Influence of Graphene and Silver Addition on Aluminum’s Thermal Conductivity and Mechanical Properties Produced by the Powder Metallurgy Technique
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation and Characterization of Powders
2.2. Sintering and Physical Properties
2.3. Microstructure of the Sintered Samples
2.4. Mechanical Properties
2.5. Thermal Conductivity
2.6. Electrical Conductivity
3. Results and Discussion
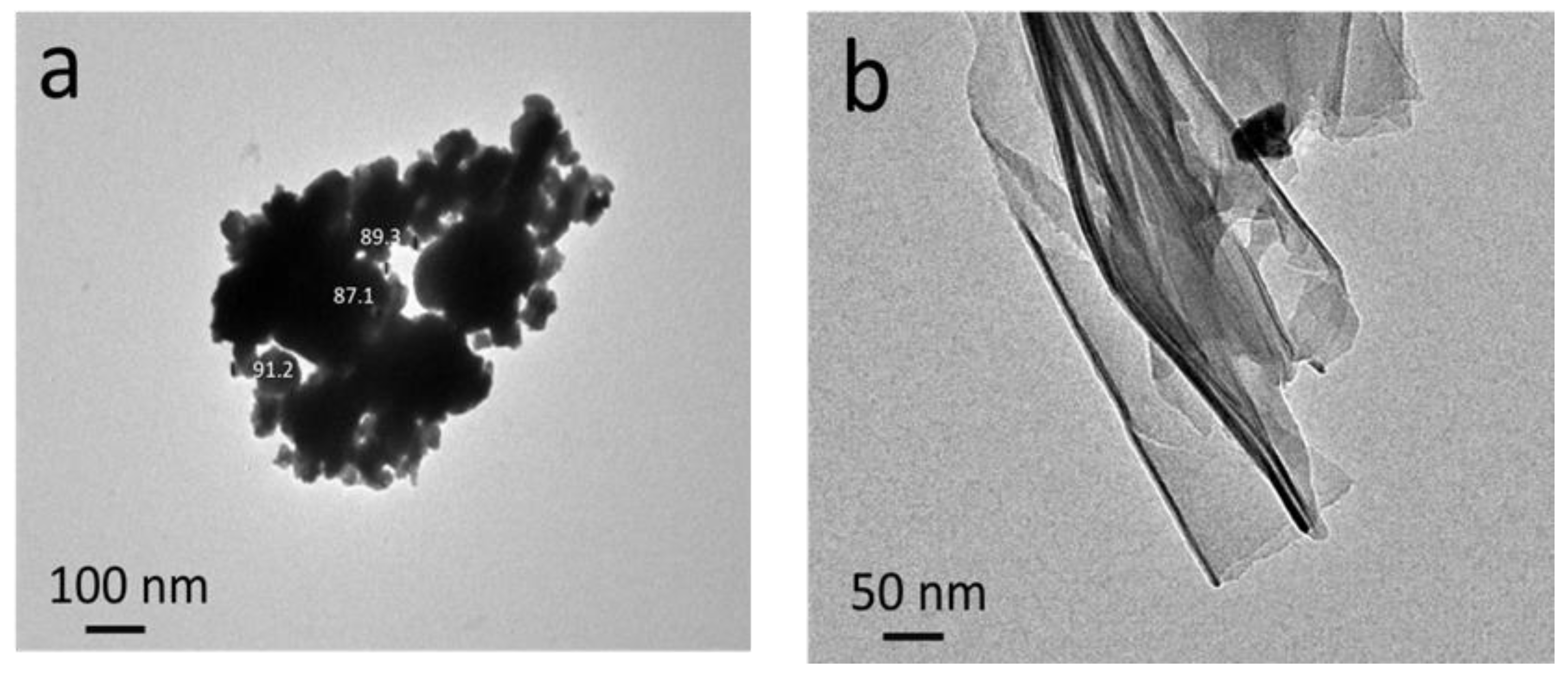
3.1. Microstructure of the Reinforcements Used
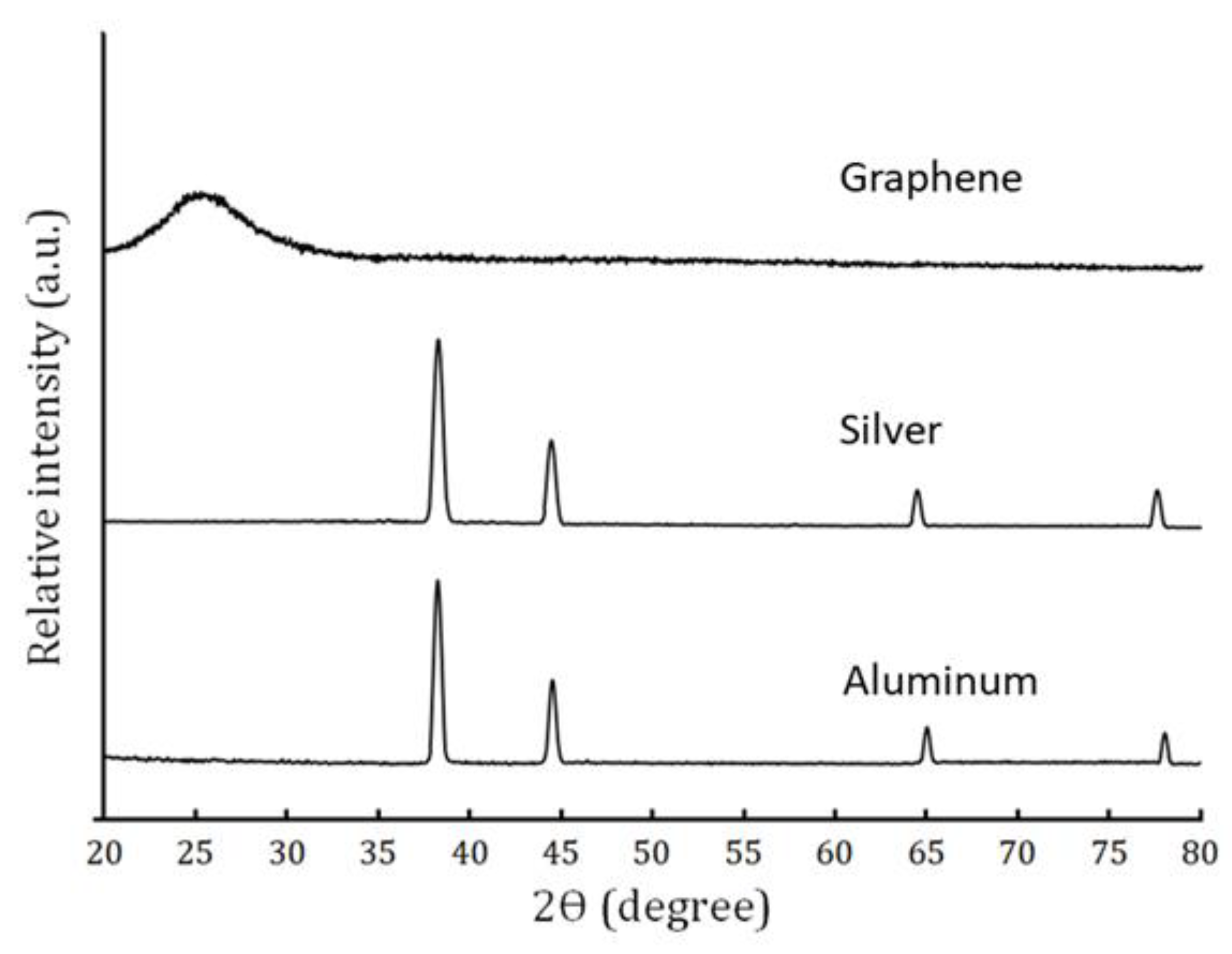
3.2. Phase Evolution
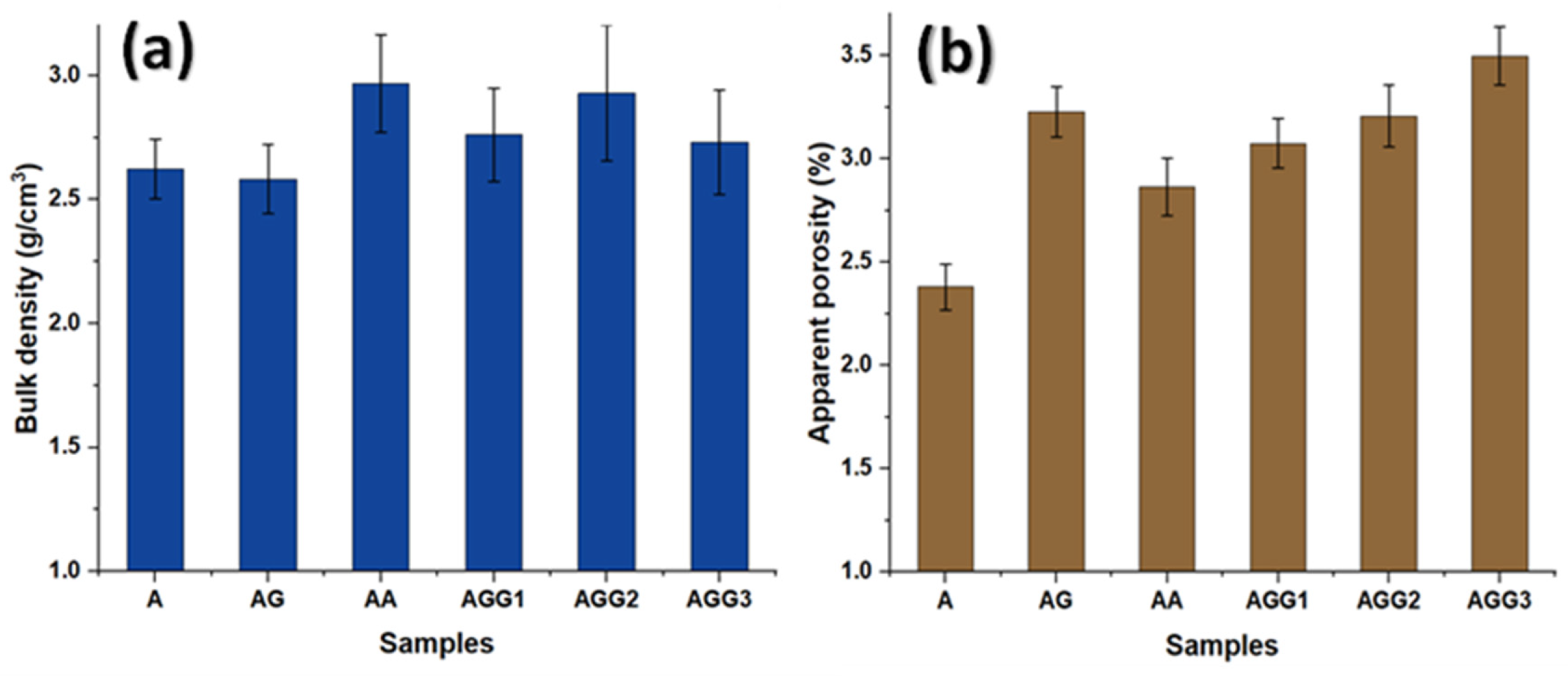
3.3. Physical Properties
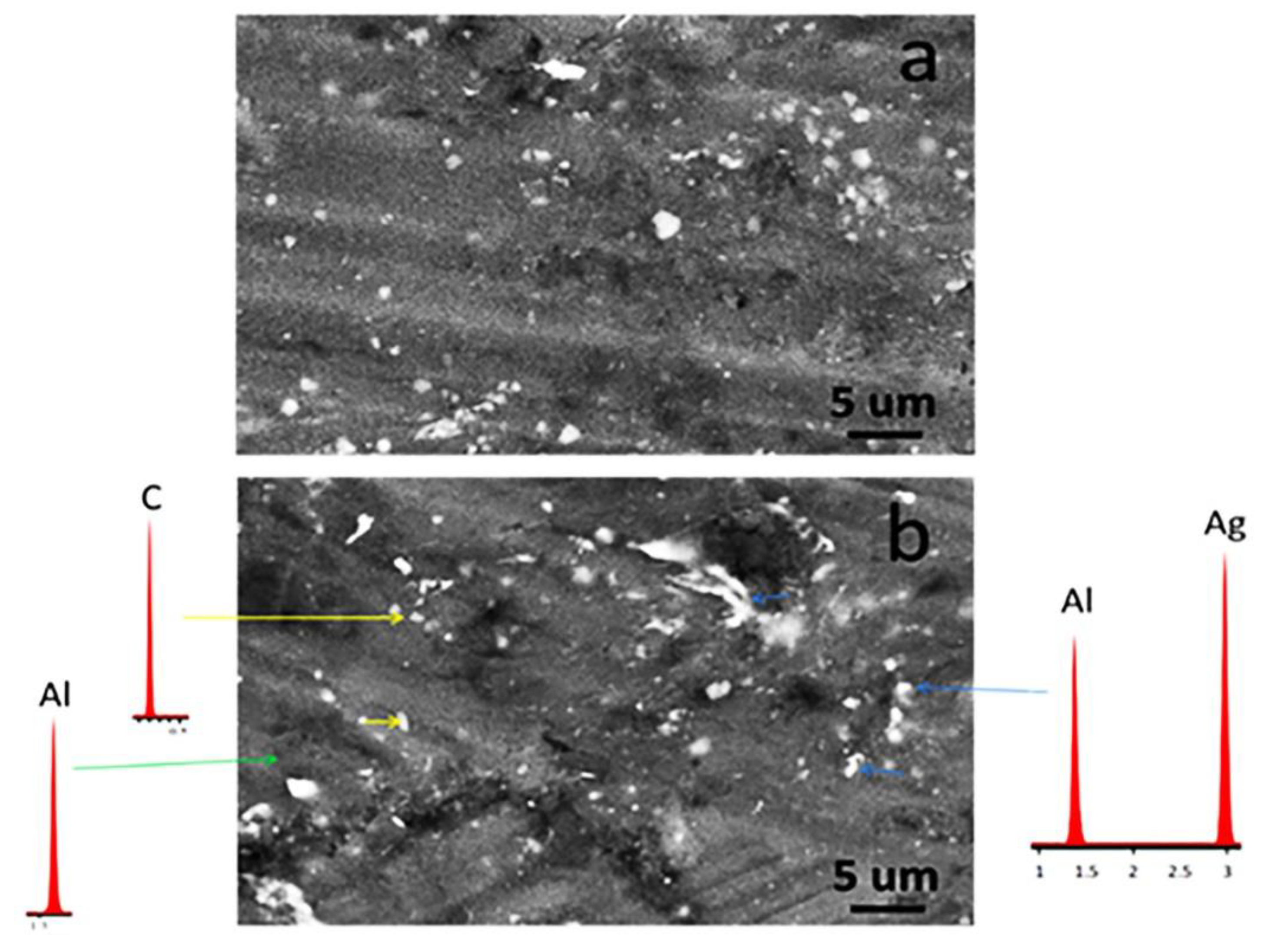
3.4. Microstructure of the Sintered Samples
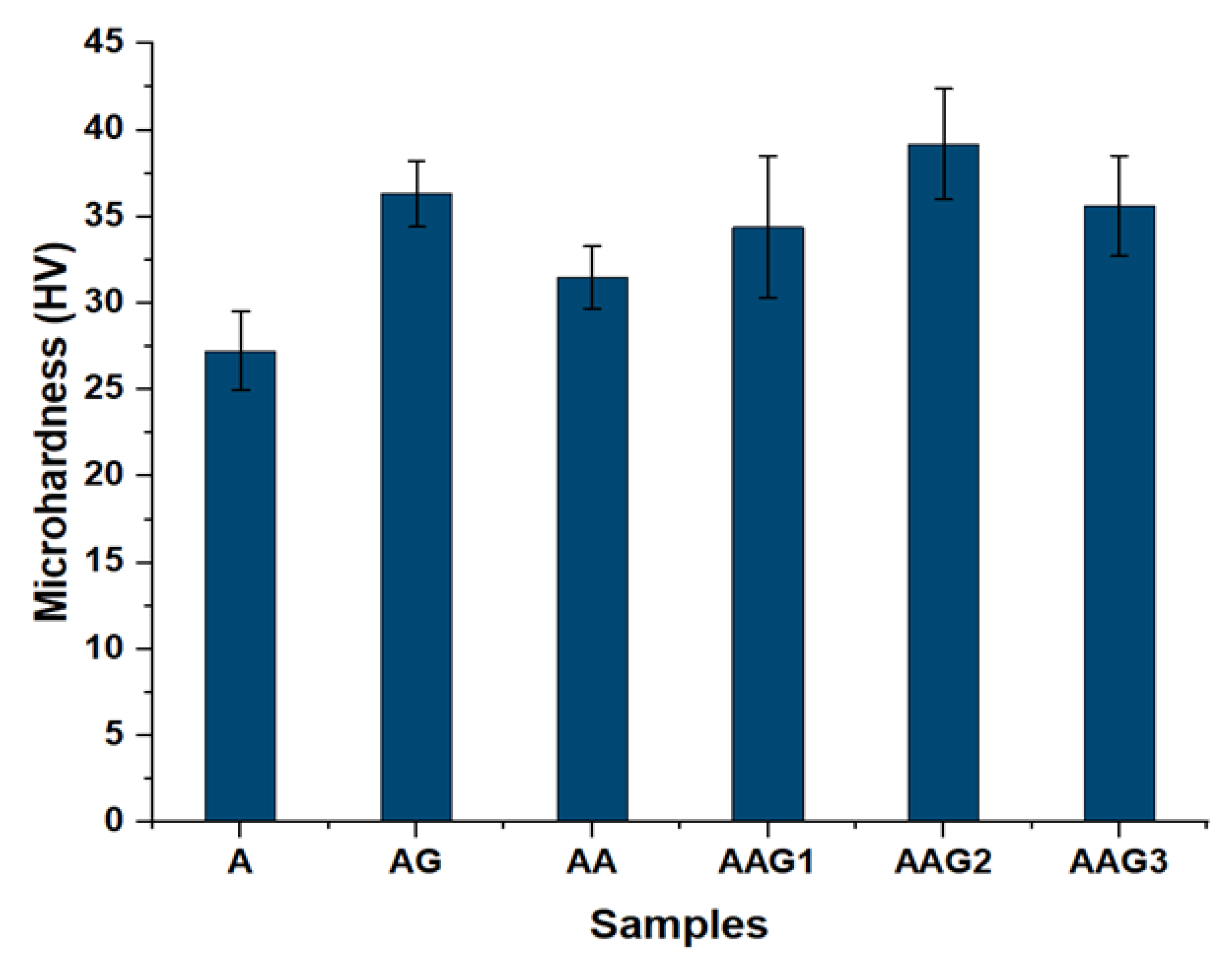
3.5. Mechanical Properties
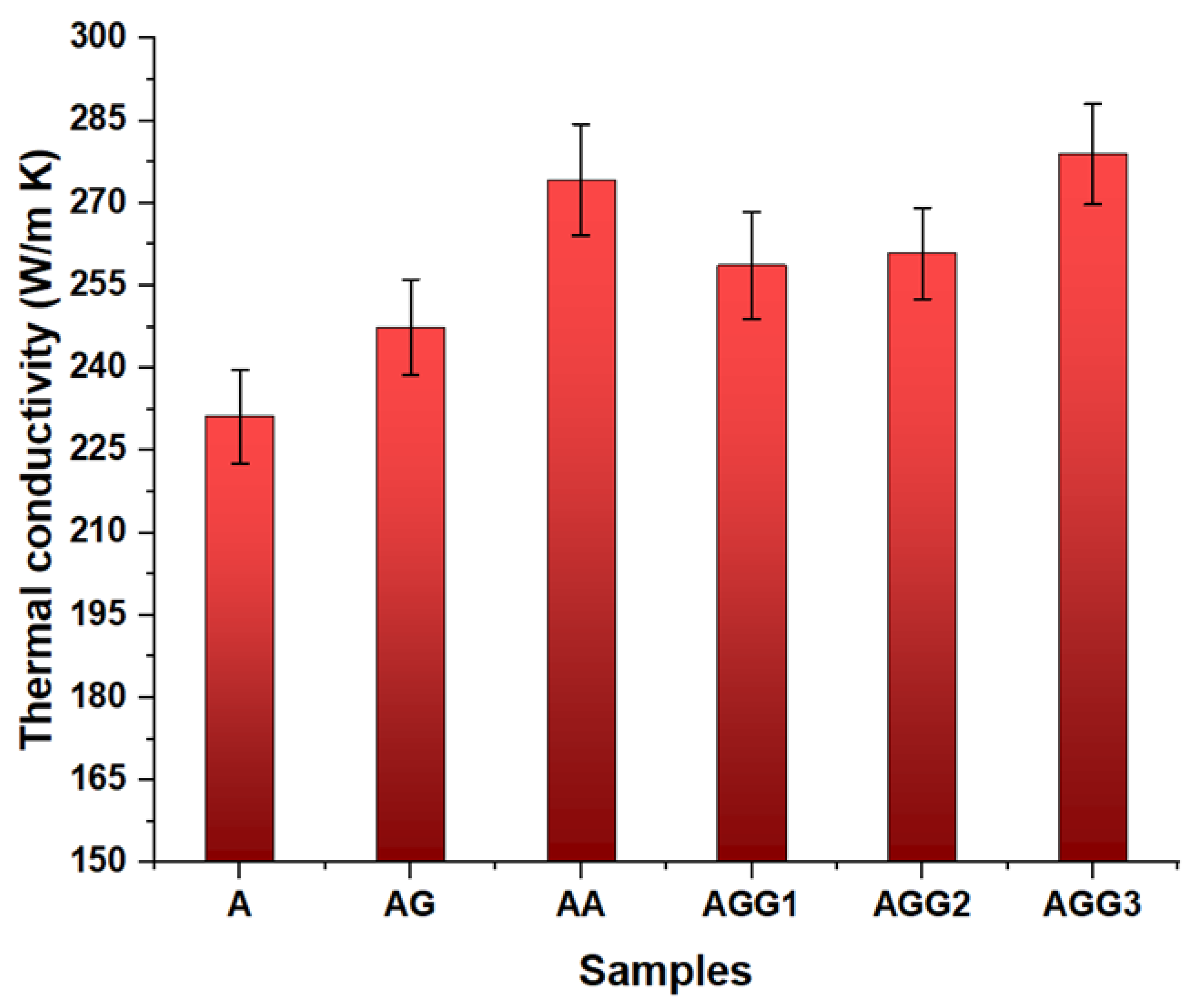
3.6. Thermal Conductivity
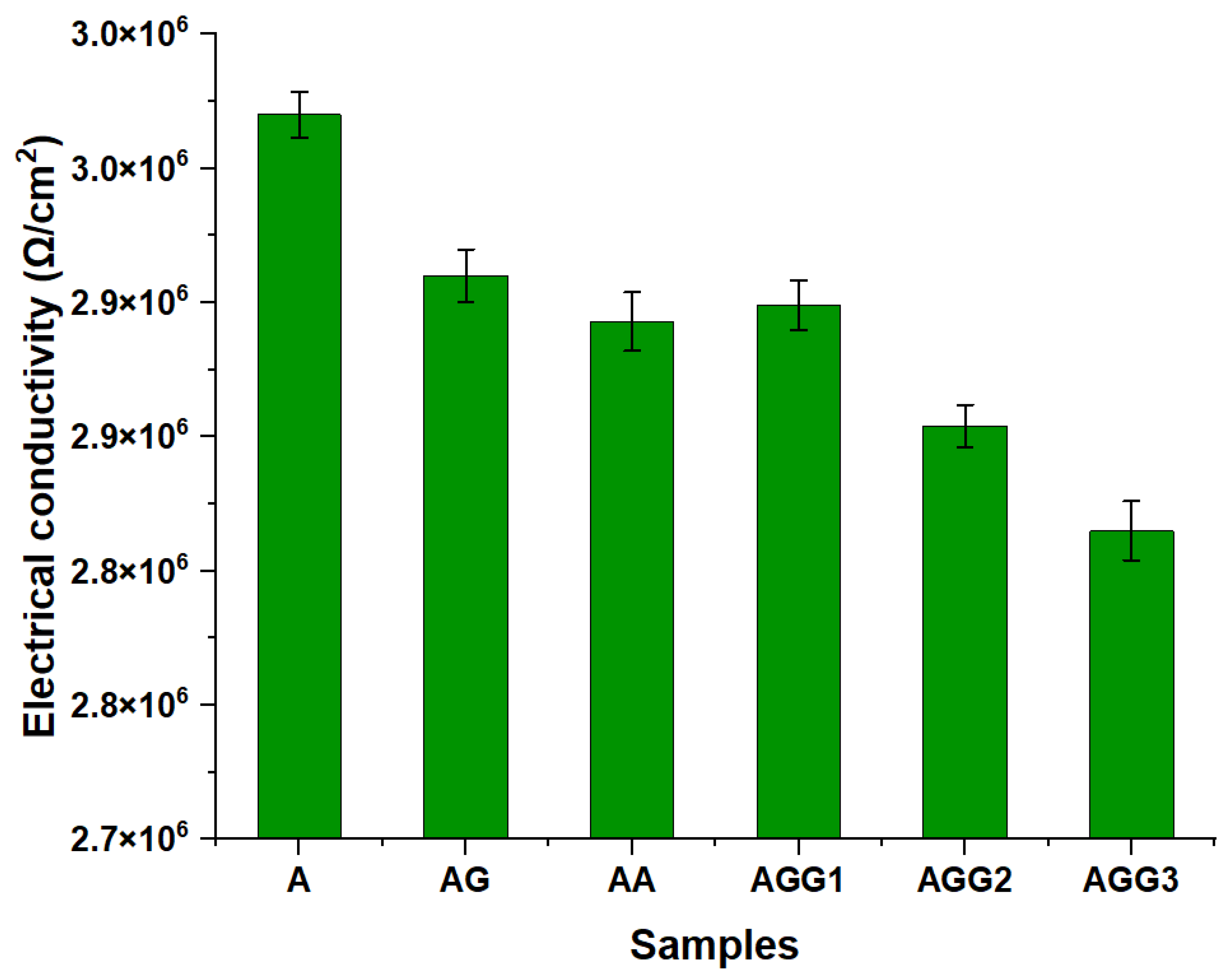
3.7. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cataldi, P.; Athanassiou, A.; Bayer, I.S. Graphene Nanoplatelets-Based Advanced Materials and Recent Progress in Sustainable Applications. Appl. Sci. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Khanna, V.; Kumar, V.; Bansal, S.A.; Prakash, C.; Ubaidullah, M.; Shaikh, S.F.; Pramanik, A.; Basak, A.; Shankar, S. Fabrication of efficient aluminium/graphene nanosheets (Al-GNP) composite by powder metallurgy for strength applications. J. Mater. Res. Technol. 2023, 22, 3402–3412. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Melaibari, A.; Alsoruji, G.; Khalil, A.M.; Mosleh, A.O. Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization. Nanotechnol. Rev. 2021, 10, 1752–1765. