Corrosion of Copper in a Tropical Marine Atmosphere Rich in H2S Resulting from the Decomposition of Sargassum Algae
Abstract
1. Introduction
2. Materials and Methods
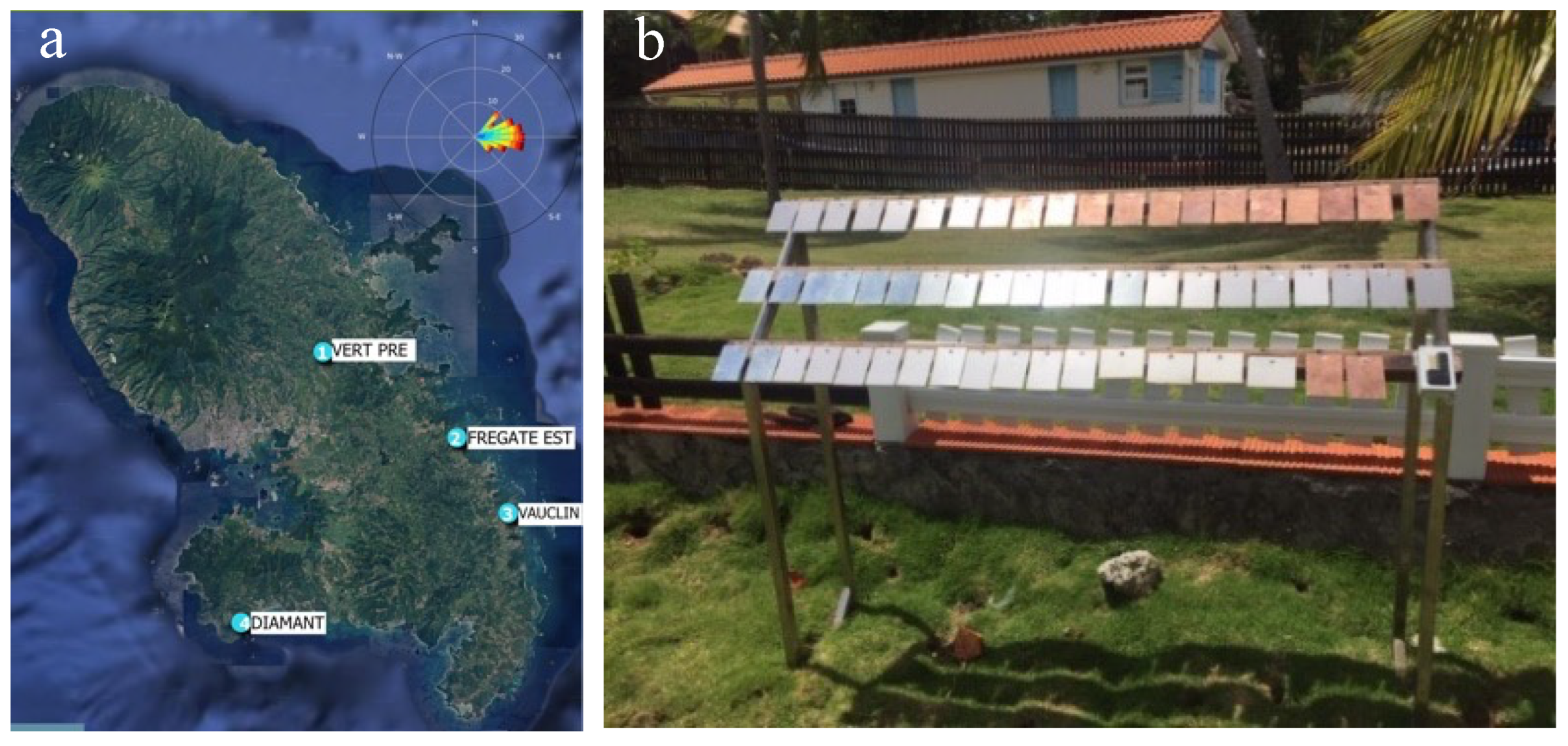
2.1. Selection of Sites, H2S and Chlorides Ion Measurement Techniques
2.2. Sample Preparation
2.3. Gravimetric Measurements
2.4. Characterization and Identification of the Corrosion Products
3. Results
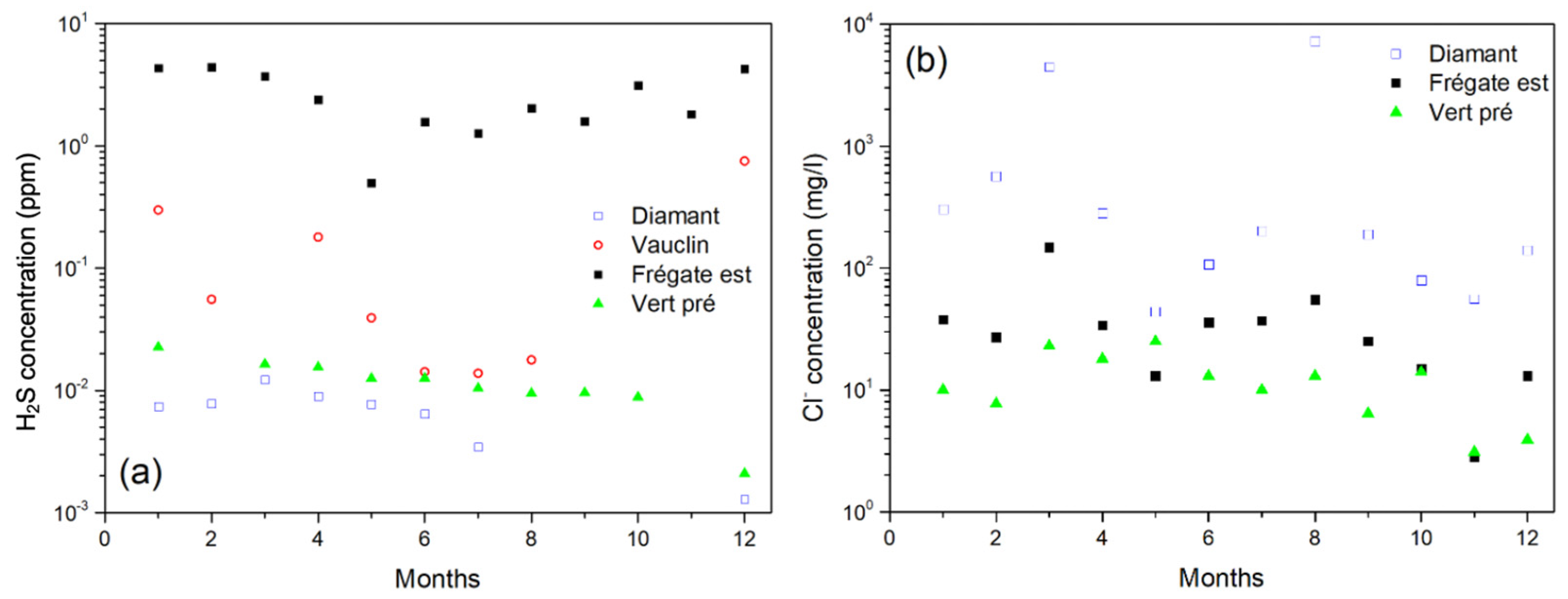
3.1. H2S and Chloride Ions Measurements
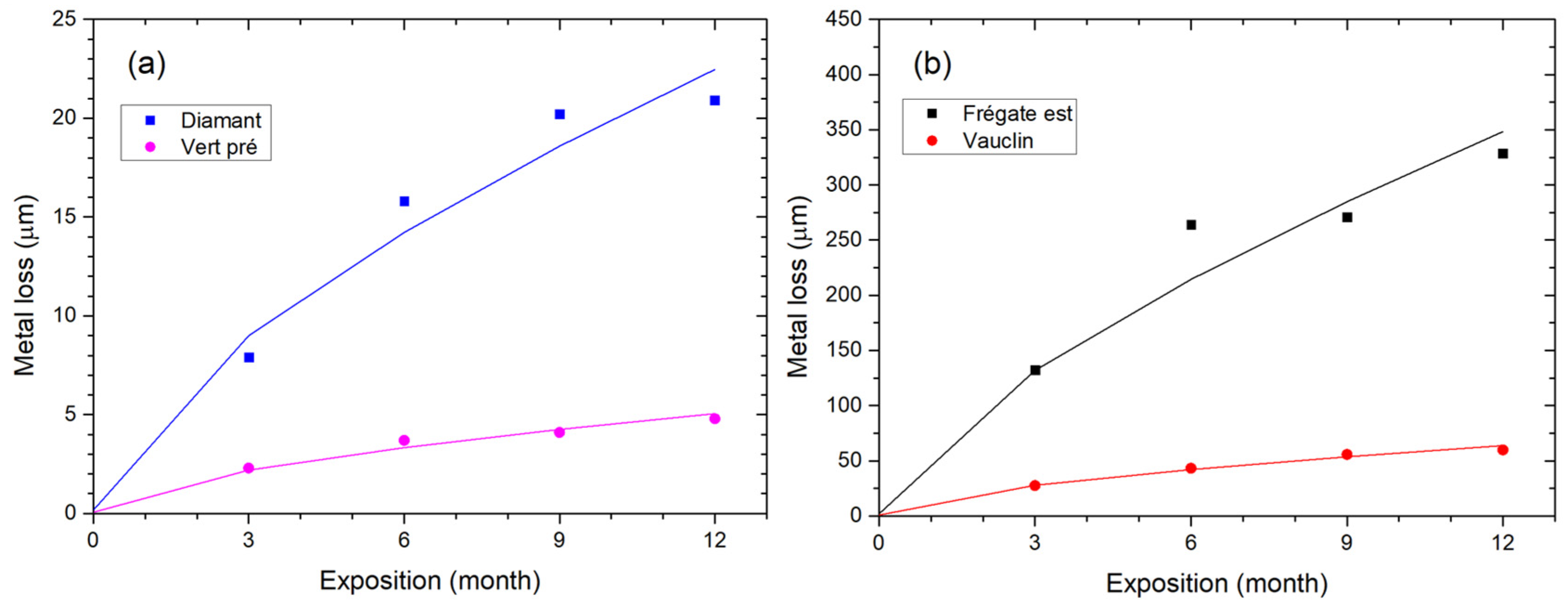
3.2. Determination of the Corrosion Rate after 1-Year Exposure
3.3. Evaluation of Copper Thickness Loss Dynamics
3.4. SEM/EDS Analysis
3.5. XRD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veleva, L.; Quintana, P.; Ramanauskas, R.; Pomes, R.; Maldonado, L. Mechanism of copper patina formation in marine environments. Electrochim. Acta 1996, 41, 1641–1645. [Google Scholar] [CrossRef]
- Aastrup, T.; Wadsak, M.; Schreiner, M.; Leygraf, C. Experimental in situ studies of copper exposed to humidified air. Corros. Sci. 2000, 42, 957–967. [Google Scholar] [CrossRef]
- El-Mahdy, G.A. Atmospheric corrosion of copper under wet/dry cyclic conditions. Corros. Sci. 2005, 47, 1370–1383. [Google Scholar] [CrossRef]
- Watanabe, M.; Tomita, M.; Ichino, T. Characterization of Corrosion Products Formed on Copper in Urban, Rural/Coastal, and Hot Spring Areas. J. Electrochem. Soc. 2001, 148, B522–B528. [Google Scholar] [CrossRef]
- Fonseca, I.T.E.; Picciochi, R.; Mendonça, M.H.; Ramos, A.C. The atmospheric corrosion of copper at two sites in Portugal: A comparative study. Corros. Sci. 2004, 46, 547–561. [Google Scholar] [CrossRef]
