Precipitation Hardening of the Electrical Conductor Aluminum Alloy 6201
Abstract
:1. Introduction
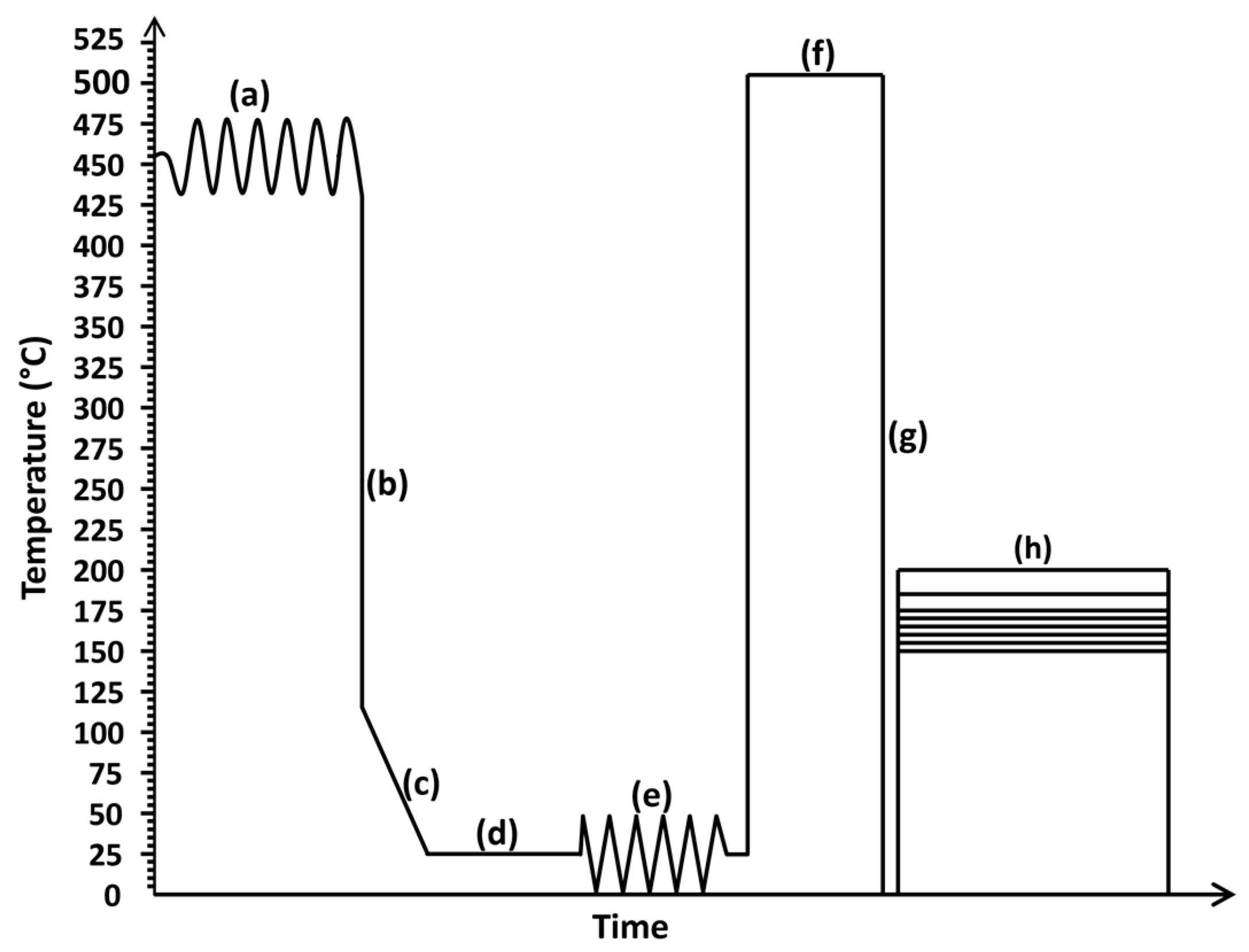
2. Materials and Methods
3. Results
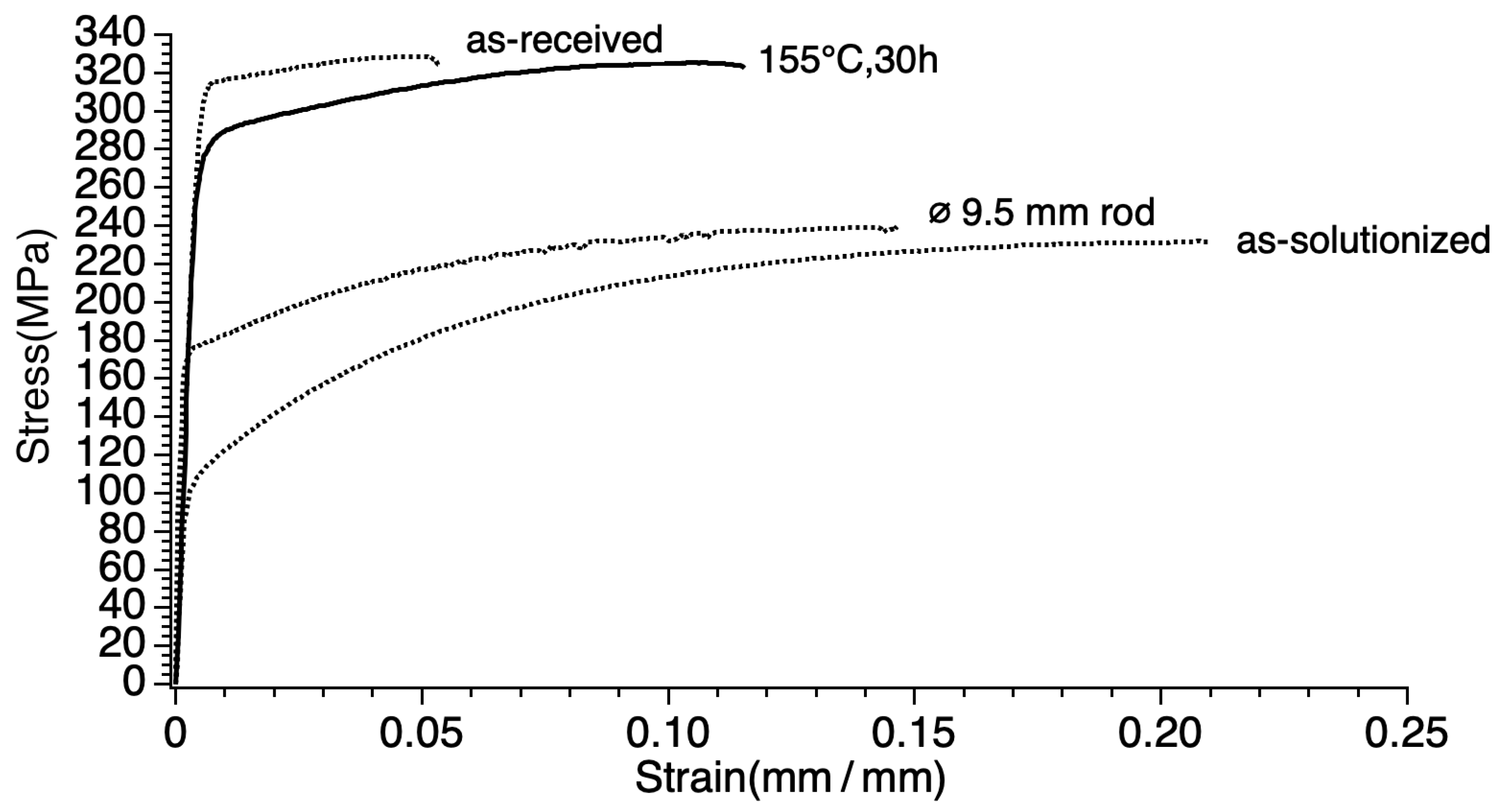
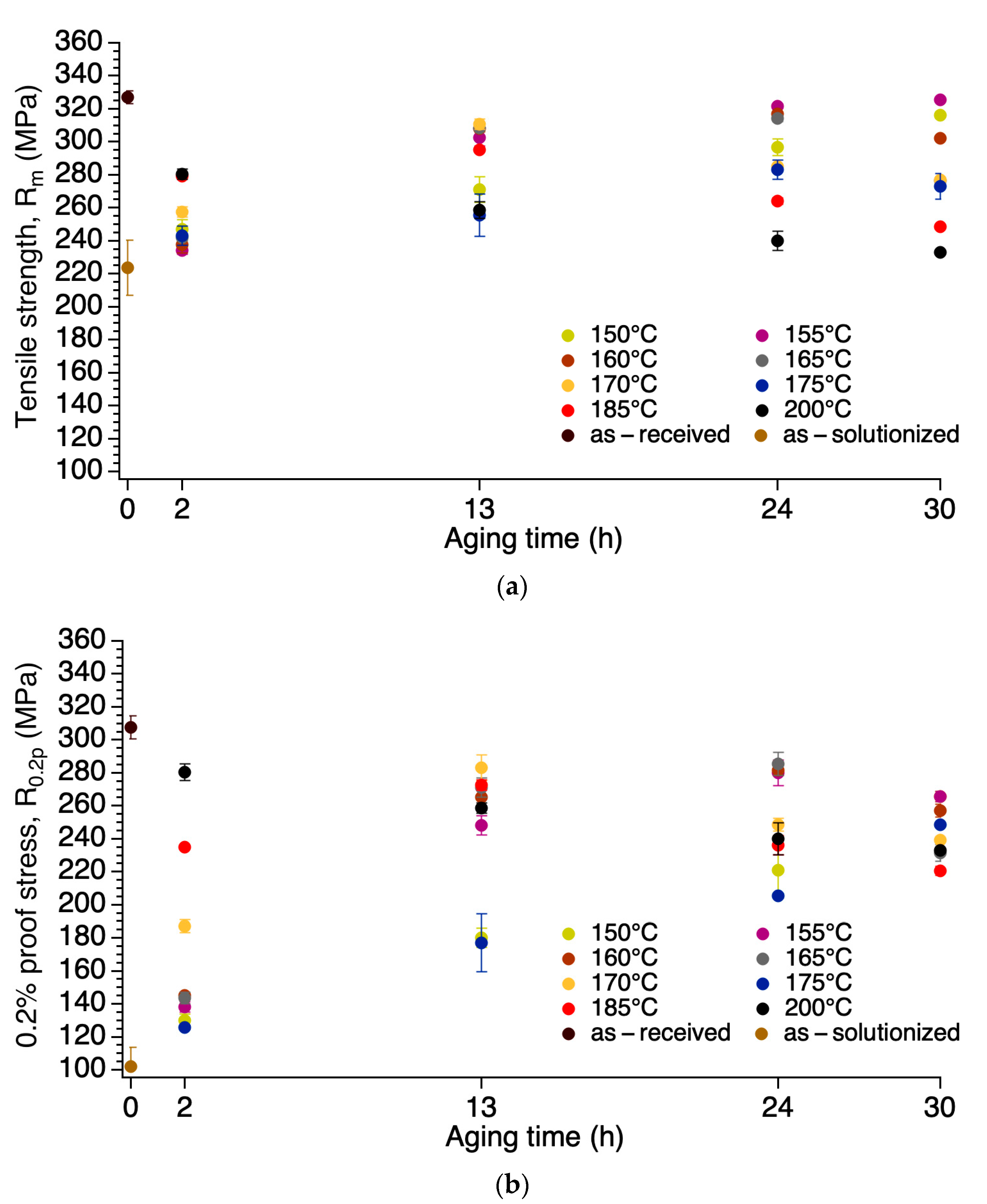
3.1. Mechanical Properties
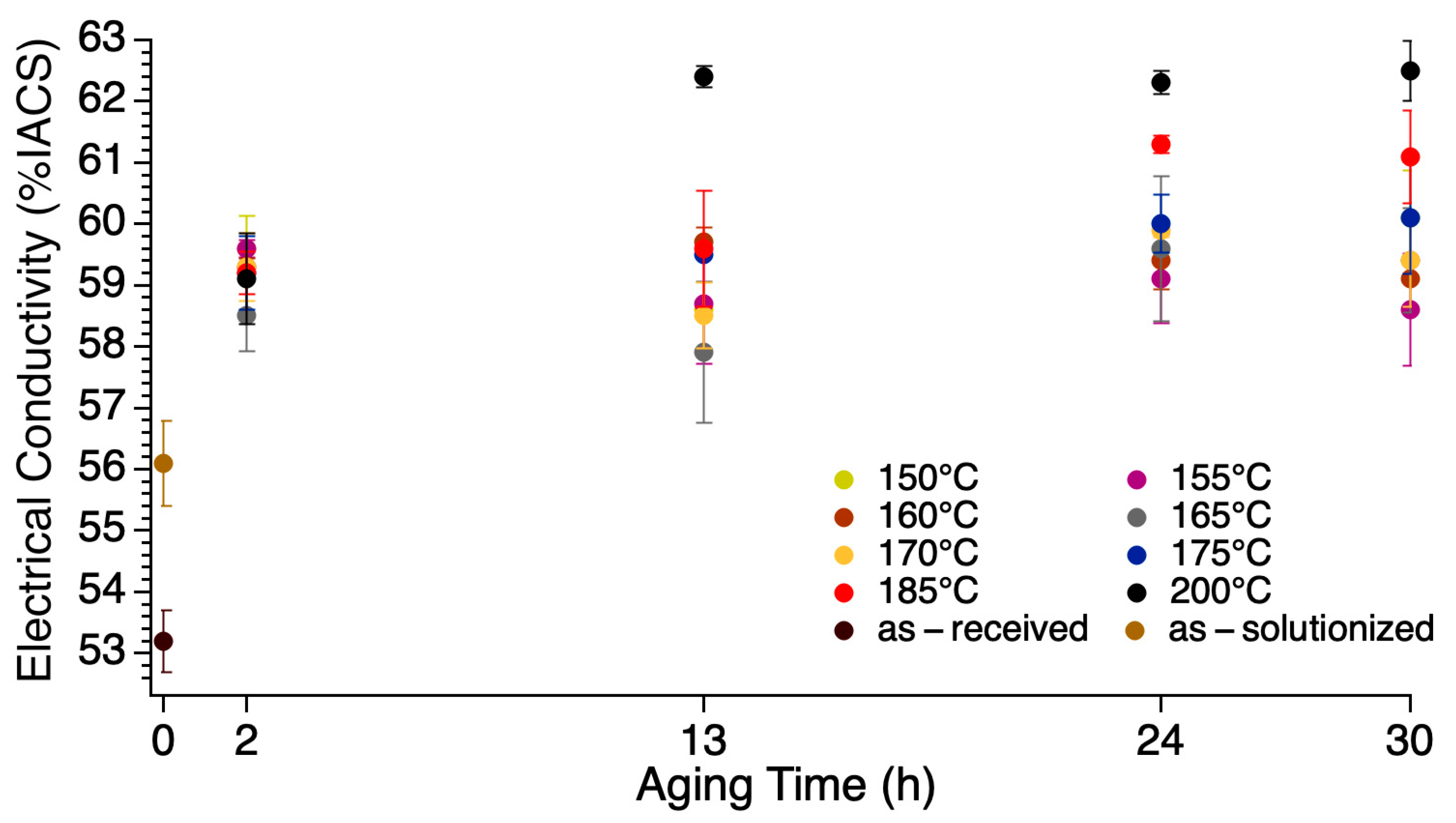
3.2. Electrical Conductivity
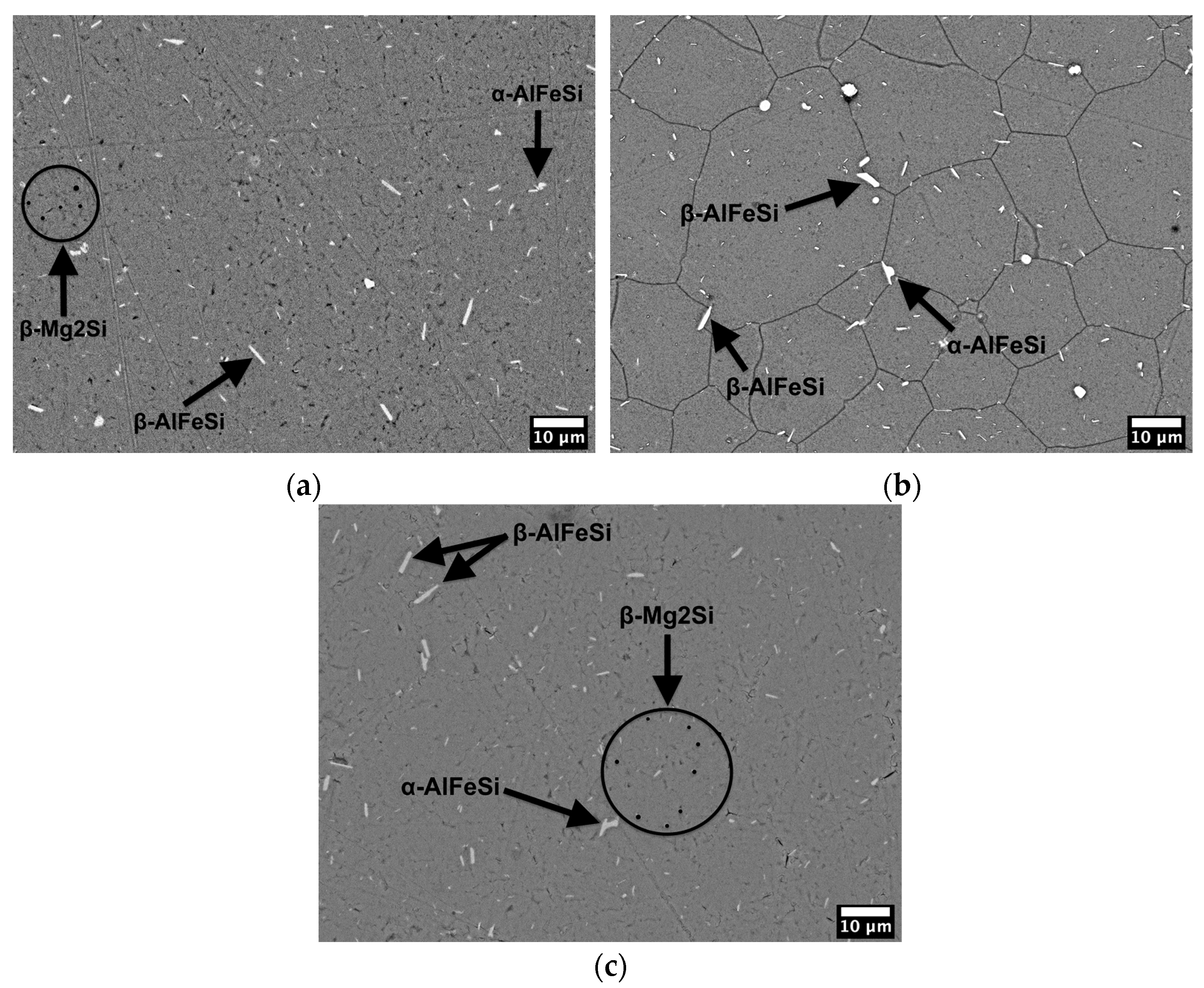
3.3. Microstructure
4. Discussion
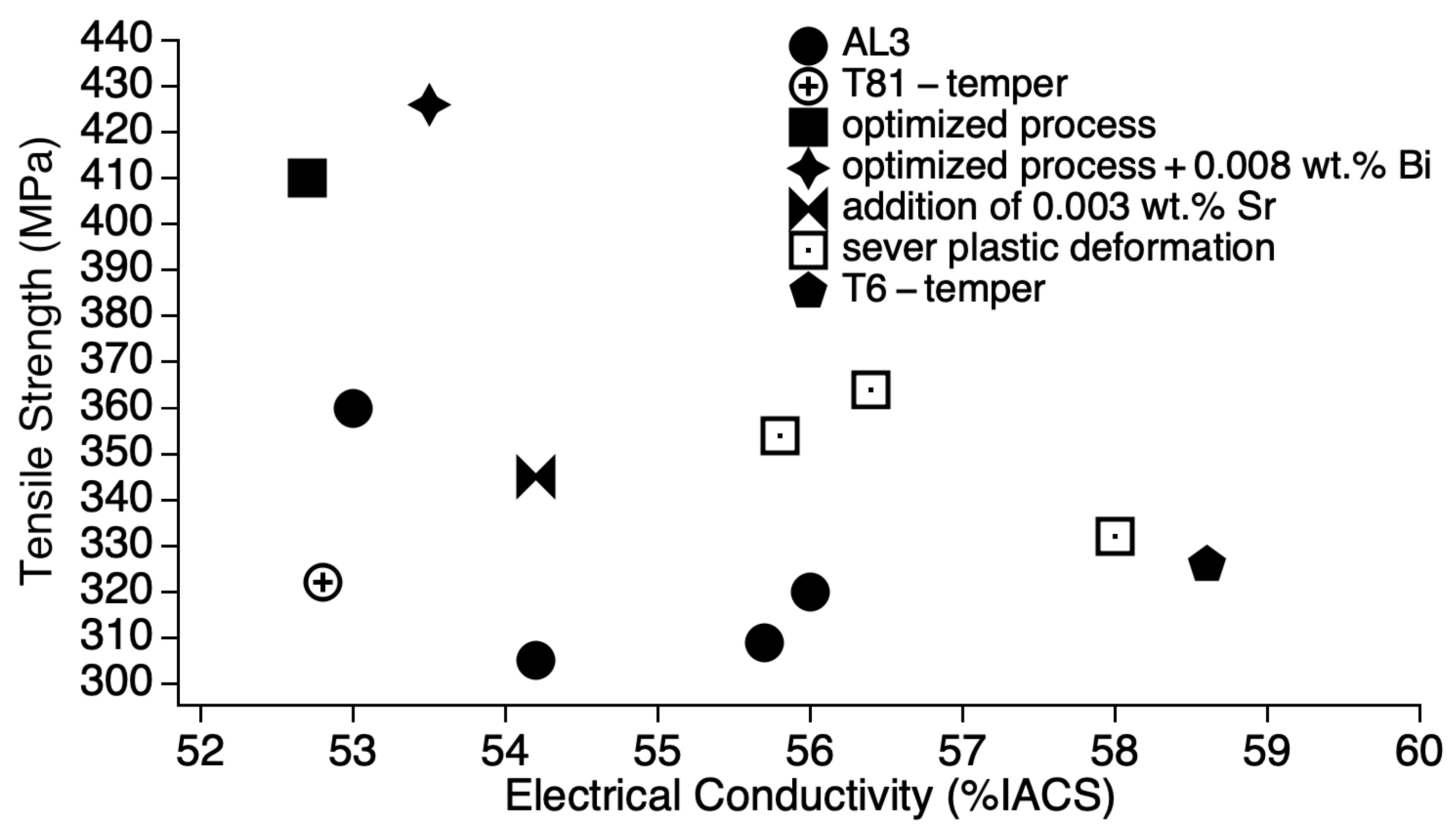
4.1. Mechanical Properties and Electrical Conductivity
4.2. Microstructure
5. Conclusions
- 1.
- Electrical conductivity persistently increased after each processing step and treatment: 53.0% IACS of the ø 9.50 mm rod, 53.2% IACS of ø 3.50 mm wire (or the as-received), 56.1% IACS of the as-solutionzed, and 62.5% IACS of the sample artificial aged at 200 °C for 30 h (the artificial aging treatment with the largest value).
- 2.
- Tensile strength did not consistently increase nor decrease, but fluctuated after each processing step and treatment: 238 MPa of the ø 9.50 mm rod, 327 MPa of the as-received, 224 MPa of the as-solutionzed, and 326 MPa of the sample artificial aged at 155 °C for 30 h (the artificial aging treatment with the largest value).
- 3.
