Characterization of Nanosized Carbide Precipitates in Multiple Microalloyed Press Hardening Steels
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mechanical and Microstructural Characteristics before Heat Treatment
3.2. Mechanical and Microstructural Characteristics after Reference Heat Treatment
3.3. Effects of Overheating Conditions
3.4. Precipitation during Paint Baking Treatment
4. Discussion
- Particle (partial) dissolution.
- Particle coarsening (Ostwald ripening).
- Loss of particle coherency.
- Particles spheroidization in the fcc matrix.
- Compositional changes in mixed microalloy carbides.
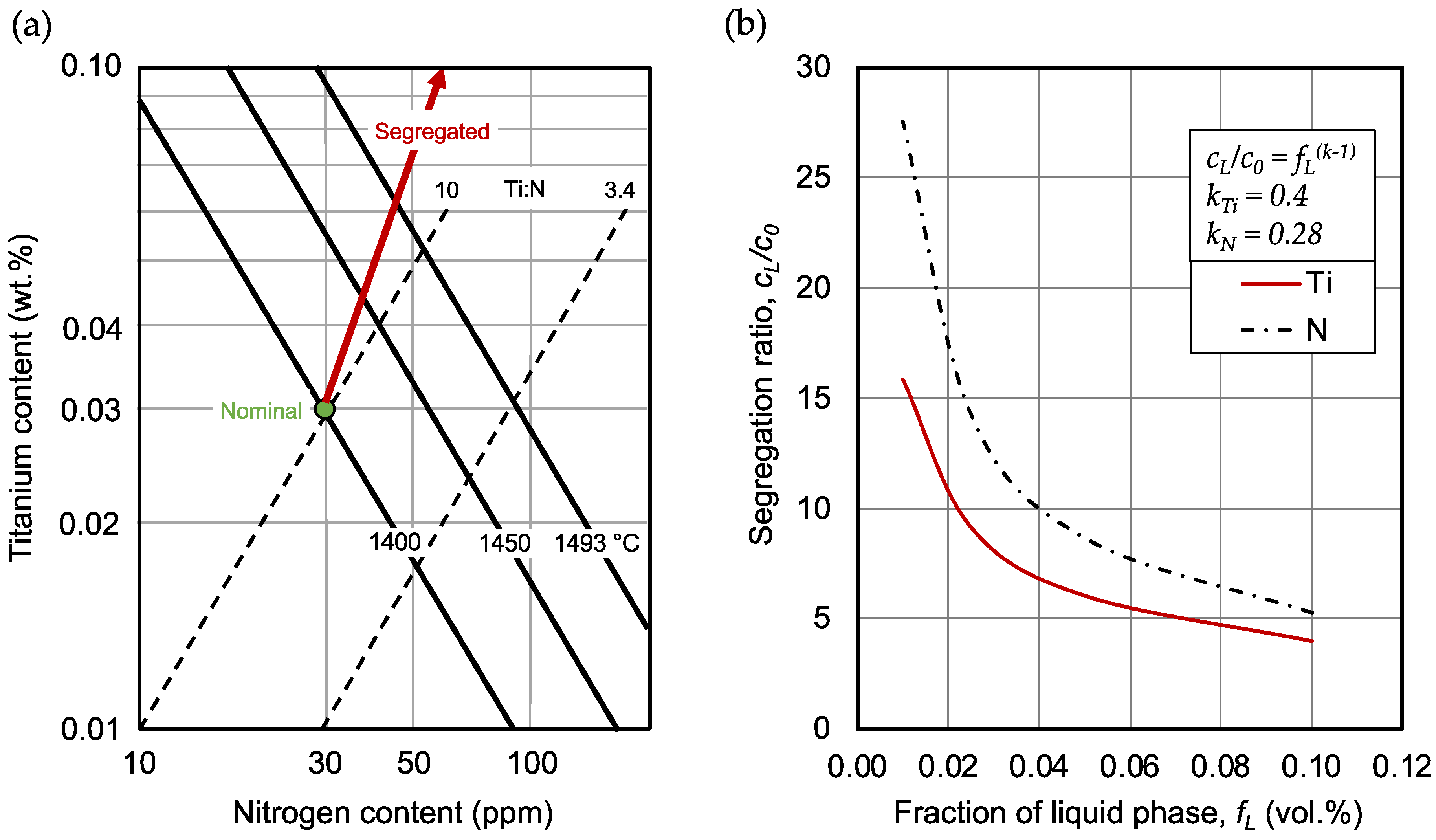
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Philippot, C.; Beauvais, M.; Sarkar, S.; Cobo, S.; Lucas, E.; Thillou, B.; Drillet, P. Advanced Cleanliness Control in the 2nd Generation of Press Hardened Steels Produced by ArcelorMittal. In Advanced High Strength Steel and Press Hardening, Proceedings of the 4th International Conference on Advanced High Strength Steel and Press Hardening (ICHSU2018), Hefei, China, 20–22 August 2018; World Scientific: Singapore, 2019; pp. 551–559. [Google Scholar]
- Pang, J.C.; Yi, H.L.; Lu, Q.; Enloe, C.M.; Wang, J.F. Effect of TiN-Particles on Fracture of Press-Hardened Steel Sheets and Components. JOM 2019, 71, 1329–1337. [Google Scholar] [CrossRef]
- Mohrbacher, H. Property Optimization in As-Quenched Martensitic Steel by Molybdenum and Niobium Alloying. Metals 2018, 8, 234. [Google Scholar] [CrossRef]
- Zheng, X.; Ghassemi-Armaki, H.; Hartwig, K.T.; Srivastava, A. Correlating Prior Austenite Grain Microstructure, Microscale Deformation and Fracture of Ultra-High Strength Martensitic Steels. Metals 2021, 11, 1013. [Google Scholar] [CrossRef]
- Enloe, C.M.; Mohrbacher, H. Influences of Martensite Morphology and Precipitation on Bendability in Press-Hardened Steels. SAE Int. J. Adv. Curr. Pract. Mobil. 2022, 4, 1181–1188. [Google Scholar] [CrossRef]
