Novel Frontiers in High-Entropy Alloys
Abstract
:1. Introduction
2. Welding and Brazing
2.1. Welding
2.2. Dissimilar Welding
2.3. Brazing
2.4. Brazing Filler Metals and Welding Interlayers
3. Nanomaterial Synthesis
3.1. Carbothermal Shock
3.2. Mechanical Alloying
3.3. Microwave Heating
3.4. Wet Chemistry
3.5. Laser Ablation Synthesis in Liquid
3.6. Other Notable Synthesis Methods
4. Catalysis
5. Marine Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent and High Entropy Alloys. Entropy 2014, 16, 4749–4768. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, P.; Nene, S.S.; Mishra, R.S. Friction Stir Welding of γ-Fcc Dominated Metastable High Entropy Alloy: Microstructural Evolution and Strength. Scr. Mater. 2021, 204, 114161. [Google Scholar] [CrossRef]
- Guo, W.; Dmowski, W.; Noh, J.Y.; Rack, P.; Liaw, P.K. Local Atomic Structure of a High-Entropy Alloy: An X-ray and Neutron Scattering Study. Metall. Mater. Trans. A 2013, 44A, 1994–1997. [Google Scholar] [CrossRef]
- Gao, M. Development of New High Entropy Alloys for Brazing of Ni-Base Superalloys. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2017. [Google Scholar]
- Murali, M.; Babu, S.P.K.; Krishna, B.J.; Vallimanalan, A. Synthesis and Characterization of AlCoCrCuFeZnx High-Entropy Alloy by Mechanical Alloying. Prog. Nat. Sci. Mater. Int. 2016, 26, 380–384. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Liu, F.; Liaw, P.K.; Zhang, Y. Recent Progress with BCC-Structured High-Entropy Alloys. Metals 2022, 12, 501. [Google Scholar] [CrossRef]
- Yan, X.; Liaw, P.K.; Zhang, Y. Ultrastrong and Ductile BCC High-Entropy Alloys with Low-Density via Dislocation Regulation and Nanoprecipitates. J. Mater. Sci. Technol. 2022, 110, 109–116. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, X.; Feng, M. Molecular Dynamics-Based Analysis of the Effect of Voids and HCP-Phase Inclusion on Deformation of Single-Crystal CoCrFeMnNi High-Entropy Alloy. Mater. Sci. Eng. A 2020, 791, 139444. [Google Scholar] [CrossRef]
- Shahmir, H.; Asghari-Rad, P.; Mehranpour, M.S.; Forghani, F.; Kim, H.S.; Nili-Ahmadabadi, M. Evidence of FCC to HCP and BCC-Martensitic Transformations in a CoCrFeNiMn High-Entropy Alloy by Severe Plastic Deformation. Mater. Sci. Eng. A 2021, 807, 140875. [Google Scholar] [CrossRef]
- Vaidya, M.; Sen, S.; Zhang, X.; Frommeyer, L.; Rogal, Ł.; Sankaran, S.; Grabowski, B.; Wilde, G.; Divinski, S.V. Phenomenon of Ultra-Fast Tracer Diffusion of Co in HCP High Entropy Alloys. Acta Mater. 2020, 196, 220–230. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic Glasses…on the Threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Swiston, A.J.; Hufnagel, T.C.; Weihs, T.P. Joining Bulk Metallic Glass Using Reactive Multilayer Foils. Scr. Mater. 2003, 48, 1575–1580. [Google Scholar] [CrossRef]
- Wu, Q.F.; Wang, Z.J.; He, F.; Wang, L.L.; Luo, J.; Li, J.J.; Wang, J.C. High Entropy Alloys: From Bulk Metallic Materials to Nanoparticles. Metall. Mater. Trans. A—Phys. Metall. Mater. Sci. 2018, 49a, 4986–4990. [Google Scholar] [CrossRef]
