Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750
Abstract
:1. Introduction
2. Materials and Methods
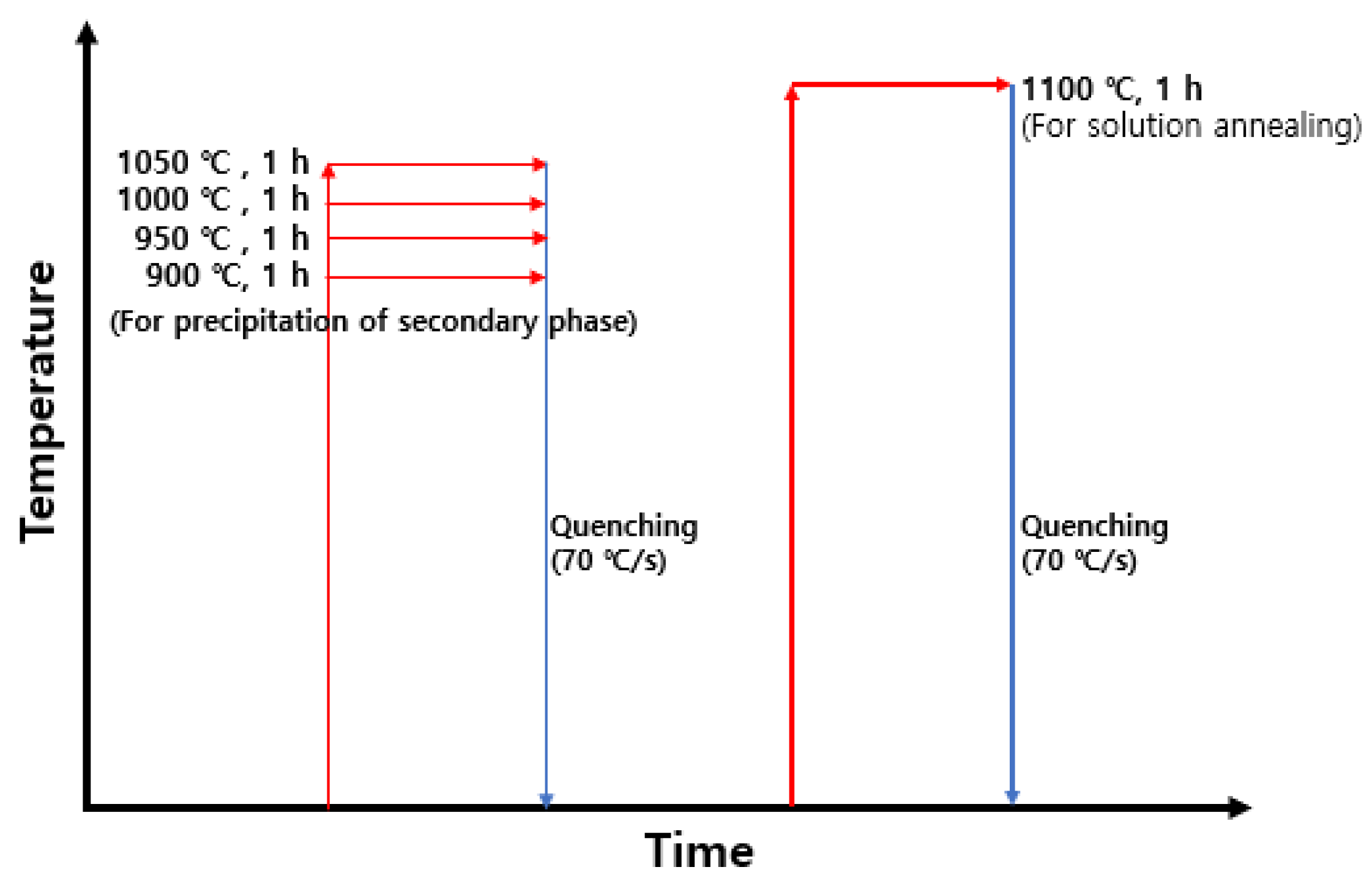
2.1. Materials and Heat Treatment