- Cai, H.L.; Wang, J.F.; Wu, D.; Yi, H.L. A Simple Methodology to Determine Fracture Strain of Press-Hardened Steels Under Plane Strain Bending. Metall. Mater. Trans. A 2021, 52, 644–654. [Google Scholar] [CrossRef]
- Gui, L.; Zhao, Y.; Feng, Y.; Ma, M.; Lu, H.; Tan, K.; Chiu, P.-H.; Guo, A.; Bian, J.; Yang, J.-R.; et al. Study on the Improving Effect of Nb-V Microalloying on the Hydrogen Induced Delayed Fracture Property of 22MnB5 Press Hardened Steel. Mater. Des. 2023, 227, 111763. [Google Scholar] [CrossRef]
- Cho, L.; Seo, E.J.; Sulistiyo, D.H.; Jo, K.R.; Kim, S.W.; Oh, J.K.; Cho, Y.R.; de Cooman, B.C. Influence of Vanadium on the Hydrogen Embrittlement of Aluminized Ultra-High Strength Press Hardening Steel. Mater. Sci. Eng. A 2018, 735, 448–455. [Google Scholar] [CrossRef]
- Mohrbacher, H. Martensitic Automotive Steel Sheet—Fundamentals and Metallurgical Optimization Strategies. Adv. Mater. Res. 2014, 1063, 130–142. [Google Scholar] [CrossRef]
- Jian, B.; Wang, L.; Mohrbacher, H.; Lu, H.Z.; Wang, W.J. Development of Niobium Alloyed Press Hardening Steel with Improved Properties for Crash Performance. Adv. Mater. Res. 2014, 1063, 7–20. [Google Scholar] [CrossRef]
- Rosenstock, D.; Gerber, T.; Castro Müller, C.; Stille, S.; Banik, J. Process Stability and Application of 1900 MPa Grade Press Hardening Steel with Reduced Hydrogen Susceptibility. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1238, 012013. [Google Scholar] [CrossRef]
- Webel, J.; Herges, A.; Britz, D.; Detemple, E.; Flaxa, V.; Mohrbacher, H.; Mücklich, F. Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning. Metals 2020, 10, 243. [Google Scholar] [CrossRef]
- Wang, J.; Enloe, C.; Singh, J.; Horvath, C. Effect of Prior Austenite Grain Size on Impact Toughness of Press Hardened Steel. SAE Int. J. Mater. Manuf. 2016, 9, 488–493. [Google Scholar] [CrossRef]