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.B.; Su, Y.; Zhang, J.; Zhang, D. Enhanced Mechanical Properties of Graphene (Reduced Graphene Oxide)/Aluminum Composites with a Bioinspired Nanolaminated Structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Parveez, B.; Kittur, M.I.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; Umarfarooq, M.A. Scientific Advancements in Composite Materials for Aircraft Applications: A Review. Polymers 2022, 14, 5007. [Google Scholar] [CrossRef]
- Zhou, H.; Ran, M.; Li, Y.; Yin, Z.; Tang, Y.; Zhang, W.; Zheng, W.; Liu, J. Improvement of thermal conductivity of diamond/Al composites by optimization of liquid-solid separation process. J. Mater. Process. Technol. 2021, 297, 117267. [Google Scholar] [CrossRef]
- Wen, C.; Gan, J.; Li, C.; Huang, Y.; Du, J. Comparative Study on Relationship Between Modification of Si Phase and Thermal Conductivity of Al–7Si Alloy Modified by Sr/RE/B/Sb Elements. Int. J. Met. 2020, 15, 194–205. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Y.; Du, J.; Wen, C.; Liu, J. Synchronous improvement in thermal conductivity and mechanical properties of Al–7Si–0.6Fe–0.5Zn cast alloy by B/La/Sr composite modification. Mater. Res. Express 2020, 7, 86501. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Sahoo, M.R.; Ratha, S.; Polai, B.; Mitra, A.; Sathpathy, B.; Sahu, A.; Kar, S.; Satyam, P.V.; Ajayan, P.M.; et al. Graphene-incorporated aluminum with enhanced thermal and mechanical properties for solar heat collectors. AIP Adv. 2020, 10, 065016. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Elsheikh, A.H.; Taha, M.A. The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging. Nanotechnol. Rev. 2022, 11, 2513–2525. