Microstructural Investigation of Some Bronze Artifacts Discovered in a Dacian Site Using Non-Destructive Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archeological Artifact Description
2.2. Investigation Methods
3. Results
3.1. Metallic Core
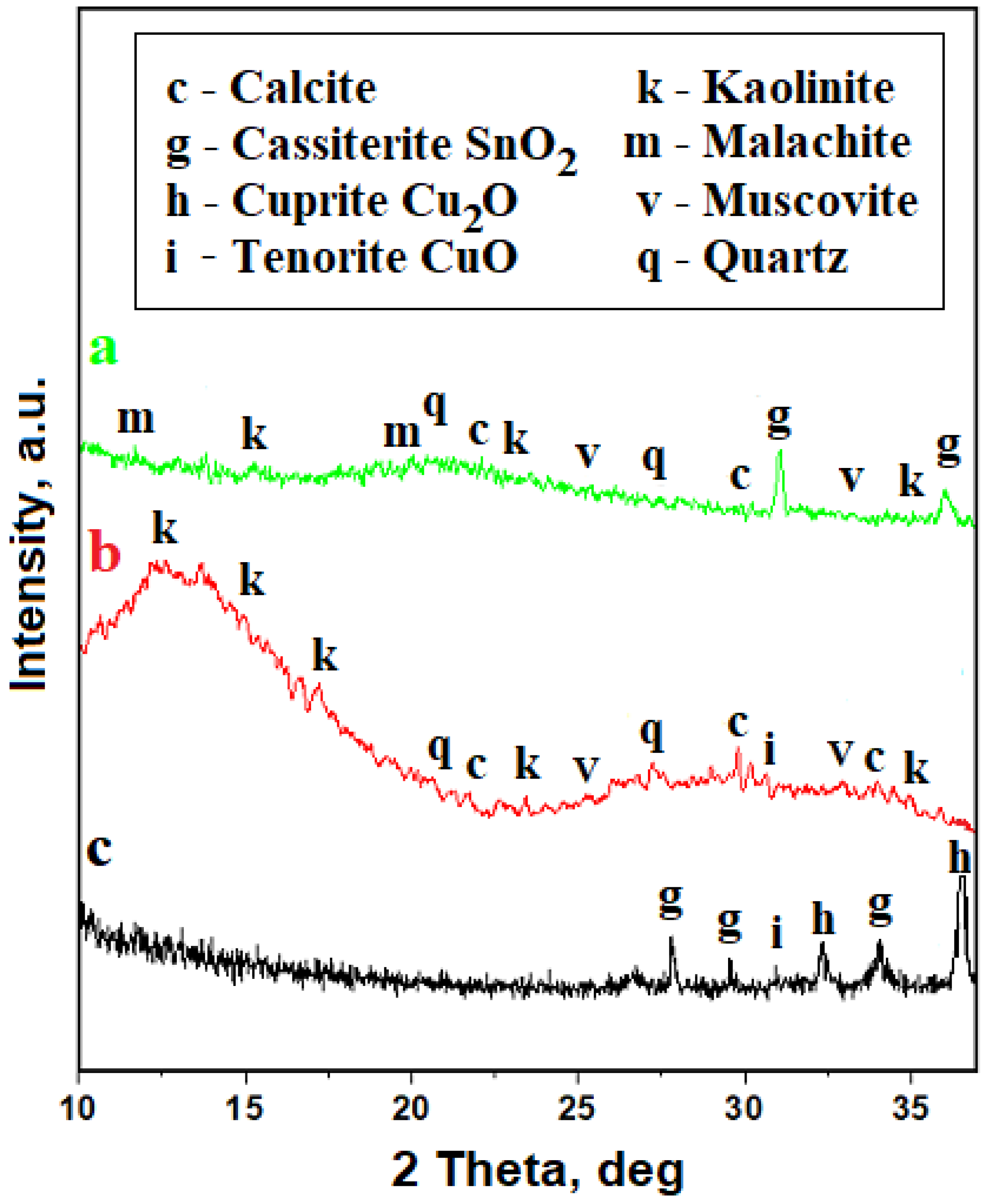
3.2. Patina
3.3. Bronze Mirror Fragment Surface Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, C.; Sellamuthu, R. The Effect of Sn Content on the Properties of Surface Refined Cu-Sn Bronze Alloys. Procedia Eng. 2014, 97, 1341–1347. [Google Scholar] [CrossRef]
- Chang, T.; Herting, G.; Goidanich, S.; Sánchez Amaya, J.M.; Arenas, M.A.; Le Bozec, N.; Jin, Y.; Leygraf, C.; Odnevall Wallinder, I. The role of Sn on the long-term atmospheric corrosion of binary Cu-Sn bronze alloys in architecture. Corros. Sci. 2019, 149, 54–67. [Google Scholar] [CrossRef]
- Kılınç, A.Ç.; Goktas, A.A.; Keskin, Ö.Y.; Köktaş, S. Extrusion-Based 3D Printing of CuSn10 Bronze Parts: Production and Characterization. Metals 2021, 11, 1774. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, R.; Xiong, W.; He, Z.; Liu, T.; Li, Y. Compressive Rheological Behavior and Microstructure Evolution of a Semi-Solid CuSn10P1 Alloy at Medium Temperature and Low Strain. Metals 2022, 12, 143. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Zhu, R.; Wang, H. Analysis of Lead Smelting Technology in the Early Bronze Age Based on Smelting Slag from the Central Plains of China. Metals 2023, 13, 435. [Google Scholar] [CrossRef]
- Xie, C.; Fu, C.; Li, S.; Liao, L.; Chen, G.; Pan, C. A Special Ancient Bronze Sword and Its Possible Manufacturing Technique from Materials Science Analysis. Materials 2022, 15, 2491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, W.; Yang, Y.; Chen, D.; Du, J.; Tang, X. Preliminary Discussion on the Highly Radiogenic Lead in Unalloyed Copper Artifacts of the Eastern Zhou Dynasty: Starting from the Huili Copper Spearheads. Metals 2020, 10, 1252. [Google Scholar] [CrossRef]