- Cobo, S.; Sturel, T.; Aouafi, A.; Allely, C.; Cornette, D. Hydrogen Embrittlement Resistance of Al-Si Coated 1.8GPa Press Hardened Steel Solutions for Body-in-White (BIW) Applications. In Proceedings of the 7th International Conference on Hot Sheet Metal Forming of High Performance Steel, Luleå, Sweden, 2–5 June 2019; pp. 179–189. [Google Scholar]
- Maikranz-Valentin, M.; Genchev, G.; Mirkovic, D. Improvement of Hydrogen Embrittlement Resistance of Press-Hardening Steels. In Proceedings of the 7th International Conference on Hot Sheet Metal Forming of High Performance Steel, Luleå, Sweden, 2–5 June 2019; pp. 611–618. [Google Scholar]
- Holzweissig, M.J.; Frost, G.; Bake, K.; Frehn, A. BTR2000—A New Uncoated Ultra-High Strength Hot Forming Steel. In Proceedings of the 7th International Conference on Hot Sheet Metal Forming of High Performance Steel, Luleå, Sweden, 2–5 June 2019; pp. 749–756. [Google Scholar]
- Zhang, Z.; Wang, X.; Sun, Q.; Yang, B.; Xiong, L.; Liu, H. Study on Microstructure and Properties of Laser Dissimilar Welded Joints of Ultra-High Strength PHS1500/PHS2000 Steel. Opt. Laser Technol. 2022, 150, 107933. [Google Scholar] [CrossRef]
- Liu, B.; Liao, X.; Tang, Y.; Si, Y.; Feng, Y.; Cao, P.; Dai, Q.; Li, K. Effects of the Addition of Nb and V on the Microstructural Evolution and Hydrogen Embrittlement Resistance of High Strength Martensitic Steels. Scanning 2022, 2022, 4040800. [Google Scholar] [CrossRef] [PubMed]
- Vander Voort, G.F. Metallography: Principles and Practice; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- de Almeida, D.T.; Clarke, T.G.R.; de Souza, J.H.C.; Haupt, W.; de Lima, M.S.F.; Mohrbacher, H. The Effect of Laser Welding on Microstructure and Mechanical Properties in Heavy-Gage Press Hardening Steel Alloys. Mater. Sci. Eng. A 2021, 821, 141341. [Google Scholar] [CrossRef]
