Effect of Brazing Temperature on Microstructure, Tensile Strength, and Oxide Film-Breaking Synergy of 5A06 Aluminum Alloy Welded by TG-TLP
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
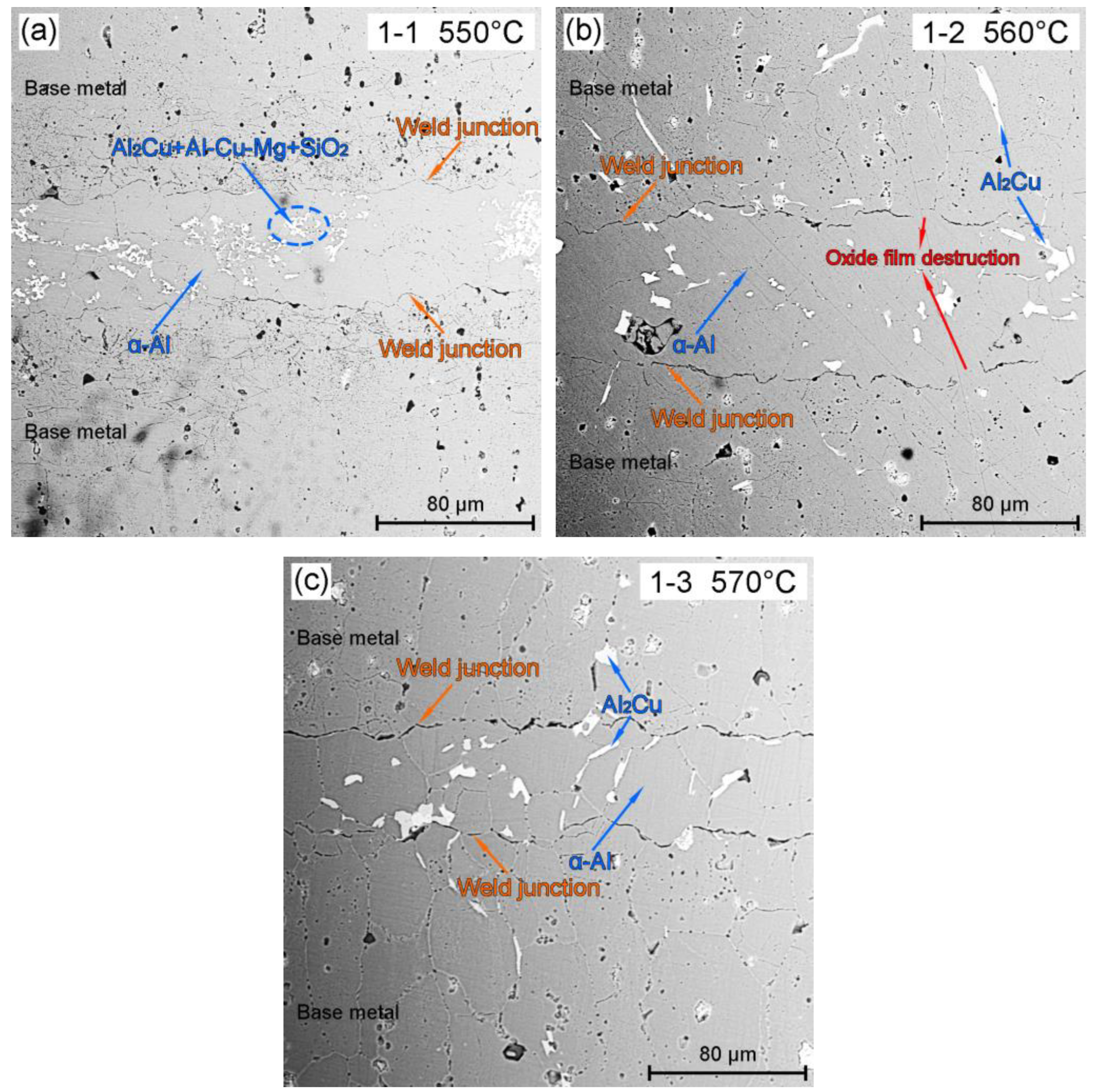
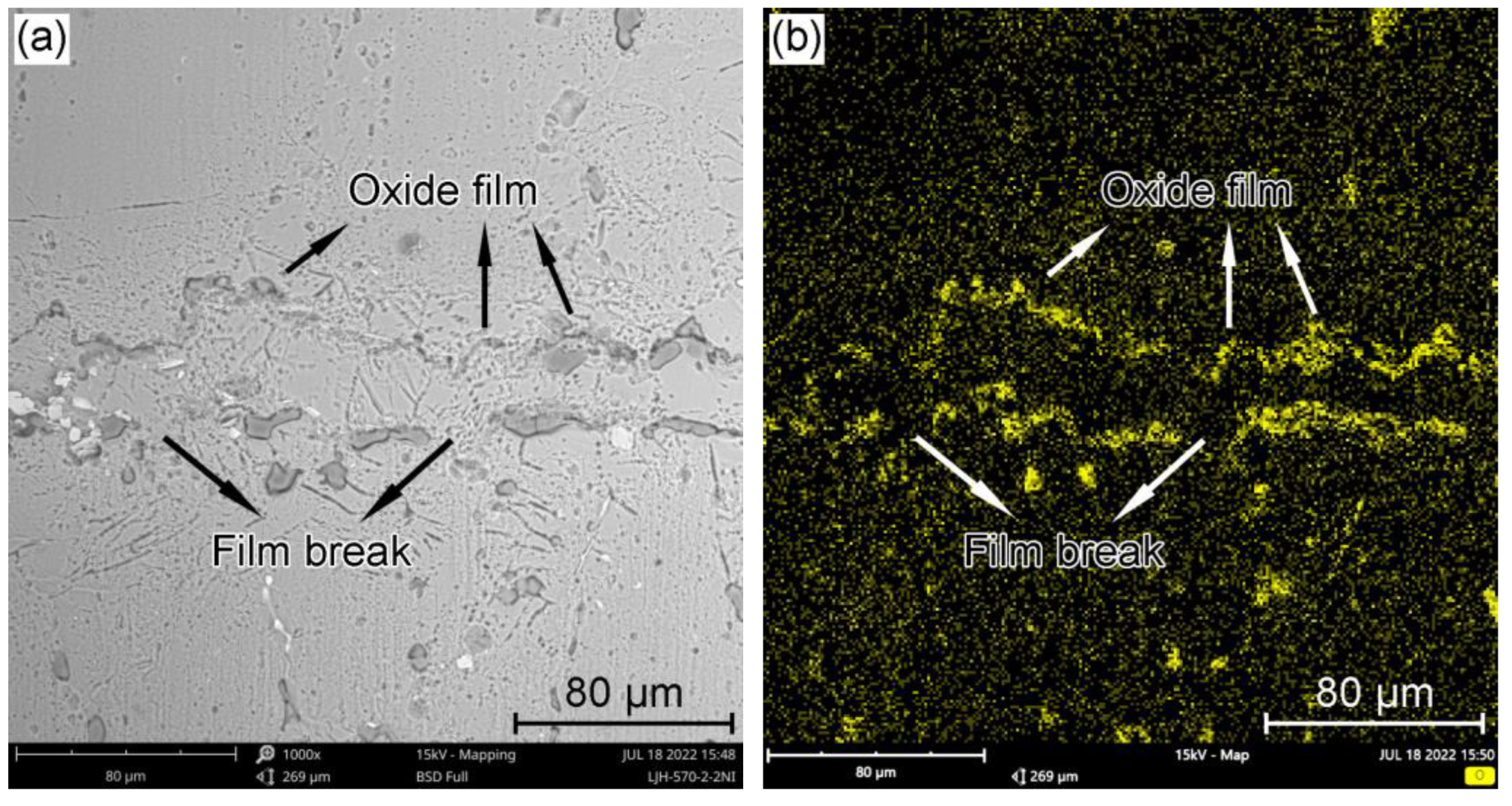
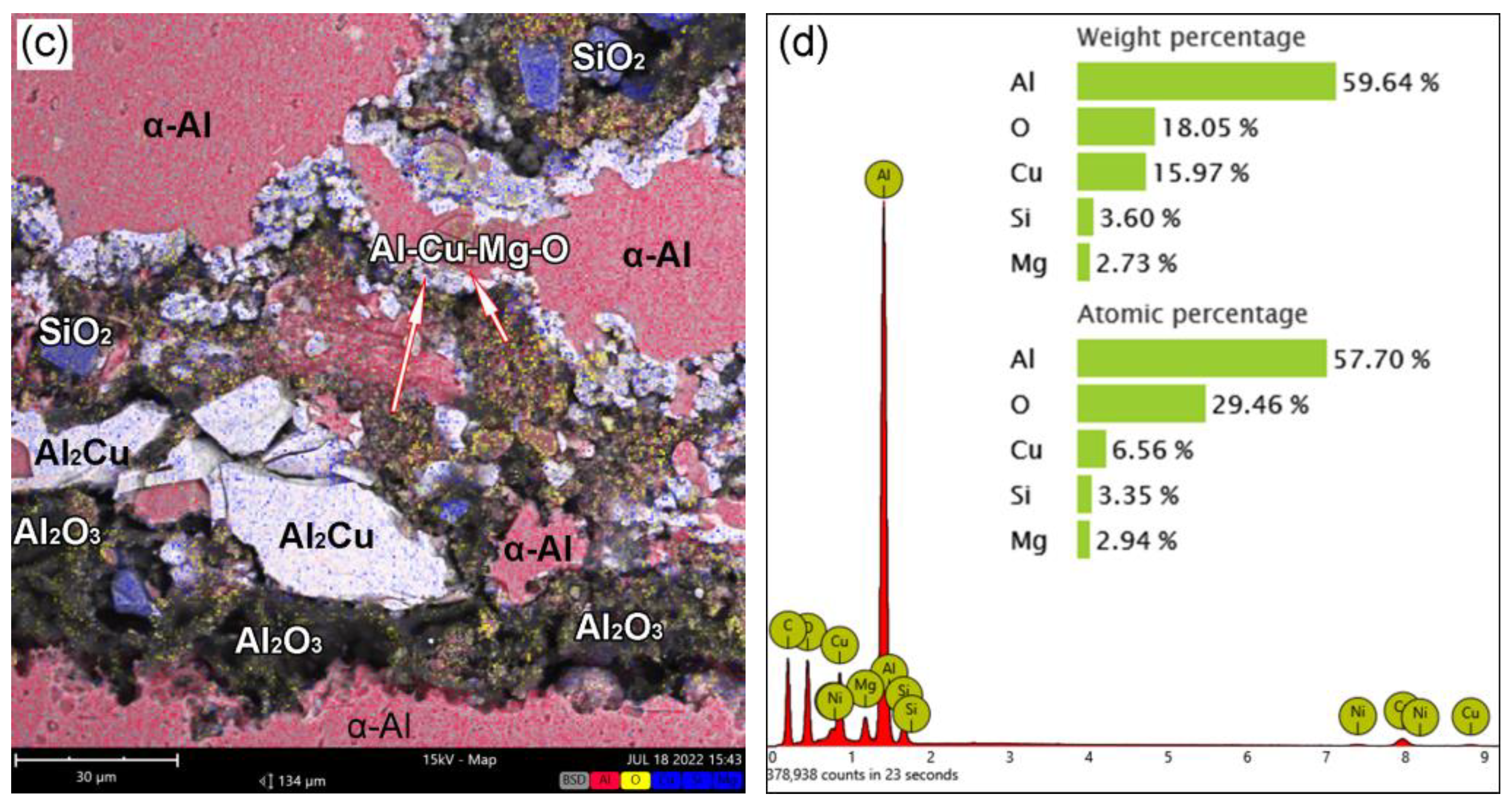
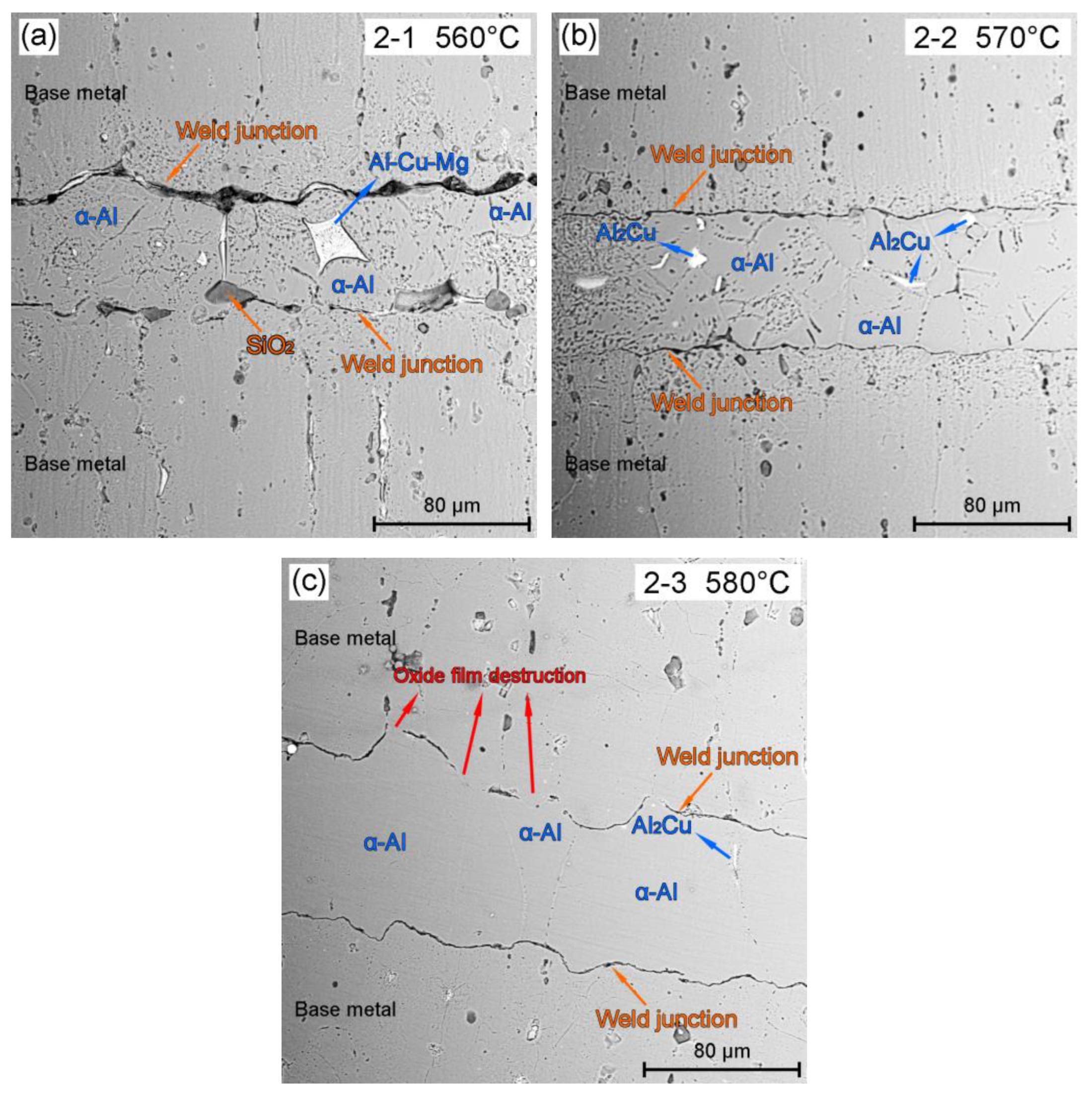
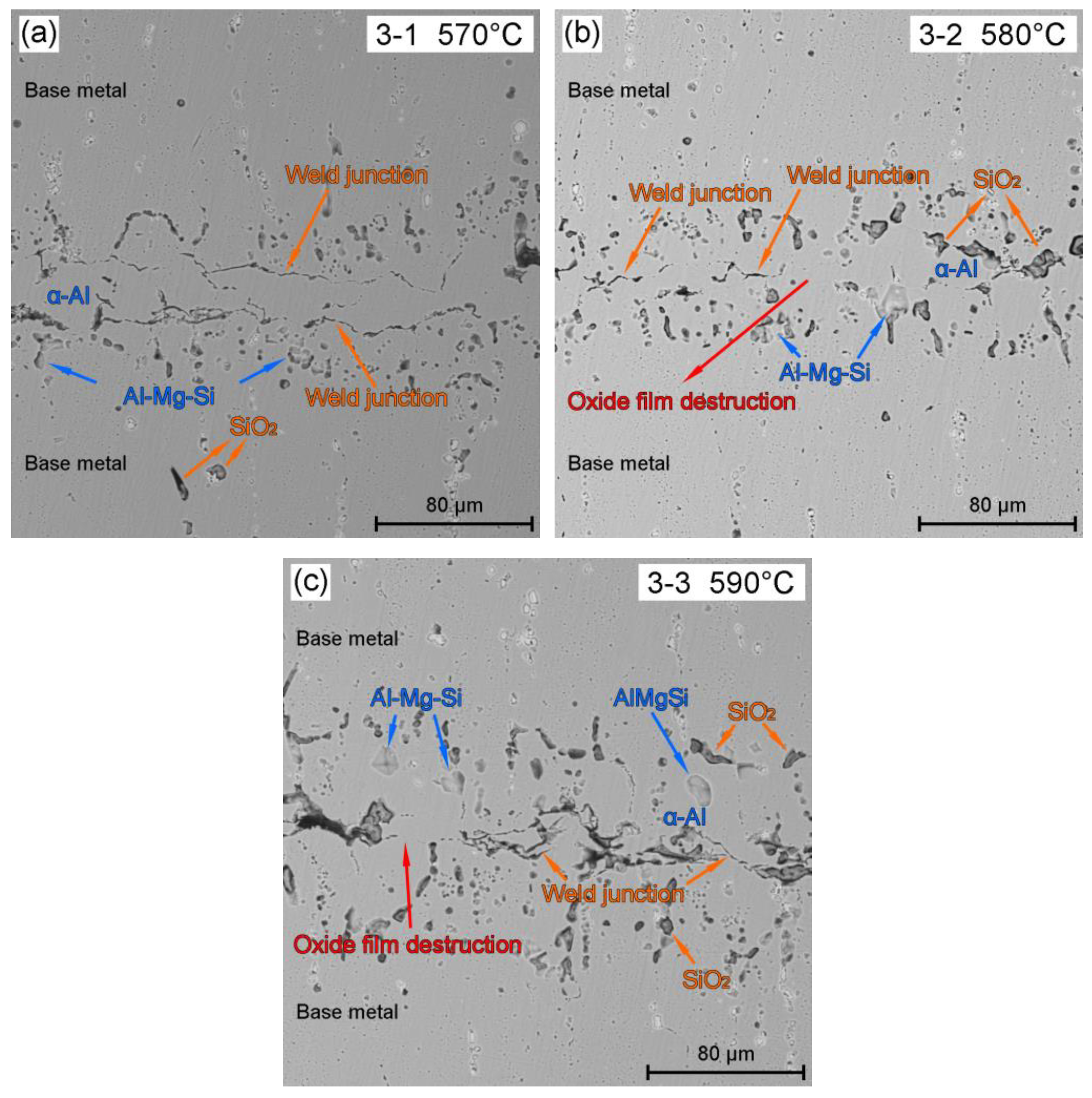
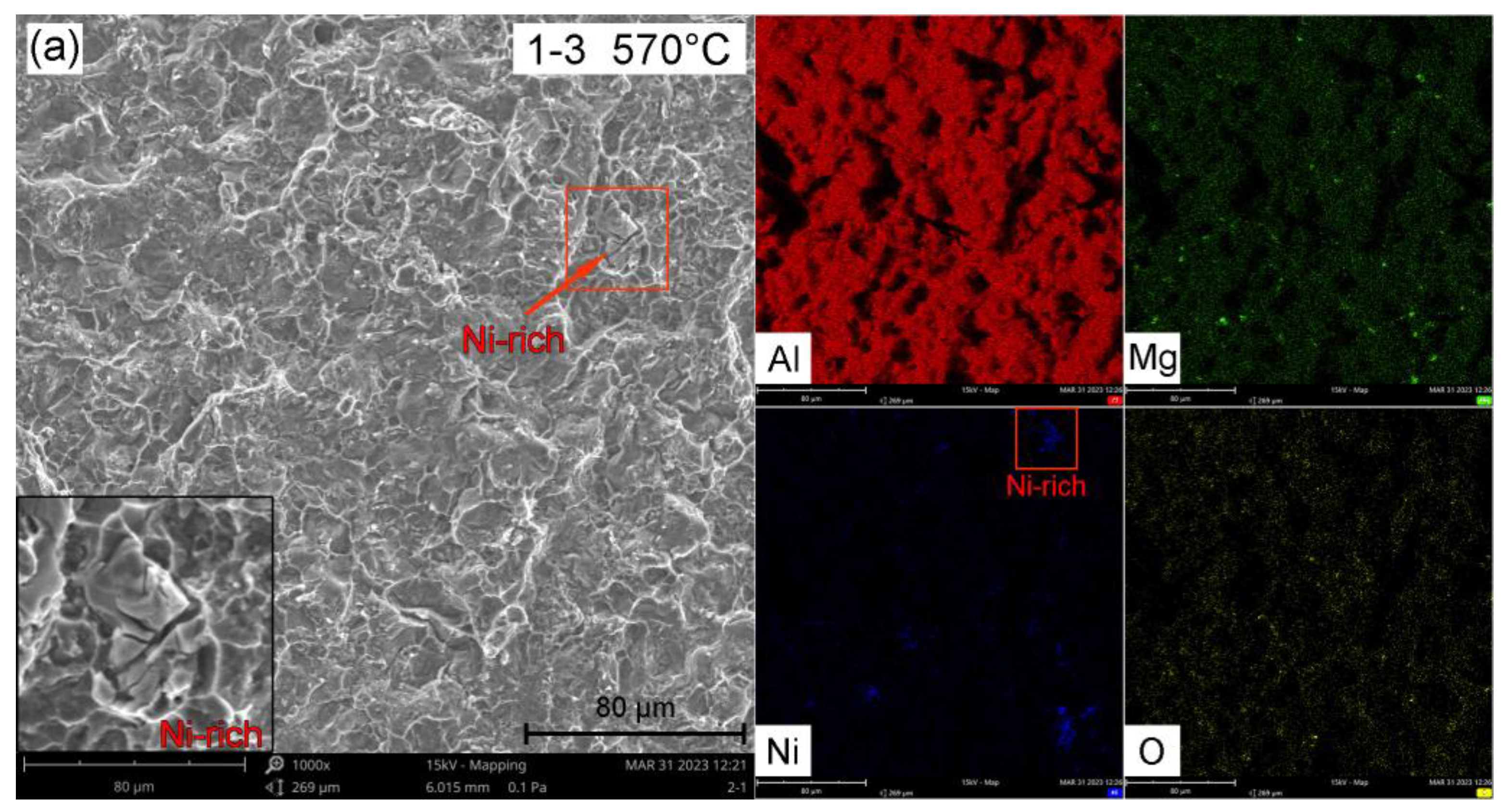
3.1. Effect of BrazingTtemperature on the Microstructures of TG-TLP Joints
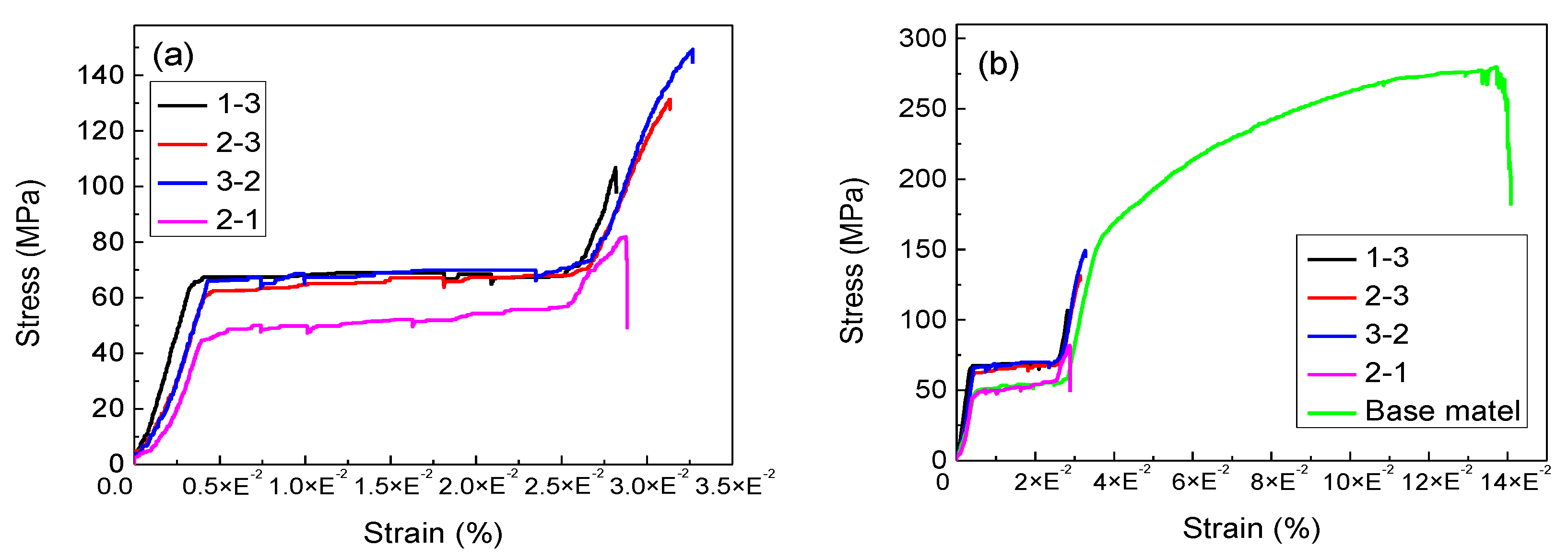
3.2. Effect of Brazing Temperature on the Tensile Properties of TG-TLP Joints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, H.; Li, M.V. Porosity suppressing and grain refining of narrow-gap rotating laser-MIG hybrid welding of 5A06 aluminum alloy. J. Manuf. Process. 2021, 68, 1100–1113. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Ye, Z. An investigation on butt joints of Ti6Al4V and 5A06 using MIG/TIG double-side arc welding-brazing. J. Manuf. Process. 2017, 27, 221–225. [Google Scholar] [CrossRef]
- Wang, Z.; Oliveira, J.P.; Zeng, Z. Laser beam oscillating welding of 5A06 aluminum alloys: Microstructure, porosity and mechanical properties. Opt. Laser. Technol. 2019, 111, 58–65. [Google Scholar] [CrossRef]