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Alazwari, M.A.; Abushanab, W.S.; Ghandourah, E.I.; Mosleh, A.O.; Ahmed, H.M.; Taha, M.A. Influence of Friction Stir Process on the Physical, Microstructural, Corrosive, and Electrical Properties of an Al–Mg Alloy Modified with Ti–B Additives. Materials 2022, 15, 835. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.F.; Fino, P. Microstructure and Thermal Conductivity of Al–Graphene Composites Fabricated by Powder Metallurgy and Hot Rolling Techniques. Acta Met. Sin. 2017, 30, 675–687. [Google Scholar] [CrossRef]
- Carreño-Gallardo, C.; Estrada-Guel, I.; López-Meléndez, C.; Ledezma-Sillas, E.; Castañeda-Balderas, R.; Pérez-Bustamante, R.; Herrera-Ramírez, J.M. B4C Particles Reinforced Al2024 Composites via Mechanical Milling. Metals 2018, 8, 647. [Google Scholar] [CrossRef]
- Nayak, K.C.; Rane, K.K.; Date, P.P.; Srivatsan, T.S. Synthesis of an Aluminum Alloy Metal Matrix Composite Using Powder Metallurgy: Role of Sintering Parameters. Appl. Sci. 2022, 12, 8843. [Google Scholar] [CrossRef]
- Fatchurrohman, N.; Mamat, A.N.B.; Yetrina, M.; Muhida, R. Investigation of Metal Matrix Composites Aluminium Reinforced Graphite Particles Produced Using Powder Metallurgy. J. Teknol. 2022, 12, 76–81. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M.A. Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 2017, 1150, 188–195. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M. Dense alumina-based carbonated fluorapatite nanobiocomposites for dental applications. Mater. Chem. Phys. 2021, 257, 123264. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Almutairi, A.M.; Albalawi, K.; Dakhilallah1, O.K.; Zakaly, H.M.H.; Ene, A.; Abulyazied, D.E.; Ahmed, S.M.; Youness, R.A.; Taha, M.A. Production of Hybrid Nanocomposites Based on Iron Waste Reinforced with Niobium Carbide/Granite Nanoparticles with Outstanding Strength and Wear Resistance for Use in Industrial Applications. Nanomaterials 2023, 13, 537. [Google Scholar] [CrossRef]