- Iannaccone, R.; Depalmas, A.; Bulla, C.; Barcellos Lins, S.A.; Brunetti, A. Characterisation of Alloy Composition of Protohistoric Small Boat Models from Sardinia (Italy). Materials 2022, 15, 1324. [Google Scholar] [CrossRef]
- Rustoiu, A. Dacian Bronze Metallurgy; Romanian Institute of Thracology: Bucharest, Romania, 1996; p. 354. (In Romanian) [Google Scholar]
- Iaroslavschi, E. Dacian Techniques; Bibliotheca Musei Napocensis: Cluj-Napoca, Romania, 1997; p. 215. (In Romanian) [Google Scholar]
- Smit, Z.; Kos, P. Elemental analysis of Celtic coins. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1984, 3, 416–418. [Google Scholar] [CrossRef]
- Northover, J.P.; Salter, C.J. Decorative metallurgy of the celts. Mater. Charact. 1990, 25, 109–123. [Google Scholar] [CrossRef]
- Ingo, G.M.; Plescia, P.; Angelini, E.; Riccucci, C.; De Caro, T. Bronze roman mirrors: The secret of brightness. Appl. Phys. A 2006, 83, 611–615. [Google Scholar] [CrossRef]
- Di Turo, F.; Montoya, N.; Piquero-Cilla, J.; De Vito, C.; Coletti, F.; Favero, G.; Doménech-Carbó, A. Archaeometric analysis of Roman bronze coins from the Magna Mater temple using solid-state voltammetry and electrochemical impedance spectroscopy. Anal. Chim. Acta 2017, 955, 36–47. [Google Scholar] [CrossRef]
- Ingo, G.M.; Angelini, E.; De Caro, T.; Bultrini, G.; Mezzi, A. Combined use of XPS and SEM + EDS for thestudy of surface microchemical structure of archaeological bronze Roman mirrors. Surf. Interface Anal. 2004, 36, 871–875. [Google Scholar] [CrossRef]
- Craddock, P. The composition of the copper alloys used by the Greek, Etruscan and Roman civilisations 2. The Archaic, Classical and Hellenistic Greeks. J. Archaeol. Sci. 1977, 4, 103–133. [Google Scholar] [CrossRef]
- Panseri, C.; Leoni, M. The manufacturing technique of Etruscan mirrors. Stud. Conserv. 1957, 3, 49–63. [Google Scholar]
