Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina
Abstract
1. Introduction
2. Methods
2.1. Corrosion Experiment on Artificial Patina
2.2. Analyses
2.2.1. Analysis of Surface Condition and Form
2.2.2. Analysis of Chromaticity and Reflectance
2.2.3. Analysis of Corrosion Products
2.2.4. Analysis of Microstructure and Composition
3. Results
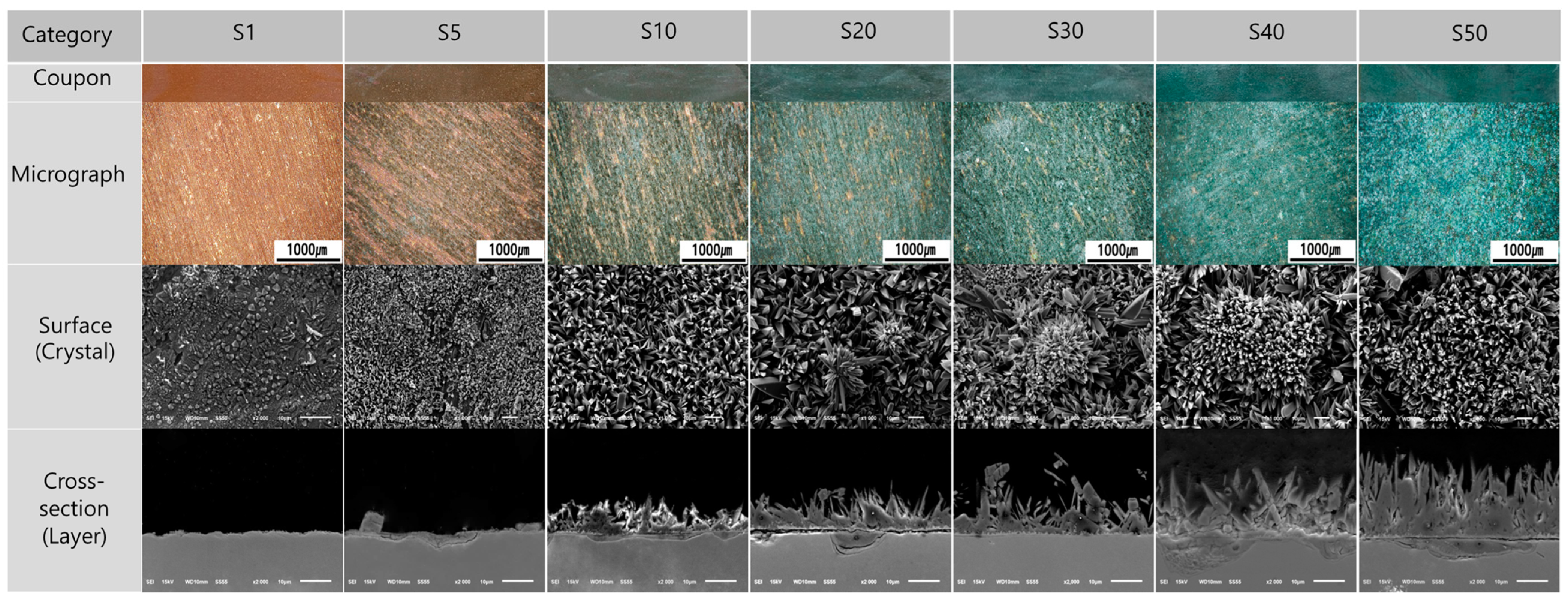
3.1. Microstructure and Material Characteristics of Specimen
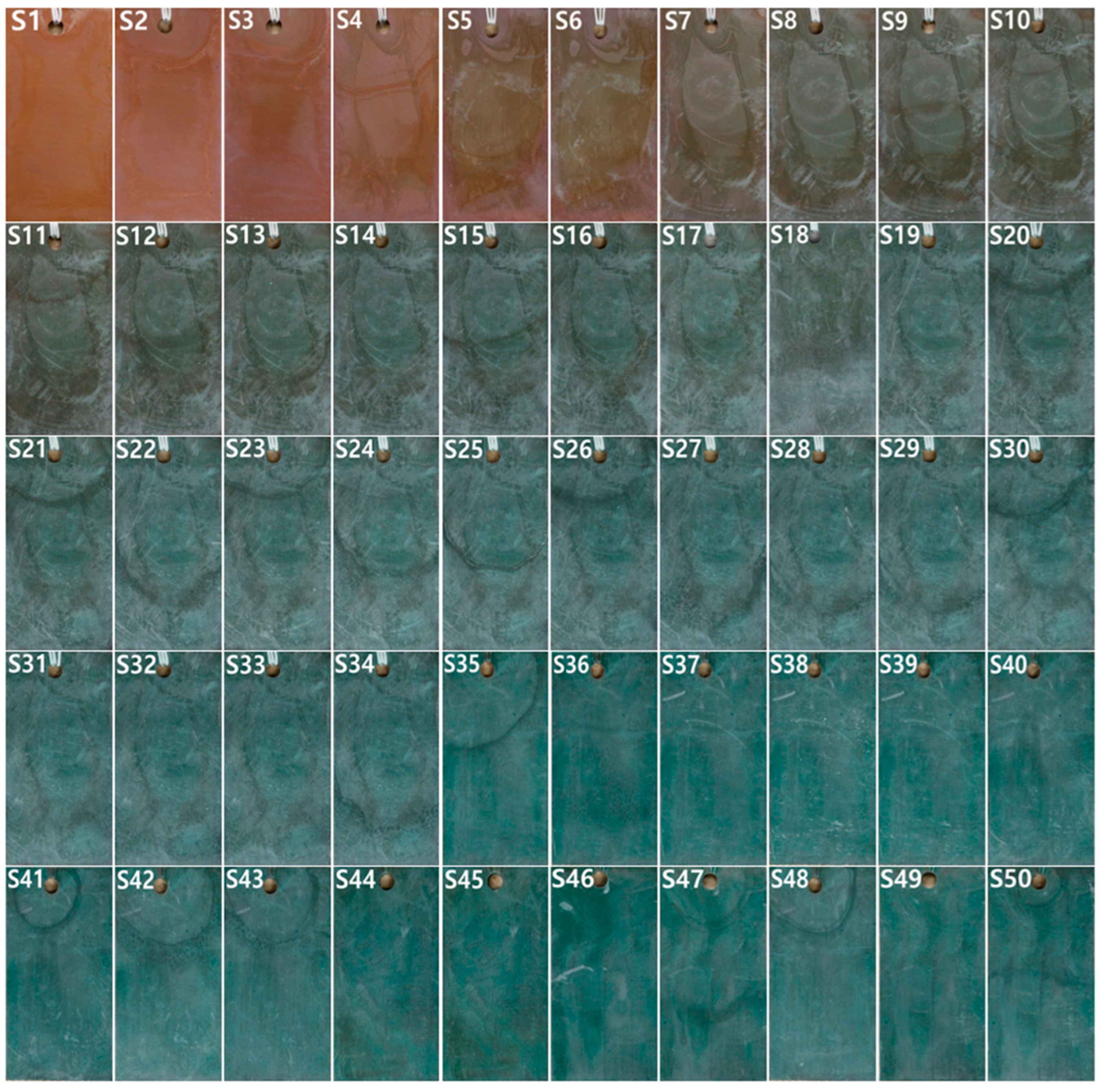
3.2. Surface Form and Condition
3.3. Chromaticity and Reflectance
3.4. Analysis of Corrosion Products
3.5. Microstructure and Composition of Corrosion Products
4. Discussion
4.1. Correlation between Color Classification and Corrosion Products of Sulfide Patina
4.2. Correlation between Composition and Corrosion Products of Sulfide Patina
4.3. Corrosion Mechanism of Sulfide Patina
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buccolieri, G.; Castellano, A.; Serra, A.; Zavarise, G.; Palmiero, E.; Buccolieri, A. Archaeometric analysis of patinas of the out-door copper statue Sant’Oronzo (Lecce, Italy) preparatory to the restoration. Microchem. J. 2020, 154, 104538. [Google Scholar] [CrossRef]
- Buccolieri, G.; Buccolieri, A.; Donati, P.; Marabelli, M.; Castellano, A. Portable EDXRF investigation of the patinas on the Riace Bronzes. Nucl. Instrum. Methods Phys. Res. B 2015, 343, 101–109. [Google Scholar] [CrossRef]
- Young, M.L.; Schnepp, S.; Casadio, F.; Lins, A.; Meighan, M.; Lambert, J.B.; Dunand, D.C. Matisse to Picasso: A compositional study of modern bronze sculptures. Anal. Bioanal. Chem. 2009, 395, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Stenger, J.; Eremin, K.; Khandekar, N.; Budny, V. Gaston Lachaise’s bronze sculpture in the Fogg Museum. J. Am. Inst. Conserv. 2010, 49, 1–26. [Google Scholar] [CrossRef]
- Ganio, M.; Leonard, A.; Plisson, J.S.; Walton, M. From sculptures to foundries: Elemental analysis to determine the prove-nance of modern bronzes. In Proceedings of the 15ème journées d’étude de la SFIIC-ICOMOS, Paris, France, 4–5 December 2014; pp. 136–144. [Google Scholar]
- Young, M.L.; Dunand, D.C. Comparing compositions of modern cast bronze sculptures: Optical emission spectroscopy versus X-ray fluorescence spectroscopy. Miner. Met. Mater. Soc. 2015, 67, 1646–1658. [Google Scholar] [CrossRef]
