1. Introduction
In the new context of the carbon-free and circular economy, steel production routes are expected to continue to play a crucial role. The hot metal manufacturing unit operation is the most carbon-intensive part of the primary metallurgy of iron production, and due to financial and production-scale considerations, the blast furnace (BF)–basic oxygen furnace (BOF) route is predominant. However, this production method is energy-intensive and requires specific quality for the raw materials, such as pellets, coke, and granular sinter. This allows for higher reduction step efficiency with an excellent gas utilization ratio, making it possible for its utilization as energetic gas for the facilities in the production route or direct use in power plants. On the other hand, employing recently developed technologies, the production of hydrogen-rich gas from the coal gasification process by means of fossil or renewable resources is in process to become economically feasible. Thus, a potential topic to be addressed is the new efficient application of rich hydrogen gas in this industry [
1,
2,
3,
4,
5,
6,
7,
8,
9]. Around 75% of the required energy in the primary steel integrated manufacturing route is consumed by the BF operation unit. In the blast furnace route, the cost of fuel and reducing agents accounts for more than 60% of the overall cost of produced pig iron. These materials are introduced in the blast furnace as pulverized coal for injection (PCI) or granular coke that is supplied via the tuyeres of the furnace. In order to make the process more ecologically friendly, efforts have been made to decrease the reducing agent rate (RAR) or, at the very least, replacing the coke consumption of the blast furnace with alternative materials injected through the tuyere [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21].
Increasing the pulverized coal injection to the desired level is a complicated matter for blast furnace technology. The main causes are the furnace’s gas flow management and an accumulation of unburned coal particles at the bottom section of the furnace, which can produce unstable solid and liquid descending motions [
4,
8,
10,
11,
12,
13,
14,
15,
16]. Alternatively, we proposed a partial replacement of the pulverized coal injection by fuel gas, which is expected to overcome these shortcomings. A detailed mathematical model formulated considering the multiphase multicomponent theory has been effectively implemented with novel features and ongoing advancements to understand the intricate phenomena in the blast furnace [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Thus, a combined injection practice of blast furnace gas (BFG) enriched with fuel hydrogen and oxygen that takes advantage of the expected increase in raceway temperature caused by the hydrogen and pulverized coal combustions is analyzed in this paper, allowing the proposal of partially replacing the fossil carbon of the pulverized coal injection practice.
