Corrosion and Formation of Surface Films on AZ31 Mg Alloy in Aqueous Solution Containing Sulfate Ions with Different pHs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. In Situ Observation and Weight Change
2.3. Electrochemical Measurements
2.4. MIM Coloring and Surface Morphologies Observation
3. Results and Discussion
3.1. In Situ Observations of the AZ31 Mg Alloy Surfaces during Immersion
3.2. Open-Circuit Potential Transients
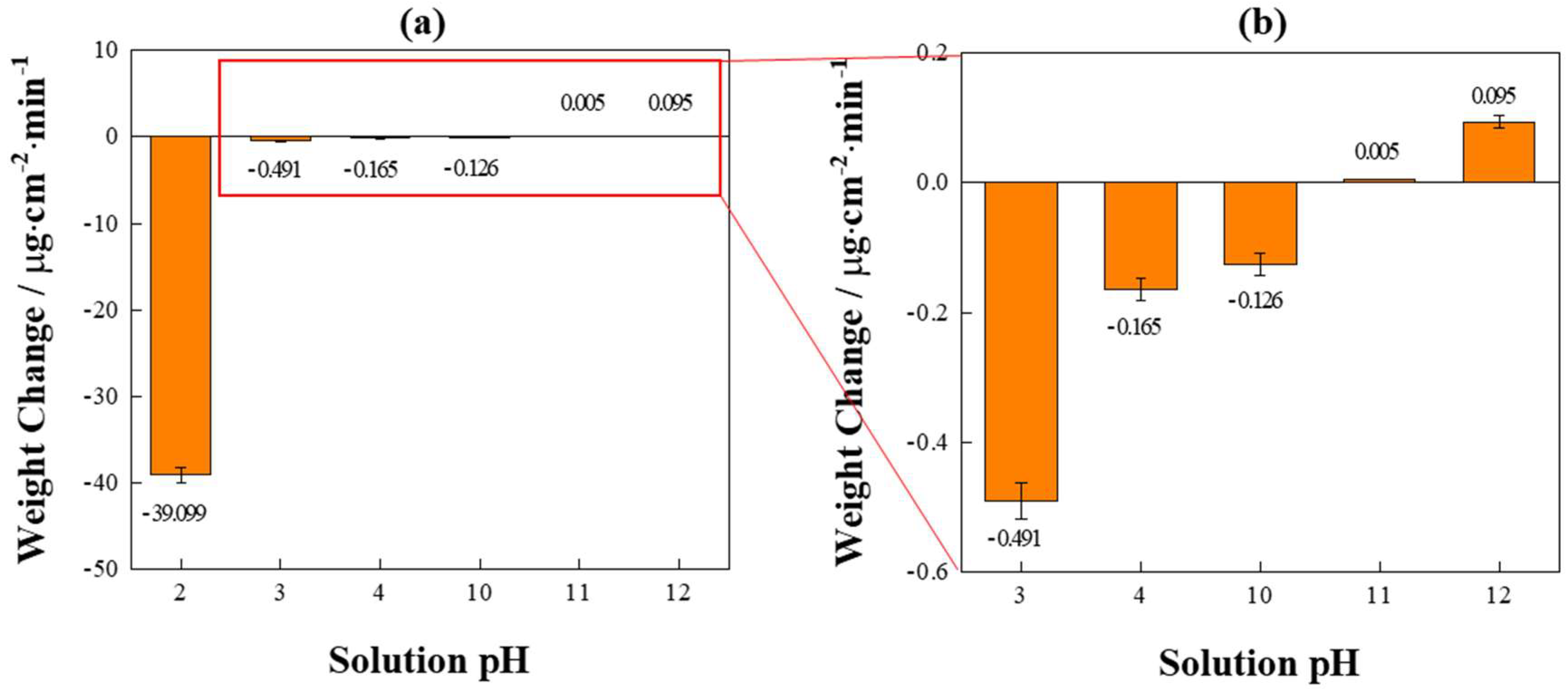
3.3. Weight Loss and Gain
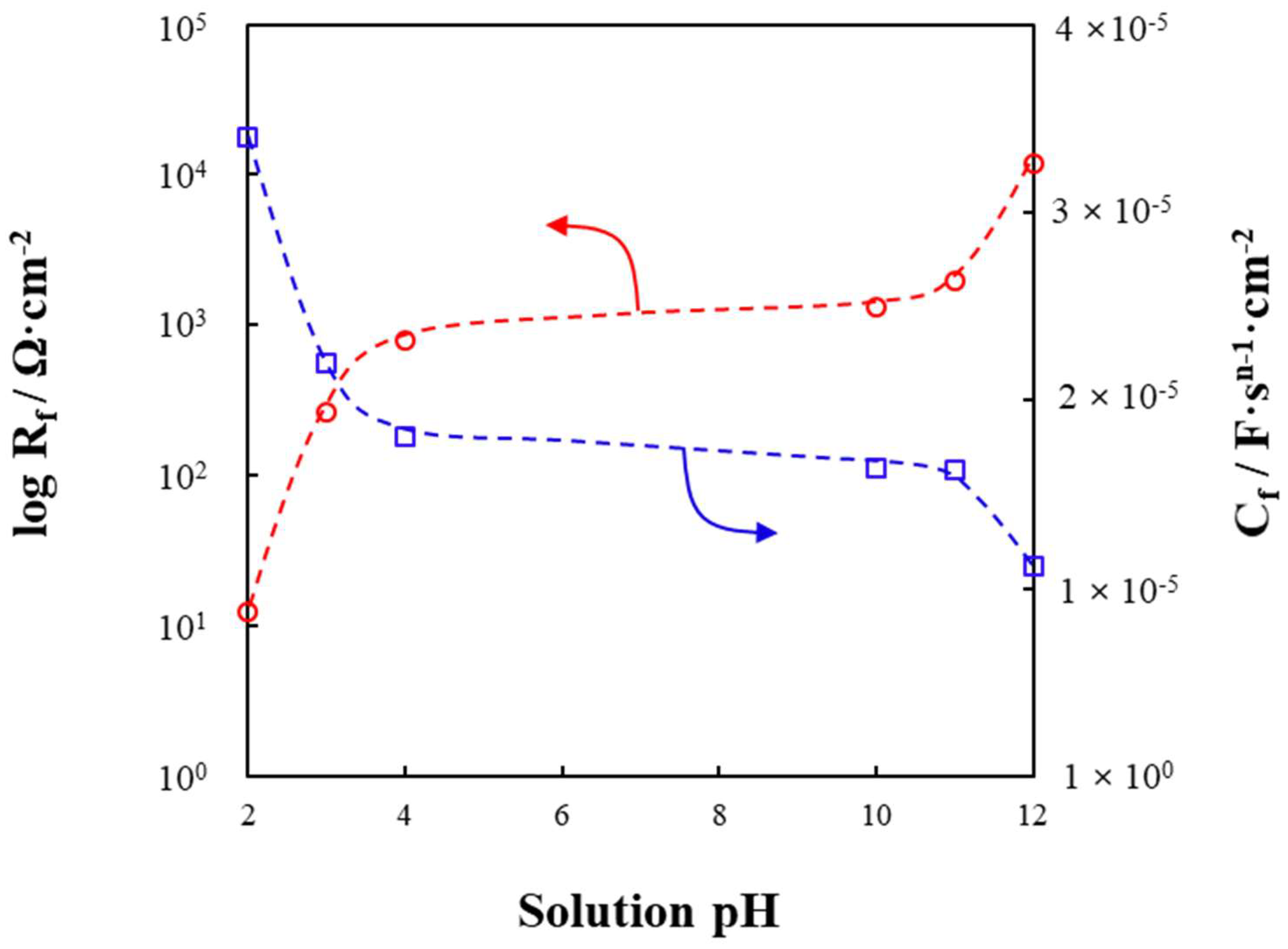
3.4. Electrochemical Impedance Spectroscopy
3.5. Surface Appearance
3.6. Surface Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Technol. 2021, 405, 126521. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy parts for automotive OEMs: A review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Yan, P.; Ying, T.; Li, Y.; Li, D.; Cao, F.; Zeng, X.; Ding, W. A novel high corrosion-resistant polytetrafluoroethylene/carbon cloth/Ag coating on magnesium alloys as bipolar plates for light-weight proton exchange membrane fuel cells. J. Power Sources 2021, 484, 29231. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- de Oliveira, M.C.L.; da Silva, R.M.P.; Souto, R.M.; Antunes, R.A. Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review. J. Magnes. Alloys 2022, 10, 2997–3030. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, C.; Zhao, K. Ultrasonic welding of AZ31B magnesium alloy and pure copper: Microstructure, mechanical properties and finite element analysis. J. Mater. Res. Technol. 2023, 23, 1273–1284. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xie, J.; Liu, S.; Fu, W.; Wu, R. Developing a Mg alloy with ultrahigh room temperature ductility via grain boundary segregation and activation of non-basal slips. Int. J. Plast. 2023, 162, 103548. [Google Scholar] [CrossRef]
- Xu, B.; Song, Y.; Yang, K.; Li, Y.; Chen, B.; Liao, X.; Jia, Q. Magnesium metal and its corrosion products: Promising materials for tumor interventional therapy. J. Magnes. Alloys 2023, 11, 763–775. [Google Scholar] [CrossRef]