- Tensile strength and electrical conductivity were found to have an inverse relationship. The sample artificially aged at 155 °C for 30 h (155-30) shows the best compromise of tensile strength and electrical conductivity; tensile strength was retained as that of the as-received (of the cold worked condition) of 326 MPa. The strain after fracture was as high as 11.5%, higher than the minimum accepted value (3.5%) and the value of the as-received. The electrical conductivity was as high as 58.6% IACS, higher than both the as-received and the as-solutionized samples as well as those of the previous studies.
- 4.
- A significant amount of second phase particles must have nucleated (precipitation), which would explain the retainment of tensile strength, as in the case of the sample artificial aged at 155 °C for 30 h, with the remarkable increase in electrical conductivity. Precipitates are made up from alloying elements; therefore, a high number of precipitates results in low number of alloying elements; the previous is responsible for such strength retainment (after the reduction during solution treatment) and the latter is responsible for the high electrical conductivity.
6. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASTM B398/B398M-15; Standard Specification for Aluminum-Alloy 6201-T81 and 6201-T83 Wire for Electrical Purposes. ASTM: West Conshohocken, PA, USA, 2015. [CrossRef]
- Karabay, S. Modification of AA-6201 Alloy for Manufacturing of High Conductivity and Extra High Conductivity Wires with Property Of. Mater. Des. 2006, 27, 821–832. [Google Scholar] [CrossRef]
