Hot Corrosion Behavior of Inconel 625 in Na2SO4 and V2O5 Molten Salt System
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Corrosion Method and Weight Loss Measurement
2.3. Surface Characterization Method
3. Results and Discussion
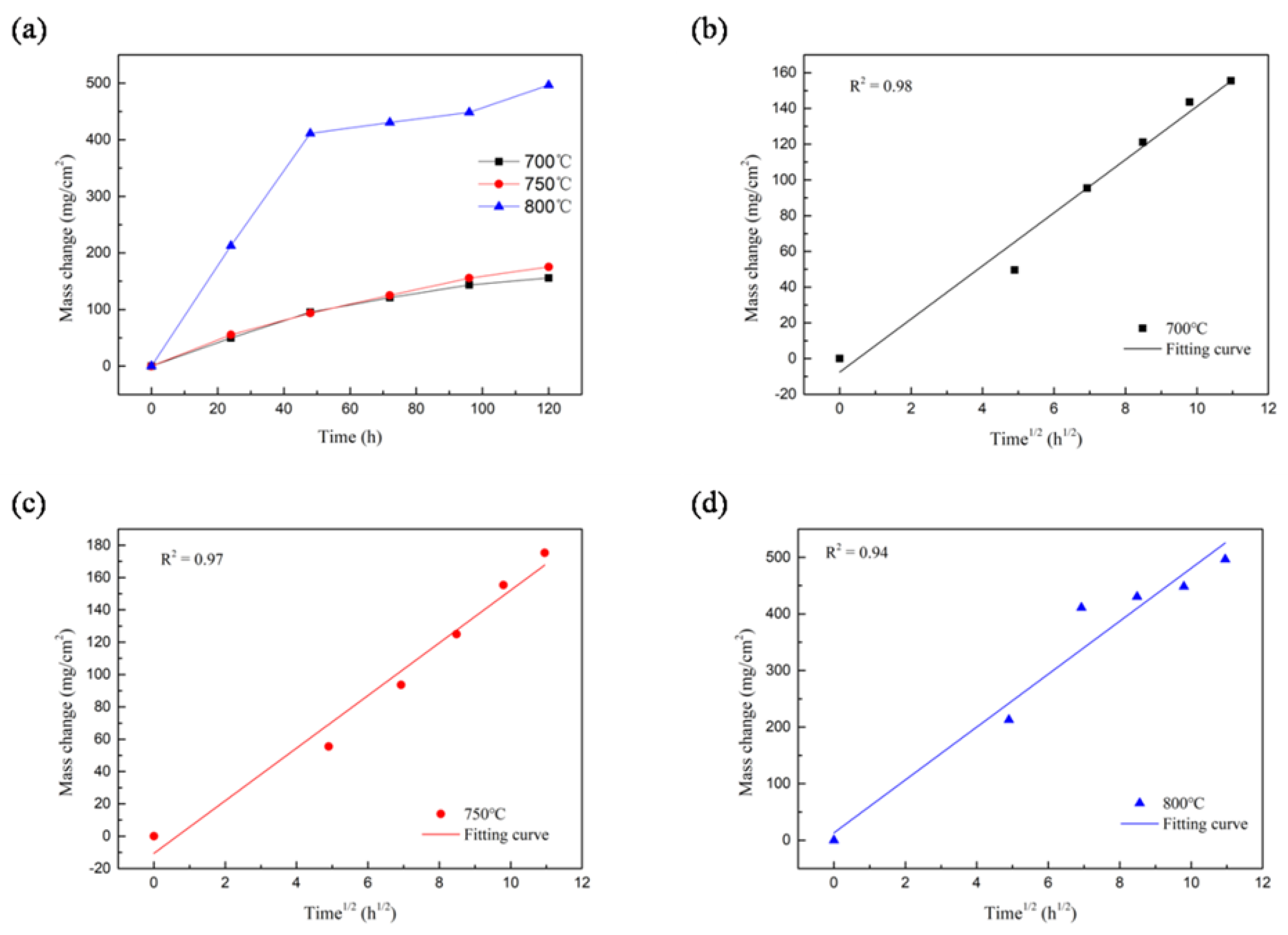
3.1. Evaluation of Hot Corrosion Rate
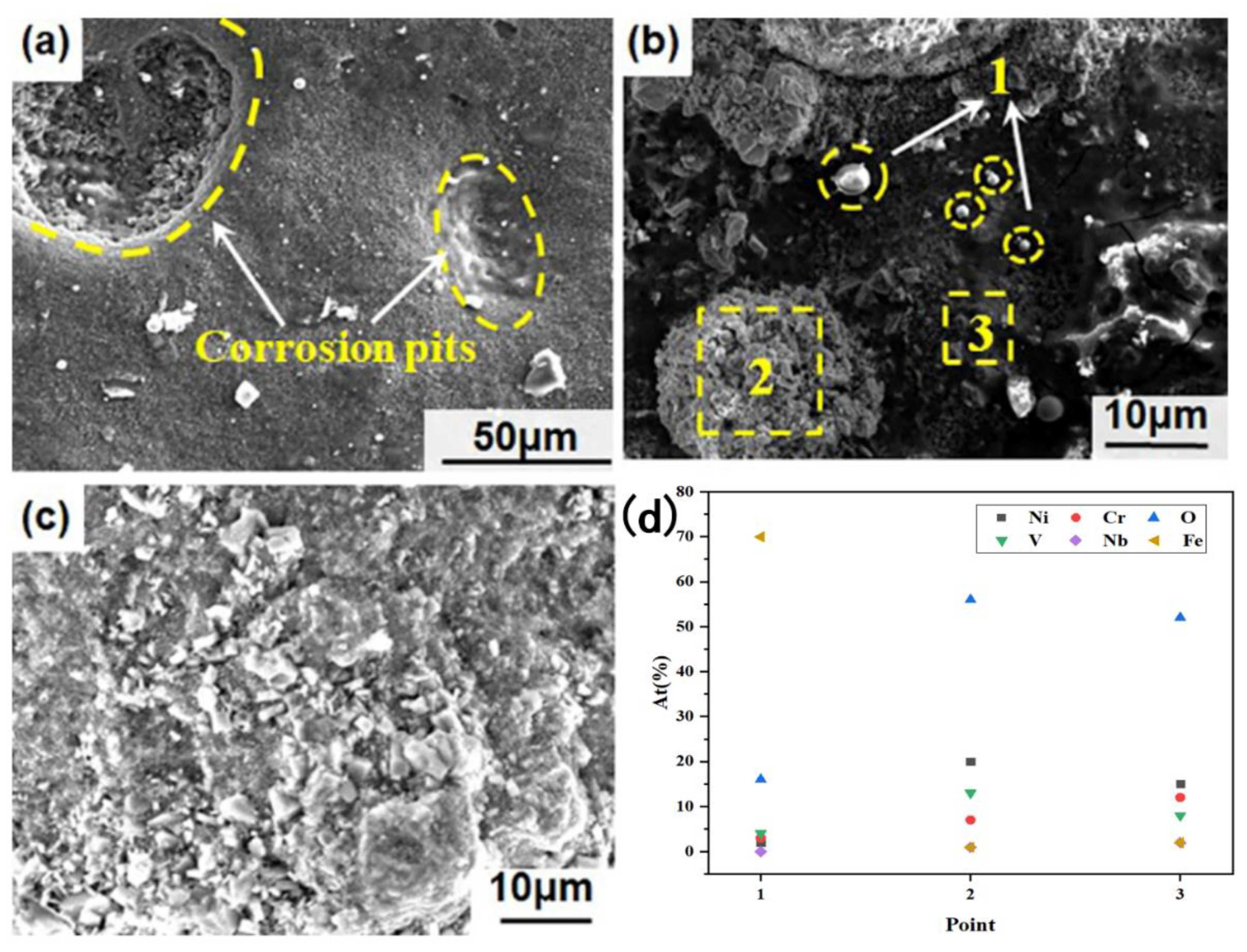
3.2. Hot Corrosion Morphology Analysis
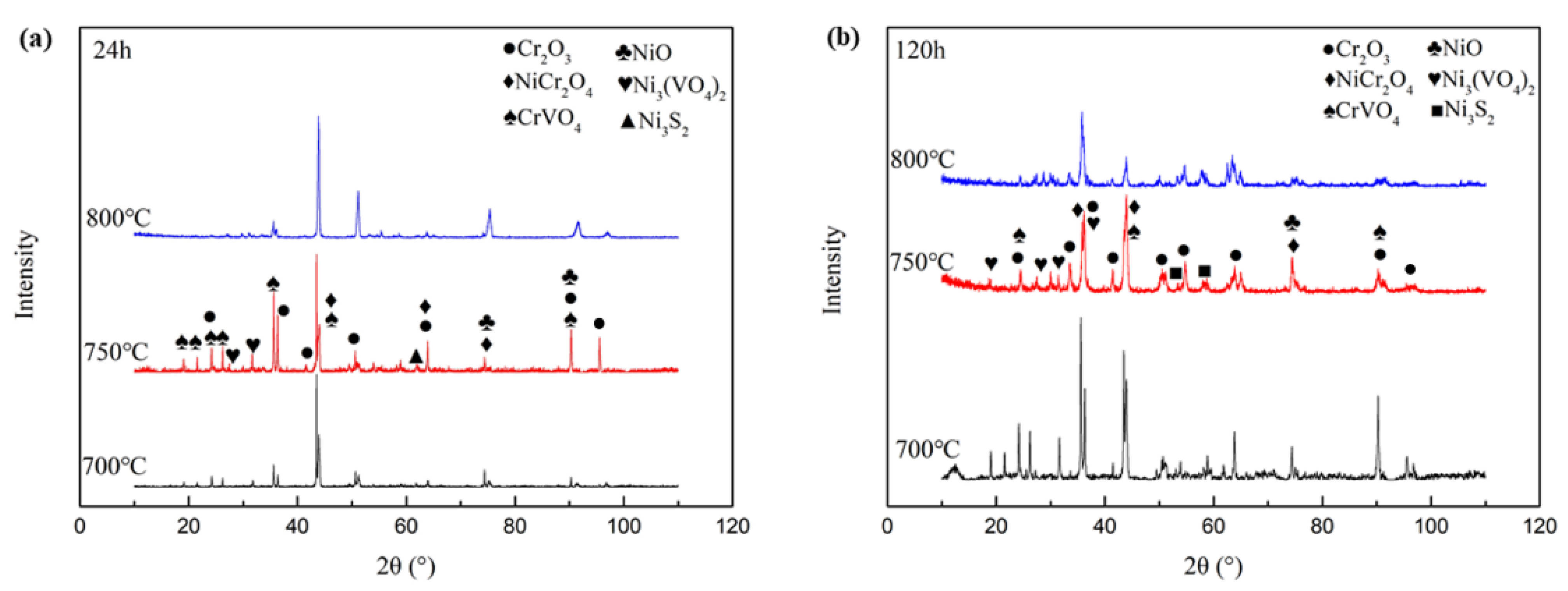
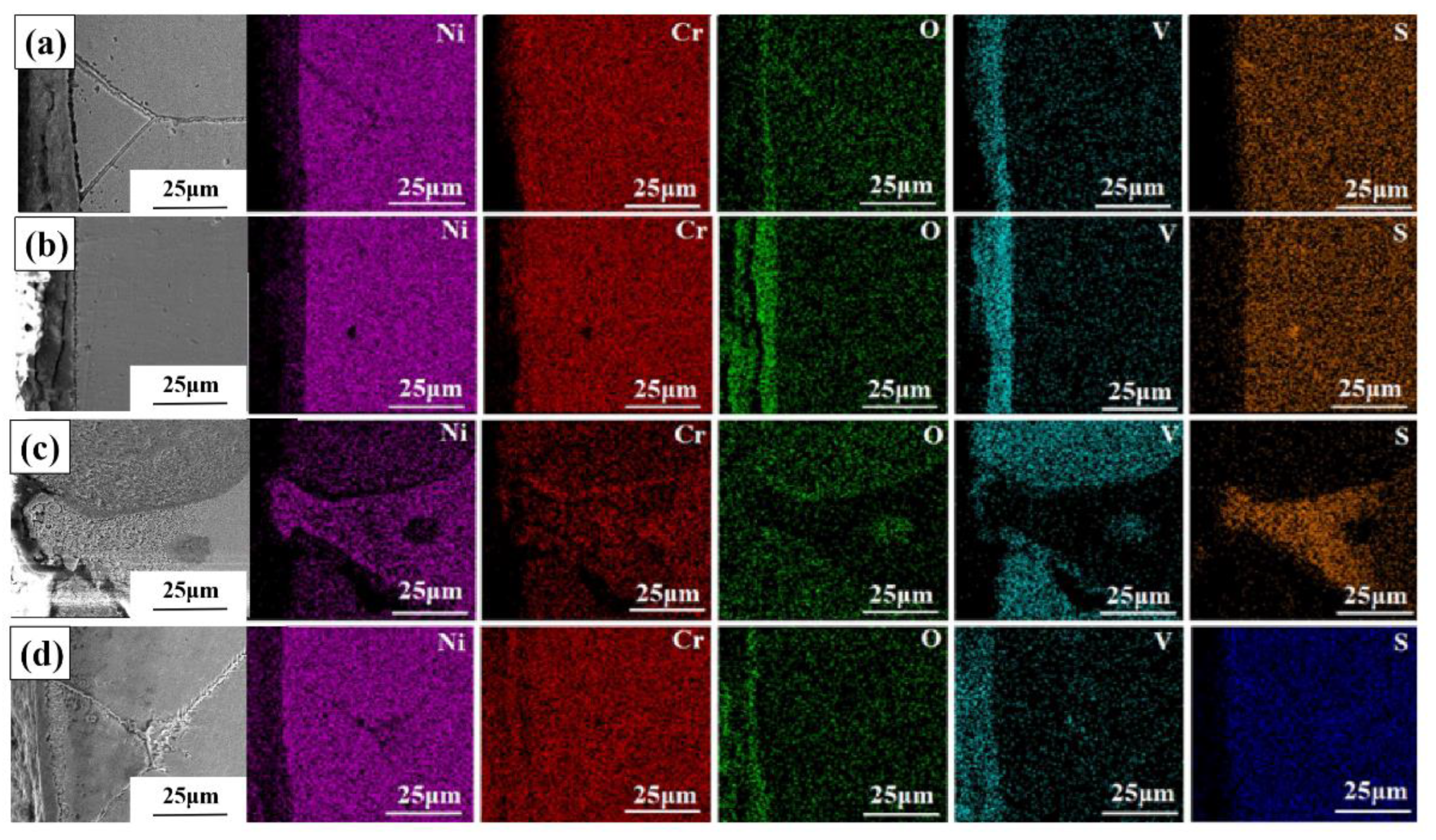
3.3. Hot Corrosion Products Analysis
3.4. Hot Corrosion Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, V. US Program on Materials Technology for Ultra-Supercritical Coal Power Plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.C.; Shang, H.; Shi, Q. Study on the Performance of Microwave Radiation Promoted Removal of Nickel and Vanadium Heavy Metals from Crude Oil. Vac. Electron. 2013, 6, 49–53. [Google Scholar] [CrossRef]
- Luo, L.T.; Gong, X.B. Microstructures and mechanical properties in powder-based additive manufacturing of a nickel-based alloy. Russ. J. Non-Ferr. Met. 2017, 58, 269–275. [Google Scholar] [CrossRef]
