Abstract
Increasing the strength of Al-based alloys is an important issue of physical metallurgy and industrial processing. Severe plastic deformation and related extension of solid solubility during mechanical alloying provide an opportunity for significant strengthening due to grain refinement, solid solution, and precipitation strengthening mechanisms. During mechanical alloying, an anomalous increase in the solid-state solubility of alloying elements occurs. The present study focuses on the investigation of the pre-milling treatment to the microstructure, phase composition, and solubility in Al-7.7 Mn-3.5 Cu (wt%) alloy processed by a high-energy ball milling of Al-14.3 Mn-6.5 Cu (wt%) master alloy diluted with Al powder. During milling, the mean granular size decreased to ~5 µm, and a strong grain refinement occurred. According to our TEM and XRD data, ball milling provided a mean grain size of 13–14 nm and a microhardness of 490–540 HV. The lattice parameter of the Al-based solid solution decreased with an increase in the milling time to 7.5–10 h, which suggested the dissolution of the alloying elements, and the lattice parameter increased at a higher milling time of 12.5–40 h, which suggested the decomposition of the solid solution. The XRD data revealed the dissolution of the Al6Mn and Al20Cu2Mn3 solidification-originated phases with a further precipitation of the Al6Mn dispersoids. Pre-milling of the master alloy entailed a significant decrease in the minimal lattice parameter value from 0.4029 nm to 0.4023 nm due to an increase in the Mn solute content from 6.2 wt% (3.3 at%) to 7.5 wt % (4.0 at%) in the studied alloy during high-energy ball milling.
1. Introduction
An important issue for aluminum-based alloys is to increase the heat resistance and elevated temperature strength. Metal matrix composite materials combine the required properties inherent in each of their components. Aluminum-matrix composites demonstrate significantly better heat resistance, hardness, and wear resistance due to ceramic reinforcements [1,2,3]. The disadvantage of ceramic-strengthened composites is a weak adhesion at the interphase boundary between the ceramic particle and the metallic matrix, which weakens the ductility, toughness, limited strength level, and composites’ corrosion resistance [4,5]. “Natural” composites strengthened by intermetallic compounds processed during liquid or solid-state reactions overcome these problems. Fine particles formed during rapid solidification or solid-state precipitation reactions exhibit clear interphase boundaries and a high interface strength [6]. An increase in the solute content and the precipitates fraction improves the solid solution, the precipitation strengthening effects, and the mechanical properties. Due to fine and stable eutectic-originated particles, Al-Ce-based and Al-Fe-based alloys processed by rapid solidification demonstrated a good combination of strength at room and elevated temperatures, corrosion properties, and ductility [7,8,9,10,11,12,13,14,15]. Similarly, as-solidified aluminum-based alloys with transition metals, e.g., Zr [16,17,18], Mn [19,20,21], and Sc [22,23,24], demonstrate a supersaturated solid solution and a high number density of nanoscale precipitates forming during their decomposition. For fine flakes, processed with a crystallization rate of about 100 K/c, 4 wt% (2.1 at%) Mn, 3 wt% (1 at%) Zr, and 3 wt% (1.9 at%) Sc are dissolved in Al. An increase in the solubility limit is also possible due to severe plastic deformation. Recent research on mechanical alloying (MA) has attracted particular attention to high solid-state solubility and the formation of various metastable and amorphous phases [25,26,27,28,29]. During mechanical alloying, the nanostructured aluminum matrix anomalously supersaturated with alloying elements is formed. Further precipitation of the nanoscale particles occurs during heat treatment and granule consolidation [30,31]. This approach helps to improve the mechanical properties of Al-based composites.
An anomalous increase in solid solubility is observed after mechanical alloying due to severe plastic deformation and the resulting high density of crystalline defects, e.g., vacancies, dislocations, and grain boundaries [29,32,33,34,35,36,37,38,39,40]. The solute content of the low-soluble elements in aluminum, Nb [41,42], Co [43], V [44,45], Zr [46,47], and Ti [26], increases, and a strong extension of the Mn [4,48] and Mg [49] solubility is observed. For the same reasons [50,51,52,53,54,55,56,57,58], solute content increased significantly after high-pressure torsion [59,60,61]. Elemental metal powders are usually used for mechanical alloying [62,63]. For Al-Zr, the dissolution of Zr during milling is studied for the Al-Zr master alloy [30], and the elemental Zr powder is mixed with Al-Cu-Mn alloy chips [46,64]. Both Al3Zr phase and elemental Zr up to 20 wt% are completely dissolved to aluminum, but the dissolution kinetics is not compared for the alloys and the elemental powders.
The Al-Cu-Mn alloys have long been of interest in terms of increased high-temperature properties of lightweight constructions. Good high-temperature strength is observed for Al-Mn-Cu-based alloys; meanwhile, the service temperatures for the materials are below ~200 °C [65,66,67,68,69]. The conventional methods and compositions provide up to 1–2% Mn content in a solid solution, and the Al20Cu2Mn3 is the main strengthening phase [70]. Mechanical alloying provided a significantly higher Mn solute in the ternary alloy, and in the presence of ~6.2 wt% (2.8 at%) Cu, ~4.4 wt% (2.3 at%) Mn dissolves in Al after high energy ball milling for 7–7.5 h [71]. The Al2O3 oxide particles refined granules and stimulated Mn and Cu dissolution but did not increase the non-equilibrium Mn solute content [72]. A higher solute manganese for the binary Al-Mn alloys is reached via high-energy ball milling by Darling et al. [4]. After milling for 200 h, the solubility reaches 3.1 at% Mn, which is almost five times higher than that of the equilibrium one. A further increase in the milling time leads to precipitation of the equilibrium intermetallic Al6Mn phase [4,72].
Pre-milling is also used as an effective technological strategy for different processes, e.g., graphene nanosheets production [73], hydrogen generation by hydrolysis of the Mg scraps [74,75], the synthesis of ceramic [76,77,78,79,80], the mechanochemical synthesis of CdSe and ZnSe [81], the reduction in Mo oxides by Zn [82], and the preparation of high drug-loaded microgranules [83]. Pre-milling provides grain refinement and improves the properties of Fe–Al intermetallic coating [84] and Cr3C2-25 NiCr coating on A516 Steel [85]. Pre-milling of Mg improves the particle refinement effect for Mg-Graphene composite [86]. A “two-step” ball milling is beneficial to the oxidation process of Mo and improves the synthesis of immiscible Cu-Mo alloys [87]. Pre-milling of the reactants stimulates the synthesis of Fe3Al matrix composite reinforced TiC nanoparticles [88] and plays a key role in the syntheses of Li-Al-OH layered double hydroxides [89], composites based on ilmenite and graphite [90], and the synthesis of nanocrystalline TiC [91]. For most materials, a refinement of the components is considered crucial to the synthesis improvement during pre-treatment. The authors of [92] reveal a strong increase in solubility for both Mg in Ti and Ti in Mg in composite materials processed by high-energy ball milling due to the pre-milling of the elemental powders of these elements. The authors of [92] suggest an increase in the stored energy in the elemental powders is a main cause of solute extension. Thus, strain-induced solubility during ball milling depends on the basic element-alloying element couple and the treatment parameters, including the components’ composition and structure. Further experiments are required to study the influence of pre-milling of the powders on the structure of the mechanically alloyed materials and the formation of anomalously supersaturated solid solutions in Al-based materials. This study aims to determine the influence of pre-milling treatment on Mn solubility and the microstructure of the Al-7.7Mn-3.5Cu alloy. We discovered a strong extension of Mn solubility in Al due to the pre-milling of the components before the final high-energy ball milling.
2. Materials and Methods
The master alloy Al—14.3 wt% Mn—6.5 wt% Cu was processed using 99.85 wt% aluminum, 99.95 wt% manganese, and 99.9 wt% copper in an Interselt induction furnace (Interselt, Saint-Petersburg, Russia) and casting into a graphite mold with a solidification rate of ~20 K/s. The alloy was annealed at 520 °C for 24 h after solidification, and chips were then prepared.
Mechanical alloying was carried out in a Retch PM400 (VERDER company) high-energy planetary mill in an argon atmosphere. The ratio of steel grinding balls to the material was 20:1. The rotation speed of the grinding drums was 300 rpm. After each 5 min of milling, the process was interrupted for 5 min to eliminate the heating effect. To provide a nominal Al—7.6 wt% Mn—3.5 wt% Cu composition, both chips of the master alloy (for powder sample A) and powder of the master alloy that was pre-milled for 20 h (for powder sample B) were mixed with Al powder (spherical AD1, 99.9 wt% Al) and were subjected to further milling for 5–40 h. To process sample B, the chips of the master alloy were pre-milled for 20 h. Therefore, sample A was processed without pre-milling, and sample B was processed with pre-milling of the master alloy.
The chemical composition was examined using spectral analysis in an Inductively Coupled Plasma-Atomic Emission Spectrometer CAP-6300 (Thermo, Waltham, MA, USA) after milling of the master alloy for 20 h and both samples A and B for 5–40 h. The data are collected in Table 1. The content of residual Fe, which was introduced into the alloy from the grinding balls, increased during milling. Iron increased from 0.15 to 0.2 wt% after milling for 5 h and from 0.27 to 0.38 wt% after milling for 10–20 h (these ranges were observed for the pre-milled master alloy and sample A), and up to 0.63 wt% after milling for 40 h (for the sample B).

Table 1.
Chemical composition of the studied materials.
The phase composition was studied with an X-ray method obtained on a D8 Discover diffractometer (Bruker Corporation, Billerica, MA, USA) in Cu Kα. The lattice parameter was determined from the X-ray diffraction patterns for the Al lines with 2θ ≤ 140° by an extrapolation method. The size of the coherent scattering regions (CSRs) was calculated using a Williamson-Hall method, as described in [9,19].