- Lombardi, G. The casting core composition and provenance of the Goljamata Kosmatka (Bulgaria) bronze head. J. Archaeol. Sci. 2009, 36, 520–527. [Google Scholar] [CrossRef]
- Daicoviciu, C. Piatra Rosie Dacian Fortress; Romanian Academy Publishing House: Bucharest, Romania, 1954. (In Romanian) [Google Scholar]
- Glodariu, I. Addenda aux “Points de repère pour la chronologie des citadelles et des établissementsdaciques de Monts d’Orăştie”. Acta Musei Napoc. 1995, 32, 119–134. [Google Scholar]
- Casalean, A.C. Artefacte din Munții Orăștiei aflate în depozitul Muzeului de Etnografie și Artă Populară din Orăștie. In ArheoVest VII. In Honorem Sabin Adrian Luca; Micle, D., Forțiu, S., Eds.; JATE Press Kiadó: Szeged, Hungary, 2019; pp. 355–364. [Google Scholar]
- Cetean, V.; Pețan, A.; Stancu, M. Historical Use of the Ashlar Limestone at Piatra Roșie Dacian Fortress; Interdisciplinary Approach in a World Heritage Site. Sustainability 2022, 14, 11818. [Google Scholar] [CrossRef]
- Yagel, O.; Ben-Yosef, E. Lead in the Levant duringthe Late Bronzeandearly Iron Ages. J. Archaeol. Rep. 2022, 46, 103649. [Google Scholar] [CrossRef]
- Vasilescu, A.; Constantinescu, B.; Stan, D.; Talmatchi, G.; Ceccato, D. XRF and micro-PIXE studies of inhomogeneity of ancient bronze and silver alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 406, 302–308. [Google Scholar] [CrossRef]
- Yakuphanoglu, F. Electrical conductivity, Seebeck coefficient and optical properties of SnO2 film deposited on ITO by dip coating. J. Alloys Compd. 2008, 470, 55–59. [Google Scholar] [CrossRef]
- Tan, C.S.; Hsu, S.C.; Ke, W.H.; Chen, L.J.; Huang, M.H. Facet-dependent electrical conductivity properties of Cu2O crystals. Nano Lett. 2015, 15, 2155–2160. [Google Scholar] [CrossRef]
- Sandu, I.G.; Stoleriu, S.; Sandu, I.; Brebu, M.; Sandu, A.V. Ancient bronze coin identification by archeological patina study. I. Composition and structure. Rev. Chim. Bucur. 2005, 56, 981–994. [Google Scholar]