- Farro, N.W.; Veleva, L.; Aguilar, P. Copper marine corrosion: I. Corrosion rates in atmospheric and seawater environments of Peruvian port. Open Corros. J. 2009, 2, 1. [Google Scholar] [CrossRef]
- Xiao, L.U.; Liu, Y.W.; Zhao, H.T.; Chen, P.A.N.; Wang, Z.Y. Corrosion behavior of copper in extremely harsh marine atmosphere in Nansha Islands, China. Trans. Nonferr. Met. Soc. China 2021, 31, 703–714. [Google Scholar]
- Mendoza, A.R.; Corvo, F.; Gómez, A.; Gómez, J. Influence of the corrosion products of copper on its atmospheric corrosion kinetics in tropical climate. Corros. Sci. 2004, 46, 1189–1200. [Google Scholar] [CrossRef]
- Syed, S. Outdoor atmospheric corrosion of copper in Saudi Arabia. Corros. Eng. Sci. Technol. 2008, 43, 267–272. [Google Scholar] [CrossRef]
- Araban, V.; Kahram, M.; Rezakhani, D. Evaluation of copper atmospheric corrosion in different environments of Iran. Corros. Eng. Sci. Technol. 2016, 51, 498–506. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; De la Fuente, D.; Simancas, J. Looking back on contributions in the field of atmospheric corrosion offered by the MICAT ibero-american testing network. Int. J. Corros. 2012, 2012, 824365. [Google Scholar] [CrossRef]
- Lopesino, P.; Alcántara, J.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Morcillo, M. Corrosion of copper in unpolluted chloride-rich atmospheres. Metals 2018, 8, 866. [Google Scholar] [CrossRef]