- Mitchell, J.; Crow, N.; Nieto, A. Effect of Surface Roughness on Pitting Corrosion of AZ31 Mg Alloy. Metals 2020, 10, 651. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Q.; Yang, Y.; Hou, X.; Yan, Y.; Hu, J.; Deng, H.; Yu, D.; Lan, W.; Pan, F. A new environmentally-friendly route to in situ form a high-corrosion-resistant nesquehonite film on pure magnesium. RSC Adv. 2020, 10, 35480–35489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Qu, Q.; Ma, J.; Wang, L.; Li, L.; Bai, W.; Ding, Z. Corrosion behaviour of AZ31B magnesium alloy in NaCl solutions saturated with CO2. Corrosion Sci. 2011, 53, 1186–1193. [Google Scholar] [CrossRef]
- Prince, L.; Rousseau, M.A.; Noirfalise, X.; Dangreau, L.; Coelho, L.B.; Olivier, M.G. Inhibitive effect of sodium carbonate on corrosion of AZ31 magnesium alloy in NaCl solution. Corrosion Sci. 2021, 179, 109131. [Google Scholar] [CrossRef]
- Soltan, A.; Dargusch, M.S.; Shi, Z.; Gerrard, D.; Al Shabibi, S.; Kuo, Y.-C.; Atrens, A. Corrosion of Mg alloys EV31A, WE43B, and ZE41A in chloride- and sulfate-containing solutions saturated with magnesium hydroxide. Mater. Corros. 2020, 71, 956–979. [Google Scholar] [CrossRef]
- Ge, F.; Cui, Z.; Liu, Y.; Lei, L.; Wang, X.; Cui, H. Influence of ammonium sulfate on the corrosion behavior of AZ31 magnesium alloy in chloride environment. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Xia, Y.; Chang, L.; Zhang, J. Localized Corrosion of Magnesium Alloys in NaCl Solutions Explored by Scanning Electrochemical Microscopy in Feedback Mode. Electrochim. Acta 2014, 132, 377–388. [Google Scholar] [CrossRef]
- Feng, Z.; Li, J.; Yang, Z.; Buchheit, R. The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials 2020, 13, 1325. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Lai, T.; Yin, Z.; Chen, Y.; Wang, K.; Yan, H.; Song, H.; Liu, R.; Luo, C.; Hu, Z. In-situ AFM and quasi-in-situ studies for localized corrosion in Mg-9Al-1Fe-(Gd) alloys under 3.5 wt.% NaCl environment. J. Magnes. Alloys, 2022; in press. [Google Scholar] [CrossRef]
- Harrison, R.; Maradze, D.; Lyons, S.; Zheng, Y.; Liu, Y. Corrosion of magnesium and magnesium–calcium alloy in biologically-simulated environment. Prog. Nat. Sci. 2014, 24, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Jia, Q.; Xu, C.; Zhang, Z.; Ren, C.; Yang, W.; Zhang, J. Research on Dynamic Corrosion Behavior and the Microstructure of Biomedical Mg–Y–Zn–Zr–Sr in Simulated Body Fluid Solution after Processing by Solution Treatment. Adv. Eng. Mater. 