2.2. Microstructure and Volume Fraction
2.3. Corrosion Properties
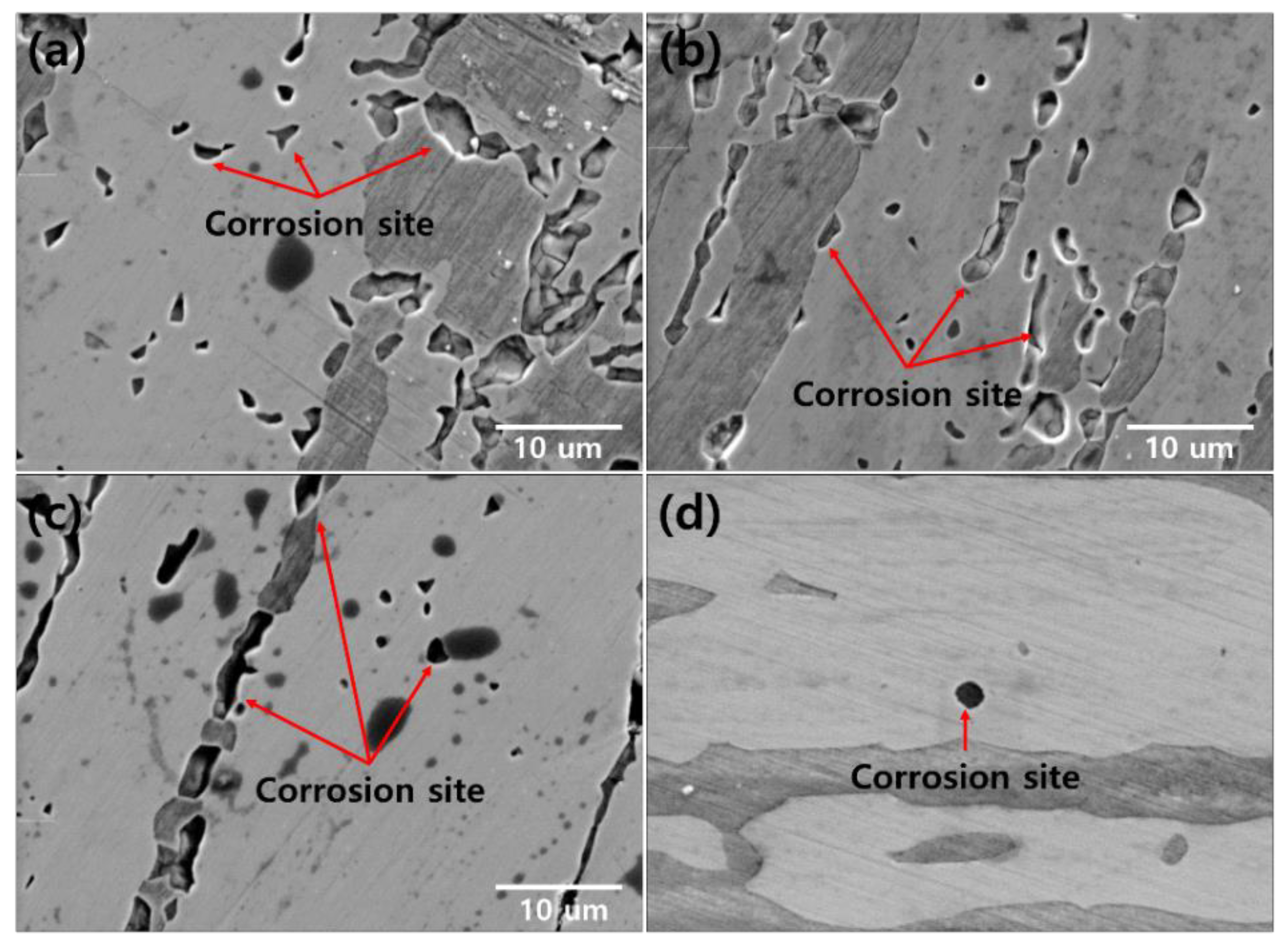
2.4. Corrosion Morphology
3. Results and Discussion
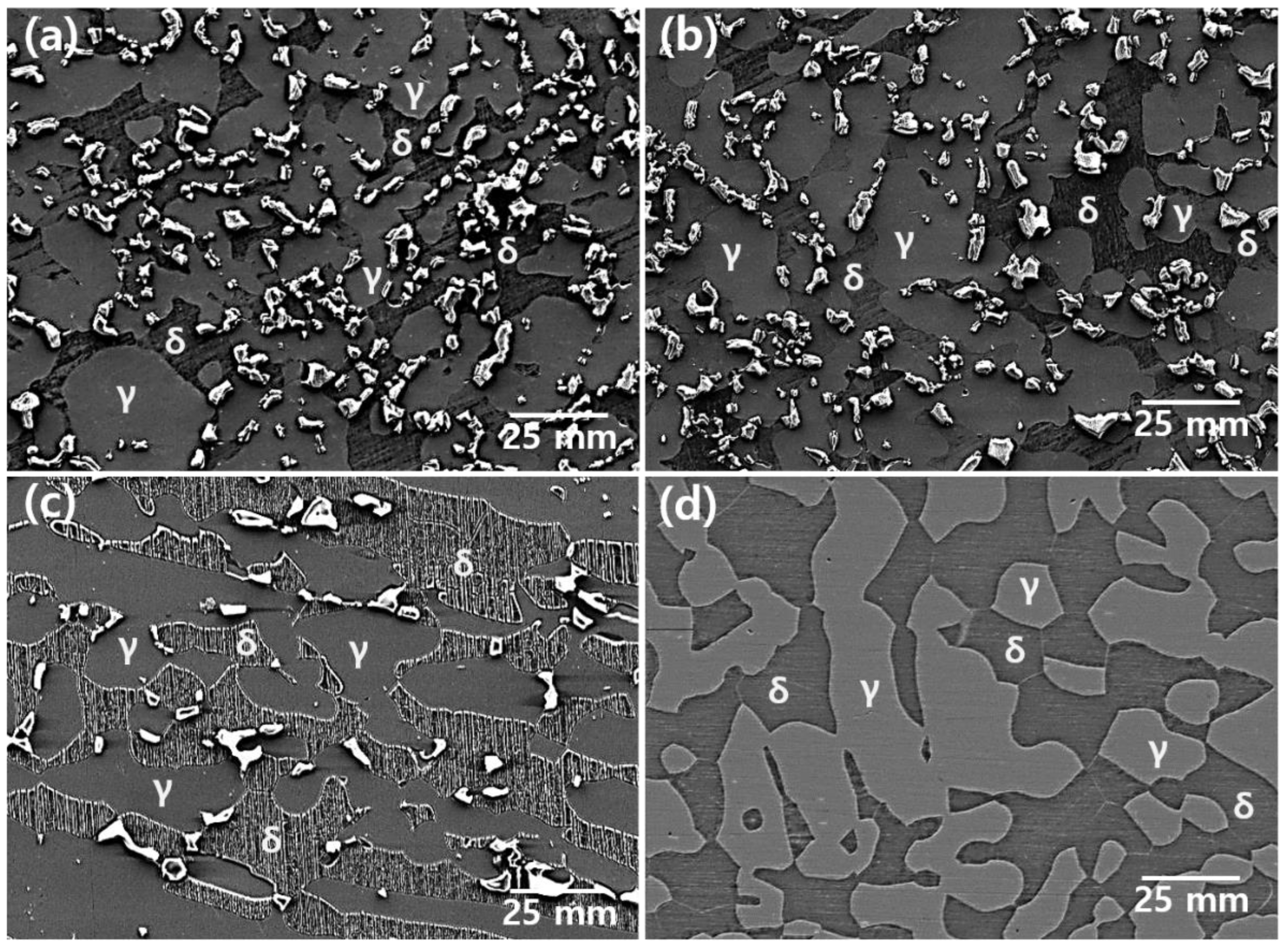