The microstructure of the master alloy and the morphology of the granules after mechanical alloying were studied with a scanning electron microscope (SEM), specifically a TESCAN VEGA 3LMH (Tescan Brno s.r.o., Kohoutovice, Czech Republic). The samples were prepared by mechanical grinding with SiC-paper of different grinds (320–4000) and polishing with silica-based suspension (OP-S) using a Struers LaboPoll-5 polishing machine (Struers APS, Ballerup, Denmark). The size of the granules was estimated from the average diameter. Microstructural analysis of the granules was performed using a JEOL-JEM 2100 (JEOL, Tokyo, Japan) transmission electron microscope (TEM).
To study microhardness, the samples were pressed with a Acrodent (Stoma, Ukraine) self-hardening substance mixed in a ratio of 1:1. The pressed specimens were mechanically polished on SiC paper of various sizes, and the microhardness was measured by the Vickers method on a 402MVD microhardness tester (Wilson & Wolpert, Fort Worth, TX, USA) at a load of 10–25 g, depending on the hardness and size of the granules. A mean value was calculated by averaging 20–25 measurements.
3. Results
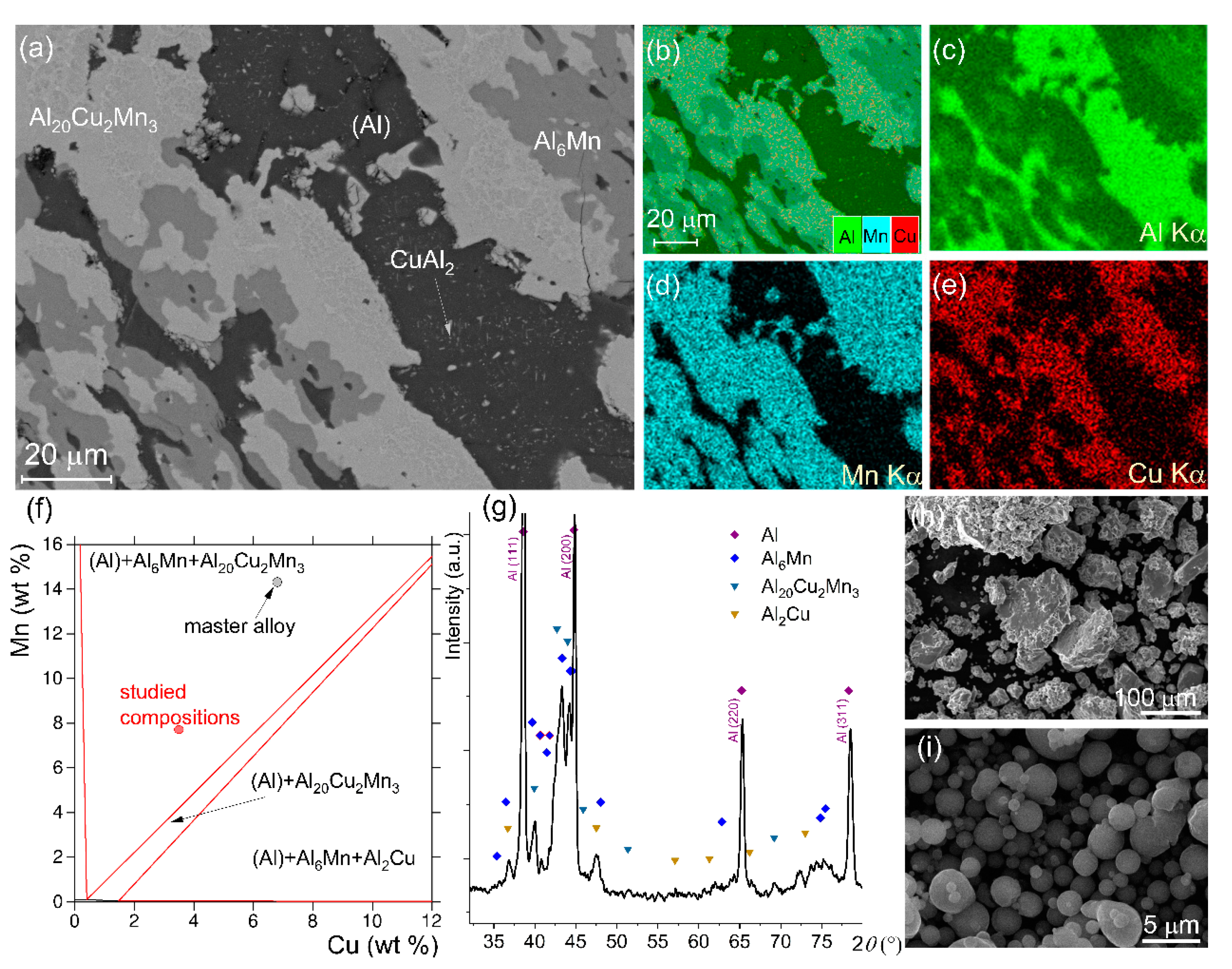
The microstructure of the annealed master alloy Al—14.3 wt% Mn—6.5 wt% Cu is presented in Figure 1a–e. The SEM-EDS maps, showing a distribution of the alloying elements, confirmed three main constituents in the microstructure: (1) an Al-enriched phase, (2) a Mn-enriched phase, and (3) both a Cu-enriched phase and an Mn-enriched phase. The XRD data (Figure 1g) agreed with the microstructural investigations and suggested that the aluminum-based solid solution (Al), the intermetallic phases of Al6Mn and Al20Cu2Mn3, and several peaks may belong to the CuAl2 phase. The secondary precipitates of the CuAl2 phase were observed in the Al matrix (Figure 1a). The XRD data and the microstructure of the master alloy agreed with the equilibrium phase composition simulated by ThermoCalc (Figure 1f). According to ThermoCalc, the volume fractions of the equilibrium Al6Mn and Al20Cu2Mn3 phases of solidification origin were 26% and 34%, respectively. The size of the milled granules of the master alloy varied in a range from 4 to 120 µm and the average granular size was 40 ± 8 µm (Figure 1h). The size of the spherical Al powders varied in a range from 0.5 to 6.1 µm with a mean size of 1.7 ± 0.2 µm (Figure 1i).
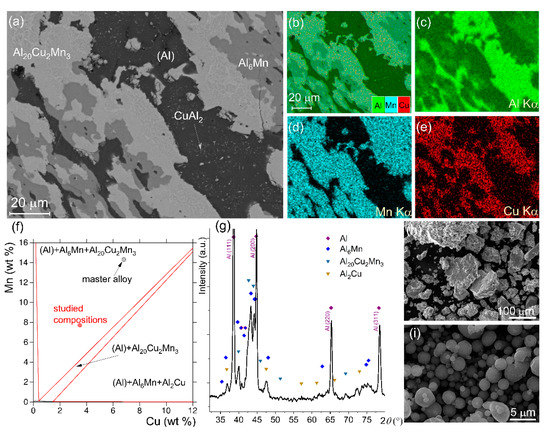
Figure 1.
(a) SEM microstructure after solidification and further annealing at 520 °C for 24 h, (b–e) element distribution SEM-EDS maps, (f) isothermal cross section at 400 °C for the Al-Mn-Cu diagram, (g) XRD spectrum, (h) granules of the Al—14.3 wt% Mn—6.5 wt% Cu master alloy after mechanical milling for 20 h, and (i) SEM micrograph for Al powder.
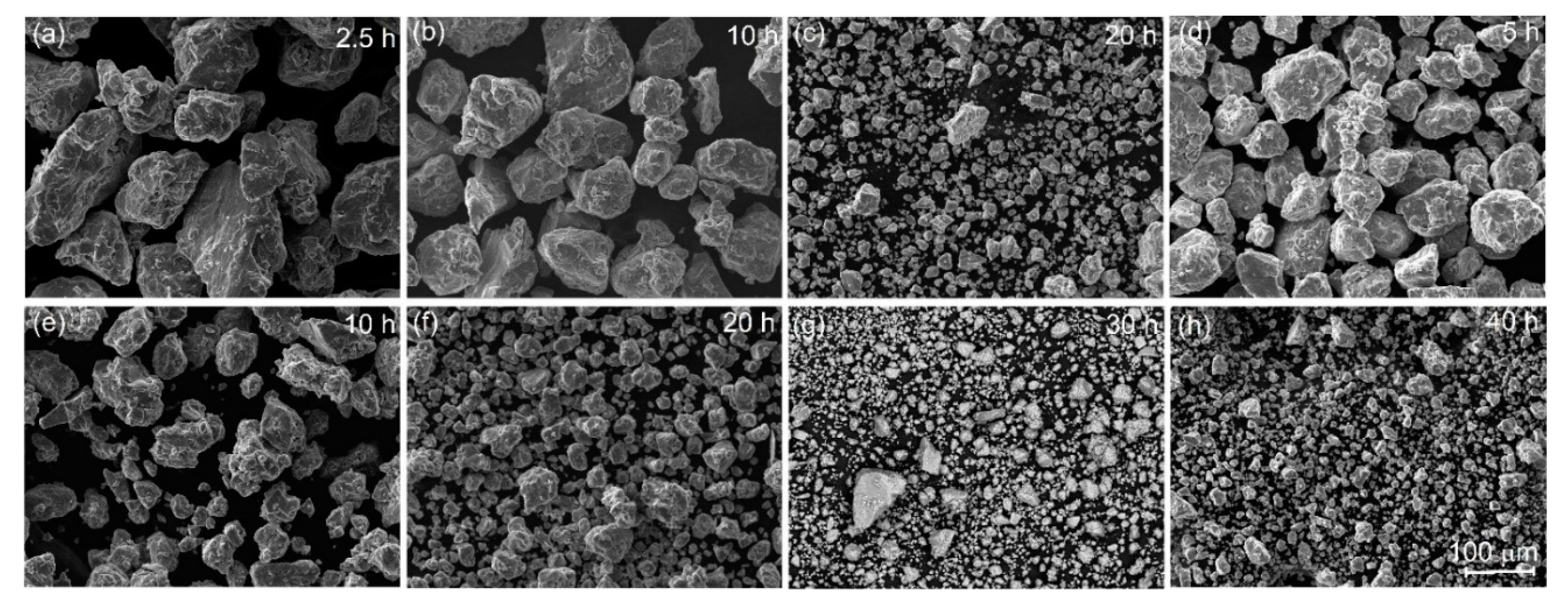
The SEM micrographs for sample A (mixture of the chips of the master alloy and Al powder) and sample B (mixture of the pre-milled granules of the master alloy and Al powder) after milling are demonstrated in Figure 2. The surface of the granules suggested a brittle fracture. According to the ThermoCalc prediction for the studied diluting alloys Al-(7.7–7.8) wt% Mn—(3.5–3.6) wt% Cu, the volume fractions of the Al6Mn, and Al20Cu2Mn3 solidification-originated phases were ~12% and ~17%, respectively. Thus, a large fraction of the intermetallic phases provided the embrittlement effect and favored granules refinement.