- Marussi, G.; Crosera, M.; Prenesti, E.; Callegher, B.; Baracchini, E.; Turco, G.; Adami, G. From Collection or Archaeological Finds? A Non-Destructive Analytical Approach to Distinguish between Two Sets of Bronze Coins of the Roman Empire. Molecules 2023, 28, 2382. [Google Scholar] [CrossRef]
- Artioli, G.; Dapiaggi, M.; Fornasini, P.; Sanson, A.; Rocca, F.; Merli, M. Negative thermal expansion in cuprite-type compounds: A combined synchrotron XRPD, EXAFS, and computational study of Cu2O and Ag2O. J. Phys. Chem. Solids 2006, 67, 1918–1922. [Google Scholar] [CrossRef]
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Álvarez-Romero, C.; Doménech-Carbó, A.; Osete-Cortina, L.; Martínez-Bazán, M.L. Microchemical surface analysis of historic copper-based coins by the combined use of FIB-FESEM-EDX, OM, FTIR spectroscopy and solid-state electrochemical techniques. Microchem. J. 2019, 148, 573–581. [Google Scholar] [CrossRef]
- Koupadi, K.; Boyatzis, S.C.; Roumpou, M.; Kalogeropoulos, N.; Kotzamani, D. Organic remains in early Christian Egyptian metal vessels: Investigation with fourier transform infrared spectroscopy and gas chromatography–mass spectrometry. Heritage 2021, 4, 3611–3629. [Google Scholar] [CrossRef]
- Rios-Reyes, C.A.; Nunez-Alarcon, E.D.; Puentes-Arguello, L.S.; Barrios Lopez, J.C.; Moreno-Gonzalez, L.; Martinez, J.A.H. Mineralogical characterization of an ancient pottery from the la Candelaria archaeological site, Santa Helena del Opón, Santander (Colombia). Mater. Sci. Eng. Int. J. 2019, 3, 118–124. [Google Scholar] [CrossRef]