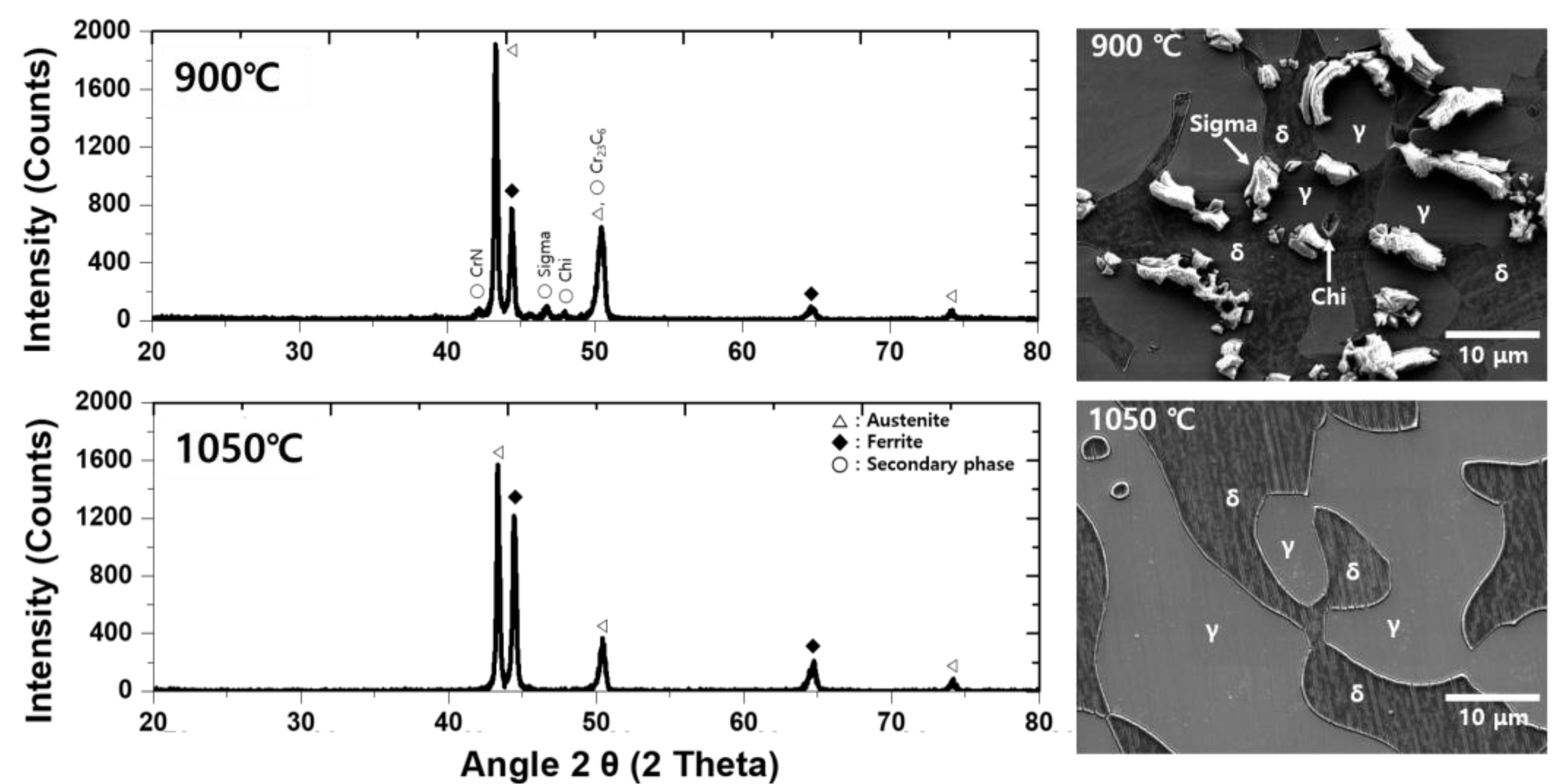
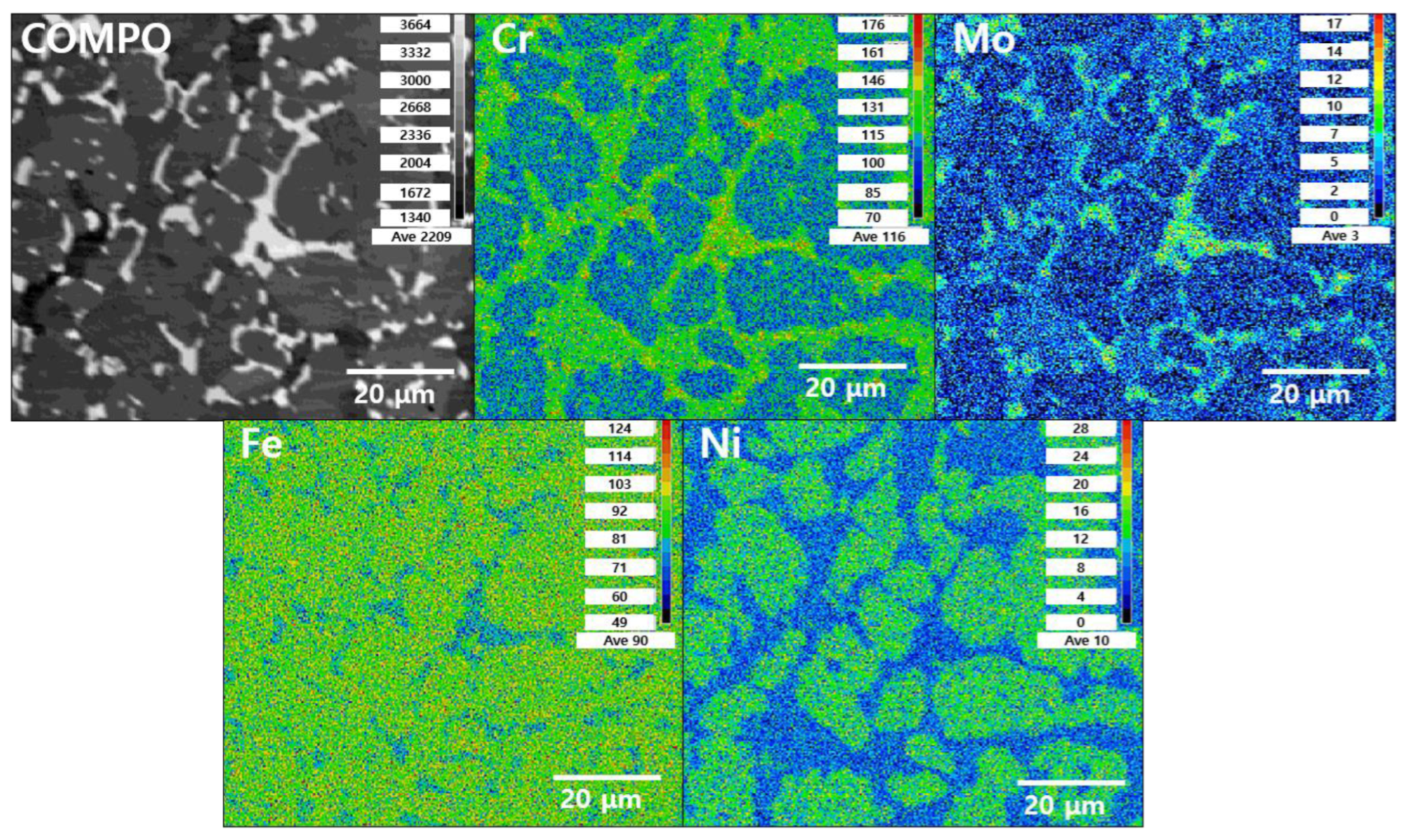
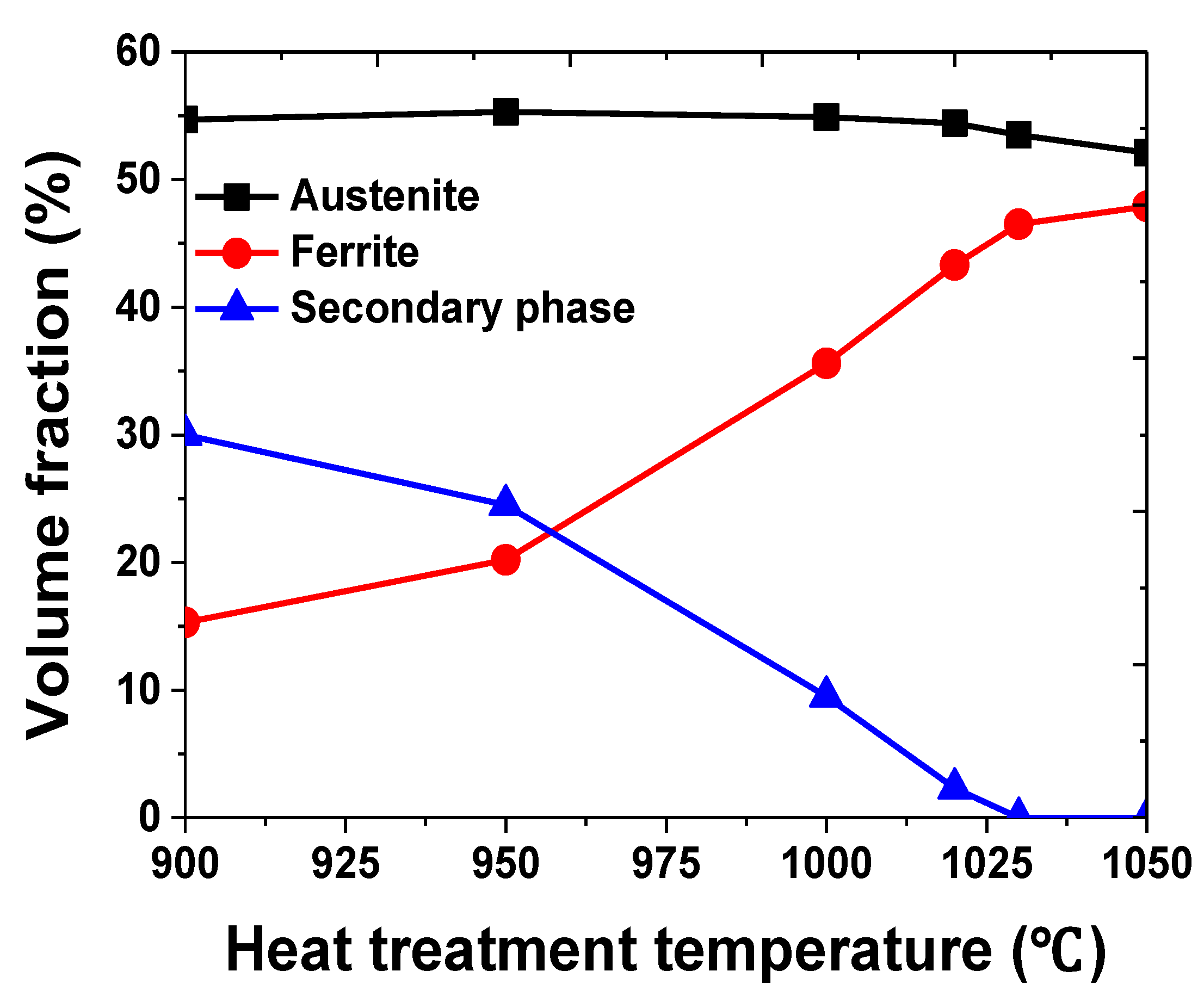
3.1. Microstructure
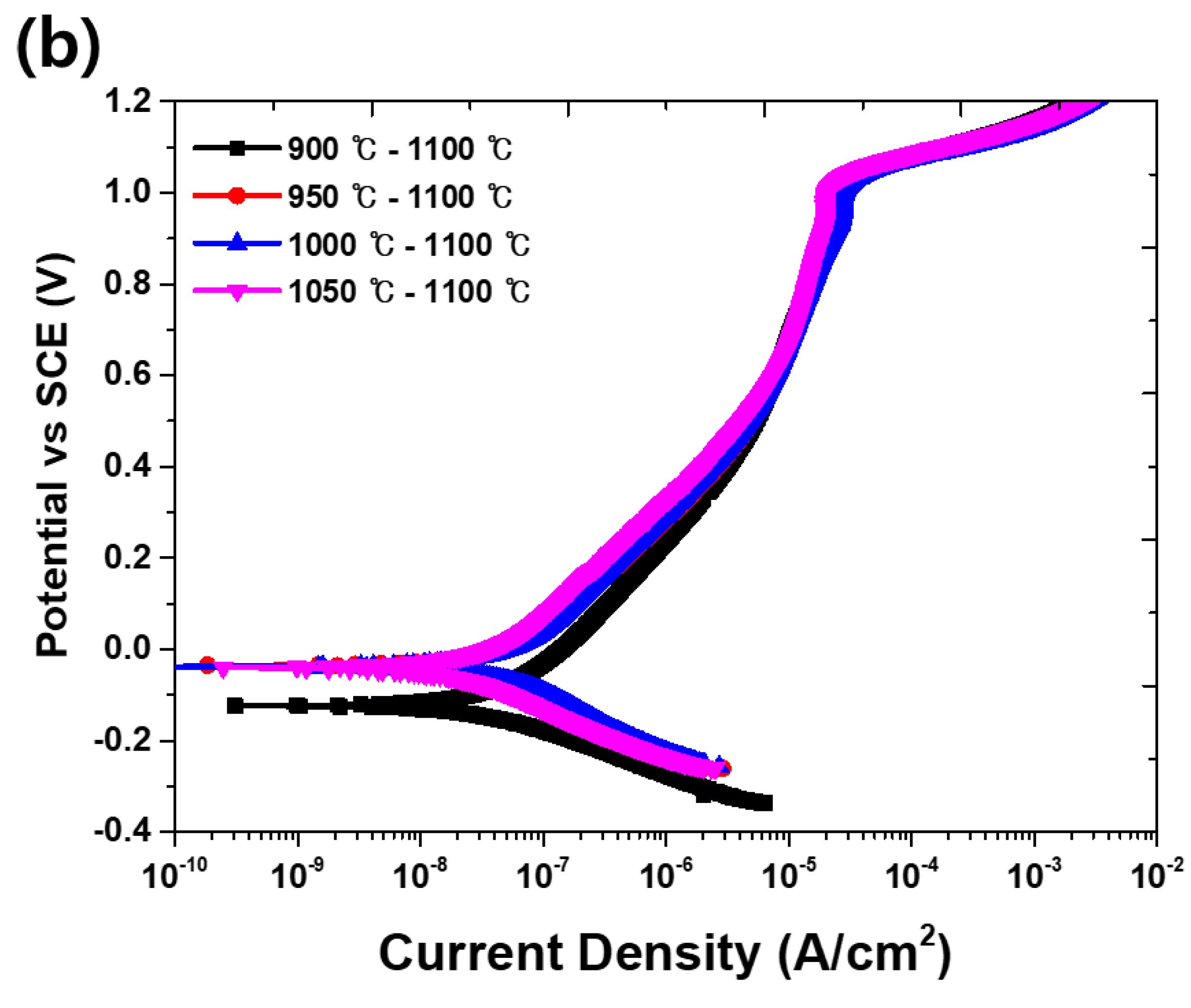