Via rapid solution loss, water gas, and gas shift reactions with the pulverized coal in the raceway, this extra energy is utilized to increase CO
2 conversion, preventing unburned coal accumulation, and enabling appropriate slagging operation [
4,
8,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Accordingly, the concept of simultaneous injection of blast furnace gas enriched with hydrogen, oxygen, and a large amount of pulverized coal injection (PCI) into the tuyere of the furnace is also proposed, as shown in
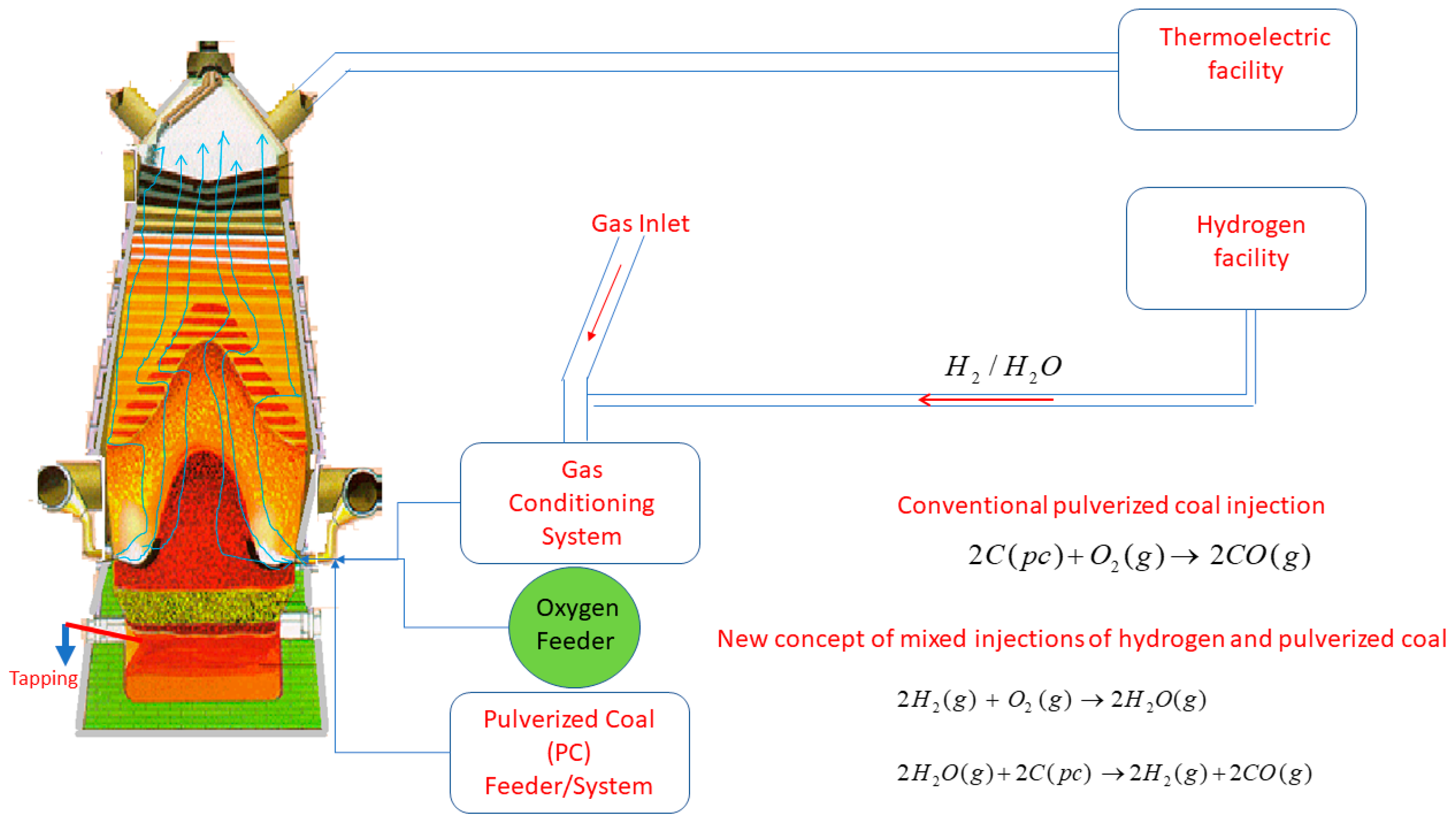
Figure 1. In this concept, the auxiliary injection system includes the hydrogen and mix gas feeder together with the oxygen and pulverized coal injection system. As observed in
Figure 1, the hydrogen takes part in the overall reactions of pulverized coal injected to produce H
2 and CO, releasing heat to the whole reactor. The reactions are simultaneously taking place. The sequence is such that H
2/PC reacts with O
2 and H
2O/CO
2 further reacts with pulverized coal. However, the H
2 and O
2 are injected together in the raceway channel and partially react, entering the raceway zone partially reacted. Moreover, the kinetics of the gas–gas reactions are much faster compared with the gas–solid reactions. The temperature of the raceway zone can be controlled by the addition of oxygen or treated blast furnace gas from the process after scrubbing and preheating.
In order to carry out this technique, it is necessary to have access to external sources of hydrogen and oxygen-rich gas, whereas blast furnace gas can be produced by cleaning the blast furnace off gas and conditioning it to be injected in the tuyeres. By doing this, the BF off gas becomes enriched and its calorific value is raised, enhancing the efficiency of the thermoelectric facility. Additionally, since the combustion of hydrogen and pulverized coal will produce extra energy, it is expected that the kinetics of pulverized coal combustion along the raceway is improved and the temperature of the bottom section of the furnace is maintained under control. Depending on the extent to which CO
2 is converted via solution loss, the enhanced water gas and solution loss reactions will then consume this extra energy. The careful regulation of these reactions will maintain the ideal temperature ranges and material flow in the blast furnace. When compared with the traditional blast furnace practice, the proposed conditions drastically change the hydrogen concentration in the granular zone and the contribution of the solid reduction by hydrogen is expected to enhance [
8,
10,
11,
12,
13,
14,
15]. Nevertheless, the reduction by hydrogen is efficient only at high temperatures compared with the carbon monoxide reduction (see
Figure 2).