- Reig, F.B.; Adelantado, J.V.G.; Moya Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Seetha, D.; Velraj, G. Spectroscopic and statistical approach of archaeological artifacts recently excavated from Tamilnadu, South India. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 149, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Filopoulou, A.; Vlachou, S.; Boyatzis, S.C. Fatty acids and their metal salts: A review of their infrared spectra in light of their presence in cultural heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef] [PubMed]
- Pięta, E.; Lekki, J.; del Hoyo-Meléndez, J.M.; Paluszkiewicz, C.; Nowakowski, M.; Matosz, M.; Kwiatek, W.M. Surface characterization of medieval silver coins minted by the early Piasts: FT-IR mapping and SEM/EDX studies. Surf. Interface Anal. 2018, 50, 78–86. [Google Scholar] [CrossRef]
- Bertolotti, G.; Bersani, D.; Lottici, P.P.; Alesiani, M.; Malcherek, T.; Schlüter, J. Micro-Raman study of copper hydroxychlorides and other corrosion products of bronze samples mimicking archaeological coins. Anal. Bioanal. Chem. 2012, 402, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Di Fazio, M.; Felici, A.C.; Catalli, F.; Doménech-Carbó, M.T.; De Vito, C.; Doménech-Carbó, A. Solid-state electrochemical characterization of emissions and authorities producing Roman brass coins. Microchem. J. 2020, 152, 104306. [Google Scholar] [CrossRef]
- Gramaticu, S.; Oberländer-Târnoveanu, E. A deposit of autonomus Istrian coins found at Calan, Hunedoara, County. Cercet. Numis. 2005, 9–11, 25–36. (In Romanian) [Google Scholar]
- Teodorescu, D.M. Ancient Fortresses in Hunedoara Mountains; Instituto de Artes Gráficas, Ed.; șiLibrarie Cartea Românească: Cluj-Napoca, Romania, 1923. (In Romanian) [Google Scholar]
- Macrea, M. Coins from Costesti Dacian Fortress. Anu. Inst. Stud. Clasice 1936, 2, 147–163. (In Romanian) [Google Scholar]
- Gazdac, C. The Coin Assembly as a Votive Deposit in Iron Age. The Case of Coins in The Ritual Complex at The Dacian Fortress of Costești-Cetățuie (Hunedoara County, Romania). J. Anc. Hist. Archaeol. 2018, 5, 55–65. [Google Scholar] [CrossRef]