- Nagataki, Y.; Tsuyama, S.; Hosoya, Y.; Okita, T. Effect of Microstructure on Bendability of Ultra-High-Strength Steel Sheet. In High-Strength Sheet Steels for the Automotive Industry; Pradhan, R., Ed.; The Iron and Steel Society: Baltimore, MD, USA, 1994; pp. 239–244. [Google Scholar]
- Taylor, K.A. Solubility Products for Titanium-, Vanadium-, and Niobium-Carbide in Ferrite. Scr. Metall. Mater. 1995, 32, 7–12. [Google Scholar] [CrossRef]
- Narita, K. Physical Chemistry of the Groups IVa (Ti, Zr), Va (V, Nb, Ta) and the Rare Earth Elements in Steel. Trans. Iron Steel Inst. Jpn. 1975, 15, 145–152. [Google Scholar] [CrossRef]
- Sekine, H.; Inoue, T.; Ogasawara, M. Solubility of V4C3 in α-Iron. Trans. Iron Steel Inst. Jpn 1968, 8, 101–102. [Google Scholar] [CrossRef]
- Ohtani, H.; Hasebe, M.; Nishizawa, T. Calculation of Fe-C, Co-C and Ni-C Phase Diagrams. Trans. Iron Steel Inst. Jpn 1984, 24, 857–864. [Google Scholar] [CrossRef]
- Nöhrer, M.; Mayer, W.; Primig, S.; Zamberger, S.; Kozeschnik, E.; Leitner, H. Influence of Deformation on the Precipitation Behavior of Nb(CN) in Austenite and Ferrite. Metall. Mater. Trans. A 2014, 45, 4210–4219. [Google Scholar] [CrossRef]
- Mohrbacher, H. Alloy Design and Processing Strategies for Grain Coarsening-Resistant Carburizing Steels. In Proceedings of the International Conference on Advances in Metallurgy of Long and Forged Products Proceedings, Online, 12–14 July 2021; Association for Iron & Steel Technology: Warrendale, PA, USA, 2021. [Google Scholar]
- Mohrbacher, H. Effects of Niobium in Galvanized Advanced High Strength Steels for Automotive Applications. In Proceedings of the 7th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Osaka, Japan, 18–22 November 2007; pp. 386–391. [Google Scholar]