- Randall, M.; Zycherman, L.; Griffith, R. Conservation of Joan Miró’s bronze sculptures at the Museum of Modern Art. AIC Objects Spec. Group Postprints 2016, 23, 233–255. [Google Scholar]
- Pouyet, E.; Ganio, M.; Motlani, A.; Saboo, A.; Casadio, F.; Walton, M. Casting light on 20th-century Parisian artistic bronze: Insights from compositional studies of sculptures using hand-held X-ray fluorescence spectroscopy. Heritage 2019, 2, 732–748. [Google Scholar] [CrossRef]
- Kwon, H.H. The Corrosion Characteristics and Applicability of Non-Destructive Investigation in the Outdoor Bronze Sculptures. Ph.D. Thesis, Kongju National University, Gongju, Republic of Korea, 2023; pp. 6–43. [Google Scholar]
- Yonehara, M.; Matsui, T.; Kihara, K.; Isono, H.; Kijima, A.; Sugibayashi, T. Experimental relationships between surface roughness, glossiness and color of chromatic colored metals. Mater. Trans. 2004, 45, 1027–1032. [Google Scholar] [CrossRef]
- Radivojević, M.; Pendić, J.; Srejić, A.; Korać, M.; Davey, C.; Benzonelli, A.; Martinón-Torres, M.; Jovanović, N.; Kamberović, Z. Experimental design of the Cu-As-Sn ternary colour diagram. J. Archaeol. Sci. 2018, 90, 106–119. [Google Scholar] [CrossRef]
- Strandberg, H. Reactions of copper patina compounds—I. Influence of some air pollutants. Atmos. Environ. 1998, 32, 3511–3520. [Google Scholar] [CrossRef]
- Krätschmer, A.; Wallinder, I.O.; Leygraf, C. The evolution of outdoor copper patina. Corros. Sci. 2002, 44, 425–449. [Google Scholar] [CrossRef]
- Bongiorno, V.; Campodonico, S.; Caffara, R.; Piccardo, P.; Carnasciali, M.M. Micro-Raman spectroscopy for the characterization of artistic patinas produced on copper-based alloys. J. Raman Spectrosc. 2012, 43, 1617–1622. [Google Scholar] [CrossRef]
- Carlo, G.D.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- Bureš, R.; Rak, P.; Stoulil, J. Long-term outdoor exposure of artificial copper patina based on brochantite. Koroze Ochr. Mater. 2020, 64, 87–94. [Google Scholar] [CrossRef]
- Moon, D.H.; Han, M.S.; Jeong, H.Y.; Go, I.H.; Cho, H.G. Mineral compositions of Korean Dancheong pigment products using quantitative XRD. J. Conserv. Sci. 2016, 32, 403–415. [Google Scholar] [CrossRef]
- Strandberg, H. Perspectives on Sculpture Conservation: Modelling Copper and Bronze Corrosion. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 1997; pp. 7–107. [Google Scholar]
