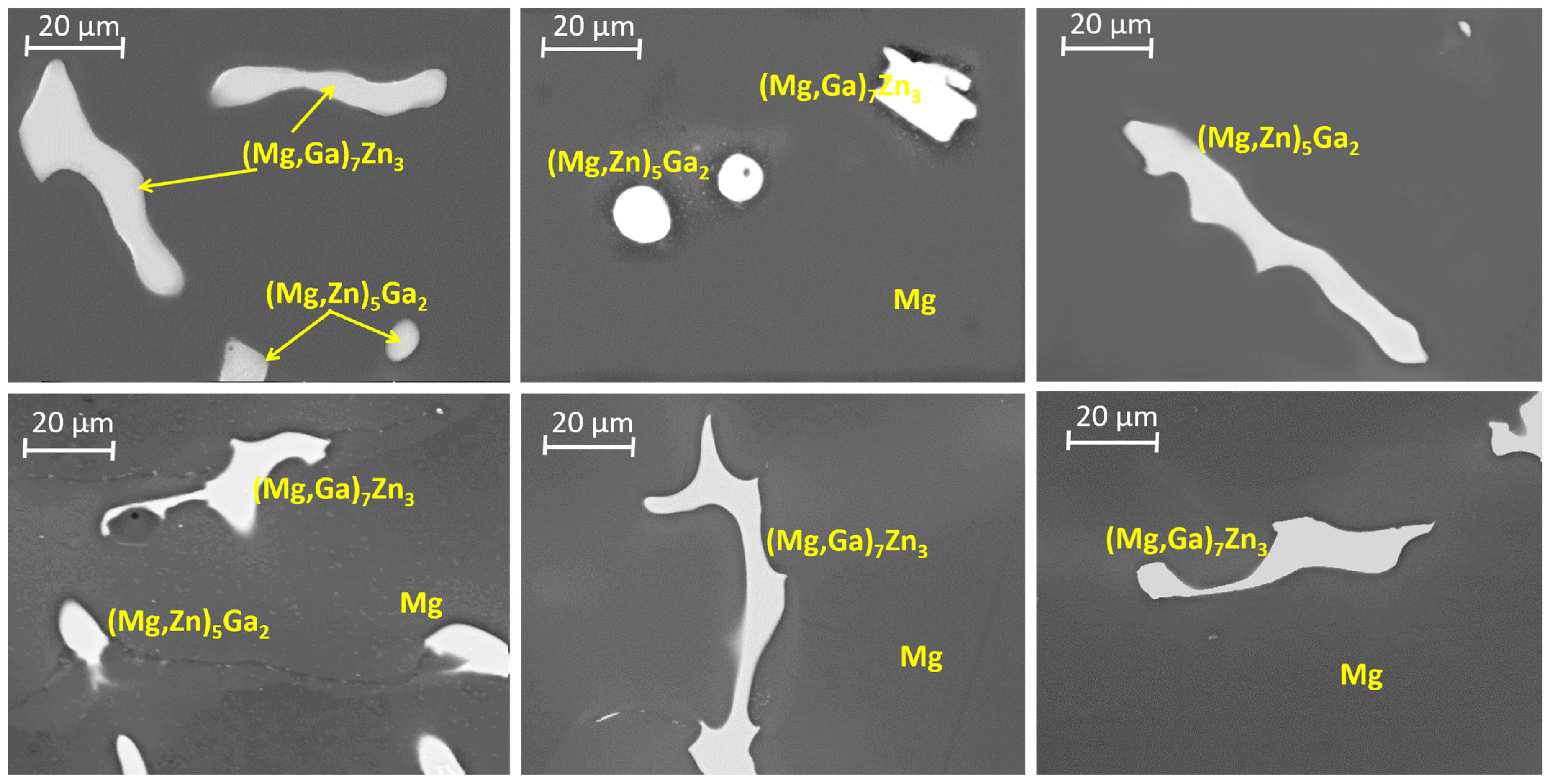

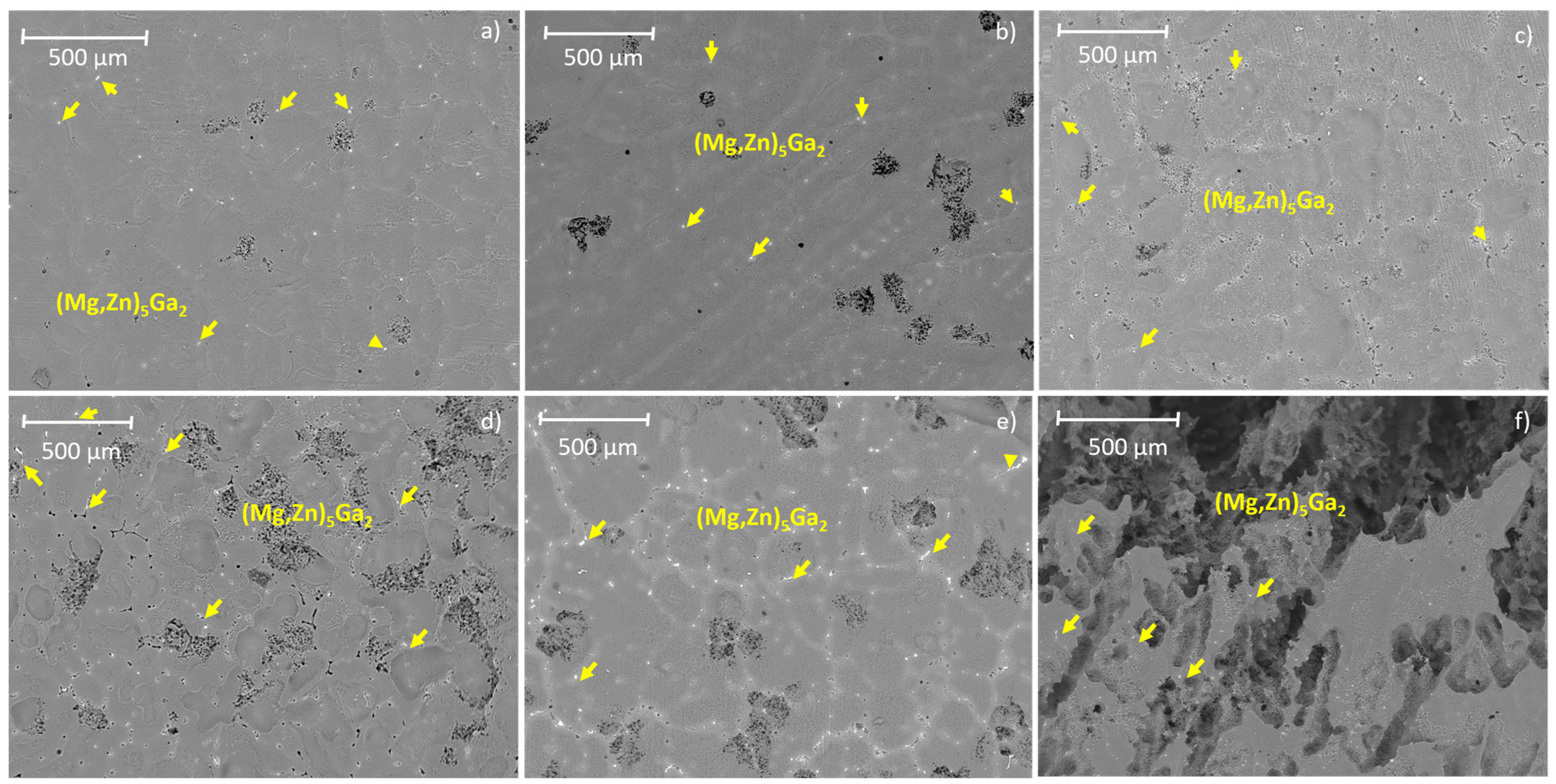

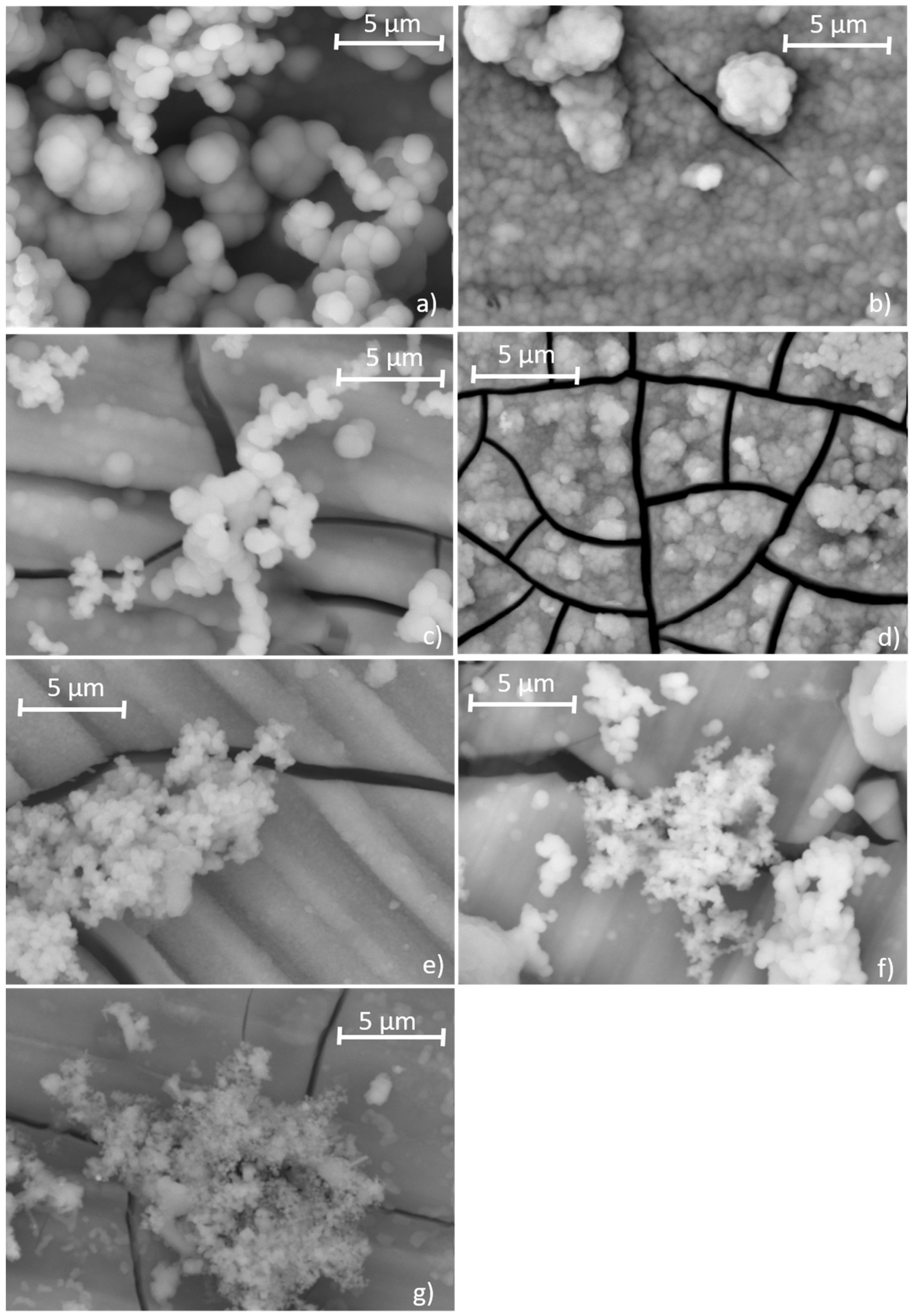
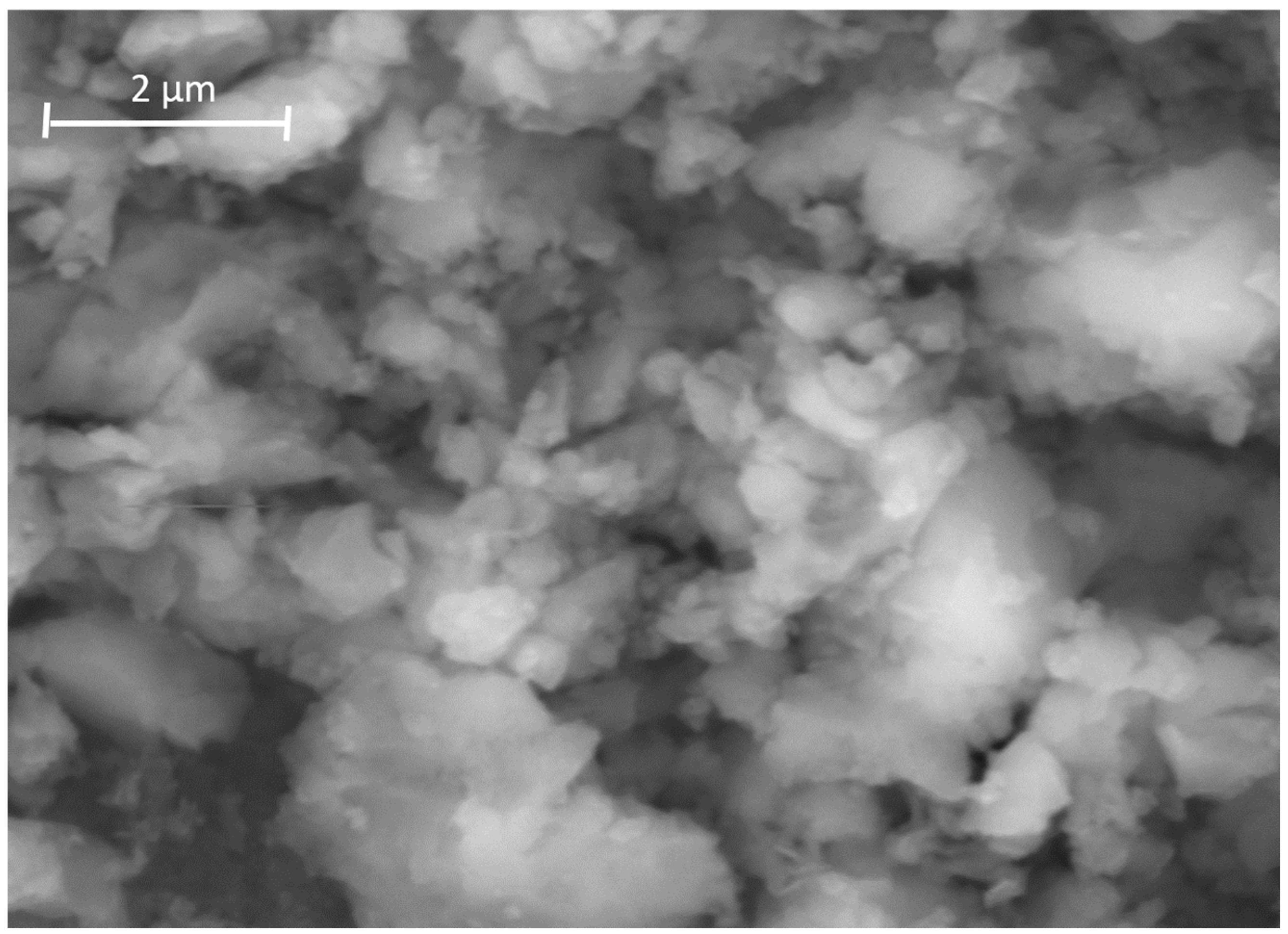
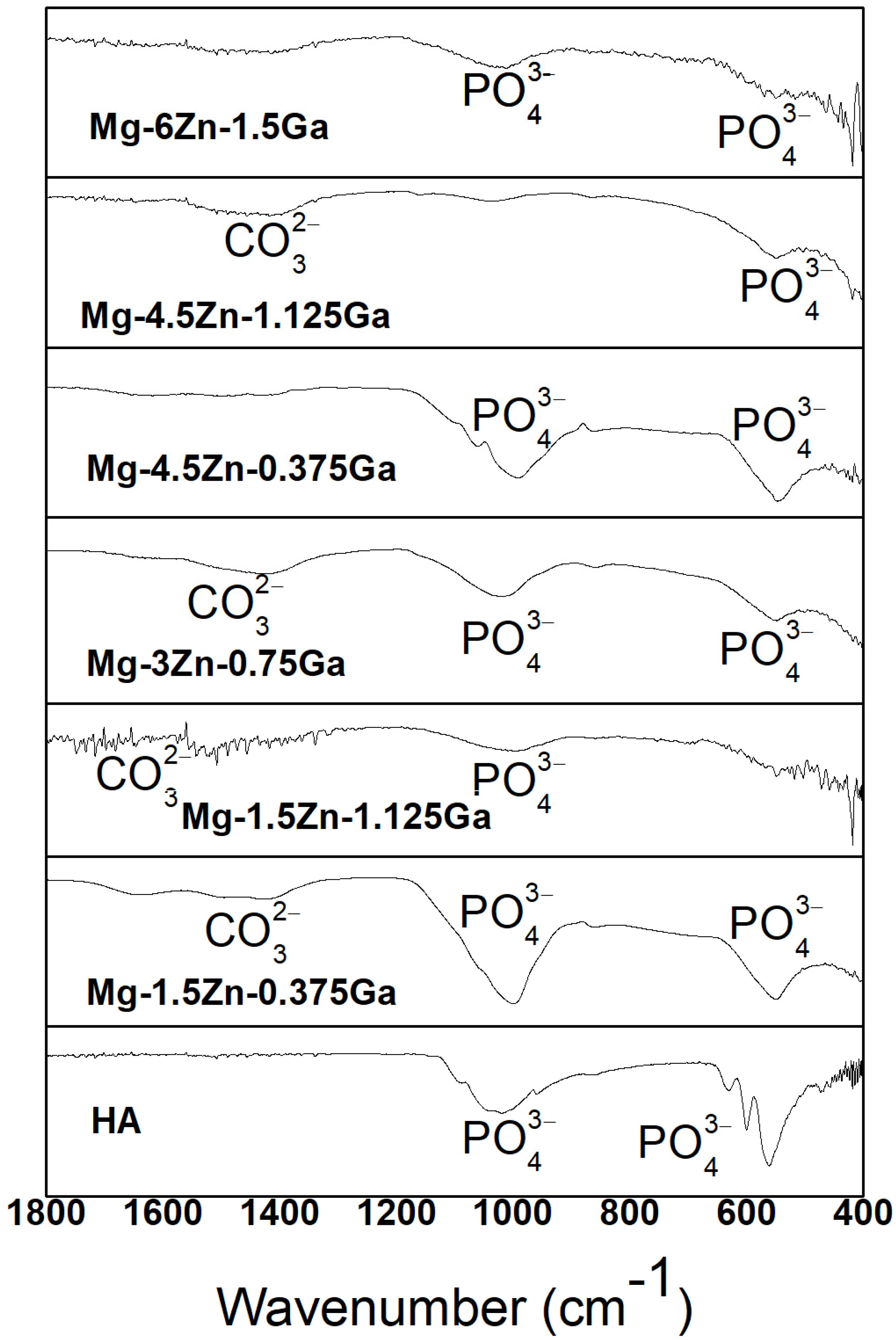
| Nominal Composition | Mg | Ga | Zn | Fe | Ni | Cu | Grain Size (μm) |
|---|---|---|---|---|---|---|---|
| pure Mg | 99.99 | 0 | ≤0.0002 | ≤0.0006 | ≤0.0002 | 530 | |
| Mg-0.38Ga-1.5Zn | 98.38 | 0.35 | 1.35 | – | – | – | 477 |