2020, 22, 1901146. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, D.; Cheng, L.; Liu, N.; Yang, L.; Hou, B. The corrosion behavior of EW75 magnesium alloy in the research vessel KEXUE during the ocean voyage. NPJ Mater. Degrad. 2022, 6, 28. [Google Scholar] [CrossRef]
- Samaniego, A.; Hurley, B.L.; Frankel, G.S. On the evidence for univalent Mg. J. Electroanal. Chem. 2015, 737, 123–128. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, L.; Wu, P.; Sun, Y.; Yu, Y.; Wei, G.; Ge, H. Effect of aggressive ions on degradation of WE43 magnesium alloy in physiological environment. Int. J. Electrochem. Sci. 2014, 9, 304–314. [Google Scholar]
- Kwon, D.; Pham, H.V.; Song, P.; Moon, S. Electrochemical behavior of AZ31 Mg alloy in neutral aqueous solutions containing various anions. J. Electrochem. Sci. Technol. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Kwon, D.; Pham, H.V.; Song, P.; Moon, S. Corrosion Behavior of the AZ31 Mg Alloy in Neutral Aqueous Solutions Containing Various Anions. Metals 2023, 13, 962. [Google Scholar] [CrossRef]
- Huang, J.; Song, G.-L.; Atrens, A.; Dargusch, M. What activates the Mg surface—A comparison of Mg dissolution mechanisms. J. Mater. Sci. Technol. 2020, 57, 204–220. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Y.; Liu, J.; Song, D.; Zhang, T.; Liu, J.; He, F.; Zhang, M.; Wang, J. Self-healing system adapted to different pH environments for active corrosion protection of magnesium alloy. J. Alloys Compd. 2020, 824, 153918. [Google Scholar] [CrossRef]
- Van Phuong, N.; Moon, S.; Chang, D.; Lee, K.H. Effect of microstructure on the zinc phosphate conversion coatings on magnesium alloy AZ91. Appl. Surf. Sci. 2013, 264, 70–78. [Google Scholar] [CrossRef]
- Fazal, B.R.; Moon, S. Electrochemical Properties of Air-Formed Oxide Film-Covered AZ31 Mg Alloy in Aqueous Solutions Containing Various Anions. J. Kor. Inst. Surf. Eng. 2017, 50, 147–154. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, L.; Xu, J.; Gong, Y.; Deng, L.; Chen, C. Study of the Corrosion and Surface Film Growth on AZ63 Magnesium Alloy in MgSO4 Solution. J. Electrochem. Soc. 2017, 164, C324. [Google Scholar] [CrossRef]