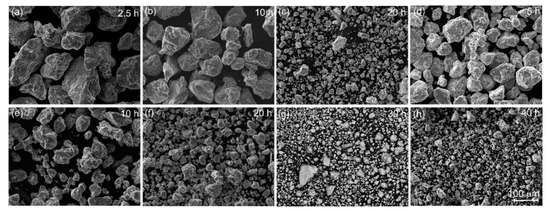
Figure 2.
Morphology of the granules of (a–c) the mixture of the master alloy chips with Al powder (sample A) and (d–h) the mixture of the pre-milled master alloyed granules with Al powder (sample B), after high-energy ball milling for 2.5–40 h (micrographs presented at the same magnification).
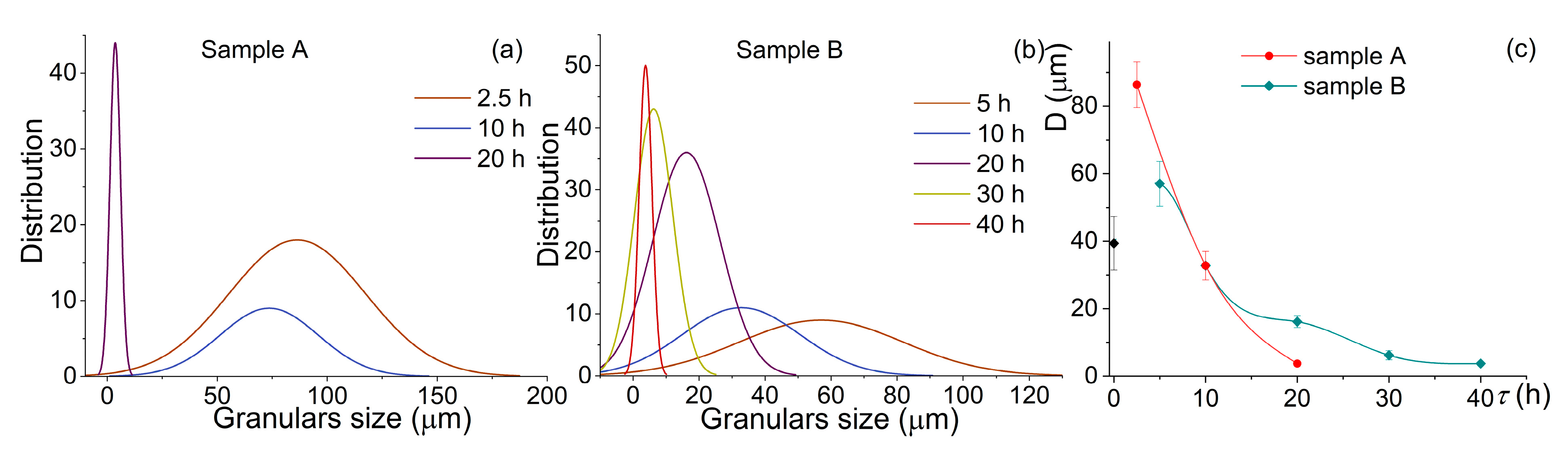
During the milling of both samples, the granules’ size decreased, and their distribution became narrow (Figure 3). For sample A, milling for 2.5 h provided a mean granular size of 86 ± 7 µm. An increase in the milling time to 10 h insignificantly refined the granules to a mean size of 74 ± 8 μm. Milling for 20 h, for sample A, provided a mean size of 5 ± 1 µm with a range of 0.9–11 µm (Figure 3a,c). For the pre-milled sample B, the granules were finer after a smaller milling time (Figure 3b,c). Milling for 5 h led to a granules size of 57 ± 7 µm, and it decreased to 33 ± 4 µm after milling for 10 h. By contrast, after milling for 20 h, a mean granular size of 16 ± 2 µm for sample B was approximately four times coarser as compared to that for sample A. An increase in the milling time to 30 and 40 h led to a significant refinement of sample B granules to 6 ± 1 µm and 5 ± 1 µm with a narrow distribution range of 0.9–25 µm and 0.9–8.4 µm, respectively. After milling for 20 h, for sample A, and milling for 40 h, for sample B, the mean sizes and the size distributions were almost the same.
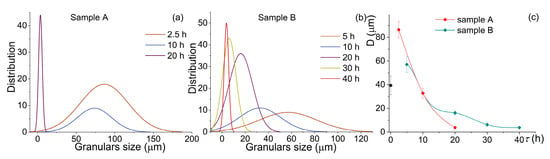
Figure 3.
Evolution of (a,b) the distribution of the granular size and (c) a mean size value during the high-energy ball milling of (a) sample A and (b) the pre-milled sample B.
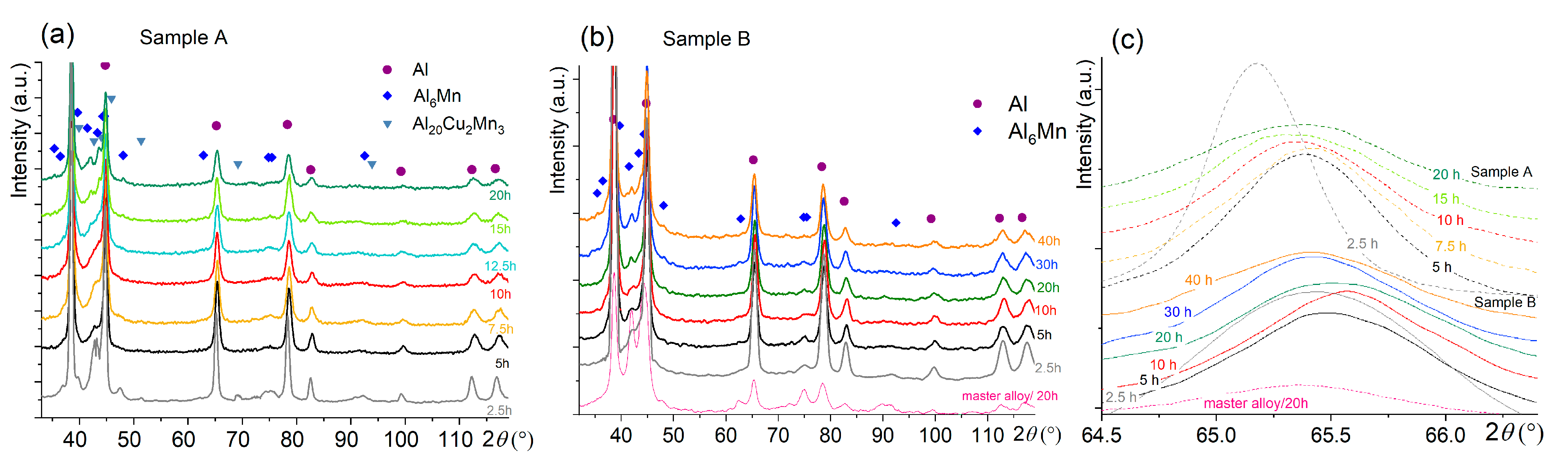
The evolution of the X-ray diffraction patterns for both samples is depicted in Figure 4. Three main phases of Al, Al6Mn, and Al20Cu2Mn3 were identified after milling for 2.5 h for sample A (Figure 4a). Two phases of Al and Al6Mn, the same as for the milled master alloy, were defined for the pre-milled sample B (Figure 4b). After mechanical alloying, the CuAl2 phase was not revealed by XRD in both samples A and B.
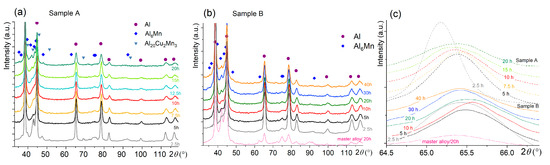
Figure 4.
Evolution of (a,b) the XRD pattern and (c) the Al solid solution peak of (a) sample A and (b) the pre-milled sample B during high-energy ball milling for 2.5–20 h.