- Karabay, S. Modification of Conductive Material AA6101 of OPGW Conductors against Lightning Strikes. Stroj. Vestn./J. Mech. Eng. 2013, 59, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Davies, G. Aluminium Alloy (6201, 6101A) Conductors. In Proceedings of the 1989 International Conference on Overhead Line Design and Construction: Theory and Practice, London, UK, 28–30 November 1988. [Google Scholar]
- Sunde, J.K.; Marioara, C.D.; Wenner, S.; Holmestad, R. On the microstructural origins of improvements in conductivity by heavy deformation and ageing of Al-Mg-Si alloy 6101. Mater. Charact. 2021, 176, 111073. [Google Scholar] [CrossRef]
- Cho, C.-H.; Cho, H. Effect of dislocation characteristics on electrical conductivity and mechanical properties of AA 6201 wires. Mater. Sci. Eng. A 2021, 809, 140811. [Google Scholar] [CrossRef]
- Al-Yagoot, O.H.; Ul-hamid, A. Comparison of Microstructure and Properties of 6201 Al Alloy Wire Obtained by Two Different Hot Rolling Methods. In Conference Proceedings: Advances in Production and Processing of Aluminium (APPA); University of Bahrain: Manama, Bahrain, 2001; pp. 11-4-1–11-4-15. [Google Scholar]