- Li, Z.; Ou, L.; Wang, Y.; Li, H. Solidification Cracking Restraining Mechanism of Al-Cu-Mg-Zn Alloy Welds Using Cold Metal Transfer Technique. Materials 2023, 16, 721. [Google Scholar] [CrossRef]
- Yi, T.; Liu, S.; Fang, C. Eliminating hole defects and improving microstructure and mechanical properties of friction stir welded joint of 2519 aluminum alloy via TIG arc. J. Mater. Process. Technol. 2022, 310, 117773. [Google Scholar] [CrossRef]
- Atieh, A.M.; Cooke, K.M.; Epstein, M. Transient liquid phase (TLP) bonding as reaction–controlled diffusion. Mater. Today Commun. 2022, 33, 104293. [Google Scholar] [CrossRef]
- Afghahi, S.S.S.; Ekrami, A.; Farahany, S. Fatigue properties of temperature gradient transient liquid phase diffusion bonded Al7075-T6 alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1073–1079. [Google Scholar] [CrossRef]
- Afghahi, S.S.S.; Jafarian, M.; Paidar, M. Diffusion bonding of Al 7075 and Mg AZ31 alloys: Process parameters, microstructural analysis and mechanical properties. Trans. Nonferrous Met. Soc. China 2016, 26, 1843–1851. [Google Scholar] [CrossRef]