During milling for 5–10 h, the intensity of the peaks of the intermetallic phases of Al6Mn and Al20Cu2Mn3 decreased. Further milling for 12.5–40 h led to the appearance of the peaks that belonged to the Al6Mn phase (Figure 4). The Al peaks shifted to the right-hand side, i.e., to higher 2θ angles with an increase in the milling time to 7.5 h for sample A and to 10–20 h for sample B (Figure 4). The same milling times corresponded to the weakest peaks of the intermetallic phases. Longer treatment shifted the Al peaks back to the left-hand side. In addition, a broadening of the Al peaks occurred with an increase in the processing time for both studied samples, which was related to grain refinement.
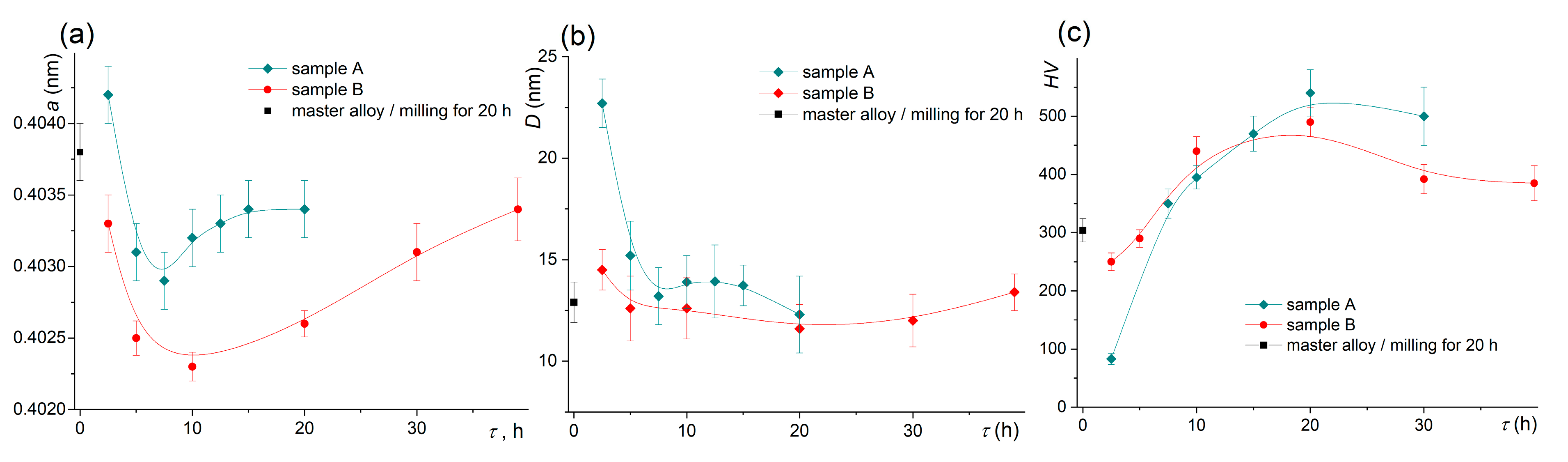
The change of the lattice parameter for both samples is presented in Figure 5a. The master alloy had a lattice parameter of 0.4038 ± 0.0001 nm after pre-milling. After milling for 7.5–10 h for sample A and 10 h for sample B, the lattice parameter decreased to 0.4023–0.4029 nm, and it increased with a further increase in the milling time. The values of the coherent scattering regions (D) that characterized the grain size decreased from 22 nm for 2.5 h to 15 nm for 5–20 h of milling for sample A (Figure 5b). The D value was 13 nm for the pre-milled master alloy and insignificantly changed for the diluted alloys.
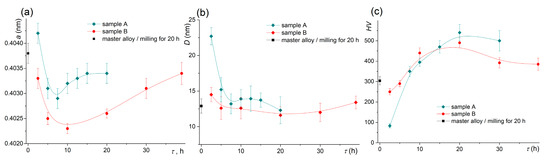
Figure 5.
Evolution of (a) the lattice parameter, (b) the coherent scattering region D, and (c) the microhardness during the high-energy ball milling of sample A and sample B.
The microhardness increased with increasing the milling time and reached the maximum of ~490–540 HV after milling for 15 h for sample A and after milling for 10 h for sample B (Figure 5c). The difference between the hardness values of the two samples was observed for a short milling time of 2.5 h; the hardness was 83 ± 10 HV for sample A and 250 ± 15 HV for the pre-treated sample B. An increase in the milling time was accompanied by a slight softening of the materials to 400–490 HV.
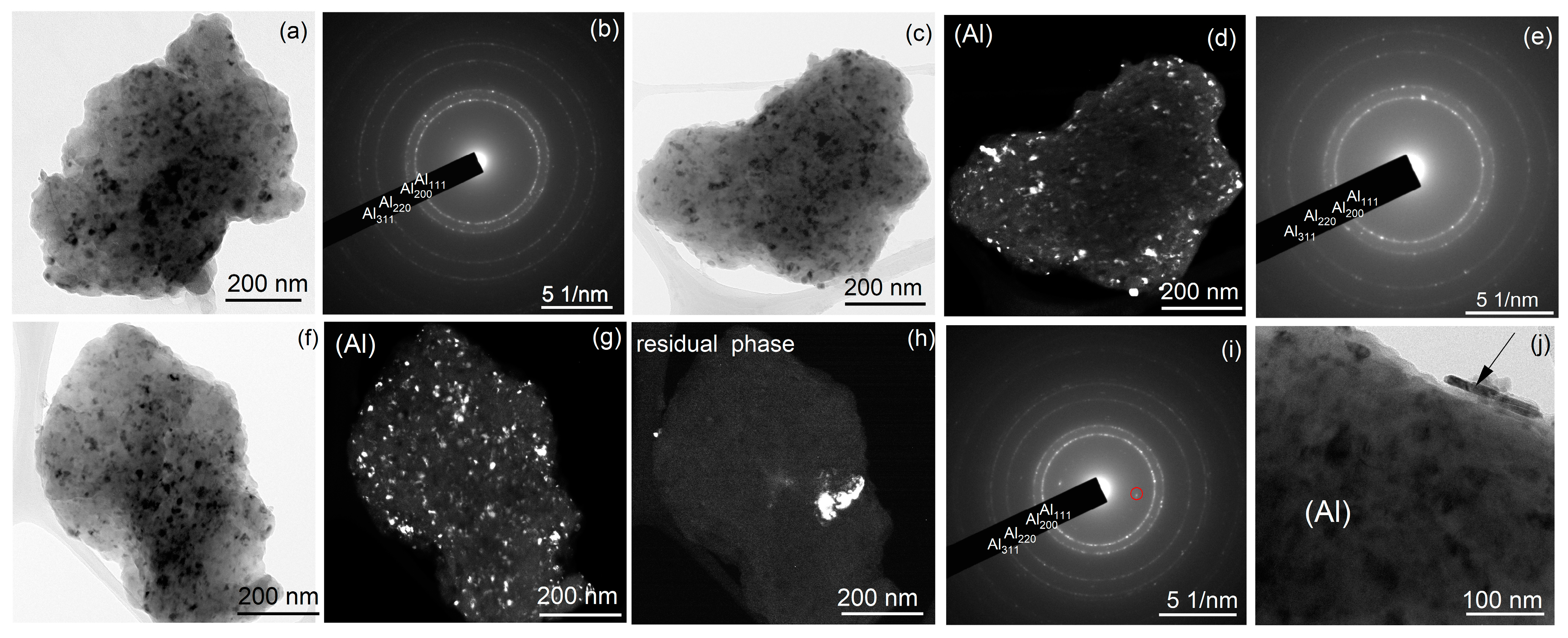
The granules were studied by TEM for sample A after milling for 20 h (Figure 6a,b and Figure 7a,b) and for sample B after milling for 20 h (Figure 6c–e) and 40 h (Figure 6f–j and Figure 7c,d). In both samples, the fine granules were nanostructured and demonstrated ring-type diffraction patterns of Al. TEM-EDS data showed 6.9–7.9 wt% Mn and 3.3–3.8 wt% Cu for both samples A and B after milling for 20 h.
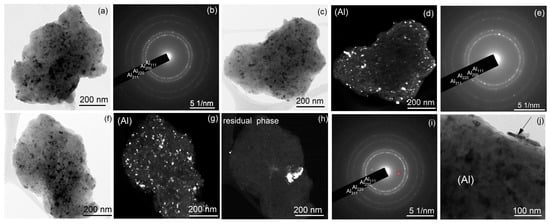
Figure 6.
TEM structure of (a,b) sample A after milling for 20 h and (c–j) the pre-milled sample B after milling for (c–e) 20 h and (f–j) 40 h; (a,c,f,j) bright field images, (d,g,h) dark field images, and (b,e,i) the corresponding SAEDs; the red-colored ring in SAED (i) is the reflection for the dark field image presented in (h).