Figure 2 shows that, when the temperature is increased, the hydrogen gives a higher thermodynamic-driven force while the carbon monoxide decreases. Overcoming these thermodynamic limitations is essential to enhance the blast furnace technology toward a more efficient and cleaner ironmaking industry.
This feature suggests that the injection of hydrogen as fuel in the tuyere level is effective for both purposes, furnishing energy by enhancing the pulverized coal combustion and flowing to the granular zone with high thermodynamic potential for burden reduction by gas–solid reactions. It is theoretically clear that this option is better than the direct injection into the shaft region for hydrogen reduction, which has a narrow range of temperature and unfavorable flowing conditions for an efficient wustite to iron reduction step, where hydrogen reduction is theoretically effective. Thus, this proposed practice is expected to improve the blast furnace performance under blast furnace gas enriched with hydrogen mixed with massive pulverized coal injections.
The current pulverized coal injection technology is well established and several blast furnaces have been operated with rates above 180 kg/tHM. Further increases in the injection rates, although viable theoretically, present additional challenges due to the combustibility of the fine coals and consequent instability of the flowing conditions of the powders and liquids in the lower part of the blast furnace, which can be minimized by the replacement of pulverized coal by enriched fuel gas which can supply the energy requirements for the reactions taking place.
Unstable conditions are caused by the accumulation of unburned powder in the lower section of the blast furnace and the high viscosity slag produced during ash melt, both of which reduce the permeability of the dropping zone [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. The temperature and gas flow inside the raceway are significantly altered when mixed gases are injected into the blast furnace. The functioning of the entire furnace is defined by these impacts on the raceway region, which results in interactions between the phases and chemical species. Particular attention should be paid to the position and shape of the cohesive zone, which have a significant impact on how efficient the granular zone is in terms of the fluid flow, heat transfer, and response rates of the descending granular burden materials. The multiphase, multicomponent model can account for these complex phenomena and quantify the yield of raw materials under stable operation conditions, indicating feasible furnace operation. In the present study, the model for an actual operation is tested with a high injection of pulverized coal (220 kg/thm) and simulated scenarios of replacement of pulverized coal injections (180, 160, and 140 kg/t
HM) by blast furnace gas enriched with hydrogen and oxygen to demonstrate the newly suggested operation taking advantage of rich hydrogen injection in the raceway region. Although hydrogen-rich gas has been previously introduced on the granular zone [
4,
8,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30], here it is proposed as a brand-new concept that not only increases the reducing zone efficiency but also makes use of the advantages in the lower furnace for thermal control by injecting blast furnace gas (BFG) and adding oxygen and hydrogen to improve the exothermic reactions balanced by the blast furnace gas to control the maximum temperature. With the help of this tiny control, the entire furnace can perform adequately and with reduced particular emissions of equivalent CO
2, making the blast furnace process technology more environmentally friendly. In this paper, we newly introduced the concept of recycling the top gas mixed with hydrogen and oxygen to improve the combustibility of the pulverized coal and substituting the fine coals injected by the rich gas to improve the flowing behavior of the powders in the dropping zone of the blast furnace reactor. These features were newly implemented in our self-developed computational code and innovative scenarios of analysis were designed. Therefore, this work represents a step towards the development of feasible technologies for the blast furnace in the context of net zero carbon dioxide emissions in the ironmaking sector.
2. Methodology
Since last century, simulation tools such as numerical techniques for studying the operation of blast furnaces have been regularly updated. The present study introduces a comprehensive multiphase, multicomponent model that considers all physical and chemical interactions, as well as intricate rate equations that dynamically couple internal state variables of the blast furnace reactor with the overall kinetics of phase transformations and chemical reactions. The thermophysical characteristics of the phases and chemical species are integrated into the model while being taken into account locally. The multiphase and multicomponent mutually interacting technique is depicted in
Figure 3. To allow the phases to coexist with their respective volume fractions expressing the number of materials filling the physical control volume, the concept links the inner state variables inherent to the process with a representative volume of the entire bed. Each phase has distinct chemical species and thermophysical characteristics that change dynamically as they flow within the reactor. Via interface phenomena, chemical reactions, and phase transitions, the phases interact with one another, transferring momentum, mass, and enthalpy. The multiphase interacting diagram (
Figure 3b) represents the phases and interactions of momentum, energy, and mass through chemical reactions taken into consideration in this model [
10,
11,
12,
13,
14,
15,
29].