- Gheorghiu, G. Dacians on the Middle of Mures River at the End of the 2nd Century BC to the Beginning of the 2nd Century AD; Mega Publishing House: Cluj-Napoca, Romania, 2005. (In Romanian) [Google Scholar]
- Daicoviciu, H. Addendum at “Dacian Settlements in Orastie Mountains”. Acta Musei Napoc. 1964, 1, 111–124. (In Romanian) [Google Scholar]
- Petan, A. The Mint at Sarmisegetusa Regia, A Re-Evaluation. In Miscellanea Historica et Archaeologica in Memoriam Dumitru Berciu; Bărbulescu, C.A., Croitoru, C., Udrescu, O.V., Eds.; Istros: Brăila-Râmnicu Vâlcea, Romania, 2020; pp. 189–207. (In Romanian) [Google Scholar]
- Stoicovici, E.; Winkler, I. Study on the composition and constitution of some antique coins by metallographic analysis. Acta Musei Napoc. 1967, 4, 449–456. (In Romanian) [Google Scholar]
- Preda, C. Geto—Dacian Coins; Romanian Academy Publishing House: Bucuresti, Romania, 1973. (In Romanian) [Google Scholar]
- Tong, Q.; Ge, J.; Rong, M.; Li, J.; Jiao, J.; Zhang, L.; Wang, J. Thermodynamic Modeling of the Ag-Cu-Sn Ternary System. Metals 2022, 12, 1557. [Google Scholar] [CrossRef]
- Constantinescu, B.; Ciută, M.M.; Popa, N.; Cristea-Stan, D.; Oberländer-Târnoveanu, E. Consideraţii privind tezaurul compus din 12 monede de tip Răduleşti-Hunedoara recuperate în urma unor săpături ilegale din zona Dealul Muncelului, vest de Sarmizegetusa Regia. In ArheoVest VI. In Memoriam Marian Gumă; JATE Press Kiadó: Szeged, Hungary, 2018; pp. 667–681. [Google Scholar]
- Pețan, A. Coin finds at Grădiştea Muncelului during the excavation campaigns of 1803–1804. Acta Musei Napoc. 2012, 47–48, 81–90. [Google Scholar]
- Schwab, R. Resources and recycling: Copper alloys and non-ferrous metalworking in the oppidum of Manching (Germany). In Proceedings of the International Symposium on the Metallurgy of the European Iron Age (SMEIA), Mannheim, Germany, 20–22 April 2010; Band 5. pp. 175–188. [Google Scholar]