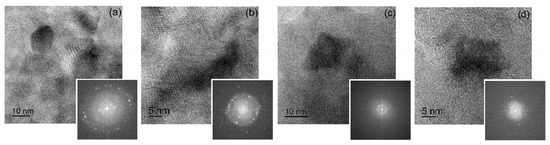
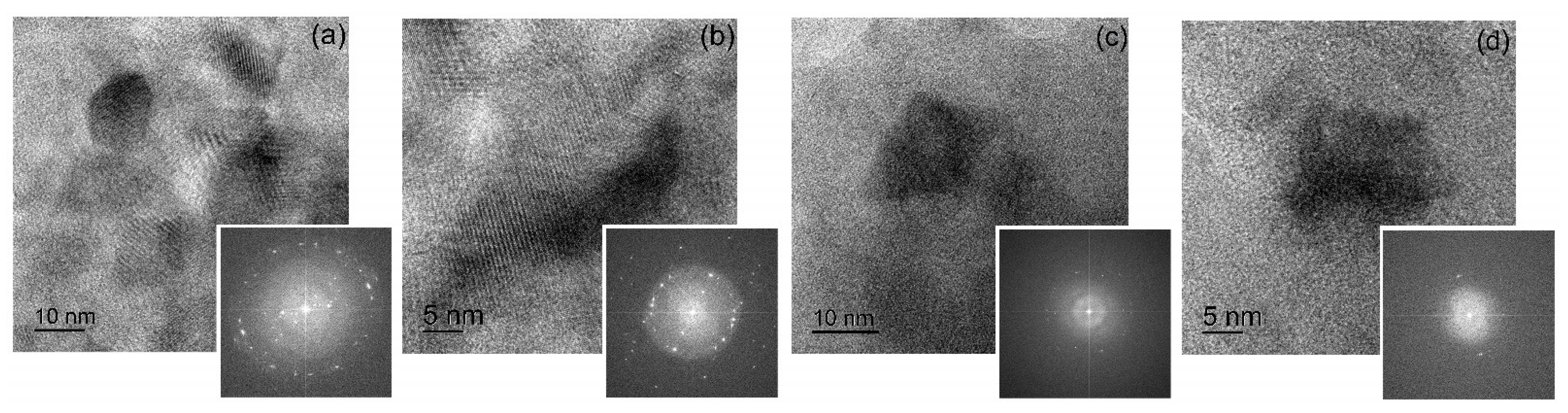
Figure 7.
High-resolution TEM images with corresponding FFT images for (a,b) sample A and (c,d) sample B.
After milling for 20 h, sample A consisted of Al (rings in SAED in Figure 6b) and secondary phase particles (extra scattered reflections in SAED in Figure 6b). High-resolution images confirmed the presence of a high number density of secondary precipitates with nanoscale size (Figure 7a,b). The Moure pattern (Figure 7a) suggested a coherency between Al grains and secondary phase precipitates. A high fraction of the granules in sample B pertained to the Al-based solid solution (Figure 6c,d and Figure 7d). However, granules with coarse residual inclusions (Figure 6g,h) were also observed even after milling for 40 h. High-resolution images for sample B revealed granules with Al grains and secondary precipitates; meanwhile, their number density was significantly lower (Figure 7c). The mean grain size of the aluminum solid solution was 15 ± 4 nm for sample A and 13 ± 3 nm for sample B, which agrees well with the values of the coherent scattering regions.
4. Discussion
Due to a large number of intermetallic compounds, the granules of the master alloy were brittle and significantly refined during pre-milling for 20 h. A comparison of the XRD data of the annealed and mechanically milled granules suggested strong refinement of the phases during mechanical alloying for the master alloy. The reflections of the Al20Cu2Mn3 phase disappeared after milling for ~5 h of sample A and were not discovered in the milled master alloy and sample B. The CuAl2 phase was also not observed by XRD in both samples A and B after mechanical alloying. Therefore, the XRD data suggest a comparatively rapid dissolution of the Cu-bearing phases during high-energy ball milling in the diluted alloys. The processes that occurred during the ball milling of the studied alloys were similar to Al-Mn-Cu alloys with the same phase composition but a higher content of the alloying elements and a larger fraction of the intermetallic phases [71,72].
The peaks of the Al6Mn phase became weaker during milling and were not clearly defined after ~10 h of milling of both samples, but further milling led to the appearance of the additional Al6Mn phase reflections in XRD. Thus, the dissolution of the Al6Mn phase followed by its further precipitation was observed during mechanical alloying. Similar behavior with dissolution and further precipitation of the intermetallic phases suggested saturation and subsequent decomposition of the solid solution during high-energy ball milling, as shown in [47,71,72].
The Cu-bearing phases, including the Al20Cu2Mn3 phase, were dissolved during pre-milling treatment. The lattice parameters after milling was 0.4038 nm, which suggested an increase in the Cu and Mn content in the Al-based solid solution. Taking into account the 6.5 wt% solute Cu content, about 2.5% Mn was dissolved in Al. According to the phase composition analysis, the maximum solute Mn should correspond to the milling time of about 10 h. The evolution of the Al lattice parameter agreed well with the change in the phase composition of the studied samples. The minimum value of the lattice parameter was observed after milling for 7.5–10 h. To estimate the Mn solubility, we considered the residual Fe that can also dissolve in the Al-based solid solution. The Fe content increased to ~0.36 wt% after 20 h of milling of sample A and to ~0.38 wt% and ~0.63 wt% after milling of alloy B for 10 h and 40 h. Considering that the Cu-bearing phases dissolved for the dilute alloys and ~3.5 wt% solute Cu, the value of 0.4029 nm suggests the dissolution of the 6.4 wt% Mn for sample A. The minimum lattice parameter of 0.4023 nm in sample B suggests an increased solute Mn to 7.4 wt%, which is similar to the total Mn content in sample B. This agrees well with the TEM data for fine granules of sample B with a size of ~1 µm that predominantly consisted of (Al) grains. The SAEDs obtained from such granules suggested single-phase Al compositions and the SEM-EDS spectra identified 7.2 ± 0.5 wt% Mn and 3.5 ± 0.2 wt% Cu, which also confirmed the possibility of such a high Mn solute. Meanwhile, the granules’ microstructure was incompletely homogeneous, and in some granules, the rarely distributed coarse residual agglomerates and platelet particles of a usual morphology for the Al6Mn phase were found. These particles may be a cause of the residual Al6Mn peaks.
An important finding of the present study is the possibility to increase the Mn solute content due to the pre-milling treatment of the components. Refinement of the granules during the pre-milling process may provide a more homogeneous distribution of the master alloy powder and fine Al powder for sample B compared to sample A containing the coarse chips of the master alloy. In addition, the accumulated vacancies, dislocations, and a high number density of grain boundaries after the pre-milling process of the master alloy may improve the mechanical alloying process at the second step of milling with Al powder. As a result, the microstructural changes in sample B were more homogeneous during milling, which should be important, especially at small treatment times. Similarly with our observations of Mn in Al, the contents of solute Mg in Ti increased significantly due to the pre-milling of the elemental powders of the elements [92]. The authors reasonably explain the phenomena by the accumulation of the strain energy during pre-milling treatment and an increase in the elemental powders’ free energy [92].
The maximum solubility of 7.5 wt% (4.0 at%) Mn in the studied alloy was higher than for 6.6 wt% (3.1 at%) Mn in the binary Al-Mn alloy [4]. For high-alloyed Al-Mn-Cu alloy that was processed without pre-milling treatment, the solute Mn was significantly lower, about 4.4 wt% [72]. It is noteworthy that the estimation of the Al lattice parameter did not consider that residual Fe can be incompletely dissolved in Al and that both Cu and Fe may substitute Mn atoms in the Al6Mn phase with a formation of the Al6(Mn,Cu,Fe) phase of the same crystalline structure [70,93,94,95,96,97]. Meanwhile, lower Cu and Fe solute suggests a higher Mn solute and therefore indicates a high Mn solubility in Al due to the pre-milling treatment.
One interesting phenomenon is an increase in the lattice parameter as a result of the Al6Mn phase precipitation after a long milling time and thus solid solution decomposition during milling. The TEM observations confirmed the presence of a large fraction of fine precipitates in sample A after milling for 20 h, which agreed with the lattice parameter growth. Oppositely, predominantly Al solid solution was revealed in sample B after the same treatment where the lattice parameter was significantly smaller. The decomposition was accompanied by an intense refinement of the granules and could have been the result of welding of the granules and friction-induced heating [27]. It should be considered that a decrease in the lattice parameter in a milling time range of 7.5–10 for sample A and 10–20 h for sample B, when the phase composition changed insignificantly, can be the result of the redistribution of the alloying elements and the formation of grain boundaries segregations [98,99]. The grain boundary segregations of Cu may decrease the solute content in the grain interior and lead to a corresponding increase in the lattice parameter of the Al-based solid solution [100].
Evidently, the maximum microhardness of the dilute alloys of 490–540 HV was 1.5–1.8 times higher than that of the high-alloyed master alloy. The hardening effect is related to the dissolution of the coarse incoherent solidification-originated particles, an increase in the solute content, and the precipitation of the fine secondary phases. Slight softening occurred at a large milling time, which can be related to the heat-induced processes accumulated during milling [27]. The Al grain size was about 13–15 nm and did not change with increasing the milling time. Thus, softening may be the result of solid solution decomposition accompanied by coarsening of the secondary precipitates.
5. Summary
The influence of pre-milling treatment on the microstructure, phase composition, and solubility in the Al-7.7 Mn-3.5 Cu (wt%) alloy processed by high-energy ball milling was studied. Two samples that consisted of the Al-14.3 Mn-6.5 Cu (wt%) master alloy chips (sample A) and pre-milled granules of the master alloy (sample B) and that were diluted with the Al powder were milled. During the mechanical alloying of both samples, the mean granular size decreased from 80–60 µm after milling for 2.5 h to 4 µm after milling for 20–40 h.
The XRD data revealed the dissolution of the Al6Mn and Al20Cu2Mn3 phases during milling for 2.5–10 h. The lattice parameter of the Al-based solid solution decreased from 0.4038 nm in the pre-milled master alloy to a minimum of 0.4029 nm for sample A and 0.4023 nm for sample B. Further milling in a time range of 12.5–40 h led to an increase in the lattice parameter and precipitation of the Mn-enriched Al6Mn phase. The dissolution of the Cu and Mn alloying elements with the formation of the highly supersaturated solid solution and its further decomposition at long-term milling with precipitation of the Al6Mn phase were observed. Pre-milling of the master alloy provided a significant decrease in the minimal lattice parameter value, which suggested an increase in the maximum solute Mn content from 6.2 wt (3.3 at%) to 7.5 wt % (4.0 at%) in the studied alloy.