The solid lines in
Figure 3b, which connect two phases, indicate all of the interactions in terms of energy, mass, and momentum. In
Figure 3b, the dotted lines indicated that, in addition to the full interactions for mass and chemical reactions, a model taking into account momentum interactions is also assumed. Chemical species from the blast as well as those produced in the reactor as a result of chemical reactions are included in the gas phase. All of the granular materials charged into the furnace via the burden materials charging mechanism are included in the solid phase (coke, lump ore, sinter, small sinter, pellets, and slag formation materials charged in the burden). The material injected through the injection lances of the tuyeres is the pulverized coal (PCI). Hot metal (HM) is the liquid substance that forms the liquid pig iron. The slag is produced with liquid oxides generated during the melting down process, which include silicon transfer, manganese, phosphorus, and also other chemical reactions with alloy components in the pig iron. The term “fines” refers to the small particles formed during the breakdown of load materials that are transported by the upward gas flow. As a result, all the chemical species existing in the load materials as well as those created during the solid transformations can lead to the formation of fines.
Table 1 provides a summary of the phases and chemical species considered in the present investigation. With a basis on local rate equations and particular mechanisms, chemical reactions comprise multiphase and multicomponent mass transfer, along with the transfer of momentum and heat.
Table 2 lists the chemical reactions and phase changes that were taken into account for this model. The authors tested and modified the available models and data from the Literature [
4,
8,
10,
11,
12,
13,
14,
15,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45] to implement the rate equations taken into account for these reactions. The local flow, composition, and temperature conditions are used to describe the exchanges of energy interphase and momentum, which are formulated with a basis on fundamental relations [
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45]. These formulations were adjusted to the blast furnace conditions [
10,
15].
In order to create a complex and coupled dynamic kinetic model, mass and energy exchange mechanisms are locally taken into account in the formulations for the rate equations. This allows the model equations to take into consideration different raw materials and operational situations. In this way, a comprehensive kinetic detailed database for a variety of basic materials has been created and incorporated into the model framework. New kinetic basic data for the chemical reactions have been regularly produced and introduced in the model considering both experimental works combined with industrial trials and statistical approaches [
10,
11,
12,
13,
14,
15,
23,
24,
25,
26,
27,
28,
29] since the model is programmed to produce an open-source own computational code. It is valuable to emphasize that novel implementations and constant experimental data acquisition for new raw materials have continuously improved the model’s predictions and its accuracy.
The principles of the kinetic models were developed using similar model structures, but considering different constant rates, and parameters modified to take into account recent experimental results acquired under controlled dimensionless parameters. As a result, the computational code and numerical simulations are open and capable of taking into account the ongoing changes in raw materials supply. Thus, a computational code for the study of the process that has been updated to take into consideration the new context of high injection rates for pulverized fuel and recirculating blast furnace gas enriched with oxygen and hydrogen as a new fuel gas in the raceway is very useful. By following this procedure, we can estimate the impacts of utilizing a new raw material source aiming at the development of a new green pig iron production route using the model built in the open computational simulation code. It is also possible to determine the operational parameters that enhance the competitive qualities, efficiency, and carbon dioxide emissions associated with new raw materials.
This study introduces additional elements including reactions for different chemical species and related new rate equations. In order to properly utilize hydrogen, it is required to establish appropriate rate equations for reactions occurring in the conditions of a blast furnace. Based on earlier research, these rate equations were modified for the parameters of the blast furnace process with injections of rich hydrogen gas and pulverized coal injection [
2,
4,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48].
The model equations are constructed under the assumption of an infinitesimal control volume, as schematically depicted in
Figure 3a. In the model’s formulation, we made the assumption that the conservation principle is true over the entire calculation domain, which is the actual operating volume of the blast furnace reactor. In order to properly compute the phase interactions, the phases are referred to as continuous and discontinuous. The model considers a collection of balance equations for energy, momentum, and chemical species of the coexisting phases accounting for the conservation principles. These equations are detailed in Equations (1)–(8).