- Fu, Z.H.; Yang, B.J.; Shan, M.L. Hydrogen embrittlement behavior of SUS301L-MT stainless steel laser-arc hybrid welded joint localized zones. Corros. Sci. 2020, 164, 108337. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, Y.L.; Gou, G.Q. Effect of heat input on interfacial characterization of the butter joint of hot-rolling CP-Ti/Q235 bimetallic sheets by Laser + CMT. Sci. Rep. 2021, 11, 10020. [Google Scholar] [CrossRef]
- Yuan, L.; Xiong, J.T.; Ren, J. Ultrastrong and ductile transient liquid phase (TLP) bonding joints reinforced by ordered multi-precipitates. Compos. Part B Eng. 2022, 231, 109568. [Google Scholar] [CrossRef]
- Bobzin, K.; Bosse, L.; Brandenburg, A. Transient Liquid Phase Bonding. In Montage Hybrider Mikrosysteme; Springer: Berlin/Heidelberg, Germany, 2005; pp. 65–78. [Google Scholar] [CrossRef]
- Guo, W.; Leng, X.; Luan, T. Ultrasonic-promoted rapid TLP bonding of fine-grained 7034 high strength aluminum alloys. Ultrason. Sonochem. 2017, 36, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Lai, Z.; Zhang, H. Mechanism of ultrasonic-assisted transient liquid phase bonding of 6061 Al alloy with cladded Zn-Al alloy in air. J. Mater. Process. Technol. 2020, 286, 116823. [Google Scholar] [CrossRef]
- Assadi, H.; Shirzadi, A.A.; Wallach, E.R. Transient liquid phase diffusion bonding under a temperature gradient: Modelling of the interface morphology. Acta Mater. 2001, 49, 31–39. [Google Scholar] [CrossRef]