- Kwon, H.H.; Kim, Y.S.; Kim, B.J.; Choi, N.Y.; Park, H.S.; Kim, J.S. A study on conservation and material characteristics of out-door bronze sculpture: Kim Chan Shikʹs ‘Feeling’. J. Conserv. Sci. 2017, 33, 155–165. [Google Scholar] [CrossRef]
- Masi, G.; Esvan, J.; Josse, C.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Gartner, N.; Kosec, T.; Robbiola, L. Characterization of typical patinas simulating bronze corrosion in outdoor conditions. Mater. Chem. Phys. 2017, 200, 308–321. [Google Scholar] [CrossRef]
- Masi, G.; Bernardi, E.; Martini, C.; Vassura, I.; Skrlep, L.; Fabjan, E.S.; Gartner, N.; Kosec, T.; Josse, C.; Esvan, J.; et al. An innovative multi-component fluoropolymer-based coating on outdoor patinated bronze for Cultural Heritage: Durability and reversibility. J. Cult. Herit. 2020, 45, 122–134. [Google Scholar] [CrossRef]
- Scott, D.A. Metallography and Microstructure of Ancient and Historic Metals; The Getty Conservation Institute: Los Angeles, CA, USA, 1991; pp. 2–10. [Google Scholar]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Graedel, T.E.; Nassau, K.; Franey, J.P. Copper patinas formed in the atmosphere—I. Introduction. Corros. Sci. 1987, 27, 639–657. [Google Scholar] [CrossRef]
- Franey, J.P.; Davis, M.E. Metallographic studies of the copper patina formed in the atmosphere. Corros. Sci. 1987, 27, 659–668. [Google Scholar] [CrossRef]
- Ropret, P.; Kosec, T. Raman investigation of artificial patinas on recent bronze—Part I: Climatic chamber exposure. J. Raman Spectrosc. 2012, 43, 1578–1586. [Google Scholar] [CrossRef]
- Kosec, T.; Ropret, P.; Legat, A. Raman investigation of artificial patinas on recent bronze—Part II: Urban rain exposure. J. Raman Spectrosc. 2012, 43, 1587–1595. [Google Scholar] [CrossRef]
- Catelli, E.; Sciutto, G.; Prati, S.; Jia, Y.; Mazzeo, R. Characterization of outdoor bronze monument patinas: The potentialities of near-infrared spectroscopic analysis. Environ. Sci. Pollut. Res. 2018, 25, 24379–24393. [Google Scholar] [CrossRef]
- Crippa, M.; Bongiorno, V.; Piccardo, P.; Carnasciali, M.M. A characterisation study on modern bronze sculpture: The artistic patinas of Nado Canuti. Stud. Conserv. 2019, 64, 16–23. [Google Scholar] [CrossRef]
- Piccardo, P.; Mille, B.; Robbiola, L. Tin and copper oxides in corroded archaeological bronzes. In Corrosion of Metallic Heritage Artefacts; Woodhead Publishing: Sawston, UK, 2007; pp. 239–262. [Google Scholar]