Momentum balance and mass conservations of continuous phases:
Balances of momentum and mass for discontinuous phases:
Conservation of the phase’s chemical species:
Conservation of the phase’s enthalpies:
The restriction of coexisting phase volumes fractions obeys Equation (7):
The variables in Equations (1)–(7) are as follows: the coordinate directions are represented by the indexes i, j, and k, whereas the physical coordinates are represented by the variable x. Effective density, volume fraction, and viscosity are the phase properties, with the phases being represented, respectively, by the indexes i and l. With the indices i and l designating the phases and j and k designating the coordinate directions, the variables u, P, and F stand for the velocity component, pressure, and interphase interaction forces, respectively. The indexes n and m stand for the chemical species and their respective chemical reactions, whereas M and r stand for the molecular weight of the chemical species and the rates of chemical reactions. Mass fractions and the effective species diffusion coefficient of chemical species inside the phases are the variables . Interphase heat exchanges, phase enthalpy, thermal conductivity, specific heat capacity, and reactions enthalpy, respectively, are denoted by ,, and .
The phases and their chemical species taken into consideration for this investigation are presented in
Table 1. The solid phase is separated into different components with distinct chemical species and compositions that go through specific kinetics of chemical reactions. In order to enable scaling up of the modeling implementations, the rate parameters acquired through experimental work are performed under similar conditions. As a result, it is possible to apply the core theoretical concepts to model all the features of the process since they are compatible with the reactor conditions.
With the use of operational data from the process monitoring, the set of partial differential equations is solved under the assumption that the boundary conditions represent the conditions of a real blast furnace operating with stable performance. As a result, the lateral walls, the burden surface profile at the top, and the slag surface at the bottom, respectively, serve as the boundary conditions in the computational domain. The solid inflow is modeled using the inflow rate provided by local solid mass consumption caused by chemical reactions, melting, or gravity-driven flows, whereas the gas phase is assumed to be a fully developed flow applying the overall conservation law. Additional oxygen, hydrogen or blast furnace gas, and pulverized coal are determined by their input rates at the tuyere injection.
The gaseous fuel injection and blast flow rate are fixed. In contrast, the injection of pulverized coal is calculated iteratively to obtain the desired injection rate, which is defined at the start of the calculation process. Mass fluxes over the sidewall are taken to be zero. In addition, by regulating the overall cooling heat transfer coefficient based on the temperature variation and flow rate of the cooling water system using the operation’s acquisition data, heat loss is allowed.
In order to assess the source terms that are present in the momentum equations, particular models based on theoretical and empirical correlations suitable for blast furnace circumstances are used. The local layer structure evolution inside the reactor is taken into account by the gas–solid momentum interactions. The change in their volume fractions quantifies the coexistence of the solid component in each representative control volume. The chemical species inside the solid component are taken into consideration using the mass fraction variables, along with their volume fractions within the solid phase components, to compute the solid phase parameters and thermophysical properties of the phase. By computing the solid individual components of the burden layer and their locally estimated volume fractions, accounting for the solid layer local motion dynamics, the layer-structured descending characteristics in the charged solids are hence locally considered.
In accordance with the additive rule, the variables
, respectively, indicate the volume fractions of the burden materials in the solid phase, lump ore, sinter, pellets, briquettes, bulk granular coke, and the total volume of the solid phase charged along the radial position in the throat. The individual volume fractions of the raw materials are locally computed using the evolution of the composition of the burden layers within the furnace and using inlet boundary conditions, determined by the solid consumption due to melting and chemical transformations, the evolution of the descending burden structure is calculated depending on the charging practice [
10,
11,
12,
13,
14,
15].
The finite volume method (FVM) is the foundation for the numerical approach used to solve the model transport equations. To take into consideration the precise geometry of the physical space domain, the formulation is built in a general non-orthogonal coordinate system [
49,
50,
51,
52]. To correctly characterize the blast furnace wall shape and physical domain while keeping the benefits of the structured grid numerical framework, the numerical mesh is built using a body-fitted coordinate system with a smooth grid applied locally [
14,
52].
The SIMPLE algorithm (Semi-Implicit Method for Pressure-Linked Equations) is used on a staggered grid frame employing covariant projections of the velocities to resolve the coupling of the governing equations of mass and momentum of the continuous phases while the volume fractions conservations are applied to account for the termed discontinuous phases. The well-known power-law formulation approach is used to calculate the coefficients of the discretized equations numerically [
51,
52]. The tridiagonal matrix solver is combined to create an iterative algorithm that solves the discretized algebraic equations line-by-line. This method enables the convergence of the tridimensional calculations with strongly nonlinear differential equations as well as iterative corrections for cell volume calculations of phase interactions, properties, rate kinetics for chemical reactions, and phase transformations, while accounting for energy exchanges [
51,
52].