- Moustafa, E.B.; AbuShanab, W.S.; Youness, R.A.; Taha, M.A. Improved mechanical properties of Cu8Ni4Sn alloy as functionally graded composites with preserving its thermal and electrical properties. Mater. Chem. Phys. 2022, 292, 126778. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A. Review on using powder metallurgy method for production of metal-based nanocomposites. Egypt. J. Chem. 2021, 64, 7215–7222. [Google Scholar]
- Youness, R.A.; Ibrahim, M.A.; Taha, M.A. Evaluation of the electrical and dielectric behavior of the apatite layer formed on the surface of hydroxyapatite/hardystonite/copper oxide hybrid nanocomposites for bone repair applications. Ceram. Int. 2022, 48, 19837–19850. [Google Scholar] [CrossRef]
- Almoselhy, R.I.; Eid, M.; Abd-Elmageed, S.M.; Youness, R. Using nanotechnology in bleaching vegetable oils. Egypt. J. Chem. 2020, 63, 2699–2706. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Ghandourah, E.; Youness, R.A.; Melaibari, A.A.; Taha, M.A. Ultralight Functionally Graded Hybrid Nanocomposites Based on Yttrium and Silica-Reinforced Mg10Li5Al Alloy: Thermal and Tribomechanical Properties. Materials 2022, 15, 9052. [Google Scholar] [CrossRef] [PubMed]
- Alazwari, M.A.; Moustafa, E.B.; Khoshaim, A.B.; Taha, M.A. Mechanical and wear evolution of in situ synthesized Ti–Cu alloy matrix hybrid composite reinforced by low-cost activated carbon and silica fume waste ceramic for industrial applications. J. Mater. Res. Technol. 2023, 22, 2284–2296. [Google Scholar] [CrossRef]