- Wallinder, I.O.; Zhang, X.; Goidanich, S.; Le Bozec, N.; Herting, G.; Leygraf, C. Corrosion and runoff rates of Cu and three Cu-alloys in marine environments with increasing chloride deposition rate. Sci. Total Environ. 2014, 472, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.C.; Farmer, J.C.; Anderson, R.J. In situ Raman Spectroscopy of Anodic Films Formed on Copper and Silver in Sodium Hydroxide Solution. J. Electrochem. Soc. 1986, 133, 739–745. [Google Scholar] [CrossRef]
- Strehblow, H.H.; Titze, B. The investigation of the passive behaviour of copper in weakly acid and alkaline solution. Electrochim. Acta 1980, 25, 839–850. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Graedel, T.E. Copper patinas formed in the atmosphere-II. Qualitative assessment of mechanisms. Corros. Sci. 1987, 27, 721–740. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Y.; Zhao, Y.; Ma, X. Atmospheric corrosion prediction: A review. Corros. Rev. 2020, 38, 299–321. [Google Scholar] [CrossRef]
- Corvo, F.; Perez, T.; Dzib, L.R.; Martin, Y.; Castañeda, A.; Gonzalez, E.; Perez, J. Outdoor–indoor corrosion of metals in tropical coastal atmospheres. Corros. Sci. 2008, 50, 220–230. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Florentin, J.; Gueye, P.; Lebrun, T.; Blateau, A.; Viguier, J.; Valentino, R.; Brouste, Y.; Kallel, H.; et al. Sargassum seaweed health menace in the Caribbean: Clinical characteristics of a population exposed to hydrogen sulfide during the 2018 massive stranding. Clin. Toxicol. 2020, 59, 215–223. [Google Scholar] [CrossRef]