- Jabbareh, M.A.; Assadi, H. Modelling of microstructure evolution in transient-liquid-phase diffusion bonding under temperature gradient. Scripta Mater. 2009, 60, 780–782. [Google Scholar] [CrossRef]
- Yu, C.H.; Zhu, Y.; Kang, H. Influence of Alloying Element to Property in Al-Si-Cu-Ni Low Melting -Point Solder. Weld. Joi. 2002, 11, 21–36. Available online: https://d.g.wanfangdata.com.cn/Periodical_hj200211007.aspx (accessed on 17 May 2023).
- Chang, S.Y.; Tsao, L.C.; Li, T.Y. Joining 6061 aluminum alloy with Al–Si–Cu filler metals. J. Alloys Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Y.; Zheng, X. Research Progress on Multi-Component Alloying and Heat Treatment of High Strength and Toughness Al–Si–Cu–Mg Cast Aluminum Alloys. Materials 2023, 16, 1065. [Google Scholar] [CrossRef]
- Lah, N.A.C.; Hussin, M.H. Role of Mg in porous oxide scale growth formation on welded Al 6061 (Al-Mg-Si). Mater. Today Proc. 2023, 75, 99–110. [Google Scholar] [CrossRef]
- Ma, C.; Xue, S.; Wang, B. Study on novel Ag-Cu-Zn-Sn brazing filler metal bearing Ga. J. Alloys Compd. 2016, 688, 854–862. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Li, H. Effect of high Cu concentration on the mechanical property and precipitation behavior of Al–Mg–Zn-(Cu) crossover alloys. J. Mater. Res. Technol. 2022, 20, 4585–4596. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, H. Liquation Cracking Susceptibility and Mechanical Properties of 7075 Aluminum Alloy GTAW Joints. Materials 2022, 15, 3651. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Feng, Y.D.; Shen, W.J. Microstructure and mechanical properties of Cu/Al joints brazed using (Cu, Ni, Zr, Er)-modified Al−Si filler alloys. Trans. Nonferrous Met. Soc. China 2022, 32, 3623–3634. [Google Scholar] [CrossRef]
- Wang, Z.J.; Gao, Z.; Wang, P. Investigation on Process of Vacuum Brazing of 6063 Aluminum Alloy. Hot W. Tech. 2019, 48, 72–76. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Liu, Q. Microstructural Characterization of the As-Cast and Homogenized Al-Cu-Mg-Ag Alloy. Materials 2023, 16, 433. [Google Scholar] [CrossRef]
- Yuan, B.; Li, G.; Guo, M. Synergy of Ni micro-alloying and thermomechanical processing in Al-Mg-Si-Cu-Zn-Fe-Mn alloys with enhanced bendability. J. Mater. Res. Technol. 2021, 15, 5059–5077. [Google Scholar] [CrossRef]
- Han, J.Q.; Wang, J.S.; Zhang, M.S. Relationship between amounts of low-melting-point eutectics and hot tearing susceptibility of ternary Al−Cu−Mg alloys during solidification. Trans. Nonferrous Met. Soc. China 2020, 30, 2311–2325. [Google Scholar] [CrossRef]