- Alshwawreh, N.; Alhamarneh, B.; Altwarah, Q.; Quandour, S.; Barghout, S.; Ayasrah, O. Electrical Resistivity and Tensile Strength Relationship in Heat-Treated All Aluminum Alloy Wire Conductors. Materials 2021, 14, 5738. [Google Scholar] [CrossRef] [PubMed]
- Khangholi, S.N.; Javidani, M.; Maltais, A.; Chen, X.-G. Optimization of mechanical properties and electrical conductivity in Al–Mg–Si 6201 alloys with different Mg/Si ratios. J. Mater. Res. 2020, 35, 2765–2776. [Google Scholar] [CrossRef]
- Flores, F.U.; Seidman, D.N.; Dunand, D.C.; Vo, N.Q. Development of High-Strength and High-Electrical-Conductivity Aluminum Alloys for Power Transmission Conductors. In TMS Annual Meeting & Exhibition; Minerals, Metals and Materials Series; Part F4; Springer: Cham, Switzerland, 2018; pp. 247–251. [Google Scholar] [CrossRef]
- Jin, H.; Guan, R.; Huang, X.; Fu, Y.; Zhang, J.; Chen, X.; Wang, Y.; Gao, F.; Tie, D. Understanding the precipitation mechanism of copper-bearing phases in Al-Mg-Si system during thermo-mechanical treatment. J. Mater. Sci. Technol. 2022, 96, 226–232. [Google Scholar] [CrossRef]
- Mulazimoglu, M.H.; Zaluska, A.; Paray, F.; Gruzleski, J.E. The effect of strontium on the Mg2Si precipitation process in 6201 aluminum alloy. Met. Mater. Trans. A Phys. Metall. Mater. Sci. 1997, 28, 1289–1295. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Fu, D.; Teng, J.; Zhang, H. Improvement of the mechanical properties of Al–Mg–Si alloys with nano-scale precipitates after repetitive continuous extrusion forming and T8 tempering. J. Mater. Res. Technol. Korean Inst. Orient. Med. 2019, 8, 5950–5960. [Google Scholar] [CrossRef]