- Tran, T.T.M.; Fiaud, C.; Sutter, E.M.M.; Villanova, A. The atmospheric corrosion of copper by hydrogen sulphide in underground conditions. Corros. Sci. 2003, 45, 2787–2802. [Google Scholar] [CrossRef]
- Tran, T.T.M.; Fiaud, C.; Sutter, E.M.M. Oxide and sulphide layers on copper exposed to H2S containing moist air. Corros. Sci. 2005, 47, 1724–1737. [Google Scholar] [CrossRef]
- Salahinejad, E.; Eslamo-Farsani, R.; Tayebi, L. Corrosion failure analysis of printed circuit boards exposed to H2S containing humid environments. Eng. Fail. Anal. 2007, 79, 538–546. [Google Scholar] [CrossRef]
- Becker, J.; Pellé, J.; Rioual, S.; Lescop, B.; Le Bozec, N.; Thierry, D. Atmospheric corrosion of silver, copper and nickel exposed to hydrogen suphide: A multi-analytical investigation approach. Corros. Sci. 2022, 209, 110726. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Wei, Q.; Wang, H.; Xie, J. Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas. Metals 2022, 12, 2015. [Google Scholar] [CrossRef]
- Guo, M.; Chen, J.; Martino, T.; Lilja, C.; Johansson, J.A.; Behazin, M.; Binns, W.J.; Keech, P.G.; Noël, J.J.; Shoesmith, D.W. The nature of the copper sulfide film grown on copper in aqueous sulfide and chloride solutions. Mater. Corros. 2021, 72, 300–307. [Google Scholar] [CrossRef]
- EN 13523-19; Coil Coated Metals—Test Methods—Part 19 Panel Design and Method of Atmospheric Exposure Testing. European committee for standardization: Brussels, Belgium, 2011.
- EN ISO-8407; Removal of Corrosion Products from Corrosion Test Specimens. iTech Standard Preview: Switzerland, 2009. Available online: https://www.iso.org/standard/42634.html (accessed on 10 April 2023).