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 162–169. [Google Scholar] [CrossRef]
- Oudbashi, O. Multianalytical study of corrosion layers in some archaeological copper alloy artefacts. Surf. Interface Anal. 2015, 47, 1138–1146. [Google Scholar] [CrossRef]
- Sebar, L.E.; Iannucci, L.; Gori, C.; Re, A.; Parvis, M.; Angelini, E.; Grassini, S. In-situ multi-analytical study of ongoing corrosion processes on bronze artworks exposed outdoors. ACTA IMEKO 2021, 10, 241–249. [Google Scholar] [CrossRef]
- Basso, E.; Pozzi, F.; Reiley, M.C. The Samuel, F.B. Morse statue in Central Park: Scientific study and laser cleaning of a 19th century American outdoor bronze monument. Herit. Sci. 2020, 8, 7–12. [Google Scholar] [CrossRef]
- Catelli, E.; Randeberg, L.L.; Strandberg, H.; Alsberg, B.K.; Maris, A.; Vikki, L. Can hyperspectral imaging be used to map corrosion products on outdoor bronze sculptures? J. Spectr. Imaging 2018, 7, a10. [Google Scholar] [CrossRef]
- Leygraf, C.; Chang, T.; Herting, G.; Wallinder, I.O. The origin and evolution of copper patina colour. Corros. Sci. 2019, 157, 337–346. [Google Scholar] [CrossRef]
- Chiavari, C.; Rahmouni, K.; Takenouti, H.; Joiret, S.; Vermaut, P.; Robbiola, L. Composition and electrochemical properties of natural patinas of outdoor bronze monuments. Electrochim. Acta 2007, 52, 7760–7769. [Google Scholar] [CrossRef]
- Robbiola, L.; Rahmouni, K.; Chiavari, C.; Martini, C.; Prandstraller, D.; Texier, A.; Takenouti, H.; Vermaut, P. New insight into the nature and properties of pale green surfaces of outdoor bronze monuments. Appl. Phys. A 2008, 92, 161–169. [Google Scholar] [CrossRef]
- Papadopoulou, O.; Delagrammatikas, M.; Vassiliou, P.; Grassini, S.; Angelini, E.; Gouda, V. Surface and interface investigation of electrochemically induced corrosion on a quaternary bronze. Surf. Interface Anal. 2014, 46, 771–775. [Google Scholar] [CrossRef]
- Fitzgerald, K.P.; Nairn, J.; Atrens, A. Surface characterization of artificial corrosion layers on copper alloy reference materials. Appl. Surf. Sci. 2002, 189, 90–101. [Google Scholar]
- Chiavari, C.; Bernardi, E.; Martini, C.; Passarini, F.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The action of stagnant rain water. Corros. Sci. 2010, 52, 3002–3010. [Google Scholar] [CrossRef]