- Mohrbacher, H.; Yang, J.-R.; Chen, Y.-W.; Rehrl, J.; Hebesberger, T. Metallurgical Effects of Niobium in Dual Phase Steel. Metals 2020, 10, 504. [Google Scholar] [CrossRef]
- Peng, J.; Jiang, H.; Zhang, G.; Chen, L.; Zhu, N.; He, Y.; Lu, X.; Li, L. In-Situ Analysis of Retained Austenite Transformation in High-Performance Micro-Alloyed TRIP Steel. J. Iron Steel Res. Int. 2017, 24, 313–320. [Google Scholar] [CrossRef]
- Geise, J.; Herzig, C. Impurity Diffusion of Vanadium and Self-Diffusion in Iron. Int. J. Mater. Res. 1987, 78, 291–294. [Google Scholar] [CrossRef]
- Klugkist, P.; Herzig, C. Tracer Diffusion of Titanium in α-Iron. Phys. Status Solidi (A) 1995, 148, 413–421. [Google Scholar] [CrossRef]
- Herzig, C.; Geise, J.; Divinski, S.V. Niobium Bulk and Grain Boundary Diffusion in Alpha-Iron. Int. J. Mater. Res. 2022, 93, 1180–1187. [Google Scholar] [CrossRef]
- Fähnle, M.; Seeger, A. On the Magnetic Contribution to the Free Enthalpy of Vacancy Formation in Ferromagnetic Crystals. Phys. Status Solidi (A) 1985, 91, 609–617. [Google Scholar] [CrossRef]
- Chung, S.-H.; Ha, H.-P.; Jung, W.-S.; Byun, J.-Y. An Ab Initio Study of the Energetics for Interfaces between Group V Transition Metal Carbides and Bcc Iron. ISIJ Int. 2006, 46, 1523–1531. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.-H.; Heo, Y.-U.; Suh, D.-W. Stability of (Ti, M)C (M = Nb, V, Mo and W) Carbide in Steels Using First-Principles Calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Zou, H.; Kirkaldy, J.S. Carbonitride Precipitate Growth in Titanium/Niobium Microalloyed Steels. Metall. Trans. A 1991, 22, 1511–1524. [Google Scholar] [CrossRef]
- Webel, J.; Mohrbacher, H.; Detemple, E.; Britz, D.; Mücklich, F. Quantitative Analysis of Mixed Niobium-Titanium Carbonitride Solubility in HSLA Steels Based on Atom Probe Tomography and Electrical Resistivity Measurements. J. Mater. Res. Technol. 2022, 18, 2048–2063. [Google Scholar] [CrossRef]