- Murashkin, M.; Medvedev, A.; Kazykhanov, V.; Krokhin, A.; Raab, G.; Enikeev, N.; Valiev, R.Z. Enhanced Mechanical Properties and Electrical Conductivity in Ultrafine-Grained Al 6101 Alloy Processed via ECAP-Conform. Metals 2015, 5, 2148–2164. [Google Scholar] [CrossRef] [Green Version]
- Majchrowicz, K.; Pakieła, Z.; Chrominski, W.; Kulczyk, M. Enhanced strength and electrical conductivity of ultrafine-grained Al-Mg-Si alloy processed by hydrostatic extrusion. Mater. Charact. 2018, 135, 104–114. [Google Scholar] [CrossRef]
- Yang, C.; Masquellier, N.; Gandiolle, C.; Sauvage, X. Multifunctional properties of composition graded Al wires. Scr. Mater. 2020, 189, 21–24. [Google Scholar] [CrossRef]
- Jin, H.; Tie, D.; Guan, R. Precipitation behavior during re-aging of Al-Mg-Si-Cu alloy. Mater. Des. 2022, 220, 110883. [Google Scholar] [CrossRef]
- Kim, Y.; Mishra, R.K.; Sachdev, A.K.; Kumar, K.S. A combined experimental-analytical modeling study of the artificial aging response of Al–Mg–Si alloys. Mater. Sci. Eng. A 2021, 820, 141566. [Google Scholar] [CrossRef]
- Jin, H.; Guan, R.; Tie, D. Mechanical and Conductive Performance of Aged 6xxx Aluminum Alloy during Rotary Swaging. Crystals 2022, 12, 530. [Google Scholar] [CrossRef]