- Yeh, A.C.; Tsao, T.K.; Chang, Y.J.; Chang, K.C.; Yeh, J.W.; Chiou, M.S.; Jian, S.R.; Kuo, C.M.; Wang, W.R.; Murakami, H. Developing New Type of High Temperature Alloys–High Entropy Superalloys. Int. J. Metall. Mater. Eng. 2015, 2015, 107. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A Review on Fundamental of High Entropy Alloys with Promising High–Temperature Properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical Performance of the AlxCoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Yurchenko, N.Y.; Zherebtsov, S.V.; Ladygin, A.N.; Salishchev, G.A.; Tikhonovsky, M.A. High Temperature Deformation Behavior and Dynamic Recrystallization in CoCrFeNiMn High Entropy Alloy. Mater. Sci. Eng. A 2015, 636, 188–195. [Google Scholar] [CrossRef]
- Jo, Y.H.; Jung, S.; Choi, W.M.; Sohn, S.S.; Kim, H.S.; Lee, B.J.; Kim, N.J.; Lee, S. Cryogenic Strength Improvement by Utilizing Room-Temperature Deformation Twinning in a Partially Recrystallized VCrMnFeCoNi High-Entropy Alloy. Nat. Commun. 2017, 8, 15719. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, J.; An, X.; Wang, H.; Zhang, Y.; Liao, X. Cryogenic-Deformation-Induced Phase Transformation in an FeCoCrNi High-Entropy Alloy. Mater. Res. Lett. 2018, 6, 236–243. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Shu, D.; Zhu, G.; Wang, D.; Sun, B. Mechanical Instability and Tensile Properties of TiZrHfNbTa High Entropy Alloy at Cryogenic Temperatures. Acta Mater. 2020, 201, 517–527. [Google Scholar] [CrossRef]
- Sokkalingam, R.; Sivaprasad, K.; Duraiselvam, M.; Muthupandi, V.; Prashanth, K.G. Novel Welding of Al0.5CoCrFeNi High-Entropy Alloy: Corrosion Behavior. J. Alloys Compd. 2020, 817, 153163. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion Behavior of an Equiatomic CoCrFeMnNi High-Entropy Alloy Compared with 304 Stainless Steel in Sulfuric Acid Solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Nene, S.S.; Frank, M.; Liu, K.; Sinha, S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Corrosion-Resistant High Entropy Alloy with High Strength and Ductility. Scr. Mater. 2019, 166, 168–172. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of High Entropy Alloys. Npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chang, Y.-J.; Murakami, H.; Sasaki, T.; Hono, K.; Li, C.-W.; Kakehi, K.; Yeh, J.-W.; Yeh, A.-C. Hierarchical Microstructure Strengthening in a Single Crystal High Entropy Superalloy. Sci. Rep. 2020, 10, 12163. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B.; Tsai, M.-H.; Senkov, O.N.; Soni, V.; Banerjee, R. Refractory High Entropy Superalloys (RSAs). Scr. Mater. 2020, 187, 445–452. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chang, Y.-J.; Murakami, H.; Gorsse, S.; Yeh, A.-C. Designing High Entropy Superalloys for Elevated Temperature Application. Scr. Mater. 2020, 187, 177–182. [Google Scholar] [CrossRef]
- Senkov, O.N.; Jensen, J.K.; Pilchak, A.L.; Miracle, D.B.; Fraser, H.L. Compositional Variation Effects on the Microstructure and Properties of a Refractory High-Entropy Superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Senkov, O.N.; Isheim, D.; Seidman, D.N.; Pilchak, A.L. Development of a Refractory High Entropy Superalloy. Entropy 2016, 18, 102. [Google Scholar] [CrossRef]
- Jain, H.; Shadangi, Y.; Chakravarty, D.; Dubey, A.K.; Mukhopadhyay, N.K. High Entropy Steel Processed through Mechanical Alloying and Spark Plasma Sintering: Alloying Behaviour, Thermal Stability and Mechanical Properties. Mater. Sci. Eng. A 2022, 856, 144029. [Google Scholar] [CrossRef]