- Hin, C.; Bréchet, Y.; Maugis, P.; Soisson, F. Kinetics of Heterogeneous Dislocation Precipitation of NbC in Alpha-Iron. Acta Mater. 2008, 56, 5535–5543. [Google Scholar] [CrossRef]
- Danoix, F.; Bémont, E.; Maugis, P.; Blavette, D. Atom Probe Tomography I. Early Stages of Precipitation of NbC and NbN in Ferritic Steels. Adv. Eng. Mater. 2006, 8, 1202–1205. [Google Scholar] [CrossRef]
- Martin, J.W. Micromechanisms in Particle-Hardened Alloys; Cahn, R.W., Thompson, M.W., Ward, I.M., Eds.; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Speich, G.R. Tempering of Low-Carbon Martensite. Trans. AIME 1969, 245, 2553–2564. [Google Scholar]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Segregation of Boron at Prior Austenite Grain Boundaries in a Quenched Martensitic Steel Studied by Atom Probe Tomography. Scr. Mater. 2015, 96, 13–16. [Google Scholar] [CrossRef]
- Mohrbacher, H.; Senuma, T. Alloy Optimization for Reducing Delayed Fracture Sensitivity of 2000 MPa Press Hardening Steel. Metals 2020, 10, 853. [Google Scholar] [CrossRef]
- Scheil, E. Bemerkungen Zur Schichtkristallbildung. Int. J. Mater. Res. 1942, 34, 70–72. [Google Scholar] [CrossRef]
- Morita, Z.; Tanaka, T. Thermodynamics of Solute Distributions between Solid and Liquid Phases in Iron-Base Ternary Alloys. Trans. Iron Steel Inst. Jpn. 1983, 23, 824–833. [Google Scholar] [CrossRef]
- Milani, A.; Philippot, C.; Lucas, E.; Cobo, S.; Gourgues, A.F. Effect of TiN Inclusions on the Crash Ductility Behaviour of Press Hardened Steels of 2nd Generation. In Proceedings of the Hot Sheet Metal Forming of High Performance Steel, Barcelona, Spain, 30 May–2 June 2022; pp. 487–494. [Google Scholar]