3.2. Electrochemical Properties
4. Discussion
5. Conclusions
- (1)
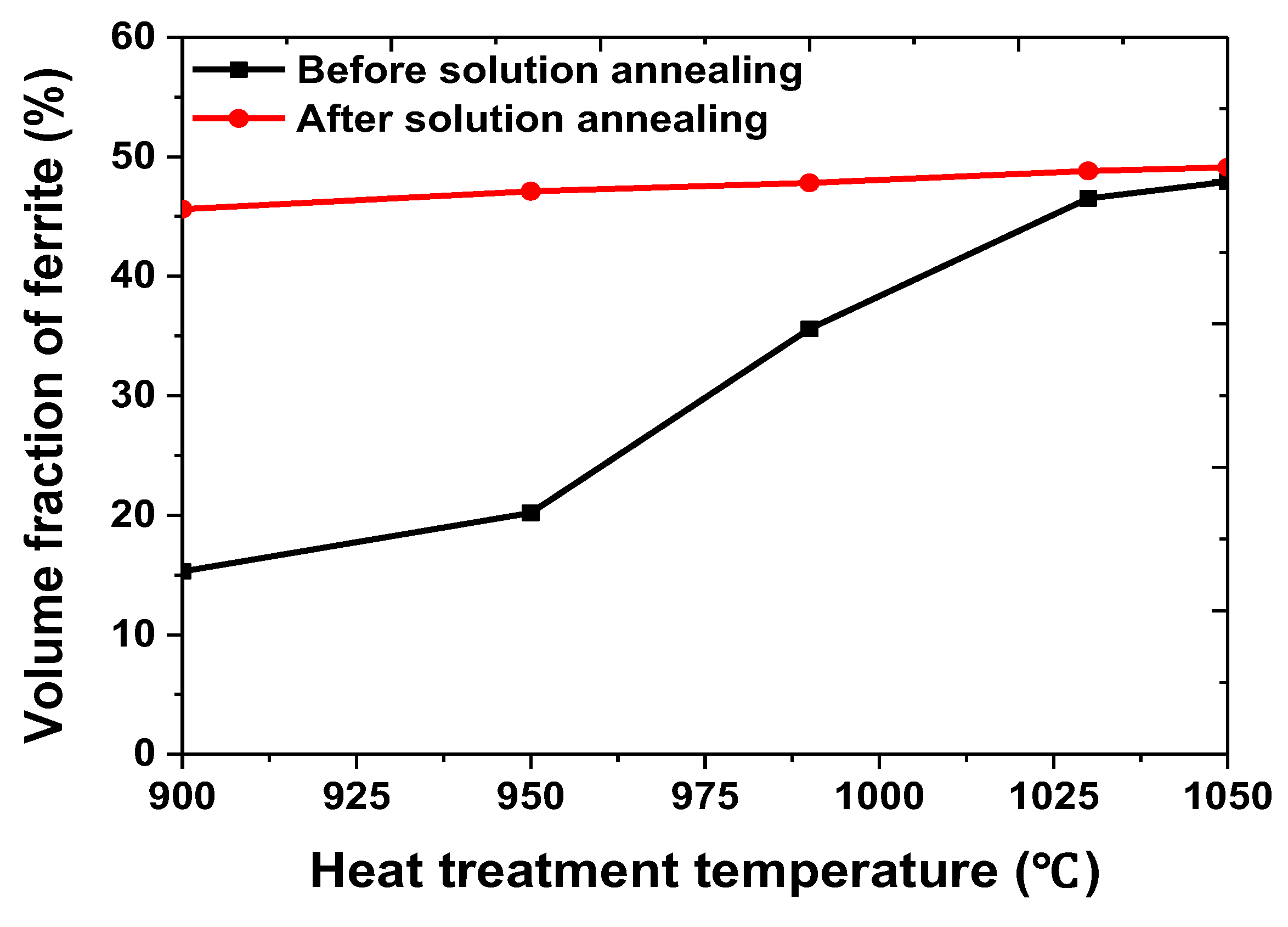
- The secondary phase precipitated at the grain boundary of austenite and grew into ferrite because of the high chemical composition of Cr and Mo. After solution annealing at 1100 °C, the secondary phase dissolved as ferrite. Solution annealing optimized the corrosion resistance by rendering the fraction of austenite and ferrite as 1:1; however, solution annealing after the precipitation of the secondary phase did not result in a volume fraction of 1:1.