- Jain, H.; Shadangi, Y.; Chakravarty, D.; Chattopadhyay, K.; Dubey, A.K.; Mukhopadhyay, N.K. Low-Density Fe40Mn19Ni15Al15Si10C1 High Entropy Steel Processed by Mechanical Alloying and Spark Plasma Sintering: Phase Evolution, Microstructure and Mechanical Properties. Mater. Sci. Eng. A 2023, 869, 144776. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, P.; Fang, Y.; Yang, Z.; Zhang, N.; Prashanth, K.G.; Jia, Y. Effect of NiCoFeAlTi High Entropy Intermetallic Reinforcement Particle Size on the Microstructure and Mechanical Properties of CoCrFeMnNi High-Entropy Alloy Composites Fabricated by Selective Laser Melting. J. Alloys Compd. 2023, 947, 169417. [Google Scholar] [CrossRef]
- Ding, Z.; Bian, J.; Shuang, S.; Liu, X.; Hu, Y.; Sun, C.; Yang, Y. High Entropy Intermetallic–Oxide Core–Shell Nanostructure as Superb Oxygen Evolution Reaction Catalyst. Adv. Sustain. Syst. 2020, 4, 1900105. [Google Scholar] [CrossRef]
- Zhou, N.; Jiang, S.; Huang, T.; Qin, M.; Hu, T.; Luo, J. Single-Phase High-Entropy Intermetallic Compounds (HEICs): Bridging High-Entropy Alloys and Ceramics. Sci. Bull. 2019, 64, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.N.; Gaytan, S.M.; Murr, L.E.; Martinez, E.; Shindo, P.W.; Hernandez, J.; Collins, S.; Medina, F. Microstructures and Mechanical Behavior of Inconel 718 Fabricated by Selective Laser Melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Liu, W.C.; Yao, M.; Chen, Z.L.; Wang, S.G. Niobium Segregation in Inconel 718. J. Mater. Sci. 1999, 34, 2583–2586. [Google Scholar] [CrossRef]
- Rajamani, D.; Ananthakumar, K.; Balasubramanian, E.; Paulo Davim, J. Experimental Investigation and Optimization of PAC Parameters on Monel 400TM Superalloy. Mater. Manuf. Process. 2018, 33, 1864–1873. [Google Scholar] [CrossRef]
- Parida, A.K.; Maity, K. Comparison the Machinability of Inconel 718, Inconel 625 and Monel 400 in Hot Turning Operation. Eng. Sci. Technol. Int. J. 2018, 21, 364–370. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A.; Mishra, R.K.; Shaz, M.A.; Yadav, T.P.; Shahi, R.R. Investigations on Phase Formation and Magnetic Properties of PromisingCo35Cr5Fe10Ni30Ti20 High Entropy Alloysynthesized through Radio Frequency Induction Melting. J. Alloys Compd. 2023, 960, 170697. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, T.P.; Mukhopadhyay, N.K. Notable Hydrogen Storage in Ti–Zr–V–Cr–Ni High Entropy Alloy. Int. J. Hydrogen Energy 2022, 47, 22893–22900. [Google Scholar] [CrossRef]
- Shadangi, Y.; Chattopadhyay, K.; Mukhopadhyay, N.K. Powder Metallurgical Processing of Al Matrix Composite Reinforced with AlSiCrMnFeNiCu High-Entropy Alloys: Microstructure, Thermal Stability, and Microhardness. J. Mater. Res. 2023, 38, 248–264. [Google Scholar] [CrossRef]
- Guo, J.; Tang, C.; Rothwell, G.; Li, L.; Wang, Y.-C.; Yang, Q.; Ren, X. Welding of High Entropy Alloys—A Review. Entropy 2019, 21, 431. [Google Scholar] [CrossRef]
- Fourmont, A.; Rogachev, A.S.; Le Gallet, S.; Politano, O.; Kovalev, D.Y.; Kochetov, N.A.; Shkodich, N.F.; Vadchenko, S.G.; Baras, F. Thermal Stability of Medium-and High-Entropy Alloys of 3d-Transition Metals. J. Phase Equilib. Diffus. 2021, 42, 720–734. [Google Scholar] [CrossRef]