- Osoba, L.O.; Oladoye, A.M.; Ogbonna, V.E. Corrosion evaluation of superalloys Haynes 282 and Inconel 718 in hydrochloric acid. J. Alloys Compd. 2019, 804, 376–384. [Google Scholar] [CrossRef]
- Kuang, W.J.; Feng, X.Y.; Han, Y.; Gary, S.W.; Hao, X.C. Insights into the roles of intergranular carbides in the initiation of intergranular stress corrosion cracking of alloy 690 in simulated PWR primary water. Corros. Sci. 2022, 196, 110048. [Google Scholar] [CrossRef]
- Liu, C.P.; Tang, X.; Cheng, L.; Leng, B.; Li, X.L.; Ye, X.X.; Huang, H.F. The characterization of corrosion layers of GH3535 and Inconel 625 alloys in molten KNO3-NaNO3 salts at 500 °C. Corros. Sci. 2022, 204, 110406. [Google Scholar] [CrossRef]
- Huang, M.; Yang, X.Y.; Liu, W.W.; Li, J.R.; Yang, S.; Qin, Y. Precipitation characteristics of a nickel-based single-crystal superalloy after long-term thermal exposure. Int. J. Mater. Res. 2018, 109, 811–818. [Google Scholar] [CrossRef]
- Ramkumar, K.D.; Abraham, W.S.; Viyash, V. Investigations on the microstructure, tensile strength and high temperature corrosion behaviour of Inconel 625 and Inconel 718 dissimilar joints. J. Manuf. Process. 2017, 25, 306–322. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Z.Y.; Zhang, L.S.; Gao, M.R.; Gao, J.; Du, M.K.; Wang, B.Y. Understanding microstructure evolution and corrosion behavior of wire arc cladding inconel 625 Superalloy by thermodynamic approaches. J. Alloys Compd. 2023, 947, 169530. [Google Scholar] [CrossRef]
- Feng, J.H.; Mao, L.; Yuan, G.C.; Zhao, Y.Y.; Vidal, J.; Liu, L. Grain size effect on corrosion behavior of Inconel 625 film against molten MgCl2-NaCl-KCl salt. Corros. Sci. 2022, 197, 110097. [Google Scholar] [CrossRef]
- Santosh, K.; Manoj, K.; Amit, H. Combating hot corrosion of boiler tubes—A study. Eng. Fail. Anal. 2018, 94, 379–395. [Google Scholar] [CrossRef]
- Bornstein, N.S.; Decrescente, M.A. The role of sodium in the accelerated oxidationphenomenon termed sulfidation. Metall. Trans. 1971, 2, 2875–2883. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Xu, Y.W.; Shi, Y.; Su, G.X.; Gu, Y.F.; Volodymyr, K. Intergranular corrosion characteristics of high-efficiency wire laser additive manufactured Inconel 625 alloys. Corros. Sci. 2022, 205, 110422. [Google Scholar] [CrossRef]