- Khangholi, S.N.; Javidani, M.; Maltais, A.; Chen, X.-G. Effects of natural aging and pre-aging on the strength and electrical conductivity in Al-Mg-Si AA6201 conductor alloys. Mater. Sci. Eng. A 2021, 820, 141538. [Google Scholar] [CrossRef]
- Sunde, J.K.; Marioara, C.D.; Holmestad, R. The effect of low Cu additions on precipitate crystal structures in overaged Al-Mg-Si(-Cu) alloys. Mater. Charact. 2020, 160, 110087. [Google Scholar] [CrossRef]
- Weng, Y.; Ding, L.; Jia, Z.; Liu, Q. Effect of combined addition of Ag and Cu on the precipitation behavior for an Al-Mg-Si alloy. Mater. Charact. 2021, 171, 110736. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Wang, B.-Y.; Wang, C.; Zha, M.; Liu, G.-J.; Yang, Z.-Z.; Wang, J.-G.; Li, J.-H.; Wang, H.-Y. Microstructure and mechanical properties of Al-Mg-Si alloy fabricated by a short process based on sub-rapid solidification. J. Mater. Sci. Technol. 2020, 41, 178–186. [Google Scholar] [CrossRef]
- Lentz, M.C.; Rengel, M.; Stray, K.; Engler, O. A modified processing route for high strength Al-Mg-Si aluminum conductors based on twin-roll cast strip. J. Mater. Process. Technol. 2020, 278, 116463. [Google Scholar] [CrossRef]
- BS EN 50183; Conductors for Overhead Lines-Aluminium-Magnesium-Silicon Alloy Wires. British Standards Institiution: London, UK, 2000; pp. 1–12.