The mean grain size was 13–15 nm according to the TEM studies, and a coherent scattering region was 12–13 nm according to the XRD data in a wide range milling time of 10–40 h. Hardness increased during mechanical alloying from ~300 HV for the master alloy to 490–540 HV for both diluted alloys. The suggested strengthening mechanisms were grain boundary, solid solution, and precipitation strengthening.
Author Contributions
Conceptualization, A.V.M. and A.G.M.; methodology, O.A.Y. and A.S.P.; software, N.Y.T.; formal analysis, A.V.M.; investigation, O.A.Y., A.S.P., N.Y.T., A.V.M. and N.B.E.; funding acquisition, O.A.Y.; supervision, A.V.M.; writing—original draft preparation, O.A.Y. and A.V.M.; writing—review and editing, A.V.M.; project administration, O.A.Y. and A.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The XRD: microstructural and hardness studies were performed in the framework of the RSF, grant number 21-79-00273. The TEM studies were partially funded by the state task to MISIS University project code of FSME-2023-0005.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Acknowledgments
For the TEM investigations, the authors thank the Collective Use Equipment Center “Material Science and Metallurgy” for the equipment modernization program represented by the Ministry of Higher Education and Science of Russian Federation (No.075-15-2021-696). The authors are also grateful to Elena Bazanova for her helpful recommendations in Academic writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Surappa, M.K. Aluminium Matrix Composites: Challenges and Opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Chelladurai, S.J.S.; Arthanari, R. Prediction of Hardness of Stir Cast LM13 Aluminum Alloy—Copper Coated Short Steel Fiber Reinforced Composites Using Response Surface Methodology. Materwiss. Werksttech. 2020, 51, 221–229. [Google Scholar] [CrossRef]
- Khelge, S.; Kumar, V.; Shetty, V.; Kumaraswamy, J. Effect of Reinforcement Particles on the Mechanical and Wear Properties of Aluminium Alloy Composites: Review. Mater. Today Proc. 2022, 52, 571–576. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Armstrong, L.; Kapoor, D.; Tschopp, M.A.; Kecskes, L.J.; Mathaudhu, S.N. Influence of Mn Solute Content on Grain Size Reduction and Improved Strength in Mechanically Alloyed Al–Mn Alloys. Mater. Sci. Eng. A 2014, 589, 57–65. [Google Scholar] [CrossRef]
- Xia, W.; Zarezadeh Mehrizi, M. Direct Synthesis of NiAl Intermetallic Matrix Composite with TiC and Al2O3 Reinforcements by Mechanical Alloying of NiO–Al–Ti–C Powder Mixture. Ceram. Int. 2021, 47, 26863–26868. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Y.; Ma, Y.; Wu, L.; Yan, H.; Liu, C.; Liu, Y.; Liu, B.; Liu, W. Microstructure and Mechanical Properties of Powder Metallurgy 2024 Aluminum Alloy during Cold Rolling. J. Mater. Res. Technol. 2021, 15, 3337–3348. [Google Scholar] [CrossRef]
- Ayer, R.; Mueller, R.R.; Scanlon, J.C.; Klein, C.F. Microstructural characterization of the dispersed phases in AL-CE-FE system. Metall. Trans. A Phys. Metall. Mater. Sci. 1988, 19A, 1645–1656. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V.; Alabin, A.N. Microstructure and Phase Composition of Al-Ce-Cu Alloys in the Al-Rich Corner. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2006; Volumes 519–521, pp. 395–400. [Google Scholar]
- Chen, Z.; Chen, P.; Li, S. Effect of Ce Addition on Microstructure of Al 20Cu 2Mn 3 Twin Phase in an Al-Cu-Mn Casting Alloy. Mater. Sci. Eng. A 2012, 532, 606–609. [Google Scholar] [CrossRef]
- Waterloo, G.; Jones, H. Microstructure and Thermal Stability of Melt-Spun Al-Nd and Al-Ce Alloy Ribbons. J. Mater. Sci. 1996, 31, 2301–2310. [Google Scholar] [CrossRef]
- Lu, Z.; Li, X.; Zhang, L. Thermodynamic Description of Al-Si-Mg-Ce Quaternary System in Al-Rich Corner and Its Experimental Validation. J. Phase Equilib. Diffus. 2018, 39, 57–67. [Google Scholar] [CrossRef]
- Gröbner, J.; Mirković, D.; Schmid-Fetzer, R. Thermodynamic Aspects of the Constitution, Grain Refining, and Solidification Enthalpies of Al-Ce-Si Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35A, 3349–3362. [Google Scholar] [CrossRef]
- Manca, D.R.; Churyumov, A.Y.; Pozdniakov, A.V.; Prosviryakov, A.S.; Ryabov, D.K.; Krokhin, A.Y.; Korolev, V.A.; Daubarayte, D.K. Microstructure and Properties of Novel Heat Resistant Al–Ce–Cu Alloy for Additive Manufacturing. Met. Mater. Int. 2019, 25, 633–640. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams: Applications for Commercial Aluminum Alloys; Elsevier B.V.: Amsterdam, The Netherlands, 2005; ISBN 9780080445373. [Google Scholar]
- Eskin, D.G.; Toropova, L.S. Tensile and Elastic Properties of Deformed Heterogeneous Aluminum Alloys at Room and Elevated Temperatures. Mater. Sci. Eng. A 1994, 183, L1–L4. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation Evolution in Al–Zr and Al–Zr–Ti Alloys during Isothermal Aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Dunand, D.C.; Seidman, D.N. Tungsten Solubility in L12-Ordered Al3Er and Al3Zr Nanoprecipitates Formed by Aging in an Aluminum Matrix. J. Alloys Compd. 2020, 820, 153383. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Yarasu, V.; Barkov, R.Y.; Yakovtseva, O.A.; Makhov, S.V.; Napalkov, V.I. Microstructure and Mechanical Properties of Novel Al-Mg-Mn-Zr-Sc-Er Alloy. Mater. Lett. 2017, 202, 116–119. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Dunand, D.C.; Seidman, D.N. Enhanced Age-Hardening Response and Creep Resistance of an Al-0.5Mn-0.3Si (at%) Alloy by Sn Inoculation. Acta Mater. 2022, 240, 118344. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Mukhamejanova, A.B.; Kotov, A.D.; Yakovtseva, O.A.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The Effect of Pre-Straining on the Annealing-Induced Precipitation Behavior of the Icosahedral I-Phase in an Aluminum-Based Alloy. Mater. Lett. 2022, 310, 131517. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mukhamejanova, A.; Kotov, A.D.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mochugovskiy, A.G. Precipitation Behavior of the Metastable Quasicrystalline I-Phase and Θ′-Phase in Al-Cu-Mn Alloy. Metals 2023, 13, 469. [Google Scholar] [CrossRef]
- Nokhrin, A.V.; Gryaznov, M.Y.; Shotin, S.V.; Nagicheva, G.S.; Chegurov, M.K.; Bobrov, A.A.; Kopylov, V.I.; Chuvil’deev, V.N. Effect of Sc, Hf, and Yb Additions on Superplasticity of a Fine-Grained Al-0.4%Zr Alloy. Metals 2023, 13, 133. [Google Scholar] [CrossRef]
- Eskin, D.G. Sc Applications in Aluminum Alloys: Overview of Russian Research in the 20th Century. In Light Metals; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; Volume Part F4, pp. 1565–1572. ISBN 9783319722832. [Google Scholar]
- Belov, N.A.; Alabin, A.N.; Eskin, D.G.; Istomin-Kastrovskii, V.V. Optimization of Hardening of Al–Zr–Sc Cast Alloys. J. Mater. Sci. 2006, 41, 5890–5899. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, H.S.; Kum, D.W. Determination of Titanium Solubility in Alpha-Aluminum during High Energy Milling. Scr. Mater. 1996, 34, 421–428. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Lavernia, E.J. Amorphization and Crystallization in Al-Ni-La during Mechanical Milling. J. Alloys Compd. 2008, 466, 189–200. [Google Scholar] [CrossRef]
- Azimi, M.; Akbari, G.H. Development of Nano-Structure Cu-Zr Alloys by the Mechanical Alloying Process. J. Alloys Compd. 2011, 509, 27–32. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Investigation of Nanostructured Al-10 Wt% Zr Material Prepared by Ball Milling for High Temperature Applications. Mater. Charact. 2017, 123, 173–177. [Google Scholar] [CrossRef]
- Senkov, O.N.; Froes, F.H.; Stolyarov, V.V.; Valiev, R.Z.; Liu, J. Microstructure and Microhardness of an Al Fe Alloy Subjected to Severe Plastic Deformation and Aging. Nanostruct. Mater. 1998, 10, 691–698. [Google Scholar] [CrossRef]