| Mg-1.12Ga-1.5Zn | 97.44 | 1.12 | 1.43 | – | – | – | 296 |
| Mg-0.75Ga-3Zn | 96.82 | 0.65 | 2.54 | – | – | – | 246 |
| Mg-0.38Ga-4.5Zn | 95.62 | 0.41 | 3.89 | – | – | – | 189 |
| Mg-1.12Ga-4.5Zn | 94.71 | 1.12 | 4.15 | – | – | – | 184 |
| Mg-1.5Ga-6Zn | 93 | 1.4 | 5.6 | – | – | – | 141 |
| Ion | SBF K-9 | Human Blood Plasma |
|---|---|---|
| Na+ | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 |
| Mg2+ | 1.5 | 1.5 |
| Ca2+ | 2.5 | 2.5 |
| Cl¯ | 147.8 | 103.0 |
| HC | 4.2 | 27.0 |
| H | 1.0 | 1.0 |
| Alloy | Ca | P | Ca/P Ratio |
|---|---|---|---|
| Mg-0.38Ga-1.5Zn | 27.86 | 16.60 | 1.28 |
| Mg-1.12Ga-1.5Zn | 27.05 | 16.38 | 1.26 |
| Mg-0.75Ga-3Zn | 27.95 | 16.26 | 1.29 |
| Mg-0.38Ga-4.5Zn | 28.02 | 16.49 | 1.30 |
| Mg-1.12Ga-4.5Zn | 26.98 | 16.22 | 1.20 |
| Mg-1.5Ga-6Zn | 28.44 | 16.17 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A. Influence of the Alloying Elements on the Corrosion Behavior of As-Cast Magnesium–Gallium–Zinc Alloys in Simulated Body Fluid. Metals 2023, 13, 743. https://doi.org/10.3390/met13040743
Hernández-Cortés AA, Escobedo-Bocardo JC, Cortés-Hernández DA. Influence of the Alloying Elements on the Corrosion Behavior of As-Cast Magnesium–Gallium–Zinc Alloys in Simulated Body Fluid. Metals. 2023; 13(4):743. https://doi.org/10.3390/met13040743
Chicago/Turabian StyleHernández-Cortés, Anabel A., José C. Escobedo-Bocardo, and Dora A. Cortés-Hernández. 2023. "Influence of the Alloying Elements on the Corrosion Behavior of As-Cast Magnesium–Gallium–Zinc Alloys in Simulated Body Fluid" Metals 13, no. 4: 743. https://doi.org/10.3390/met13040743