The phase volume fractions are iteratively calculated using the total mass conservation of phases, and the momentum equations calculations are employed to couple the discontinuous phase motions. The power-law formulation for the momentum equations coupled with the upwind scheme for the volume fraction are iteratively linked to determine the numerical coefficients in the iterative procedure of the momentum and volume fraction momentum equations based on the mass balance of the assessed phase. The numerical technique employs the power-law scheme for the iterative computations for all chemical species within the phase calculations, coupling the iterative calculations of all phases simultaneously [
14,
50,
51,
52].
The rate equations for the chemical processes are used to generate the enthalpy conservation equations for each phase, coupling the mass and enthalpy exchanges. Using the law of mixture, a polynomial mathematical expression relating the phase enthalpy and its temperature is created as a function of the iterative calculation of the local composition. By assuming the additive rule, the specific heat capacities of the chemical species are locally determined. The real roots of the polynomial equations are obtained using the secant method to determine the temperature of each phase [
14].
We updated the characteristics and chemical reactions in our earlier model to take into account coupled phenomena involving all of the phases and chemical species indicated in
Table 1 and
Table 2. Experimental data and previously published rate equations [
4,
8,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48] were used to obtain the rate equations for the chemical processes. Four cases of blast furnace gas (BFG) enriched with oxygen and hydrogen at the raceway were proposed to substitute the pulverized coal injections (PCI) in order to examine these novel conditions. These proposed scenarios were contrasted with a reference case of actual operation in a blast furnace of 3200 m
3 working volume, with extensive experience operating with high rates of pulverized coal and natural gas injection leading to a high production rate.
3. Results and Discussion
In this study, we investigate the possibilities of the replacement of pulverized fossil coal injections by blast furnace gas enriched with hydrogen and oxygen organized into four scenarios of calculated operations. The actual operation with recorded data is used to compare the estimated outcomes after first adjustments with the reference case using the overall operation outputs, giving a good correlation with the overall phenomena of the furnace operating under such conditions. The first stage in ensuring the model’s consistency and sensitivity to controllable operational factors, such as blast, burden materials, and proper boundary conditions, is to create a good, accurate reference scenario prediction. Three additional analysis case possibilities were taken into account. These proposed cases covered the possibility of increasing the amount of substitution of pulverized coal injections in the raceway and needed adjustments on the oxygen and hydrogen supply to compensate the effects of the blast furnace gas recirculation, enhancing the overall specific emissions of CO2.
Granular coke, sinter, pellets, dolomite/quartz, and lump ore constitute the burden materials. The operational procedure and the material’s accessibility determine the charging method and the quantities of the materials used to create the layer structure. The calculations in this study were based on the same ratio employed in the reference scenario (sinter: 46%, pellets: 42%, and lump ore: 12%).
The oxygen enrichment in the reference example was 8.3%, with a fixed blast flow rate and blast temperature of 1090 °C. To confirm the reference case’s reliability and conformity to current industrial practice, iterative calculation is a crucial task. The burden descending materials thickness determination for the metallic and coke layers is initially adjusted using the reference scenario calculation.
Using the initial top radial burden probe profiler, and measurements of the temperature and gas composition, refinement is carried out by an iterative process. We use a straightforward optimization approach to repeatedly adjust the layer thickness so that the measured gas composition and predicted temperature reasonably agree with one another. The predicted normalized burden layer thickness and averaged size distribution variation along the radial direction is shown in
Figure 4a and b, respectively.
The raw materials chemical composition of the charged bulk materials used is listed in
Table 3. The chemical analysis was obtained during the industrial trial of the reference scenario’s monitoring and acquisition data.
The injection temperatures were maintained at 1095 °C for all calculations. The process of the blast furnace is treated, heated, and enhanced with hydrogen that has calorific values comparable to a medium-value fuel of conventional pulverized coal, but with minimal impurities and regulated humidity.