- Callister, W.D. Materials Science and Engineering: An Introduction; Materials & Design; John Wiley & Sons: New York, NY, USA, 2009; Volume 12, p. 992. [Google Scholar]
- Cofer, D.B.; Bass, J.A. Continuous Casting and Rolling of 6201 Aluminum Alloy. 1971. Available online: https://pdfpiw.uspto.gov/.piw?Docid=03613767&homeurl=http%3A%2F%2Fpatft.uspto.gov%2Fnetacgi%2Fnph-Parser%3FSect1%3DPTO1%2526Sect2%3DHITOFF%2526d%3DPALL%2526p%3D1%2526u%3D%25252Fnetahtml%25252FPTO%25252Fsrchnum.htm%2526r%3D1%2526f%3DG%2526l%3D50%2526s1%3D3 (accessed on 18 February 2020).
- B918/B918M-20a; Standard Practice for Heat Treatment of Wrought Aluminum Alloys. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- B557-15; Standard Test Methods for Tension Testing Wrought and Cast Aluminum- and Magnesium-Alloy Products (Metric). ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- B193-20; Standard Test Method for Resistivity of Electrical Conductor Materials. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM E112-13; Standard Test Methods for Determining Average Grain Size. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- B230/B230M-22; Standard Specification for Aluminum 1350-H19 Wire for Electrical Purposes. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Alexopoulos, N.D.; Robson, J.D.; Stefanou, G.; Stergiou, V.; Karanika, A.; Kourkoulis, S.K. Microstructural and mechanical characterization of the aging response of wrought 6156 (Al-Mg-Si) aluminum alloy. Alloys 2022, 1, 180–195. [Google Scholar] [CrossRef]

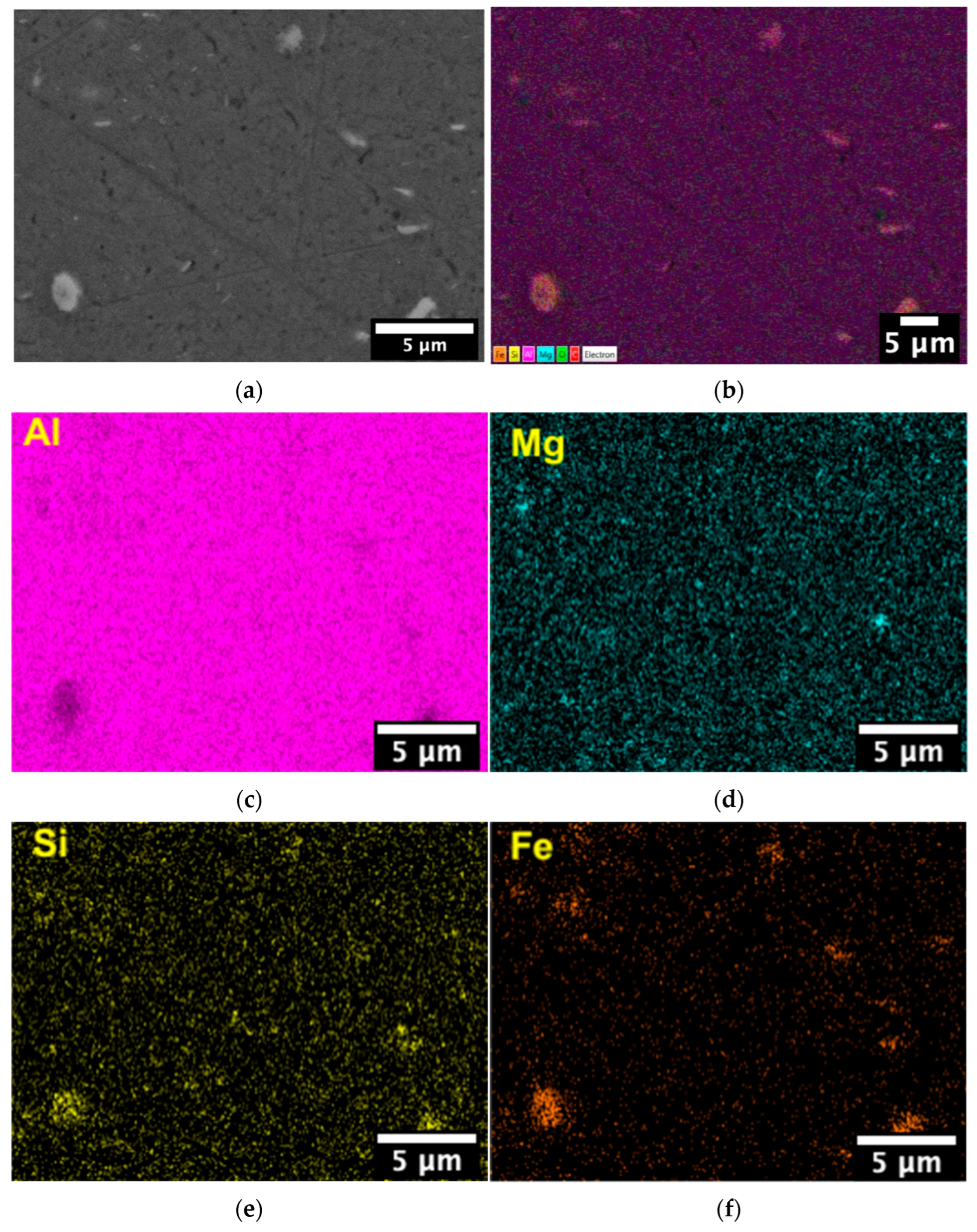

| Al | Mg | Si | Fe | Cu | Other Elements |
|---|---|---|---|---|---|
| 98.3 | 0.63 | 0.55 | 0.21 | 0.08 | 0.23 |
| Specimen | Percentage Cold Working (%) | Solution Heat Treatment | Precipitation Heat Treatment | Temper Designation | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Temperature (°C) | Time (h) | |||
| As-Received | 86 | - | - | - | - | - |
| As-Solutionized | 510 | 1 | - | - | O | |
| 150-2, 150-13, 150-24, 150-30 | 150 | 2, 13, 24, 30 | T6 | |||
| 155-2, 155-13, 155-24, 155-30 | 155 | 2, 13, 24, 30 | ||||
| 160-2, 160-13, 160-24, 160-30 | 160 | 2, 13, 24, 30 | ||||
| 165-2, 165-13, 165-24, 165-30 | 165 | 2, 13, 24, 30 | ||||
| 170-2, 170-13, 170-24, 170-30 | 170 | 2, 13, 24, 30 | ||||
| 175-2, 175-13, 175-24, 175-30 | 175 | 2, 13, 24, 30 | ||||
| 185-2, 185-13, 185-24, 185-30 | 185 | 2, 13, 24, 30 | ||||
| 200-2, 200-13, 200-24, 200-30 | 200 | 2, 13, 24, 30 | ||||