- Youness, R.A.; Amer, M.S.; Taha, M.A. Tribo-mechanical measurements and in vivo performance of zirconia-containing biphasic calcium phosphate material implanted in a rat model for bone replacement applications. Mater. Chem. Phys. 2022, 285, 126085. [Google Scholar] [CrossRef]
- Abushanab, W.S.; Moustafa, E.B.; Youness, R.A. Evaluation of the dynamic behavior, elastic properties, and in vitro bioactivity of some borophosphosilicate glasses for orthopedic applications. J. Non-Cryst. Solids 2022, 586, 121539. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Alturki, A.M.; Youness, R.A.; Abomostafa, H.M. Synthesis, structural and biomedical characterization of hydroxyapatite/borosilicate bioactive glass nanocomposites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4077–4092. [Google Scholar] [CrossRef]
- Youness, R.A.; Zawrah, M.F.; Taha, M.A. Synthesis of ZnO containing calcium silicate nano powders: A study on sinterability, mechanical and electrical properties. Silicon 2023. [Google Scholar] [CrossRef]
- Abushanab, W.S.; Moustafa, E.B.; Ghandourah, E.I.; Hussein, H.; Taha, M.A.; Mosleh, A.O. impact of hard and soft reinforcements on the microstructure, mechanical, and physical properties of the surface composite matrix manufactured by friction stir processing. Coatings 2023, 13, 284. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Zawrah, M. Review on nanocomposites fabricated by mechanical alloying. Int. J. Miner. Met. Mater. 2019, 26, 1047–1058. [Google Scholar] [CrossRef]
- Rajkovic, V.; Bozic, D.; Jovanovic, M.T. Properties of copper matrix reinforced with various size and amount of Al2O3 particles. J. Mater. Process. Technol. 2008, 200, 106–114. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M. Effect of nano ZrO2 on strengthening and electrical properties of Cu-matrix nanocomposits prepared by mechanical alloying. Ceram. Int. 2017, 43, 12698–12704. [Google Scholar] [CrossRef]