- Zhang, S.; Huang, Y.; Sun, B.; Liao, Q.; Lu, H.; Jian, B.; Mohrbacher, H.; Zhang, W.; Guo, A.; Zhang, Y. Effect of Nb on Hydrogen-Induced Delayed Fracture in High Strength Hot Stamping Steels. Mater. Sci. Eng. A 2015, 626, 136–143. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, J.; Zhao, Q.; Liu, J.; Huang, F.; Huang, Y.; Li, X. Dual Role of Nanosized NbC Precipitates in Hydrogen Embrittlement Susceptibility of Lath Martensitic Steel. Corros. Sci. 2020, 164, 108345. [Google Scholar] [CrossRef]
- Graux, A.; Cazottes, S.; de Castro, D.; San Martín, D.; Capdevila, C.; Cabrera, J.M.; Molas, S.; Schreiber, S.; Mirković, D.; Danoix, F.; et al. Precipitation and Grain Growth Modelling in Ti-Nb Microalloyed Steels. Materialia 2019, 5, 100233. [Google Scholar] [CrossRef]
- Turkdogan, E.T. Causes and Effects of Nitride and Carbonitride Precipitation During Continuous Casting. ISS Trans. 1990, 11, 39–48. [Google Scholar]

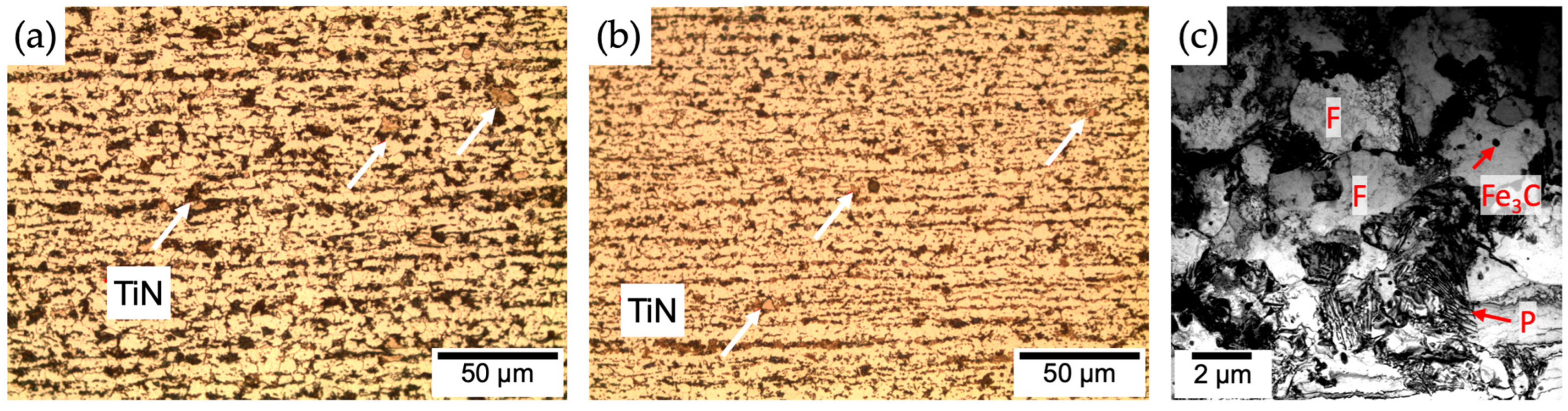
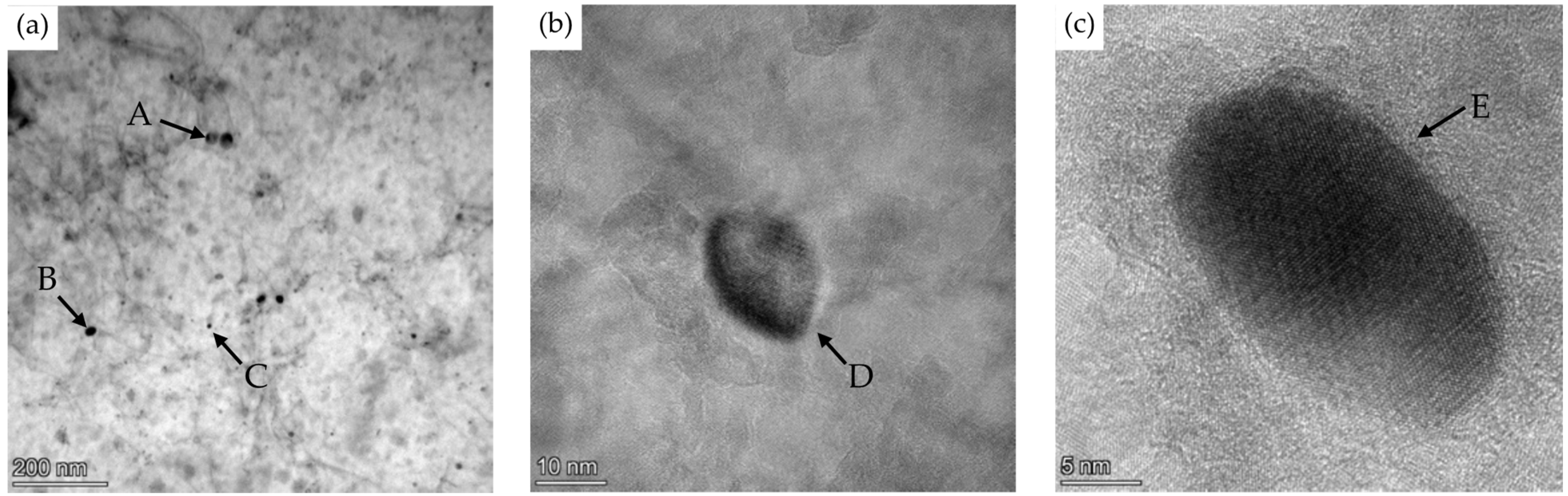
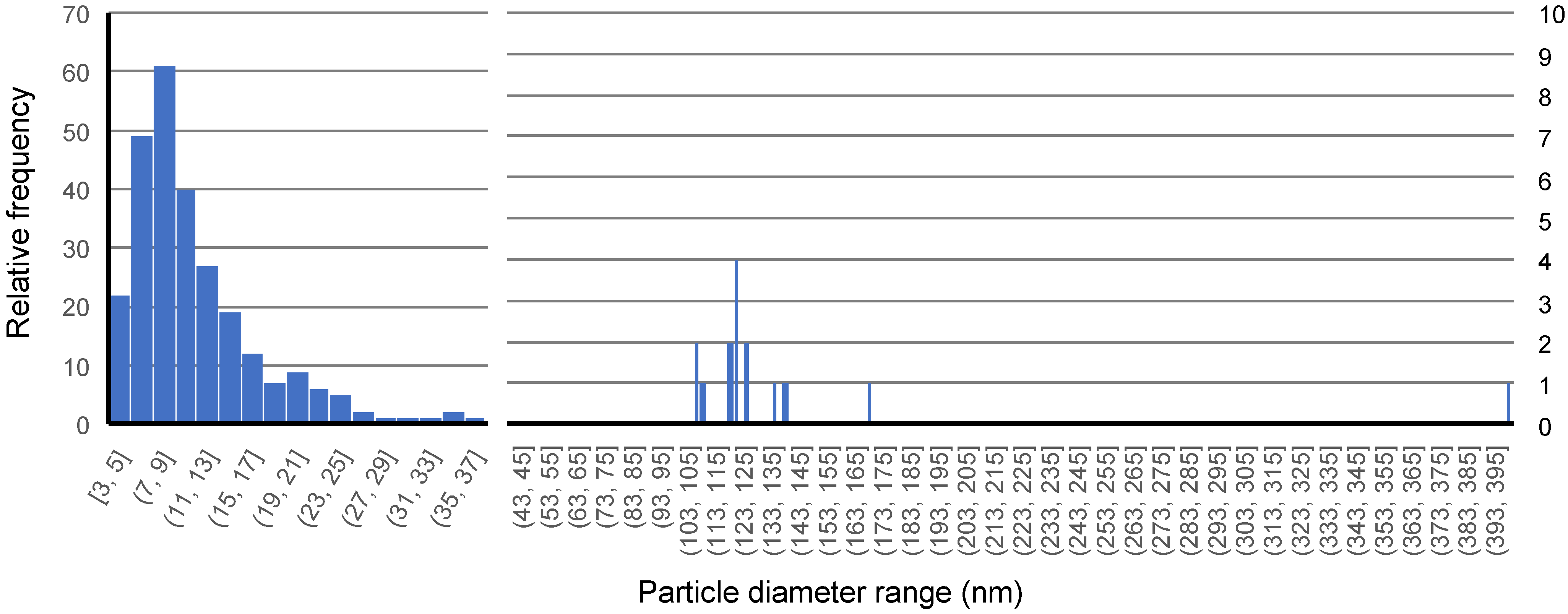
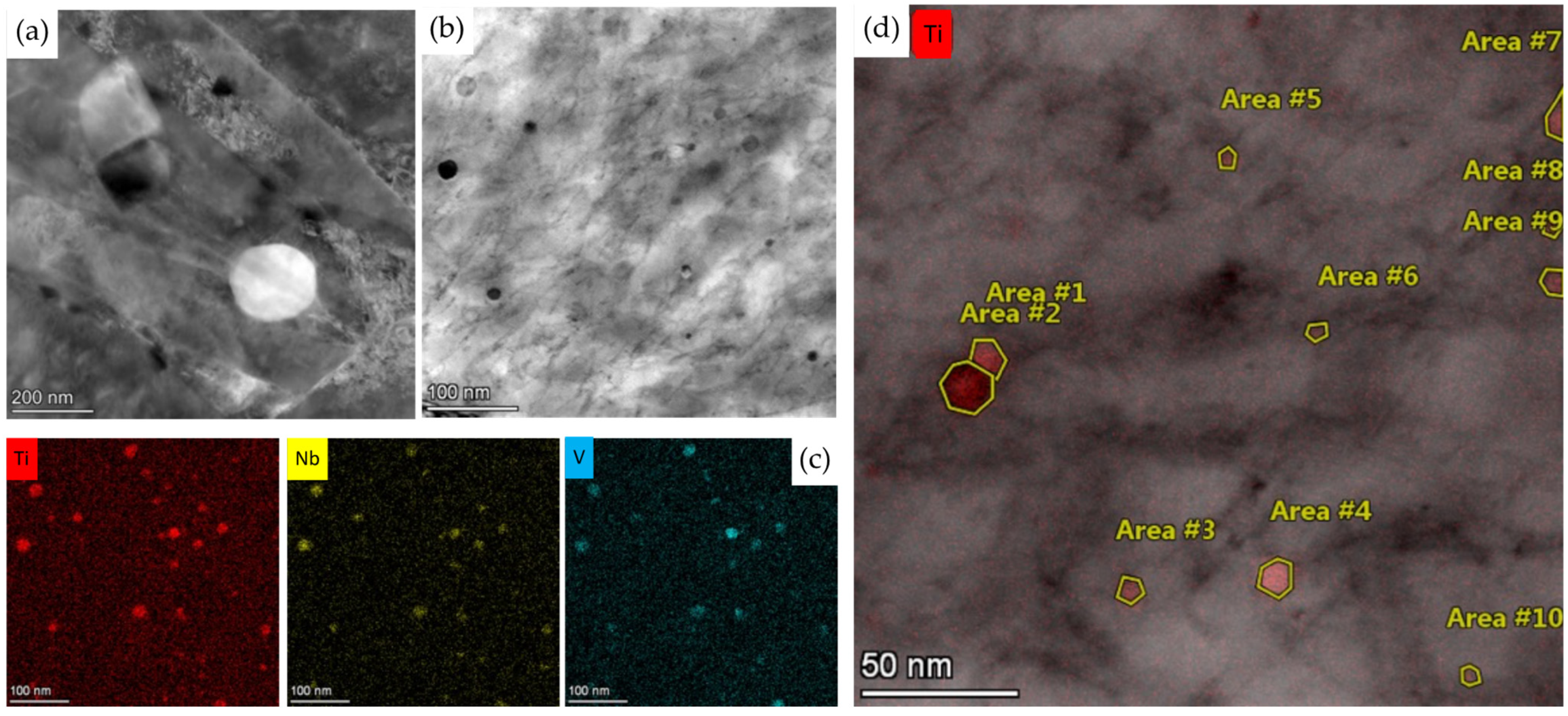
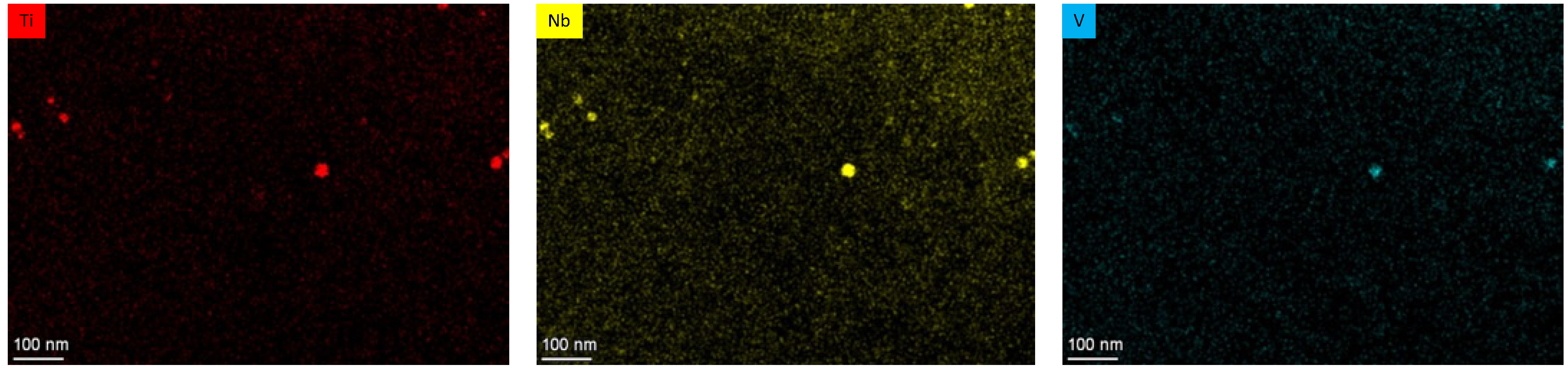
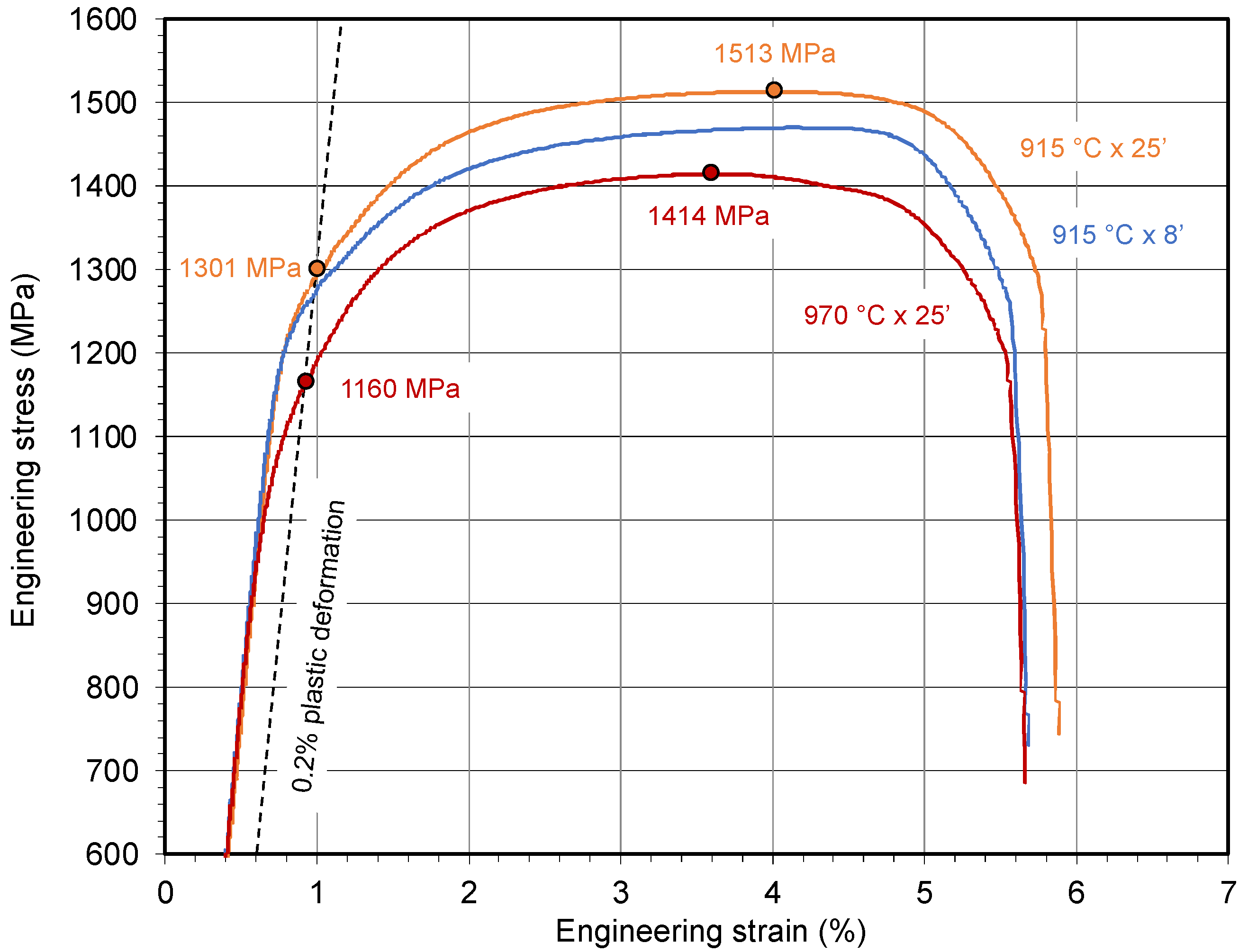




| C | Si | Mn | Cr | N | Ti | Nb | V | B | P | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard PHS | 0.24 | 0.25 | 1.31 | 0.18 | 0.003 | 0.03 | n.a. | n.a. | 0.002 | 0.01 | 0.001 |
| NbV PHS | 0.20 | 0.28 | 1.21 | 0.17 | 0.003 | 0.03 | 0.04 | 0.04 | 0.002 | 0.01 | 0.001 |
| Particle | Ti | Nb | V | Mo | Sum |
|---|---|---|---|---|---|
| A | 0.29 | 0.17 | 0.26 | 0.12 | 0.84 |
| B | 0.35 | 0.23 | 0.15 | 0.09 | 0.82 |
| C | 0.16 | 0.13 | 0.12 | 0.06 | 0.47 |
| D | 0.40 | 0.31 | 0.77 | 0.04 | 1.52 |
| E | 0.42 | 0.39 | 0.24 | 0.00 | 1.05 |
| Standard PHS | NbV PHS | |||
|---|---|---|---|---|
| Test Direction | Longitudinal | Transverse | Longitudinal | Transverse |
| Yield strength Rp0.2 (MPa) | 462 | 445 | 454 | 452 |
| Tensile strength Rm (MPa) | 665 | 652 | 668 | 679 |
| Total elongation A80 (%) | 19 | 16 | 20 | 22 |
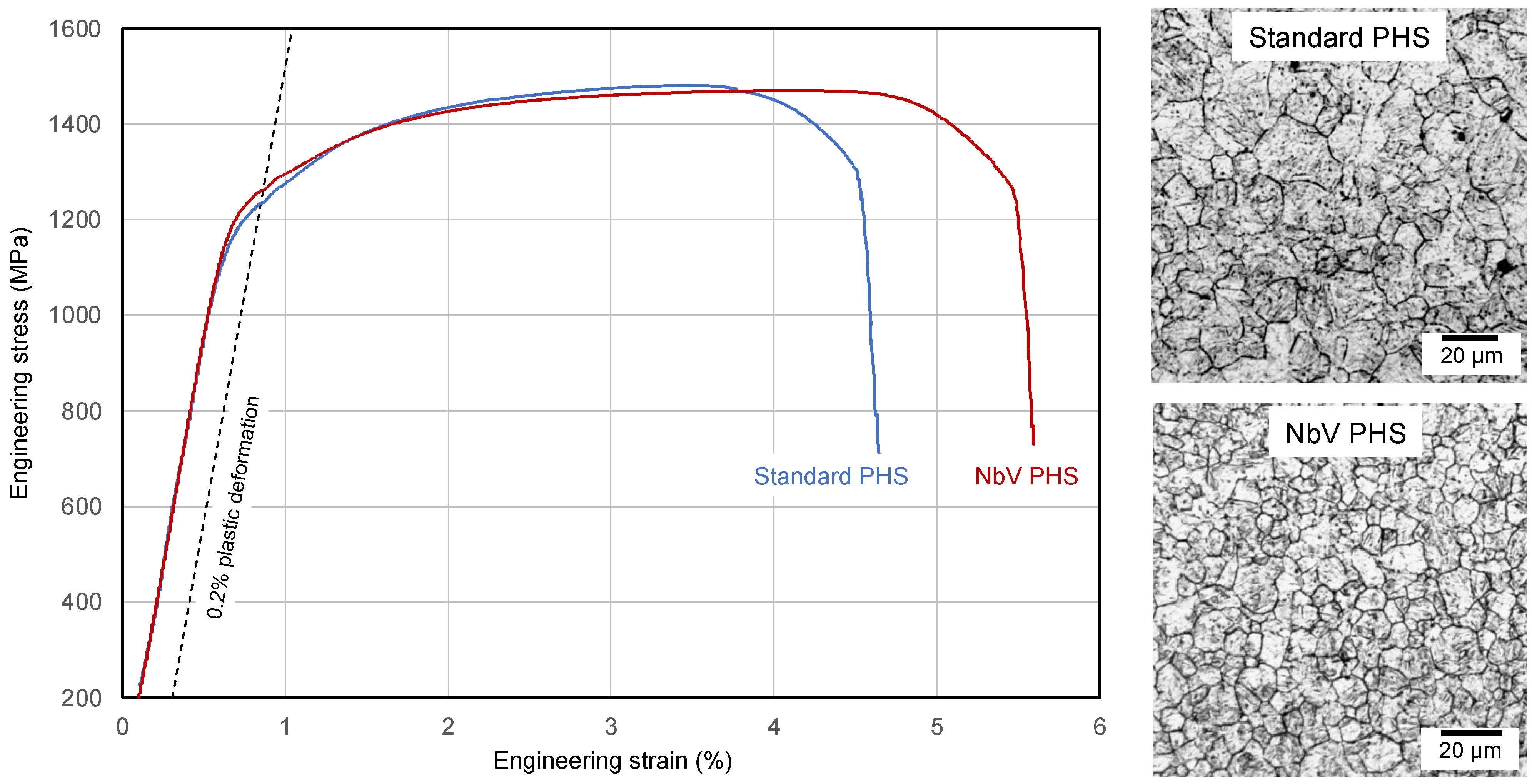
| Standard PHS | NbV PHS | |