- Deng, H.B.; Chen, Y.H.; Jia, Y.L. Microstructure and mechanical properties of dissimilar NiTi/Ti6Al4V joints via back-heating assisted friction stir welding. J Manuf. Process. 2021, 64, 379–391. [Google Scholar] [CrossRef]
- Chen, Y.H.; Sun, S.W.; Zhang, T.M. Effects of post-weld heat treatment on the microstructure and mechanical properties of laser-welded NiTi/304SS joint with Ni filler. Mat. Sci. Eng. A 2020, 771, 138545. [Google Scholar] [CrossRef]
- Chen, Y.H.; Mao, Y.Q.; Lu, W.W. Investigation of welding crack in micro laser welded NiTiNb shape memory alloy and Ti6Al4V alloy dissimilar metals joints. Opt. Laser Technol. 2017, 91, 197–202. [Google Scholar] [CrossRef]
- Sun, D.Q.; Cu, X.Y.; Liu, W.H. Transient liquid phase bonding of magnesium alloy (Mg–3Al–1Zn) using aluminium interlayer. Mat. Sci. Eng. A 2005, 391, 29–33. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Mosallanejad, M.H.; Atapour, M. Hybrid additive manufacturing of an electron beam powder bed fused Ti6Al4V by transient liquid phase bonding. J. Mater. Res. Technol. 2022, 20, 180–194. [Google Scholar] [CrossRef]
- Fang, J.X.; Wang, J.X.; He, H.T. Microstructure evolution and deformation behavior during stretching of a compositionally inhomogeneous TWIP-TRIP cantor-like alloy by laser powder deposition. Mat. Sci. Eng. A 2022, 847, 143319. [Google Scholar] [CrossRef]