- Liu, H.F.; Tan, C.K.I.; Meng, T.L.; Teo, S.L.; Liu, J.Y.; Cao, J.; Wei, Y.F.; Tan, D.C.C.; Lee, C.J.J.; Suwardi, A.; et al. Hot corrosion and internal spallation of laser-cladded inconel 625 superalloy coatings in molten sulfate salts. Corros. Sci. 2021, 193, 109869. [Google Scholar] [CrossRef]
- Garcia-Fresnillo, L.; Chyrkin, A.; Bhme, C.; Barnikel, J.; Quadakkers, W.J. Oxidation behaviour and microstructural stability of Inconel 625 during long-term exposure in steam. J. Mater. Sci. 2014, 49, 6127–6142. [Google Scholar] [CrossRef]
- Pooja, M.; Ravishankar, K.S.; Madav, V. High temperature corrosion behaviour of stainless steels and Inconel 625 in hydroxide salt. Mater. Today 2021, 46, 2612–2615. [Google Scholar] [CrossRef]
- Guo, M.J.; Lu, Y.X.; Zhu, R.Z.; Zheng, X.G. The properties of several Fe based and Ni based alloys at 25Na2SO4-75K2SO4 Corrosion behavior under the action of 4 (wt.%) mixed salt. Oxid Met. 1991, 11, 188–192. [Google Scholar]
- Xu, Z.H.; Guan, B.; Wei, X.L.; Lu, J.F.; Ding, J.; Wang, W.L. Evaluation of microstructure, nanoindentation and corrosion behavior of laser cladded Stellite-6 alloy on Inconel-625 substrate. Sol. Energy 2022, 238, 216–225. [Google Scholar] [CrossRef]