- Shen, J.; Agrawal, P.; Rodrigues, T.A.; Lopes, J.G.; Schell, N.; Zeng, Z.; Mishra, R.S.; Oliveira, J.P. Gas Tungsten Arc Welding of As-Cast AlCoCrFeNi2.1 Eutectic High Entropy Alloy. Mater. Des. 2022, 223, 111176. [Google Scholar] [CrossRef]
- Martin, A.C.; Oliveira, J.P.; Fink, C. Elemental Effects on Weld Cracking Susceptibility in AlxCoCrCuyFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2020, 51, 778–787. [Google Scholar] [CrossRef]
- Lin, P.-T.; Liu, H.-C.; Hsieh, P.-Y.; Wei, C.-Y.; Tsai, C.-W.; Sato, Y.S.; Chen, S.-C.; Yen, H.-W.; Lu, N.-H.; Chen, C.-H. Heterogeneous Structure-Induced Strength-Ductility Synergy by Partial Recrystallization during Friction Stir Welding of a High-Entropy Alloy. Mater. Des. 2021, 197, 109238. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Curado, T.M.; Zeng, Z.; Lopes, J.G.; Rossinyol, E.; Park, J.M.; Schell, N.; Braz Fernandes, F.M.; Kim, H.S. Gas Tungsten Arc Welding of As-Rolled CrMnFeCoNi High Entropy Alloy. Mater. Des. 2020, 189, 108505. [Google Scholar] [CrossRef]
- Wu, Z.; David, S.A.; Feng, Z.; Bei, H. Weldability of a High Entropy CrMnFeCoNi Alloy. Scr. Mater. 2016, 124, 81–85. [Google Scholar] [CrossRef]
- Nam, H.; Park, S.; Park, N.; Na, Y.; Kim, H.; Yoo, S.-J.; Moon, Y.-H.; Kang, N. Weldability of Cast CoCrFeMnNi High-Entropy Alloys Using Various Filler Metals for Cryogenic Applications. J. Alloys Compd. 2020, 819, 153278. [Google Scholar] [CrossRef]
- Shen, J.; Gonçalves, R.; Choi, Y.T.; Lopes, J.G.; Yang, J.; Schell, N.; Kim, H.S.; Oliveira, J.P. Microstructure and Mechanical Properties of Gas Metal Arc Welded CoCrFeMnNi Joints Using a 410 Stainless Steel Filler Metal. Mater. Sci. Eng. A 2022, 857, 144025. [Google Scholar] [CrossRef]
- Nam, H.; Yoo, S.; Ha, J.W.; Lee, B.-J.; Song, S.; Na, Y.; Kang, N. Enhancement of Tensile Properties of Gas Tungsten Arc Welds Using Cu-Coated CoCrFeMnNi Filler and Post–Weld Heat Treatment. J. Mater. Res. Technol. 2022, 19, 4857–4866. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Z.; Hu, W.; Lu, Y.; Zhu, Z.; Ji, Y.; Lu, Y.; Qin, Z.; Lu, X. In Situ Strengthening of CrMnFeCoNi High-Entropy Alloy with Al Realized by Laser Additive Manufacturing. J. Alloys Compd. 2020, 847, 156563. [Google Scholar] [CrossRef]
- Nam, H.; Park, S.; Chun, E.-J.; Kim, H.; Na, Y.; Kang, N. Laser Dissimilar Weldability of Cast and Rolled CoCrFeMnNi High-Entropy Alloys for Cryogenic Applications. Sci. Technol. Weld. Join. 2020, 25, 127–134. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Shen, J.; Zeng, Z.; Park, J.M.; Choi, Y.T.; Schell, N.; Maawad, E.; Zhou, N.; Kim, H.S. Dissimilar Laser Welding of a CoCrFeMnNi High Entropy Alloy to 316 Stainless Steel. Scr. Mater. 2022, 206, 114219. [Google Scholar] [CrossRef]
- Adomako, N.K.; Shin, G.; Park, N.; Park, K.; Kim, J.H. Laser Dissimilar Welding of CoCrFeMnNi-High Entropy Alloy and Duplex Stainless Steel. J. Mater. Sci. Technol. 2021, 85, 95–105. [Google Scholar] [CrossRef]
- Bellmann, J.; Roder, K.; Zimmermann, M.; Beyer, E.; Kroll, L.; Nestler, D. Influence of Copper Interlayers on the Magnetic Pulse Welding Process between Aluminum and Steel. Metals 2021, 11, 868. [Google Scholar] [CrossRef]
- Sadeghian, A.; Iqbal, N. A Review on Dissimilar Laser Welding of Steel-Copper, Steel-Aluminum, Aluminum-Copper, and Steel-Nickel for Electric Vehicle Battery Manufacturing. Opt. Laser Technol. 2022, 146, 107595. [Google Scholar] [CrossRef]