- García-García, V.; Mejía, I.; Hernández-Belmontes, H. Computational simulation of thermo-mechanical field and fluid flow and their effect on the solidification process in TWIP steel welds. J. Manuf. Process. 2022, 84, 100–120. [Google Scholar] [CrossRef]
- Tan, C.; Zang, C.; Xia, H. Influence of Al additions in Zn–based filler metals on laser welding–brazing of Al/steel. J. Manuf. Process. 2018, 34, 251–263. [Google Scholar] [CrossRef]

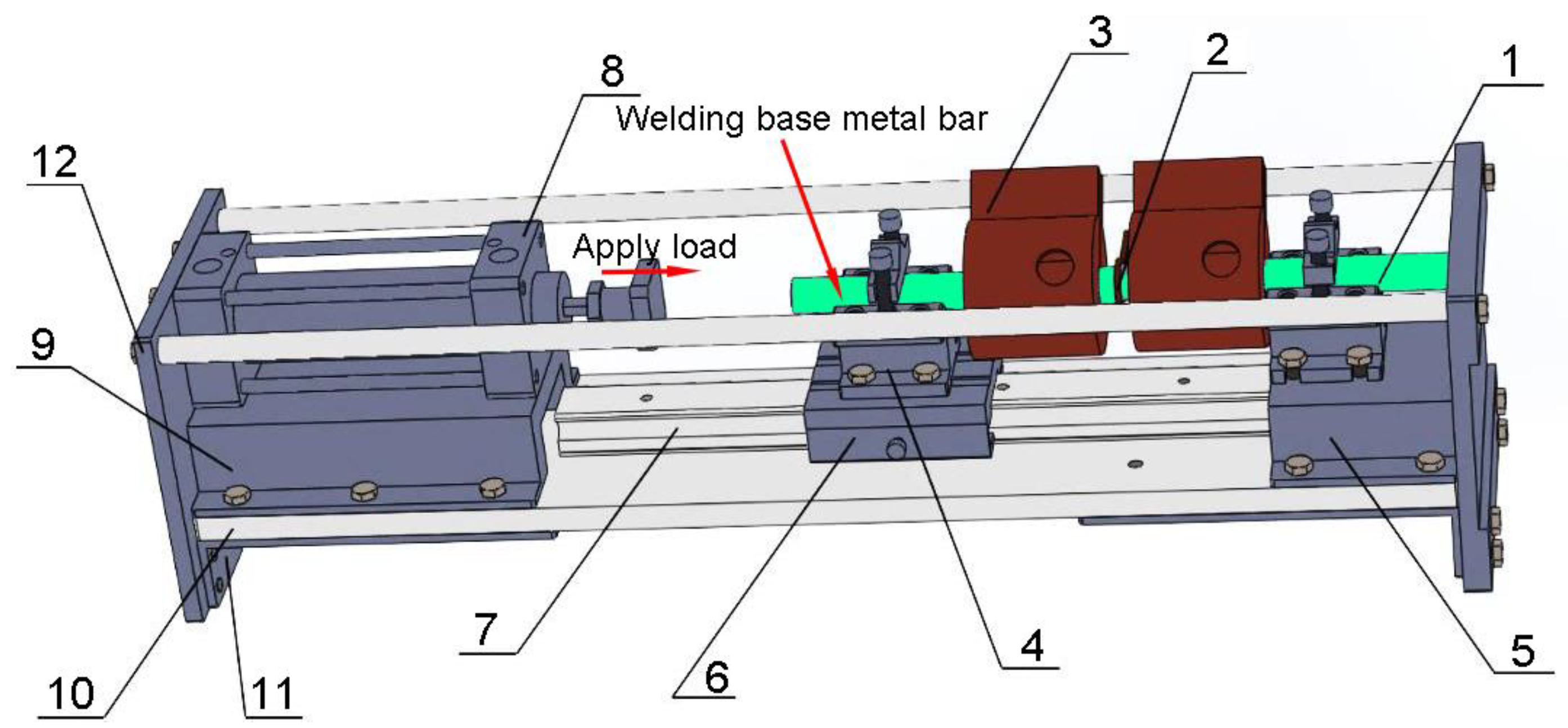
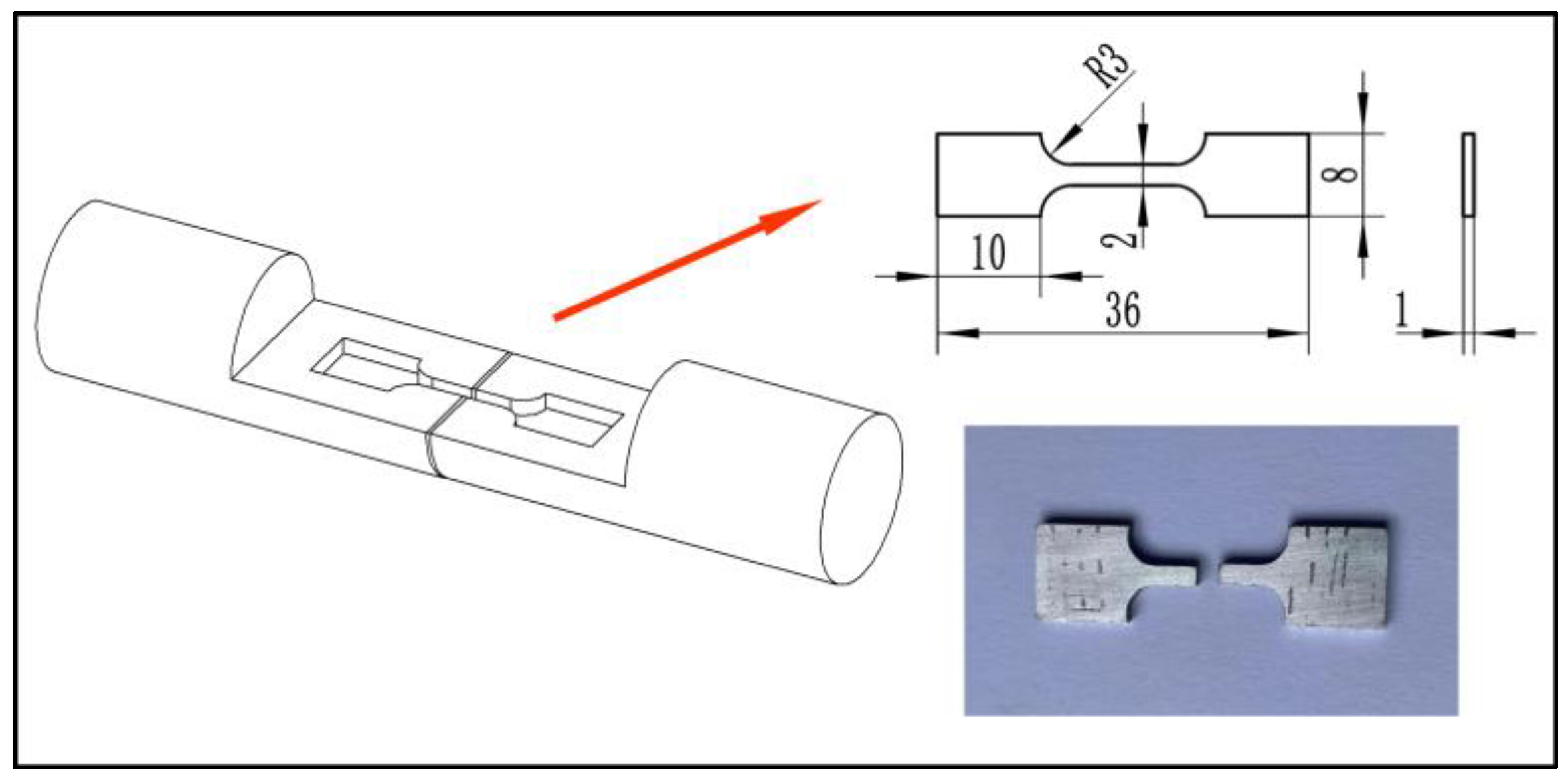




| Composition | Si | Fe | Cu | Mn | Mg | Al | Zn | Ti | Ni | Ga |
|---|---|---|---|---|---|---|---|---|---|---|
| 5A06 | ≤0.40 | 0~0.4 | ≤0.10 | 0.5 ~0.8 | 5.8 ~6.8 | Bal. | ≤0.10 | 0.02 ~0.10 | – | – |
| 1# solder | 5.5 ~6.0 | – | 19.0 ~20.0 | – | – | Bal. | – | – | 2.0 ~2.5 | – |
| 2# solder | 9.0 ~10.0 | – | 9.0 ~10.0 | – | 2.5 ~3.0 | Bal. | – | – | – | 1.0 ~1.2 |
| 3# solder | 9.0 ~10.0 | – | 5.0 ~6.0 | – | 1.5 ~2.0 | Bal. | 9.0 ~10.0 | – | – | – |
| No. | Composition (wt.%) | Brazing Temperature (°C) |
|---|---|---|
| 1-1 | Al-20Cu-6Si-2Ni | 550 |
| 1-2 | Al-20Cu-6Si-2Ni | 560 |
| 1-3 | Al-20Cu-6Si-2Ni | 570 |
| 2-1 | Al-10Cu-10Si-3Mg-1Ga | 560 |
| 2-2 | Al-10Cu-10Si-3Mg-1Ga | 570 |
| 2-3 | Al-10Cu-10Si-3Mg-1Ga | 580 |