- Zhushchikhovskaya, I.S.; Buravlev, I.Y. Ancient Ceramic Casting Molds from the Southern Russian Far East: Identification of Alloy Traces via Application of Nondestructive SEM-EDS and pXRF Methods. Heritage 2021, 4, 2643–2667. [Google Scholar] [CrossRef]
- Joy, J. Iron AgeMirrors: A Biographical Approach; BAR British Series 518; Archaeopress: Oxford, UK, 2010. [Google Scholar]
- Picon, M.; Boucher, S.; Condamin, J. Recherches techniques sur des bronzes de Gaule romaine. Gallia 1966, 24, 189–215. [Google Scholar] [CrossRef]
- Meeks, N. A technical study of Roman bronze mirrors. In Proceedings of the Acta of the 12th International Congress of Ancient Bronzes, Nijmegen, The Netherlands, 1–4 June 1992; Gerhartl-Witteveen, A., Ed.; 1995; pp. 179–193. [Google Scholar]
- Crișan, I.H. Ziridava—The Excavation from “Șanțul Mare” from 1960, 1961, 1962, 1964; Cultural Comity Publishing: Arad, Romania, 1978. (In Romanian) [Google Scholar]

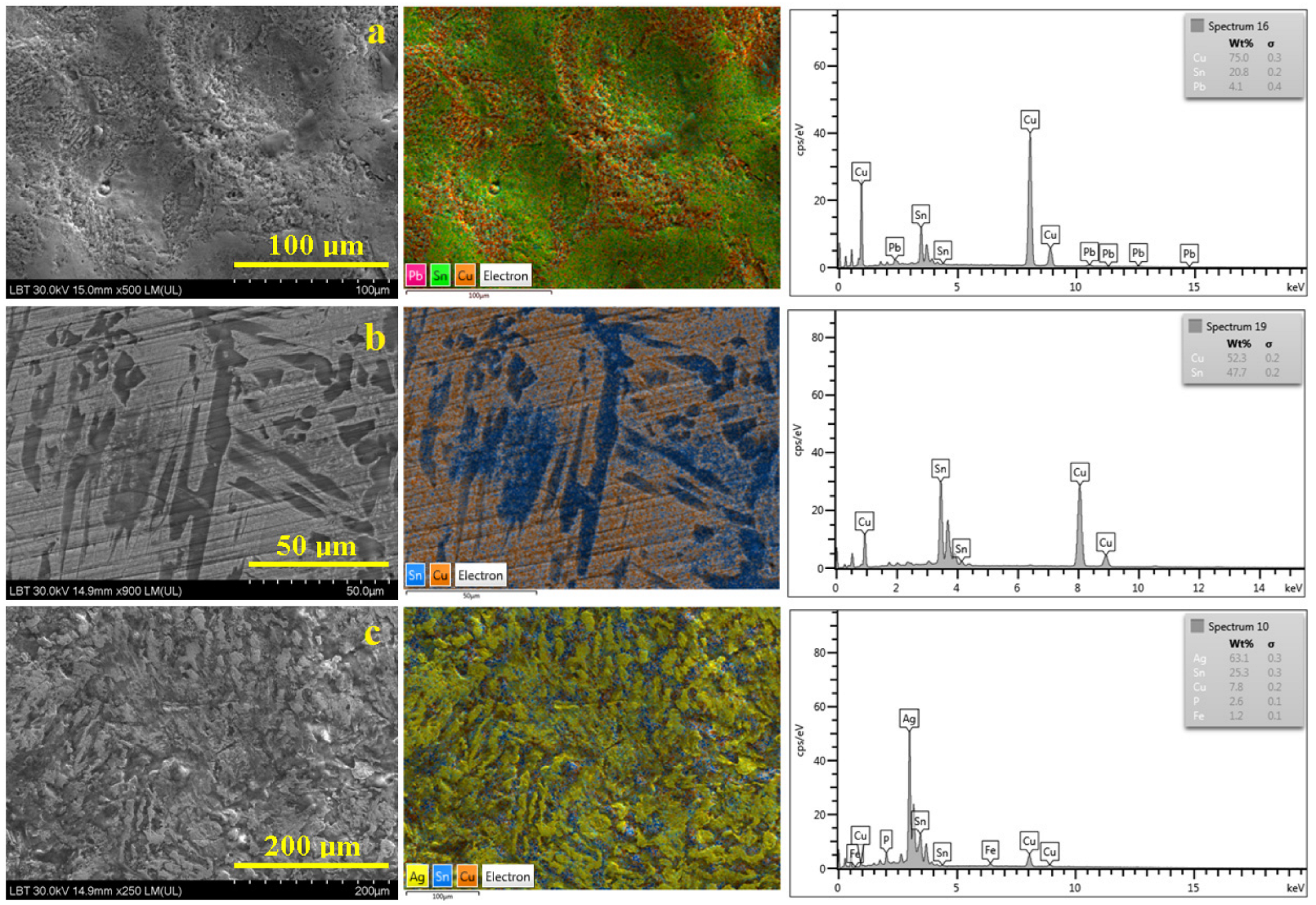

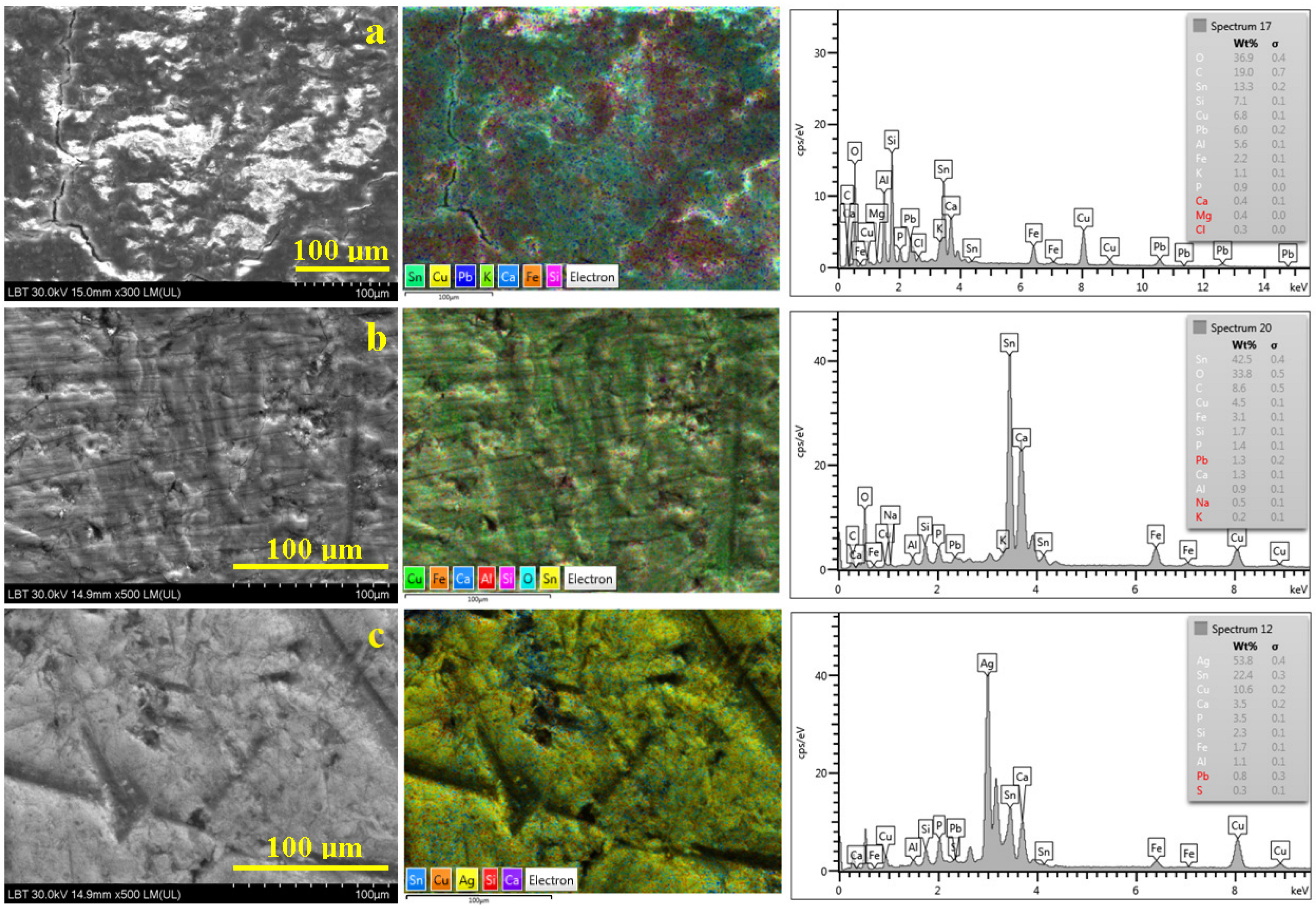
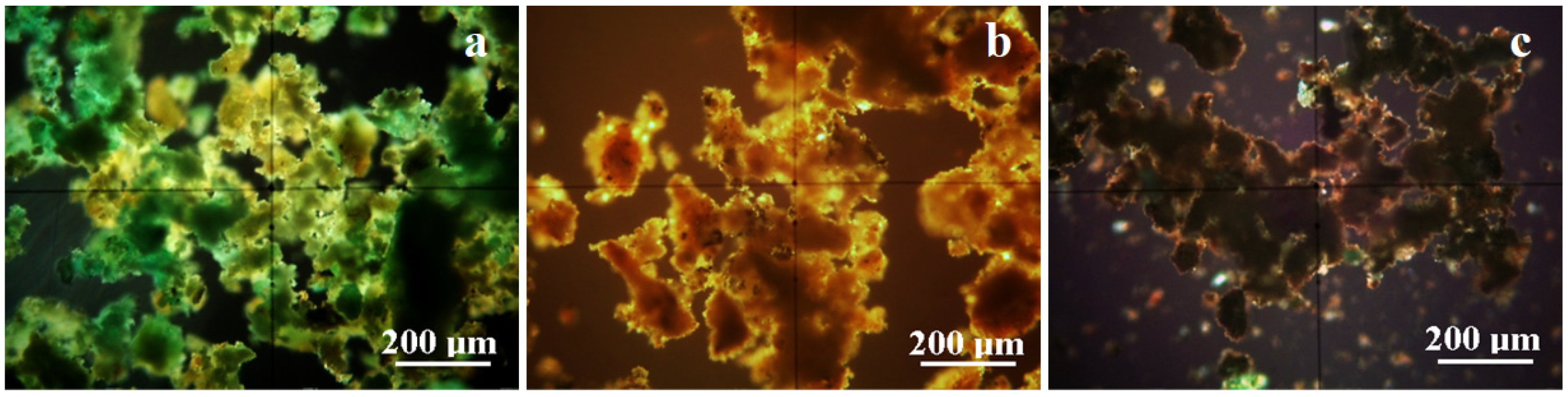

| Elements Amount, wt.% | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | C | Sn | Si | Cu | Pb | Ag | Al | Fe | Na | K | Ca | Mg | Cl | P | S | |
| a | 36.9 | 19.0 | 13.3 | 7.1 | 6.8 | 6.0 | - | 5.6 | 2.2 | - | 1.1 | 0.4 | 0.4 | 0.3 | - | - |
| b | 33.8 | 8.6 | 42.5 | 1.7 | 4.5 | 1.3 | - | 0.9 | - | 0.5 | 0.2 | 1.3 | - | - | 1.4 | - |
| c | - | - | 22.4 | 2.3 | 10.6 | 0.8 | 53.8 | 1.1 | 1.7 | - | - | 3.5 | - | - | 3.5 | 0.3 |
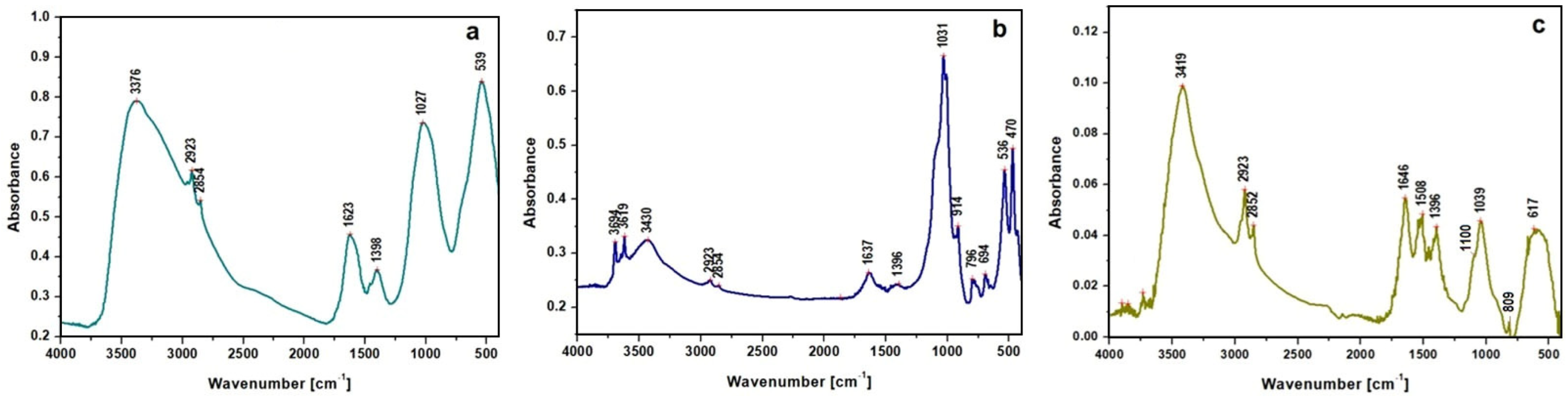
| Compound/ Functional Group | Peak Wavenumber [cm−1] | Vibration Assignment | References | ||
|---|---|---|---|---|---|
| Histria Coin | Mirror | Silver Coin | |||
| Clay minerals (Silicates) | - | 3694 | - | OH stretching Absorbed water (free) | [30,31] |
| - | 3619 | - | |||
| - | 3430 | 3419 | |||
| 3376 | - | - | |||
| 1623 | 1637 | 1646 | OH deformation of water Adsorbed water in silicates | [32,33] | |