- Yang, L.; Shi, X.; Tian, X.; Xue, Y.; Wang, J.; Qi, L. Influence of pH value on the microstructure and corrosion behavior of carbon fiber reinforced magnesium matrix composites. J. Mater. Res. Technol. 2022, 17, 412–424. [Google Scholar] [CrossRef]
- Kim, Y.H.; Rahman, M.A.; Hwang, J.S.; Ko, H.; Huh, J.-Y.; Byun, J.Y. Reflection color tuning of a metal–insulator–metal cavity structure using arc plasma deposition of gold nanoparticles. Appl. Surf. Sci. 2021, 562, 150140. [Google Scholar] [CrossRef]
- Merson, E.; Myagkikh, P.; Poluyanov, V.; Merson, D.; Vinogradov, A. On the role of hydrogen in stress corrosion cracking of magnesium and its alloys: Gas-analysis study. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 748, 337–346. [Google Scholar] [CrossRef]
- Maltseva, A.; Shkirskiy, V.; Lefèvre, G.; Volovitch, P. Effect of pH on Mg(OH)2 film evolution on corroding Mg by in situ kinetic Raman mapping (KRM). Corrosion Sci. 2019, 153, 272–282. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Anovitz, L.M.; Littrell, K.C.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.L.; Thomson, J.K.; Gallego, N.C.; Davis, B. Film Breakdown and Nano-Porous Mg(OH)2 Formation from Corrosion of Magnesium Alloys in Salt Solutions. J. Electrochem. Soc. 2015, 162, C140. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Pham, H.V.; Song, P.; Moon, S. Corrosion and Formation of Surface Films on AZ31 Mg Alloy in Aqueous Solution Containing Sulfate Ions with Different pHs. Metals 2023, 13, 1150. https://doi.org/10.3390/met13071150
Kwon D, Pham HV, Song P, Moon S. Corrosion and Formation of Surface Films on AZ31 Mg Alloy in Aqueous Solution Containing Sulfate Ions with Different pHs. Metals. 2023; 13(7):1150. https://doi.org/10.3390/met13071150
Chicago/Turabian StyleKwon, Duyoung, Hien Van Pham, Pungkeun Song, and Sungmo Moon. 2023. "Corrosion and Formation of Surface Films on AZ31 Mg Alloy in Aqueous Solution Containing Sulfate Ions with Different pHs" Metals 13, no. 7: 1150. https://doi.org/10.3390/met13071150
APA StyleKwon, D., Pham, H. V., Song, P., & Moon, S. (2023). Corrosion and Formation of Surface Films on AZ31 Mg Alloy in Aqueous Solution Containing Sulfate Ions with Different pHs. Metals, 13(7), 1150. https://doi.org/10.3390/met13071150