- Bergk, B.; Mühle, U.; Kieback, B.; Koutná, N.; Holec, D.; Clemens, H. Nanocrystalline Alloys of Molybdenum with Sodium and Yttrium Obtained by Mechanical Alloying. In Proceedings of the Proceedings Euro PM 2017: International Powder Metallurgy Congress and Exhibition, Milan, Italy, 1–5 October 2017. [Google Scholar]
- Pasebani, S.; Samimi, P.; Saber, M. Effects of Scandium and Hafnium Solute Additions on Microstructure Thermal Stability in Nanostructured Ferritic Alloys. Mater. Charact. 2019, 151, 216–220. [Google Scholar] [CrossRef]
- Kumar, A.P.; Muthaiah, V.M.S.; Mula, S. Effect of Nb, Y and Zr on Thermal Stability of Nanocrystalline Al-4.5 Wt% Cu Alloy Prepared by Mechanical Alloying. J. Alloys Compd. 2017, 722, 617–627. [Google Scholar] [CrossRef]
- Ashrafi, H.; Emadi, R.; Enayati, M.H. Microstructural and Hardness Changes during Isothermal Annealing of Nanostructured Al-11.6Fe-1.3V-2.3Si Alloy. J. Mater. Eng. Perform. 2015, 24, 1026–1030. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gupta, A.K.; Roy, D.; Basu Mallick, A. Nanomechanical Properties of Mechanically Alloyed and Spark Plasma Sintered W-Nanoparticulate Dispersed Cu-Nb Alloys. Mater. Lett. 2020, 274, 128004. [Google Scholar] [CrossRef]
- Schuler, T.; Nastar, M.; Soisson, F. Vacancy-Induced Dissolution of Precipitates in out-of-Equilibrium Systems: A Test Case of FeX (X = C,N,O) Alloys. Phys. Rev. B 2017, 95, 014113. [Google Scholar] [CrossRef]
- Gupta, R.K.; Murty, B.S.; Birbilis, N. Future Work and Possible Applications of Nanocrystalline Al Alloys as Produced by High-Energy Ball Milling; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Olynik, N.; Cheng, B.; Sprouster, D.J.; Parish, C.M.; Trelewicz, J.R. Microstructural Transitions during Powder Metallurgical Processing of Solute Stabilized Nanostructured Tungsten Alloys. Metals 2022, 12, 159. [Google Scholar] [CrossRef]
- Tejeda-Ochoa, A.; Kametani, N.; Carreño-Gallardo, C.; Ledezma-Sillas, J.E.; Adachi, N.; Todaka, Y.; Herrera-Ramirez, J.M. Formation of a Metastable Fcc Phase and High Mg Solubility in the Ti-Mg System by Mechanical Alloying. Powder Technol. 2020, 374, 348–352. [Google Scholar] [CrossRef]
- Christudasjustus, J.; Larimian, T.; Esquivel, J.; Gupta, S.; Darwish, A.A.; Borkar, T.; Gupta, R.K. Aluminum Alloys with High Elastic Modulus. Mater. Lett. 2022, 320, 132292. [Google Scholar] [CrossRef]
- Witharamage, C.S.; Christudasjustus, J.; Gupta, R.K. The Effect of Milling Time and Speed on Solid Solubility, Grain Size, and Hardness of Al-V Alloys. J. Mater. Eng. Perform. 2021, 30, 3144–3158. [Google Scholar] [CrossRef]
- Sui, H.X.; Zhu, M.; Qi, M.; Li, G.B.; Yang, D.Z. The Enhancement of Solid Solubility Limits of AlCo Intermetallic Compound by High-energy Ball Milling. J. Appl. Phys. 1992, 71, 2945–2949. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Influence of the V Content on Microstructure and Hardness of High-Energy Ball Milled Nanocrystalline Al-V Alloys. J. Alloys Compd. 2018, 760, 63–70. [Google Scholar] [CrossRef]
- Christudasjustus, J.; Witharamage, C.S.; Walunj, G.; Borkar, T.; Gupta, R.K. The Influence of Spark Plasma Sintering Temperatures on the Microstructure, Hardness, and Elastic Modulus of the Nanocrystalline Al-XV Alloys Produced by High-Energy Ball Milling. J. Mater. Sci. Technol. 2022, 122, 68–76. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D. Strengthening of Mechanically Alloyed Al-Based Alloy with High Zr Contents. Mater. Sci. Eng. A 2018, 713, 174–179. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Catalano, J.E.; Tschopp, M.A.; Kecskes, L.J. Effect of Processing Parameters on the Microstructure of Mechanically Alloyed Nanostructured Al-Mn Alloys. In Advanced Composites for Aerospace, Marine, and Land Applications II; Springer Nature: Cham, Switzerland, 2016; pp. 3–11. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Sundaresan, R. Metastable Phases in Mechanically Alloyed Al Mn Powder Mixtures. Mater. Sci. Eng. A 1991, 131, 237–242. [Google Scholar] [CrossRef]
- Scudino, S.; Sakaliyska, M.; Surreddi, K.B.; Eckert, J. Mechanical Alloying and Milling of Al–Mg Alloys. J. Alloys Compd. 2009, 483, 2–7. [Google Scholar] [CrossRef]
- Mohammadi, A.; Enikeev, N.A.; Murashkin, M.Y.; Arita, M.; Edalati, K. Developing Age-Hardenable Al-Zr Alloy by Ultra-Severe Plastic Deformation: Significance of Supersaturation, Segregation and Precipitation on Hardening and Electrical Conductivity. Acta Mater. 2021, 203, 116503. [Google Scholar] [CrossRef]
- Gubicza, J. Lattice Defects and Their Influence on the Mechanical Properties of Bulk Materials Processed by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1230–1242. [Google Scholar] [CrossRef]
- Straumal, B.; Kilmametov, A.; Korneva, A.; Zięba, P.; Zavorotnev, Y.; Metlov, L.; Popova, O.; Baretzky, B. The Enrichment of (Cu, Sn) Solid Solution Driven by High-Pressure Torsion. Crystals 2021, 11, 766. [Google Scholar] [CrossRef]
- Tolmachev, T.P.; Pilyugin, V.P.; Ancharov, A.I.; Chernyshov, E.G.; Patselov, A.M. The Formation, Structure, and Properties of the Au–Co Alloys Produced by Severe Plastic Deformation under Pressure. Phys. Met. Metallogr. 2016, 117, 135–142. [Google Scholar] [CrossRef]
- Korneva, A.; Straumal, B.; Kilmametov, A.; Chulist, R.; Cios, G.; Baretzky, B.; Zięba, P. Dissolution of Ag Precipitates in the Cu–8wt%Ag Alloy Deformed by High Pressure Torsion. Materials 2019, 12, 447. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Baretzky, B.; Kogtenkova, O.A.; Straumal, P.B.; Lityńska-Dobrzyńska, L.; Chulist, R.; Korneva, A.; Zięba, P. High Pressure Torsion of Cu–Ag and Cu–Sn Alloys: Limits for Solubility and Dissolution. Acta Mater. 2020, 195, 184–198. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; Horita, Z. Strengthening via Microstructure Refinement in Bulk Al–4 Mass% Fe Alloy Using High-Pressure Torsion. Mater. Trans. 2012, 53, 46–55. [Google Scholar] [CrossRef]
- Bachmaier, A.; Rathmayr, G.B.; Bartosik, M.; Apel, D.; Zhang, Z.; Pippan, R. New Insights on the Formation of Supersaturated Solid Solutions in the Cu–Cr System Deformed by High-Pressure Torsion. Acta Mater. 2014, 69, 301–313. [Google Scholar] [CrossRef]
- Bachmaier, A.; Kerber, M.; Setman, D.; Pippan, R. The Formation of Supersaturated Solid Solutions in Fe–Cu Alloys Deformed by High-Pressure Torsion. Acta Mater. 2012, 60, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.X.; Kawasaki, M.; Huang, Y.; Langdon, T.G. An Examination of Microstructural Evolution in a Pb–Sn Eutectic Alloy Processed by High-Pressure Torsion and Subsequent Self-Annealing. Mater. Sci. Eng. A 2021, 802, 140653. [Google Scholar] [CrossRef]
- Dobromyslov, A.V.; Taluts, N.I.; Pilyugin, V.P.; Tolmachev, T.P. Mechanical Alloying of Al–Fe Alloys Using Severe Deformation by High-Pressure Torsion. Phys. Met. Metallogr. 2015, 116, 942–950. [Google Scholar] [CrossRef]
- Verma, N.; Pant, N.; Beach, J.A.; Ivanisenko, J.; Ashkenazy, Y.; Dillon, S.; Bellon, P.; Averback, R.S. Effects of Ternary Alloy Additions on the Microstructure of Highly Immiscible Cu Alloys Subjected to Severe Plastic Deformation: An Evaluation of the Effective Temperature Model. Acta Mater. 2019, 170, 218–230. [Google Scholar] [CrossRef]
- Zhou, E.; Suryanarayana, C.; Froes, F.H. Solid Solubility Extension of Magnesium in Titanium by Mechanical Alloying. In Proceedings of the TMS Annual Meeting, Las Vegas, NV, USA, 12–16 February 1995; Volume 23, pp. 43–51. [Google Scholar]
- Esquivel, J.; Murdoch, H.A.; Darling, K.A.; Gupta, R.K. Excellent Corrosion Resistance and Hardness in Al Alloys by Extended Solid Solubility and Nanocrystalline Structure. Mater. Res. Lett. 2018, 6, 79–83. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Microstructural Characterization of Mechanically Alloyed Al–Cu–Mn Alloy with Zirconium. Mater. Sci. Eng. A 2015, 623, 109–113. [Google Scholar] [CrossRef]