| Carbonate minerals | 617 | - | 617 | asymmetric deformation carbonate group | [34,35] |
| 914 | 914 | C–O–H out-of-plane bending COO− symmetric carboxylate stretching | [36] | ||
| Lipids | 2923 | 2923 | 2923 | Aliphatic CH2 asymmetric stretching | [31,32] |
| 2854 | 2854 | 2852 | Aliphatic CH2 symmetric stretching | ||
| 1398 | 1396 | 1396 | Copper and calcium oxalates | ||
| 694 | Long alkyl chain of fatty acids and salts | ||||
| Silicates | 1027 | 1031 | 1039 | Si-O-Si asymmetric stretch | [32,33] |
| 470 | Si-O-Si deformation | ||||
| 796 | 809 | Si-O stretching of quartz | [32] | ||
| Sulfide/sulfate ions | ~1100 | Ag2S and/or Ag2SO4 | [37] | ||
| Copper oxides | 470 | O–Cu–O symmetric stretching | [38,39] | ||
| 539 | 536 | Cuprite Cu2O/tenorite CuO | |||
| Surface Parameter | Obverse Smooth Surface | |
|---|---|---|
| Oxidized Side | Metallic Side | |
| Height, nm | 458 ± 22.9 | 51.0 ± 2.55 |
| Ra, nm | 264 ± 13.2 | 33.7 ± 1.68 |
| Abrasion track width, nm | 60–90 | 400–600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petan, A.; Petean, I.; Paltinean, G.A.; Filip, M.R.; Borodi, G.; Tudoran, L.B. Microstructural Investigation of Some Bronze Artifacts Discovered in a Dacian Site Using Non-Destructive Methods. Metals 2023, 13, 863. https://doi.org/10.3390/met13050863
Petan A, Petean I, Paltinean GA, Filip MR, Borodi G, Tudoran LB. Microstructural Investigation of Some Bronze Artifacts Discovered in a Dacian Site Using Non-Destructive Methods. Metals. 2023; 13(5):863. https://doi.org/10.3390/met13050863
Chicago/Turabian StylePetan, Aurora, Ioan Petean, Gertrud Alexandra Paltinean, Miuta Rafila Filip, Gheorghe Borodi, and Lucian Barbu Tudoran. 2023. "Microstructural Investigation of Some Bronze Artifacts Discovered in a Dacian Site Using Non-Destructive Methods" Metals 13, no. 5: 863. https://doi.org/10.3390/met13050863