|---|---|---|
| Yield strength Rp0.2 (MPa) | 1231 | 1262 |
| Tensile strength Rm (MPa) | 1470 | 1462 |
| Total elongation A80 (%) | 4.6 | 5.4 |
| PAGS (µm) | 15–20 (ASTM G 8.5) | 10–15 (ASTM G 9.5) |
| Area | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | Ave. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| V | 0.39 | 0.41 | 0.73 | 0.39 | 0.50 | 0.34 | 0.30 | 0.40 | 0.48 | 0.52 | 0.45 |
| Nb | 0.98 | 1.02 | 1.05 | 1.00 | 0.92 | 1.39 | 1.16 | 0.84 | 0.69 | 1.14 | 1.02 |
| HT condition | 915 °C × 8′ | 915 °C × 25′ | 970 °C × 8′ | 970 °C × 25′ |
| Hp parameter | 22.7 | 23.3 | 23.8 | 24.4 |
| Standard PHS: PAGS range (µm) | 15–20 | 30–40 | 30–55 | 130–150 |
| NbV PHS: PAGS range (µm) | 10–15 | 20–30 | 15–35 | 50–70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohrbacher, H.; Bacchi, L.; Ischia, G.; Gialanella, S.; Tedesco, M.; D'Aiuto, F.; Valentini, R. Characterization of Nanosized Carbide Precipitates in Multiple Microalloyed Press Hardening Steels. Metals 2023, 13, 894. https://doi.org/10.3390/met13050894
Mohrbacher H, Bacchi L, Ischia G, Gialanella S, Tedesco M, D'Aiuto F, Valentini R. Characterization of Nanosized Carbide Precipitates in Multiple Microalloyed Press Hardening Steels. Metals. 2023; 13(5):894. https://doi.org/10.3390/met13050894
Chicago/Turabian StyleMohrbacher, Hardy, Linda Bacchi, Gloria Ischia, Stefano Gialanella, Michele Tedesco, Fabio D'Aiuto, and Renzo Valentini. 2023. "Characterization of Nanosized Carbide Precipitates in Multiple Microalloyed Press Hardening Steels" Metals 13, no. 5: 894. https://doi.org/10.3390/met13050894
APA StyleMohrbacher, H., Bacchi, L., Ischia, G., Gialanella, S., Tedesco, M., D'Aiuto, F., & Valentini, R. (2023). Characterization of Nanosized Carbide Precipitates in Multiple Microalloyed Press Hardening Steels. Metals, 13(5), 894. https://doi.org/10.3390/met13050894