- Nassif, L.A.; Rioual, S.; Farah, W.; Fauchon, M.; Toueix, Y.; Hellio, C.; Abboud, M.; Lescop, B. Electrophoretic deposition of zinc alginate coatings on stainless steel for marine antifouling applications. J. Environ. Chem. Eng. 2020, 8, 104246. [Google Scholar] [CrossRef]
- Vera, R.; Araya, R.; Bagnara, M.; Diaz-Gomez, A.; Ossandon, S. Atmospheric corrosion of copper exposed to different environments in the region of Valparaiso, Chile. Mater. Corros. 2017, 68, 316–328. [Google Scholar] [CrossRef]
- Vera, R.; Valverde, B.; Olave, E.; Díaz, A.; Sánchez, R.; Muñoz, L.; Martínez, C.; Rojas, P. Corrosion behavior of copper exposed in marine tropical atmosphere in Rapa Nui (Easter Island) Chile 20 years after MICAT. Metals 2022, 12, 2082. [Google Scholar] [CrossRef]
- Schindelholz, E.J.; Cong, H.; Jove-Colon, C.F.; Li, S.; Ohlhausen, J.A.; Moffat, H.K. Electrochemical aspects of copper atmospheric corrosion in the presence of sodium chloride. Electrochim. Acta 2018, 276, 194–206. [Google Scholar] [CrossRef]
- Krätschmer, A.; Wallinder, I.O.; Leygraf, C. The evolution of outdoor copper patina. Corros. Sci. 2002, 44, 425–450. [Google Scholar] [CrossRef]
- Zhang, X.; Wallinder, I.O.; Leygraff, C. Mechanistic studies of corrosion product flaking on copper and copper-based alloys in marine environments. Corros. Sci. 2014, 85, 15–25. [Google Scholar] [CrossRef]
- Salas, B.V.; Wiener, M.S.; Koytchev, R.Z.; Badilla, G.L.; Irigoyen, R.R.; Beltrán, M.C.; Nedev, N.R.; Alvarez, M.C.; Gonzalez, N.R.; Rull, J.M.B. Copper corrosion by atmospheric pollutants in the electronics industry. Int. Sch. Res. Not. 2013, 2013, 846405. [Google Scholar]
- Zaafarany, I.; Boller, H. Corrosion of copper electrode in sodium sulfide solution. J. Saudi Chem. Soc. 2010, 14, 183–189. [Google Scholar] [CrossRef]
- Zittlau, A.H.; Shi, Q.; Boerio-Goates, J.; Woodfield, B.F.; Majzlan, J. Thermodynamics of the basic copper sulfate antlerite, posnjakite and brochantite. Geochemistry 2013, 73, 39–50. [Google Scholar] [CrossRef]
- Zhang, R.; Schmidt, R.; Gilbert, J.; Zhang, J. Effects of Gaseous Pollution and Thermal Conditions on the Corrosion Rates of Copper and Silver in Data Centre Environment: A Literature Review. In Proceedings of the 7th International Building Physics Conference, Syracuse, NY, USA, 23–26 September 2018; pp. 1365–1370. [Google Scholar]


| Cu | O | Cl | S | C | Si | Mg | Al | Ca | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vert pré | 34.4 | 48.9 | 6.6 | 0.8 | 7.0 | 0.8 | 0.6 | 0.7 | 0.1 | 0.1 |
| Diamant | 22.6 | 57.6 | 6.0 | 0.7 | 7.6 | 0.3 | 3.5 | 0.5 | 1.0 | 0.2 |
| Vauclin | 28.5 | 33.7 | 1.9 | 15.1 | 17.7 | 1.5 | 0.3 | 0.8 | 0.1 | 0.4 |
| Frégate est | 28.1 | 36.4 | 1.6 | 22.4 | 7.6 | 2.5 | 0.2 | 1.0 | 0.0 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.S.; Lebrini, M.; Lescop, B.; Pellé, J.; Rioual, S.; Amintas, O.; Boullanger, C.; Roos, C. Corrosion of Copper in a Tropical Marine Atmosphere Rich in H2S Resulting from the Decomposition of Sargassum Algae. Metals 2023, 13, 982. https://doi.org/10.3390/met13050982
Ahmed MS, Lebrini M, Lescop B, Pellé J, Rioual S, Amintas O, Boullanger C, Roos C. Corrosion of Copper in a Tropical Marine Atmosphere Rich in H2S Resulting from the Decomposition of Sargassum Algae. Metals. 2023; 13(5):982. https://doi.org/10.3390/met13050982
Chicago/Turabian StyleAhmed, Mahado Said, Mounim Lebrini, Benoit Lescop, Julien Pellé, Stéphane Rioual, Olivia Amintas, Carole Boullanger, and Christophe Roos. 2023. "Corrosion of Copper in a Tropical Marine Atmosphere Rich in H2S Resulting from the Decomposition of Sargassum Algae" Metals 13, no. 5: 982. https://doi.org/10.3390/met13050982
APA StyleAhmed, M. S., Lebrini, M., Lescop, B., Pellé, J., Rioual, S., Amintas, O., Boullanger, C., & Roos, C. (2023). Corrosion of Copper in a Tropical Marine Atmosphere Rich in H2S Resulting from the Decomposition of Sargassum Algae. Metals, 13(5), 982. https://doi.org/10.3390/met13050982