- Robbiola, L.; Hurtel, L.P. New contribution to the study of corrosion mechanisms of outdoor bronzes: Characterization of the corroding surfaces of Rodin’s bronzes. Mem. Etudes Sci. Rev. Metall. 1991, 88, 809–823. [Google Scholar]
- Robbiola, L.; Fiaud, C.; Pennec, S. New model of outdoor bronze corrosion and its implications for conservation. In ICOM Committee for Conservation 10th Triennial Meeting; HAL Open Science: Lyon, France, 1993; pp. 796–801. [Google Scholar]
- Strandberg, H.; Johansson, G.; Rosvall, J. Outdoor bronze sculptures—A conservation view on the examination of the state of preservation. In Proceedings of the ICOM Committee for Conservation 11th Triennial Meeting, Edinburgh, Scotland, 1–6 September 1996; pp. 894–900. [Google Scholar]
- Selwyn, L.S.; Binnie, N.E.; Poitras, J.; Laver, M.E.; Downham, D.A. Outdoor bronze statues: Analysis of metal and surface samples. Stud. Conserv. 1996, 41, 205–228. [Google Scholar]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants and Conservation; Getty Publication: Los Angeles, CA, USA, 2002; pp. 43–59, 81–99, 122–167. [Google Scholar]
- Fuente, D.; Simancas, J.; Morcillo, M. Morphological study of 16-year patinas formed on copper in a wide range of atmospheric exposures. Corros. Sci. 2008, 50, 268–285. [Google Scholar] [CrossRef]
- Gianni, L. Corrosion Behavior of Bronze Alloys Exposed to Urban and Marine Environment: An Innovative Approach to Corrosion Process Understanding and to Graphical Results Presentation. Ph.D. Thesis, University of Ghent, Ghent, Belgium, 2011; pp. 7–27. [Google Scholar]












| Composition (wt%) | ||||
|---|---|---|---|---|
| Cu | Zn | Sn | Pb | Total |
| 88.8 | 5.1 | 3.1 | 3 | 100 |
| Patina | Sulfide Patina | |
|---|---|---|
| Category | ||
| Corrosive solution | Aqueous solution of 50 mM CuSO4 (in deionized water) | |
| Specimen fabrication | 50 cycles of (24 h deposition → 24 h natural drying) | |
| No. | Position | Composition (wt%) | Layer | |||||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Sn | Pb | O | S | |||
| S1 | 1 | 88.80 | 7.97 | 2.93 | 0.12 | - | - | bronze |
| 2 | 80.11 | 1.90 | 1.46 | 0.90 | 15.40 | 0.23 | cuprite | |
| 3 | 79.01 | 1.11 | 1.69 | 0.69 | 16.87 | 0.63 | ||
| 4 | 77.76 | 0.94 | 1.2 | 0.51 | 19.57 | - | ||
| S5 | 1 | 88.58 | 7.90 | 3.00 | 0.12 | 0.40 | - | bronze |
| 2 | 63.94 | 1.83 | 12.26 | 0.80 | 20.30 | 0.87 | cuprite, cassiterite | |
| 3 | 64.93 | 1.04 | 12.07 | 0.97 | 19.98 | 1.00 | ||
| 4 | 69.75 | 2.96 | 8.54 | 0.97 | 17.06 | 0.71 | ||