- Lu, X.D.; Tian, S.G.; Chen, T.; Guo, C.A.; Li, G.R. Study on internal oxidation and internal sulfidation behavior of high chromium nickel base alloy during molten sulfate hot corrosion. Rare Metal. Mat. Eng. 2014, 43, 79–84. [Google Scholar]
- Ye, Y.; He, G.Q.; Dai, L.Q.; Yuan, M.F.; Liu, X.S.; Lu, S.Q. Study on the thermal corrosion behavior of Inconel 625 alloy under sulfate condition. Met. Funct. Mater. 2016, 23, 33–38. [Google Scholar] [CrossRef]
- Tiwari, S.N.; Prakash, S. Literature review Magnesiumoxide as inhibitor of hot oil ash corrosion. Mater. Sci. Tech.-Lond. 1998, 14, 467–492. [Google Scholar] [CrossRef]
- Foster, W.K.; Leipold, M.H.; Shevlin, T.S. A Simple Phase Equilibrium Approach to the Problem of Oil-Ash Corrosion. Corrosion 1956, 12, 23–32. [Google Scholar] [CrossRef]
- Cunningham, G.W.; Brasunas, A.D.S. The effects of contamination by vanadium and sodium compounds on the air-corrosion of stainless steel. Corrosion 1956, 12, 35–42. [Google Scholar] [CrossRef]
- ASTM Standards G1-03; Practice for Preparing, Cleaning, And Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011. Available online: https://www.astm.org (accessed on 8 March 2023).