The overall field calculation for the reference scenario of actual blast furnace operation is shown in
Figure 5 for the pig iron, slag, solid, and gas phase flowing conditions, respectively. The liquid volume fractions and the flowing directions are affected by the gas flow due to strong momentum interactions near the raceway region. As can be observed, in the vicinity of the raceway region, which comprises a two-raceway sector, the temperature and liquid volume fractions vary strongly. The stability of the operation strongly depends on the shape of this region and it is assumed to be an important feature to visually decide whether the simulated case is accepted as operationally feasible. The solid flow pattern follows the materials consumption due to chemical reactions and melting, while the temperature of the gas phase is determined by the local heat generation or consumption due to the chemical reactions and phase transformations. It is possible to observe a stable formation of the cohesive zone for the reference case, which is responsible for smooth operation of the blast furnace process.
Figure 6 compares the numerical predictions to the temperature and composition of the gas as measured and monitored by the analysis probe.
These numbers are the result of a continued, iterative search for the simultaneous measurement of the composition and temperature of the gas phase.
Figure 6 depicts a good agreement of the computed findings and the averaged measured data. The parameters measured during the stable operation were averaged for a data set of 10 continuous heats of stable operation.
The operational data for the blast furnace is compared in
Table 4 between model predictions and measured values. Except for the temperature of the outlet gas, the computed findings agree with the averaged measured data with an error of less than 5%. This discrepancy can be attributed to the humidity of the samples collected, which strongly influence the measured temperature.
The computational effort required for validation and verification consumes the most calculation time due to the iterative determination of the bulk charging distribution adjustments that best match the initial charging system estimation positions for the average thickness of the burden layers and the settled materials obtained from the dynamics of descending granular flow and upward gas velocities. The only method to achieve this is to use a multiphase, multicomponent comprehensive model approach. Using this method, we can consider the effects of multifactorial operational parameters as shape factors and burden materials lump solids rolling characteristics and locally average solid granulometric distributions, and also the shaft gas flowing pattern coupled with variable gas and solids thermophysical properties.
As observed in
Figure 6, the model’s prediction closely matches the measured data that were gathered during an actual process operation of a typical operational practice. In order to examine new gas injection scenarios and assess their feasibility and potential for switching the ironmaking industry from a carbon to a hydrogen base, the developed model was employed.
The comparison of inner temperature distributions of the gas phase for increasing replacement of pulverized coal by blast furnace gas enriched with hydrogen and oxygen is shown in
Figure 7. The temperature patterns are strongly affected by the gas injections in the lower part of the blast furnace for the scenarios proposed. Increasing temperature was observed within the granular zone, while in the dropping zone the overall temperature decreased as the amount of replacement of the pulverized coal was increased. These patterns confirmed that the endothermic reactions are compensated by the enhancement of the exothermic reactions with an increase in replacement of pulverized coal by gas injections.
The overall parameters of the operation are shown in
Table 5. As can be observed, the partial replacement of the pulverized coal injection by the injection of blast furnace gas enriched with hydrogen and oxygen allowed productivity to significantly increase (the best scenario predicted an increase of 20.5%). The increase in productivity was due to the correspondent oxygen enrichment needed to keep the thermal conditions at the bottom of the furnace. The coke rate was kept nearly constant while the fuel rate decreased continuously as the replacement of the pulverized coal injections was increased. The material balance of the burden and slag slightly changed to attain the overall mass balance. The oxygen enrichment changed from 8.3% to 14.2% to compensate for gas reactions and furnish energy to keep the blast furnace operation stable. It was possible to inject up to 210.3 kg of BFG per tonne of pig iron produced in the best scenario analyzed. Additionally, we predicted a significant decrease in the specific equivalent CO
2 emissions when partial replacement of the pulverized coal injection was analyzed (the best scenario indicated a 14.8% decrease in specific CO
2 emission).
The cohesive zone stability in the blast furnace operation plays an important role in the performance of the blast furnace. The shape and thickness of the cohesive zone indicate the smooth burden descending pattern and hence the gas–solid efficiency of the reduction reactions.
Figure 8 shows comparatively the solid flow patterns and the cohesive zone locations for the partial replacement of pulverized coal of 40, 60, and 80 kg/t
HM, respectively. As can be observed in
Figure 8, the cohesive zone positions slightly changed but still kept similar shapes compared with the reference case of 220 kg/t
HM of pulverized coal injection. The descending velocity of the burden materials slightly increased to account for the increase in productivity, as observed by the time interval for the particle trajectories, shown in
Figure 8a–d. It is important to mention that the calculations were carried out for a two-raceway angular sector to compute nonsymmetrical solid flows near the raceway region which were not possible to capture using two-dimensional symmetry assumptions as usually carried out in the Literature [
24,
29,
32]. The stability of the whole operation strongly depends on the liquid flow patterns, temperature, and holdup in the lower part of the blast furnace. A good visualization of these parameters can be obtained by the temperature associated with the liquid trajectories.