- Michi, R.A.; Bahl, S.; Fancher, C.M.; Sisco, K.; Allard, L.F.; An, K.; Yu, D.; Dehoff, R.R.; Plotkowski, A.; Shyam, A. Load Shuffling during Creep Deformation of an Additively Manufactured AlCuMnZr Alloy. Acta Mater. 2023, 244, 118557. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, H.; Wang, R.; Peng, C.; Feng, Y.; Wang, X. Microstructure and Mechanical Properties of the Extruded Al-Cu-Mn-Sc-Zr Alloy during Single-Stage and Two-Stage Aging. J. Mater. Eng. Perform. 2022, 32, 185–198. [Google Scholar] [CrossRef]
- Belov, N.A.; Korotkova, N.O.; Shurkin, P.K.; Aksenov, A.A. Substantiation of the Copper Concentration in Thermally Stable Wrought Aluminum Alloys Containing 2 wt% of Mn. Phys. Met. Metallogr. 2020, 121, 1211–1219. [Google Scholar] [CrossRef]
- Belov, N.A.; Akopyan, T.K.; Shurkin, P.K.; Korotkova, N.O. Comparative Analysis of Structure Evolution and Thermal Stability of Commercial AA2219 and Model Al-2 wt%Mn-2 wt%Cu Cold Rolled Alloys. J. Alloys Compd. 2021, 864, 158823. [Google Scholar] [CrossRef]
- Zupanič, F.; Bončina, T. Heat-Resistant Al-Alloys with Quasicrystalline and L12- Precipitates. Solid State Phenom. 2022, 327, 26–32. [Google Scholar] [CrossRef]
- Glazoff, M.V.; Khvan, A.; Zolotorevsky, V.S.; Belov, N.A.; Dinsdale, A. Casting Aluminum Alloys; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128118054. [Google Scholar]
- Yakovtseva, O.A.; Prosviryakov, A.S.; Cheverikin, V.V.; Zanaeva, E.N.; Mikhaylovskaya, A.V. Influence of High-Energy Ball Milling on the Microstructure, Phase Composition, and Microhardness of the Al–Mn–Cu Alloy. Russ. J. Non-Ferrous Met. 2022, 63, 426–433. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Bazlov, A.I.; Prosviryakov, A.S.; Emelina, N.B.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The Influence of the Al2O3 Particles on the Microstructure of the Mechanically Alloyed Al-Mn-Cu Alloy. J. Alloys Compd. 2023, 930, 167452. [Google Scholar] [CrossRef]
- Ghanbari, H.; Shafikhani, M.A.; Daryalaal, M. Graphene Nanosheets Production Using Liquid-Phase Exfoliation of Pre-Milled Graphite in Dimethylformamide and Structural Defects Evaluation. Ceram. Int. 2019, 45, 20051–20057. [Google Scholar] [CrossRef]
- Al Bacha, S.; Awad, A.S.; El Asmar, E.; Tayeh, T.; Bobet, J.-L.; Nakhl, M.; Zakhour, M. Hydrogen Generation via Hydrolysis of Ball Milled WE43 Magnesium Waste. Int. J. Hydrogen Energy 2019, 44, 17515–17524. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Liu, Y.; Zhang, Y.; Zhu, Y.; Li, L. Purity of MgH2 Improved by the Process of Pre-Milling Assisted Hydriding of Mg Powder under a Hydrogen Pressure of 0.5 MPa. Russ. J. Phys. Chem. A 2019, 93, 665–673. [Google Scholar] [CrossRef]
- Dan-Dan, S.; Jia-Liang, Z.; Yan-Qing, W.; Zhong-Qiu, Z.; Da-Kang, L. Raw-Material Pre-Milling on Physical Property of BaTiO$lt;Inf$gt;3$lt;/Inf$gt; Piezoelectric Ceramics. J. Inorg. Mater. 2017, 32, 615. [Google Scholar] [CrossRef]
- Yoon, M.-S.; Khansur, N.H.; Choi, B.-K.; Lee, Y.-G.; Ur, S.-C. The Effect of Nano-Sized BNBT on Microstructure and Dielectric/Piezoelectric Properties. Ceram. Int. 2009, 35, 3027–3036. [Google Scholar] [CrossRef]
- Aminikia, B. Investigation of the Pre-Milling Effect on Synthesis of Nanocrystalline TiB2–TiC Composite Prepared by SHS Method. Powder Technol. 2012, 232, 78–86. [Google Scholar] [CrossRef]
- Kasraee, K.; Tayebifard, A.; Salahi, E. Investigation of Pre-Milling Effect on Synthesis of Ti5Si3 Prepared by MASHS, SHS, and MA. J. Mater. Eng. Perform. 2013, 22, 3742–3748. [Google Scholar] [CrossRef]
- Lee, Y.G.; Ur, S.C.; Mahmud, I.; Yoon, M.S. Effects of Mechanically Activated Milling and Calcination Process on the Phase Stability and Particle Morphology of Monoclinic Zirconia Synthesized by Hydrolysis of ZrOCl2 Solution. Korean J. Mater. Res. 2013, 23, 543–549. [Google Scholar] [CrossRef]
- Ohtani, T.; Kusano, Y.; Ishimaru, K.; Morimoto, T.; Togano, A.; Yoshioka, T. Pre-Milling Effects on Self-Propagating Reactions in Mechanochemical Synthesis of CdSe and ZnSe. Chem. Lett. 2015, 44, 1234–1236. [Google Scholar] [CrossRef]
- Hoseinpur, A.; Jalaly, M.; Bafghi, M.S.; Vahdati Khaki, J.; Sakaki, M. The Effect of Preliminary Mechanical Activation on the Zinc Loss Control in Combustive Reduction of MoO3 by Zn. Int. J. Refract. Met. Hard Mater. 2016, 54, 251–259. [Google Scholar] [CrossRef]
- Cho, C.-H.; Hwang, K.-M.; Hwang, K.-M.; Seok, S.H.; Kim, S.-H.; Seo, J.-W.; Park, E.-S. Preparation and Characterization of High Drug-Loaded Microgranules: Particle Sizing and Mechanical Properties. Powder Technol. 2018, 326, 344–355. [Google Scholar] [CrossRef]
- Canakci, A.; Erdemir, F.; Varol, T.; Dalmış, R.; Ozkaya, S. Effects of a New Pre-Milling Coating Process on the Formation and Properties of an Fe–Al Intermetallic Coating. Powder Technol. 2014, 268, 110–117. [Google Scholar] [CrossRef]
- Oliveira, R.D.P.S.; Cogo, G.R.; Nascimento, B.L.; Reis, M.M.S.; Takimi, A.; Griza, S.; Bergmann, C.P. Influence of Pre-Milling of Cr3C2-25 NiCr Spray Powder on the Fatigue Life of HVOF-Sprayed Coating on ASTM A516 Steel Substrate. Materials 2023, 16, 1593. [Google Scholar] [CrossRef]
- Kwak, Y.J.; Choi, E.; Song, M.Y. Milling Processes and Hydrogen Storage Properties of Mg-Graphene Composites. Mater. Sci. 2019, 25, 286–291. [Google Scholar] [CrossRef]
- Shang, X.; Wang, X.; Chen, S. Effects of Ball Milling Processing Conditions and Alloy Components on the Synthesis of Cu-Nb and Cu-Mo Alloys. Materials 2019, 12, 1224. [Google Scholar] [CrossRef]
- Ghazanfari, H.; Blais, C.; Alamdari, H.; Gariépy, M.; Schulz, R. Mechanically Activated Combustion Synthesis of Fe3Al Composite Powders Reinforced with Sub-Micrometer TiC Particles. J. Alloys Compd. 2018, 761, 71–79. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, W. Mechano-Hydrothermal Preparation of Li-Al-OH Layered Double Hydroxides. Solid State Sci. 2018, 79, 93–98. [Google Scholar] [CrossRef]
- Chen, Y.; Hwang, T.; Marsh, M.; Williams, J.S. Study on Mechanism of Mechanical Activation. Mater. Sci. Eng. A 1997, 226–228, 95–98. [Google Scholar] [CrossRef]
- Xiang, D.P.; Li, C.; Liu, C.J.; Ding, L. Vacuum Synthesis of Nanocrystalline TiC at a Low Carbothermal Reduction Temperature. Adv. Mater. Res. 2013, 774–776, 881–886. [Google Scholar] [CrossRef]
- Zhou, E.; Suryanarayana, C.; Froes, F.H.S. Effect of Premilling Elemental Powders on Solid Solubility Extension of Magnesium in Titanium by Mechanical Alloying. Mater. Lett. 1995, 23, 27–31. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation Crystallography of Plate-Shaped Al 6(Mn,Fe) Dispersoids in AA5182 Alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Engler, O.; Laptyeva, G.; Wang, N. Impact of Homogenization on Microchemistry and Recrystallization of the Al-Fe-Mn Alloy AA 8006. Mater. Charact. 2013, 79, 60–75. [Google Scholar] [CrossRef]
- Engler, O.; Liu, Z.; Kuhnke, K. Impact of Homogenization on Particles in the Al-Mg-Mn Alloy AA 5454-Experiment and Simulation. J. Alloys Compd. 2013, 560, 111–122. [Google Scholar] [CrossRef]
- Engler, O.; Miller-Jupp, S. Control of Second-Phase Particles in the Al-Mg-Mn Alloy AA 5083. J. Alloys Compd. 2016, 689, 998–1010. [Google Scholar] [CrossRef]
- Dupuy, L.; Blandin, J.J. Damage Sensitivity in a Commercial Al Alloy Processed by Equal Channel Angular Extrusion. Acta Mater. 2002, 50, 3253–3266. [Google Scholar] [CrossRef]
- Feng, Z.; Luo, X.; Chen, Y.; Chen, N.; Wu, G. Surface Severe Plastic Deformation Induced Solute and Precipitate Redistribution in an Al-Cu-Mg Alloy. J. Alloys Compd. 2019, 773, 585–596. [Google Scholar] [CrossRef]
- Sauvage, X.; Ganeev, A.; Ivanisenko, Y.; Enikeev, N.; Murashkin, M.; Valiev, R. Grain Boundary Segregation in UFG Alloys Processed by Severe Plastic Deformation. Adv. Eng. Mater. 2012, 14, 968–974. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Enikeev, N.A.; Murashkin, M.Y.; Kazykhanov, V.U.; Sauvage, X. On the Origin of the Extremely High Strength of Ultrafine-Grained Al Alloys Produced by Severe Plastic Deformation. Scr. Mater. 2010, 63, 949–952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).