| 5 | 61.01 | 0.97 | 12.90 | 0.95 | 23.54 | 0.63 | ||
| 6 | 78.98 | 1.94 | 2.69 | 0.32 | 15.73 | 0.35 | cuprite | |
| 7 | 81.58 | 1.48 | 1.17 | 0.54 | 14.67 | 0.56 | ||
| 8 | 84.23 | 1.39 | 0.98 | 0.52 | 12.87 | - | ||
| 9 | 61.96 | 0.27 | 0.88 | 0.71 | 30.08 | 6.09 | brochantite | |
| 10 | 60.49 | 0.64 | 0.74 | 0.21 | 31.85 | 6.08 | ||
| S20 | 1 | 87.72 | 7.40 | 4.57 | - | 0.31 | - | bronze |
| 2 | 61.71 | 0.10 | 13.51 | 1.47 | 22.46 | 0.75 | cuprite, cassiterite | |
| 3 | 59.53 | 0.45 | 9.07 | 1.39 | 25.76 | 3.80 | ||
| 4 | 83.59 | 0.87 | 0.68 | 0.83 | 14.02 | 0.01 | cuprite | |
| 5 | 84.21 | 0.92 | 0.96 | 0.65 | 13.26 | - | ||
| 6 | 85.27 | 0.91 | 1.17 | 0.15 | 12.51 | - | ||
| 7 | 81.13 | 1.39 | 0.67 | 0.47 | 15.92 | 0.43 | ||
| 8 | 60.96 | - | 0.39 | 1.31 | 28.67 | 8.68 | brochantite | |
| 9 | 61.57 | 0.12 | 0.65 | 1.26 | 28.75 | 7.64 | ||
| 10 | 62.10 | 0.13 | 0.51 | 1.79 | 27.23 | 8.24 | ||
| 11 | 64.62 | 0.03 | 0.56 | 1.15 | 26.27 | 7.37 | ||
| S50 | 1 | 82.10 | 6.80 | 4.14 | 6.11 | 0.84 | - | bronze |
| 2 | 36.05 | 0.58 | 29.42 | 1.95 | 29.60 | 2.39 | cassiterite | |
| 3 | 47.90 | - | 13.35 | 2.05 | 27.91 | 8.79 | ||
| 4 | 55.71 | 0.04 | 6.07 | 1.37 | 27.59 | 9.23 | cuprite, cassiterite | |
| 5 | 84.38 | 0.39 | 0.33 | 0.51 | 14.37 | 0.03 | cuprite | |
| 6 | 83.64 | 0.66 | 0.92 | 0.37 | 14.41 | 0.01 | ||
| 7 | 79.33 | 0.69 | 0.52 | 0.82 | 17.20 | 1.44 | ||
| 8 | 66.52 | 0.47 | 0.62 | 1.07 | 23.98 | 7.34 | brochantite | |
| 9 | 68.93 | 0.46 | 0.51 | 0.91 | 22.96 | 6.22 | ||
| 10 | 68.58 | - | 0.35 | 1.18 | 23.56 | 6.33 | ||
| 11 | 66.03 | - | 0.24 | 0.79 | 25.13 | 7.80 | ||
| 12 | 67.31 | - | 0.14 | 1.08 | 25.62 | 5.85 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Cho, N. Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina. Metals 2023, 13, 1101. https://doi.org/10.3390/met13061101
Kwon H, Cho N. Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina. Metals. 2023; 13(6):1101. https://doi.org/10.3390/met13061101
Chicago/Turabian StyleKwon, Heehong, and Namchul Cho. 2023. "Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina" Metals 13, no. 6: 1101. https://doi.org/10.3390/met13061101
APA StyleKwon, H., & Cho, N. (2023). Corrosion Behaviors of Outdoor Bronze Sculptures in an Urban–Industrial Environment: Corrosion Experiment on Artificial Sulfide Patina. Metals, 13(6), 1101. https://doi.org/10.3390/met13061101