- Wang, L.Y.; Li, H.P.; Liu, Q.Y.; Xu, L.P.; Lin, S.; Zheng, K. Effect of sodium chloride on the electrochemical corrosion of Inconel 625 at high temperature and pressure. J. Alloys Compd. 2017, 703, 523–529. [Google Scholar] [CrossRef]
- Malafaia, A.M.S.; Oliveira, R.B.; Latu-Romain, L.; Wouters, Y.; Baldan, R. Isothermal oxidation of Inconel 625 superalloy at 800 and 1000 °C: Microstructure and oxide layer characterization. Mater. Charact. 2020, 161, 110–121. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C. Sulfate–Vanadate-Induced Corrosion of Different Alloys. Oxid. Met. 2018, 89, 499–516. [Google Scholar] [CrossRef]
- Zahrani, E.M.; Alfantazi, A.M. Hot corrosion of Inconel 625 wrought alloy and weld overlay on carbon steel by gas metal arc welding in 47 PbSO4-23 ZnO-13 Pb3O4-7 PbCl2-5 CdO-5 Fe2O3 molten salt mixture. Corros. Sci. 2021, 183, 109348. [Google Scholar]
- Kumar, M.P.; Manikandan, M. Insights into the surface behavior of Inconel 617 and Inconel 625 material in molten salt. Mater. Let. 2023, 333, 133679. [Google Scholar]
- Lim, Y.S.; Kim, D.J.; Hwang, S.S. M23C6 precipitation behavior and grain boundary serration in Ni-based Alloy 690. Mater. Charact. 2014, 96, 28–39. [Google Scholar] [CrossRef]
- Zahrani, E.M.; Alfantazi, A.M. Molten salt induced corrosion of Inconel 625 superalloy in PbSO4–Pb3O4–PbCl2–Fe2O3–ZnO environment. Corros. Sci. 2012, 65, 340–359. [Google Scholar] [CrossRef]




| C | Cr | Ni | Co | Mo | Al | Ti | Fe | Nb | Si | Mn | S | P | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.042 | 21.77 | 60.63 | 0.19 | 8.79 | 0.21 | 0.40 | 3.68 | 3.75 | 0.12 | 0.2 | 0.0006 | 0.006 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, L.; Zhang, G.; Xue, H.; Cui, M.; Wang, W.; Liu, D. Hot Corrosion Behavior of Inconel 625 in Na2SO4 and V2O5 Molten Salt System. Metals 2023, 13, 1069. https://doi.org/10.3390/met13061069
Li L, Li L, Zhang G, Xue H, Cui M, Wang W, Liu D. Hot Corrosion Behavior of Inconel 625 in Na2SO4 and V2O5 Molten Salt System. Metals. 2023; 13(6):1069. https://doi.org/10.3390/met13061069
Chicago/Turabian StyleLi, Liang, Lanfeng Li, Guofeng Zhang, Hongdi Xue, Maomao Cui, Wenxu Wang, and Dexue Liu. 2023. "Hot Corrosion Behavior of Inconel 625 in Na2SO4 and V2O5 Molten Salt System" Metals 13, no. 6: 1069. https://doi.org/10.3390/met13061069