| Specimen | Tensile Strength, Rm (MPa) | Percentage Total Elongation at after Fracture on 25 mm, At (%) | 0.2% Proof Stress, Non-Proportional Extension a, R0.2p (MPa) | Strength Coefficient, K (MPa) | Strain Hardening Exponent, n | Vickers Hardness, HV/0.5 | Electrical Conductivity nΩ·m (% IACS) | |
|---|---|---|---|---|---|---|---|---|
| ø 9.50 mm (rod) | 238 ± 5.0 | 14.5 ± 0 | 159 ± 32.25 | 374 ± 0 | 0.166 ± 0.0118 | 64.8 ± 2.1 | 53.0 ± 0.37 | |
| ø 3.5 mm (wire) | as-received | 327 ± 3.92 | 5.0 ± 0.49 | 308 ± 6.86 | 387 ± 10.78 | 0.04 ± 0.009 | 92 ± 8.3 | 53.2 ± 0.51 |
| as-solutionized | 224 ± 16.66 | 21 ± 1.47 | 102 ± 11.76 | 448 ± 23.52 | 0.298 ± 0.0127 | 70.1 ± 1.1 | 56.1 ± 0.70 | |
| 155-30 | 326 ± 0.98 | 11.5 ± 0 | 266 ± 2.94 | 439 ± 4.9 | 0.09 ± 0.005 | 84.5 ± 2.1 | 58.6 ± 0.91 | |
| Pre-Treatment | Precipitation Heat Treatment Temperature and Time | Post-Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Diameter/Side Length | Tensile Strength | Elongation | Electrical Conductivity | Hardness, HV | Tensile Strength | Elongation | Electrical Conductivity | Hardness, HV | |
| (mm) | (MPa) | (%) | (% IACS) | (°C, h) | (MPa) | (%) | (% IACS) | |||
| [This work] | 3.5 | 327 ± 3.92 | 5.0 ± 0.49 | 53.2 ± 0.51 | 92 ± 8.3 | 155, 30 | 326 ± 0.98 | 11.5 ± 0.0 | 58.6 ± 0.91 | 84.5 ± 2.1 |
| [7] | 4.0 | 283 | 3.5 | 51 | 86 | 170, 7 | 309 | 8.3 | 55.7 | 102 |
| [8] | 1.70 | 327 | - | 49.3 | - | 150, 5 | 305 | - | 54.2 | - |
| 3.50 | 333 | - | 49.3 | - | 170, 1.5 | 320 | - | 56 | - | |
| 130, 5 | 360 | - | 53.5 | - | ||||||
| [9] | 4.7 | 256 | 1.3 | 48 | 102 | 180, 4 | 322 | 4.75 | 52.8 | 110 |
| [10] | 2.65 | - | - | - | - | 200, 16 | 410 | - | 53.5 | - |
| 200, 7 | 426 | - | 52.7 | - | ||||||
| [12] | 2.8 | - | - | - | - | 175, 3 | 345 | 9.9 | 54.2 | - |
| [13] | 2.97 | - | - | - | - | 120, 3 | 345 | 7.6 | - | - |
| [14] | 3.2 | - | - | - | - | 170, 12 | 364 | 3.5 | 56.4 | - |
| [15] | 4.0 | 305 | 7.8 | 50.9 | 87 | 180, 7 | 332 | 8.8 | 58 | 91 |
| 3.0 | 305 | 6.1 | 50.6 | 89 | 180, 2 | 354 | 5.5 | 55.8 | 95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allamki, A.; Al-Maharbi, M.; Qamar, S.Z.; Al-Jahwari, F. Precipitation Hardening of the Electrical Conductor Aluminum Alloy 6201. Metals 2023, 13, 1111. https://doi.org/10.3390/met13061111
Allamki A, Al-Maharbi M, Qamar SZ, Al-Jahwari F. Precipitation Hardening of the Electrical Conductor Aluminum Alloy 6201. Metals. 2023; 13(6):1111. https://doi.org/10.3390/met13061111
Chicago/Turabian StyleAllamki, Alyaqadhan, Majid Al-Maharbi, Sayyad Zahid Qamar, and Farooq Al-Jahwari. 2023. "Precipitation Hardening of the Electrical Conductor Aluminum Alloy 6201" Metals 13, no. 6: 1111. https://doi.org/10.3390/met13061111