- Gullino, A.; Matteis, P.; D’Aiuto, F. Review of Aluminum-To-Steel Welding Technologies for Car-Body Applications. Metals 2019, 9, 315. [Google Scholar] [CrossRef]
- Sokoluk, M.; Cao, C.; Pan, S.; Li, X. Nanoparticle-Enabled Phase Control for Arc Welding of Unweldable Aluminum Alloy 7075. Nat. Commun. 2019, 10, 98. [Google Scholar] [CrossRef]
- Luebbe, M.; Duan, J.; Zhang, F.; Poplawsky, J.; Pommeranke, H.; Arivu, M.; Hoffman, A.; Buchely, M.; Wen, H. A High-Strength Precipitation Hardened Cobalt-Free High-Entropy Alloy. Mater. Sci. Eng. A 2023, 870, 144848. [Google Scholar] [CrossRef]
- Nautiyal, P.; Jain, J.; Agarwal, A. Influence of Loading Path and Precipitates on Indentation Creep Behavior of Wrought Mg–6 Wt% Al–1 Wt% Zn Magnesium Alloy. Mater. Sci. Eng. A 2016, 650, 183–189. [Google Scholar] [CrossRef]
- Ye, X.; Hua, X.M.; Wu, Y.X.; Lou, S.N. Precipitates in Coarse-Grained Heat-Affected Zone of Ni-Based 718 Superalloy Produced by Tungsten Inert Gas Welding. J. Mater. Process. Technol. 2015, 217, 13–20. [Google Scholar] [CrossRef]
- Wang, J.; Peng, F.; Zhou, L.; Luo, Y.; Zhang, W.; Wu, Z. High-Strength Ductility Joining of Multicomponent Alloy to 304 Stainless Steel Using Laser Welding Technique. Materials 2023, 16, 2374. [Google Scholar] [CrossRef]
- Arab, A.; Guo, Y.; Zhou, Q.; Chen, P. Joining AlCoCrFeNi High Entropy Alloys and Al-6061 by Explosive Welding Method. Vacuum 2020, 174, 109221. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Shi, J.; Du, Y.; Peng, Y.; Jin, F.; Xiong, J.; Zhang, F. Microstructure and Mechanical Properties of the Brazed Region in the AlCoCrFeNi High-Entropy Alloy and FGH98 Superalloy Joint. Mater. Sci. Eng. A 2021, 804, 140714. [Google Scholar] [CrossRef]
- Lin, C.; Shiue, R.-K.; Wu, S.-K.; Lin, Y.-S. Dissimilar Infrared Brazing of CoCrFe(Mn)Ni Equiatomic High Entropy Alloys and 316 Stainless Steel. Crystals 2019, 9, 518. [Google Scholar] [CrossRef]
- Li, H.; Shen, W.; Chen, W.; Wang, W.; Liu, G.; Lu, C.; Zheng, W.; Ma, Y.; Yang, J.; Ding, Z.; et al. Microstructural Evolution and Mechanical Properties of AlCoCrFeNi High-Entropy Alloy Joints Brazed Using a Novel Ni-Based Filler. J. Alloys Compd. 2021, 860, 157926. [Google Scholar] [CrossRef]
- Ren, H.S.; Feng, H.L.; Ren, X.Y.; Pang, S.J.; Cheng, Y.Y.; Xiong, H.P. Joining of TiAl-Based Alloy and a Ni-Based Superalloy with a NiCoFeCuSiB High Entropy Filler Metal. Weld World 2022, 66, 557–565. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, J.; Song, X.G.; Yang, M.X.; Yang, T.L.; Yin, J. Eutectic-Reaction Brazing of Al0.3CoCrFeNi High-Entropy Alloys Using Ni/Nb/Ni Interlayers. J. Mater. Sci. Technol. 2022, 121, 245–255. [Google Scholar] [CrossRef]
- Song, X.G.; Sun, J.; Wang, Z.H.; Hu, S.P.; Lin, D.Y.; Chen, N.B.; Liu, D.; Long, W.M. Brazing of SiC Ceramic to Al0.3CoCrFeNi High Entropy Alloy by Graphene Nanoplates Reinforced AgCuTi Composite Fillers. Ceram. Int. 2023, 49, 19216–19226. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Wang, M.; He, R.; Tan, C.; Cao, W.; Xu, H. Brazing ZrB2-SiC Ceramics to Nb with a Novel CoFeNiCrCu High Entropy Alloy. J. Eur. Ceram. Soc. 2021, 41, 54–61. [Google Scholar] [CrossRef]