| 3-1 | Al-6Cu-10Si-2Mg-10Zn | 570 |
| 3-2 | Al-6Cu-10Si-2Mg-10Zn | 580 |
| 3-3 | Al-6Cu-10Si-2Mg-10Zn | 590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Q.; Xia, P.; Lin, T.; Zhang, C.; Zhou, N.; Huang, Y. Effect of Brazing Temperature on Microstructure, Tensile Strength, and Oxide Film-Breaking Synergy of 5A06 Aluminum Alloy Welded by TG-TLP. Metals 2023, 13, 1048. https://doi.org/10.3390/met13061048
Chen Y, Liu Q, Xia P, Lin T, Zhang C, Zhou N, Huang Y. Effect of Brazing Temperature on Microstructure, Tensile Strength, and Oxide Film-Breaking Synergy of 5A06 Aluminum Alloy Welded by TG-TLP. Metals. 2023; 13(6):1048. https://doi.org/10.3390/met13061048
Chicago/Turabian StyleChen, Yi, Qiang Liu, Peiyun Xia, Tiesong Lin, Chengcong Zhang, Nengtao Zhou, and Yongde Huang. 2023. "Effect of Brazing Temperature on Microstructure, Tensile Strength, and Oxide Film-Breaking Synergy of 5A06 Aluminum Alloy Welded by TG-TLP" Metals 13, no. 6: 1048. https://doi.org/10.3390/met13061048
APA StyleChen, Y., Liu, Q., Xia, P., Lin, T., Zhang, C., Zhou, N., & Huang, Y. (2023). Effect of Brazing Temperature on Microstructure, Tensile Strength, and Oxide Film-Breaking Synergy of 5A06 Aluminum Alloy Welded by TG-TLP. Metals, 13(6), 1048. https://doi.org/10.3390/met13061048