- Ramadan, S.; El-Meligy, W.M.; Saudi, H.A.; Zawrah, M.F.; Taha, M.A. Influence of Graphene Content on Sinterability and Physico-Mechanical Characteristics of Al/Graphene Composites Prepared via Powder Metallurgy. Biointerface Res. Appl. Chem. 2022, 13, 192. [Google Scholar]
- Niteesh Kumar, S.J.; Keshavamurthy, R.; Haseebuddin, M.R.; Koppad, P.G. mechanical properties of aluminium-graphene composite synthesized by powder metallurgy and hot extrusion. Trans. Indian Inst. Met. 2017, 70, 605–613. [Google Scholar] [CrossRef]
- Alsoruji, G.; Moustafa, E.B.; Alzahrani, M.A.; Taha, M.A. Preparation of Silicon Bronze-Based Hybrid Nanocomposites with Excellent Mechanical, Electrical, and Wear Properties by Adding the Ti3AlC2 MAX Phase and Granite Via Powder Metallurgy. Silicon 2022. [Google Scholar] [CrossRef]
- Nourouzi, S.; Damavandi, E.; Rabiee, S.M. Microstructural and mechanical properties of Al-Al2O3 composites focus on experimental techniques. Int. J. Microstruct. Mater. Prop. 2016, 11, 383–395. [Google Scholar]
- Youness, R.A.; Taha, M.A. Study of mechanical properties and wear behavior of nano-ZrO2-hardened Al2024 matrix composites prepared by stir cast method. Egypt. J. Chem. 2021, 65, 307–313. [Google Scholar]
- AbuShanab, W.S.; Moustafa, E.B.; Ghandourah, E.; Taha, M.A. Effect of graphene nanoparticles on the physical and mechanical properties of the Al2024-graphene nanocomposites fabricated by powder metallurgy. Results Phys. 2020, 19, 103343. [Google Scholar] [CrossRef]
- Kumar, C.A.V.; Rajadurai, J.S. Influence of rutile (TiO2) content on wear and microhardness characteristics of aluminium-based hybrid composites synthesized by powder metallurgy. Trans. Nonferrous Met. Soc. China 2016, 26, 63–73. [Google Scholar] [CrossRef]