Figure 9 and
Figure 10 show the temperature and flow patterns for the liquid metal (pig iron) and slag, respectively.
Figure 9a–d show the 3D temperature distributions for the scenarios of the replacement of pulverized coal injections (PCI) by blast furnace gas (BFG) enriched with hydrogen and oxygen in the lower part of the blast furnace (dropping zone). As can be observed in these figures, the temperatures are higher in the vicinity of the raceways, followed by a decrease and partial recovering at the center of the deadman region.
The reasons for such behavior are due to the heat transfer at the higher temperature near the raceway and the silicon transfer mechanism prevailing at the central region of the dropping zone. The slag exchanges heat by different mechanisms in this region with gas, solid, and hot metal. The model considers simultaneously these mechanisms. When the gas injection rate is increased with a high H2 concentration, the averaged heat capacity of the gas phase increases and hence improves the potential to exchange heat. Similar behavior in the slag temperature distributions is observed in
Figure 10a–d. The observed differences of the temperature levels and flow directions are due to the strong effects on the momentum interaction attributed to the particular contact area and thermophysical properties, which play important roles on the momentum and heat transfer during the local flow of slag and hot metal in this region. As can be observed, the slag flow is preferentially directed toward to the central region, explained by the strong interaction with the increase in the gas flow in the vicinity of the raceway region.
Another important aspect of the expected behavior of the inner phenomena of the blast furnace process under such new operational conditions is emphasized by the gas temperature and compositions at the outlet surface of the blast furnace (surface of the burden materials).
Figure 11a,b evidence the flow conditions and shaft performance. As can be observed, the average temperatures are moved upward, repleting the higher gas volume passing through the furnace shaft and consistently increasing the concentrations of the CO and H
2 in the outlet gas. These patterns are expected due to the increasing contribution of the hydrogen reduction of the gas–solid reactions.
Table 6 confirmed these expectations by showing a consistent decrease in the contribution of the CO reduction while the contributions of the hydrogen reduction in the gas–solid reactions increased. It is important to emphasize that the results are consistent with the thermodynamics-driven forces of the reduction steps shown in
Figure 2, and that the hydrogen contributes to the gas–solid reduction only during the final step of the reduction at higher temperatures, as evidenced in
Table 6, where the predicted contributions of the hydrogen gas–solid reduction continuously increased as the amount of replaced pulverized coal increased. The predicted values of the contribution of the first stage of reduction of hematite to magnetite are considered negligible by hydrogen compared with carbon monoxide. In
Table 6 we included important gasification reaction contributions that are affected by the proposed practice of replacement of pulverized coal injection (PCI) by blast furnace gas injection enriched with hydrogen and oxygen. The amount of carbon gasification consuming coke or pulverized coal depends on the gas flow and composition, and the local availability of pulverized coal, which preferentially reacts with the gas species due to its reactivity and fine particles.
The overall results of the proposed scenarios of replacement of pulverized coal by blast furnace gas enriched with hydrogen and oxygen indicated feasible operational practice. The increase in the productivity and decrease in the equivalent CO
2 emissions are clearly predicted, as expected. The internal state variables were calculated, and indicated stable distribution patterns compatible with the actual operation of the blast furnace process. Therefore, we are confident to recommend this technological innovation to the process operation, with predicted reduction in equivalent CO
2 emission and replacement of fossil fuel utilization in the blast furnace process. Detailed behavior of the flow patterns for the gas phase are presented in the
video supplementary materials calculated for the stable operation of the reference case (
Supplementary Materials Video S1) and for the replacement scenarios of 40 kg/tHM (
Supplementary Materials Video S2) and 80 kg/tHM (
Supplementary Materials Video S3). The animation of the solid flow dynamics and cohesive zone position for the best scenario of 80 kg/tHM are shown in
Supplementary Materials Video S4. A slice animation of the vertical plane is shown in
Supplementary Materials Video S5 where the wustite to iron reduction location region is highlighted. As can be observed, the inner pattern of the reactor is consistently modified due to the new kinematic fluid dynamics, heat transfer and reactions coupling.