- (2)
- The secondary phase is formed by the segregation of Cr and Mo, which decreased the pitting corrosion resistance, as confirmed by the potentiodynamic polarization curve and CPT test analysis. The solution annealing after the precipitation of secondary phase fully dissolved the secondary phase but did not result in the growth of ferrite or optimization of the pitting corrosion resistance. To improve the pitting corrosion resistance by the dissolution of the secondary phase and growth of ferrite, solution annealing after precipitation of the secondary phase should be performed at a higher temperature than the conventional temperature (1100 °C).
- (3)
- SDSS2507 is manufactured by high-temperature forging because it is used in valves and pipes in offshore plants. However, owing to the difference in the cooling rate between the inside and outside, a secondary phase is formed inside and hot cracks occur. To stabilize the microstructure and improve the corrosion resistance, the solution annealing of SDSS2507 at a temperature higher than that of the solution heat treatment can prevent high-temperature cracking because the high temperature over the solution annealing temperature at the same cooling rate can prevent the precipitation of the secondary phase.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, D.; Ramsay, H.; Moller, G.; Watt, C. Observation on foods of kiore (Rattus exulans) found in husking stations on northern offshore islands of New Zealand. N. Z. J. Ecol. 1984, 7, 131–138. [Google Scholar]
- Park, J.; Woo, J. A Study on Process Management Method of Offshore Plant Piping Material. JSNAK 2018, 56, 143–151. [Google Scholar] [CrossRef]
- Kaldellis, J.K.; Apostolou, D. Life cycle energy and carbon footprint of offshore wind energy. Comparison with onshore counterpart. Renew. Energy 2017, 108, 72–84. [Google Scholar] [CrossRef]
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Shin, B.; Park, J.; Heo, S.; Chung, W. Effect of cooling rate after heat treatment on pitting corrosion of super duplex stainless steel UNS S 32750. Anti-Corros. Methods Mater. 2018, 65, 492–498. [Google Scholar] [CrossRef]
- Kosea, C.; Topalb, C. Texture, microstructure and mechanical properties of laser beam welded AISI 2507 super duplex stainless steel. Matter. Chem. Phys. 2022, 289, 126490. [Google Scholar] [CrossRef]
- Iacoviello, F.; Cocco, V.; Franzese, E.; Natali, S. High temperature embrittled duplex stainless steels: Influence of the chemical composition on the fatigue crack propagation. Procedia Struct. Integr. 2017, 3, 308–315. [Google Scholar] [CrossRef]
- Ha, H.; Jo, H.; Lee, J.; Kim, S.; Moon, J.; Jang, J.; Lee, T.; Lee, C. Effect of combined addition of N and C on high temperature deformation behavior of UNS S32101 type lean duplex stainless steels. Mater. Today Commun. 2021, 29, 102749. [Google Scholar] [CrossRef]
- Westin, E.; Putz, A.; Maderthoner, A.; Pilhagen, J. Solidification cracking in duplex stainless steel flux-cored arc welds Part 1—Cracking in 30-mm-thick material welded under high restraint. Weld. World 2022, 66, 2405–2423. [Google Scholar] [CrossRef]
- Tahchieva, A.; Chatterjee, D.; Helvoort, A.; Isern, N.; Cabrera, J. Effect of the nano structuring by high-pressure torsion process on the secondary phase precipitation in UNS S32750 Superduplex stainless steel. Mater. Charact. 2022, 183, 111639. [Google Scholar] [CrossRef]
- Kalandyk, B.; Zapala, R.; Palka, P. Effect of Isothermal Holding at 750 °C and 900 °C on Microstructure and Properties of Cast Duplex Stainlesss Steel Containg 24%Cr-5%Ni-2.5%Mo-2.5%Cu. Materials 2022, 15, 8569. [Google Scholar] [CrossRef]
- Assumpcao, R.; Ferreira, M.; Sousa, M.; Santos, D.; Sicupira, D. Effect of strain-induced martensite reverse transformation on microstructure evolution and electrochemical behavior of 2304 lean duplex stainless steel. Corros. Eng. Sci. Technol. 2022, 57, 499–508. [Google Scholar] [CrossRef]
- Videira, A.; Mendes, W.; Ventrella, V.; Calliari, I. Increasing the Corrosion Resistance in the UNS S32750 Super Duplex Steel Welded Joints through Hybrid GTAW-Laser Welding and Nitrogen. Materials 2023, 16, 543. [Google Scholar] [CrossRef]
- Shin, B.; Kim, D.; Park, S.; Hwang, H.; Park, J.; Chung, W. Precipitation condition and effect of volume fraction on corrosion properties of secondary phase on casted super-duplex stainless steel UNS S32750. Anti-Corros. Methods Mater. 2018, 66, 61–66. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S32750. Int. J. Electrochem. Sci. 2021, 16. [Google Scholar] [CrossRef]