- Ashok, N.; Shanmughasundaram, P. Effect of particles size on the mechanical properties of SiC-reinforced aluminium 8011 composites. Mater. Tehnol. 2017, 51, 667–672. [Google Scholar] [CrossRef]
- Marioara, C.D.; Nakamura, J.; Matsuda, K.; Andersen, S.J.; Holmestad, R.; Sato, T.; Kawabata, T. HAADF-STEM study of β′-type precipitates in an over-aged Al–Mg–Si–Ag alloy. Philos. Mag. 2012, 92, 1149–1158. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Zhang, H. Influences of Ag Addition on the Microstructure and Mechanical Properties of Al-4Mg Alloy. Materials 2022, 15, 7705. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Tak, W.-S.; Mun, S.Y.; Nam, S.; Moon, S.Y.; Kim, W.S. Graphene Encapsulated Al Particles for Improvement of Thermal Conductivity in Composites. Materials 2020, 13, 3602. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhou, C.; Li, Z.; Ou, B.; Zhao, K.; Yu, P.; Li, S.; Zhao, J. Thermal conductivity of Aluminum/Graphene metal-matrix composites: From the thermal boundary conductance to thermal regulation. Mater. Today Commun. 2022, 30, 103147. [Google Scholar] [CrossRef]
- Mamala, A.; Knych, T.; Kwaśniewski, P.; Kawecki, A.; Kiesiewicz, G.; Sieja-Smaga, E.; Ściężor, W.; Gniełczyk, M.; Kowal, R. New Al-Ag Alloys for Electrical Conductors with Increased Current Carrying Capacity. Arch. Met. Mater. 2016, 61, 1875–1880. [Google Scholar] [CrossRef]
- Khoshaim, A.B.; Moustafa, E.B.; Alazwari, M.A.; Taha, M.A. An Investigation of the Mechanical, Thermal and Electrical Properties of an AA7075 Alloy Reinforced with Hybrid Ceramic Nanoparticles Using Friction Stir Processing. Metals 2023, 13, 124. [Google Scholar] [CrossRef]




| Sample | Composition (vol.%) | ||
|---|---|---|---|
| Al | G | Ag | |
| A | 100 | ----- | ----- |
| AG | 99.5 | 0.5 | ----- |
| AA | 95 | ----- | 5 |
| AGG1 | 97.25 | 0.25 | 2.5 |
| AGG2 | 97 | 0.5 | 2.5 |
| AGG3 | 94.75 | 0.25 | 5 |
| Element | Mg | Si | Fe | Cu | Zn | Mn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | 0.08 | 0.04 | 0.04 | 0.06 | 0.05 | 0.01 | 0.02 | 0.01 | balance |
| Sample Code | Crystal Size (nm) ± 0.1 | Lattice Strain (%) ± 0.001 |
|---|---|---|
| A | 18.04 | 0.4813 |
| AG | 16.62 | 0.5157 |
| AA | 12.86 | 0.6450 |
| AGG1 | 15.28 | 0.5544 |
| AGG2 | 15.06 | 0.5611 |
| AGG3 | 12.58 | 0.6619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa, E.B.; Abdel Aziz, S.S.; Taha, M.A.; Saber, A.-H. Influence of Graphene and Silver Addition on Aluminum’s Thermal Conductivity and Mechanical Properties Produced by the Powder Metallurgy Technique. Metals 2023, 13, 836. https://doi.org/10.3390/met13050836
Moustafa EB, Abdel Aziz SS, Taha MA, Saber A-H. Influence of Graphene and Silver Addition on Aluminum’s Thermal Conductivity and Mechanical Properties Produced by the Powder Metallurgy Technique. Metals. 2023; 13(5):836. https://doi.org/10.3390/met13050836
Chicago/Turabian StyleMoustafa, Essam B., Salem S. Abdel Aziz, Mohammed A. Taha, and Abdel-Halim Saber. 2023. "Influence of Graphene and Silver Addition on Aluminum’s Thermal Conductivity and Mechanical Properties Produced by the Powder Metallurgy Technique" Metals 13, no. 5: 836. https://doi.org/10.3390/met13050836
APA StyleMoustafa, E. B., Abdel Aziz, S. S., Taha, M. A., & Saber, A.-H. (2023). Influence of Graphene and Silver Addition on Aluminum’s Thermal Conductivity and Mechanical Properties Produced by the Powder Metallurgy Technique. Metals, 13(5), 836. https://doi.org/10.3390/met13050836