- Liu, J.; Das, Y.; King, S.; Jonsson, J.; Wessman, S.; Hedstrom, H. Effect of Cooling Rate after Solution Treatment on Subsequent Phase Separation Evolution in Super Duplex Stainless Steel 25Cr-7Ni (wt.%). Metals 2022, 12, 890. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Park, J.; Chung, W.; Shin, B. Microstructure and corrosion performance of high-entropy alloy and austenite and super duplex stainless steels in 3.5% NaCl solution. J. Electrochem. Sci. 2023, 18, 100074. [Google Scholar] [CrossRef]
- Calliari, I.; Brunelli, K.; Dabala, M.; Ramous, E. Measuring secondary phase in duplex stainless steels. JOM 2009, 61, 80–83. [Google Scholar] [CrossRef]
- Torres, C.; Johnsen, R.; Iannuzzi, M. Crevice corrosion of solution annealed 25Cr duplex stainless steels: Effect of W on critical temperature. Corros. Sci. 2021, 178, 109053. [Google Scholar] [CrossRef]
- Yoon, B.; Ahn, Y. Effect of Aging on Pitting Corrosion Resistance of 21Cr Lean Duplex Stainless Steel with Different Molybdenum Contents. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Barros, B.; Pecly, P.; Pardal, J.; Gonzaga, A.; Tavares, S. Comparison Between Hot Rolled and Powder Metallurgy Hot Isostatic Pressing(PM-HIP) Processed Duplex Stainless Steel UNS S32205. J. Mater. Eng. Perform. 2022, 31, 5504–5510. [Google Scholar] [CrossRef]
- Laleh, M.; Haghdadi, N.; Hughes, A.; Primig, S.; Tan, E. Enhancing the repassivation ability and localized corrosion resistance of an additively manufactured duplex stainless steel by post-proceeding heat treatment. Corros. Sci. 2023, 198, 110106. [Google Scholar] [CrossRef]
- Andrade, R.A.; Magnabosco, R. Computational Simulation of Duplex Stainless Steel Continuous Cooling Transformation Curves Using DICTRA. Mater. Res. 2022, 25. [Google Scholar] [CrossRef]
- Magnabosco, R.; Costa Morias, L.; Santos, D. Use of composition profiles near sigma phase for assessment of localized corrosion resistance in a duplex stainless steel. Calphad 2019, 64, 126–130. [Google Scholar] [CrossRef]
- Costa Morias, L.; Magnabosco, R. Experimental investigations and DICTRA simulation of sigma phase formation in a duplex stainless steel. Calphad 2017, 58, 214–218. [Google Scholar] [CrossRef]
- Li, M.; Zou, D.; Li, Y.; Tong, L. Effect of Cooling Rate on Pitting Corrosion Behavior of 904L Austenitic Stainless Steel in a Simulated Flue Gas Desulfurization Solution. Met. Mater. Int. 2023, 29, 730–747. [Google Scholar] [CrossRef]
- Kong, Y.; Zhao, L.; Zhu, L.; Huang, H. The selection of laser beam diameter in directed energy deposition of austenitic stainless steel: A comprehensive assessment. Addit. Manuf. 2022, 52, 102646. [Google Scholar] [CrossRef]





| C | N | Mn | Ni | Cr | Mo | Fe | PRE | |
|---|---|---|---|---|---|---|---|---|
| SDSS2507 | 0.01 | 0.3 | 0.79 | 6.8 | 25.0 | 3.8 | Bal | 42 |
| Chemical Composition (wt.%) | Sigma | Chi |
|---|---|---|
| Cr | 30.1 ± 2.1 | 22.1 ± 1.5 |
| Mo | 8.8 ± 0.6 | 2.2 ± 0.1 |
| Ni | 4.6 ± 0.4 | 9.5 ± 0.3 |
| Mn | 1.1 ± 0.1 | 1.0 ± 0.1 |
| Fe | Bal | Bal |
| (a) | (b) | |||||
|---|---|---|---|---|---|---|
| Ecorr (mV) | Icorr (A/cm2) | EPit (mV) | Ecorr (mV) | Icorr (A/cm2) | EPit (mV) | |
| 900 °C | −150 ± 10 | 9 × 10−7 | 990 ± 10 | −100 ± 5 | 1 × 10−7 | 1020 ± 10 |
| 950 °C | −150 ± 10 | 9 × 10−7 | 1000 ± 10 | −40 ± 5 | 1 × 10−7 | 1020 ± 10 |
| 1000 °C | −140 ± 10 | 8 × 10−7 | 1010 ± 10 | −40 ± 5 | 1 × 10−7 | 1020 ± 10 |
| 1050 °C | −80 ± 10 | 2 × 10−7 | 1030 ± 10 | −40 ± 5 | 1 × 10−7 | 1030 ± 10 |
| Phase | Austenite (%) | Ferrite (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Chemical Composition | Cr | Mo | N | Fe | Cr | Mo | N | Fe |
| 23.2 | 3.1 | 0.48 | Bal | 26.3 | 5.0 | 0.05 | Bal | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Chung, W.; Shin, B.-H. Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750. Metals 2023, 13, 957. https://doi.org/10.3390/met13050957
Kim D, Chung W, Shin B-H. Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750. Metals. 2023; 13(5):957. https://doi.org/10.3390/met13050957
Chicago/Turabian StyleKim, Dohyung, Wonsub Chung, and Byung-Hyun Shin. 2023. "Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750" Metals 13, no. 5: 957. https://doi.org/10.3390/met13050957