- Bridges, D.; Zhang, S.; Lang, S.; Gao, M.; Yu, Z.; Feng, Z.; Hu, A. Laser Brazing of a Nickel-Based Superalloy Using a Ni-Mn-Fe-Co-Cu High Entropy Alloy Filler Metal. Mater. Lett. 2018, 215, 11–14. [Google Scholar] [CrossRef]
- Mathieu, A.; Shabadi, R.; Deschamps, A.; Suery, M.; Matteï, S.; Grevey, D.; Cicala, E. Dissimilar Material Joining Using Laser (Aluminum to Steel Using Zinc-Based Filler Wire). Opt. Laser Technol. 2007, 39, 652–661. [Google Scholar] [CrossRef]
- Mohan, D.G.; Tomków, J.; Karganroudi, S.S. Laser Welding of UNS S33207 Hyper-Duplex Stainless Steel to 6061 Aluminum Alloy Using High Entropy Alloy as a Filler Material. Appl. Sci. 2022, 12, 2849. [Google Scholar] [CrossRef]
- Azhari-Saray, H.; Sarkari-Khorrami, M.; Nademi-Babahadi, A.; Kashani-Bozorg, S.F. Dissimilar Resistance Spot Welding of 6061-T6 Aluminum Alloy/St-12 Carbon Steel Using a High Entropy Alloy Interlayer. Intermetallics 2020, 124, 106876. [Google Scholar] [CrossRef]
- Liu, J.; Lin, D.; Hu, J.; Xi, X.; Tang, Z.; Liu, Z.; Hu, S.; Bian, H.; Song, X. Microstructure and Mechanical Properties of a GH3536/SS304 Joint Brazed with a Co25Fe25Mn5Ni25Ti20 Eutectic High-Entropy Alloy Filler. Mater. Charact. 2022, 193, 112305. [Google Scholar] [CrossRef]
- Bridges, D.; Ma, C.; Palmer, Z.; Wang, S.; Feng, Z.; Hu, A. Laser Brazing of Inconel® 718 Using Ag and Cu-Ag Nanopastes as Brazing Materials. J. Mater. Process. Technol. 2017, 249, 313–324. [Google Scholar] [CrossRef]
- Bridges, D.; Nielsen, B.; Zhang, L.; Zhang, S.; Xu, R.; Hu, A. Wettability, Diffusion Behaviors, and Modeling of Ni Nanoparticles and Nanowires in Brazing Inconel 718. Adv. Eng. Mater. 2020, 23, 2001053. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, X.X.; Chen, H.Z.; Zhang, L.X. Brazing of TiAl and Ti2AlNb Alloys Using High-Entropy Braze Fillers. Mater. Charact. 2022, 186, 111814. [Google Scholar] [CrossRef]
- Wu, B.; Yu, W.; Hu, Z.; Sun, X. Microstructural Evolution and Mechanical Properties in the Self-Propagating Brazing Joint of Al0.1CoCrFeNi HEAs and 304 SS Using Reactive Multilayer Nanofoils. Mater. Charact. 2023, 196, 112572. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, G.; Zhao, Y.; Tan, C.; He, R. Brazing SiC Ceramics and Zr with CoCrFeNiCuSn High Entropy Alloy. Ceram. Int. 2022, 48, 23325–23333. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, H.; Nai, X.; Wang, P.; Deng, H.; Wen, G.; Liu, F.; Li, W. Microstructure and Mechanical Properties of SiCf/SiC Composites/GH536 Superalloy Joints Brazed with CoFeNiCrCu High-Entropy Alloy Filler. Mater. Charact. 2022, 194, 112419. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Zhao, Y.; Wang, M.; He, R.; Tan, C.; Wang, W.; Zhou, X. Joining of SiC Using CoFeCrNiCuTi High Entropy Alloy Filler by Electric Current Field Assisted Sintering. J. Eur. Ceram. Soc. 2022, 42, 1995–2003. [Google Scholar] [CrossRef]
- Hu, J.; Lin, D.; Li, X.; Li, H.; Li, J.; Tang, Z.; Xi, X.; Song, X.; Fu, W. Interfacial Strengthening Mechanism of C/C/Nb0.74CoCrFeNi2/Nb Brazed Joints: Stress-Relieving Effects of the FCC Phase. Mater. Sci. Eng. A 2022, 854, 143895. [Google Scholar] [CrossRef]
- Way, M.; Luo, D.; Tuley, R.; Goodall, R. A New High Entropy Alloy Brazing Filler Metal Design for Joining Skutterudite Thermoelectrics to Copper. J. Alloys Compd. 2021, 858, 157750. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Bloom, B.P.; Wei, J.; Li, H.; Li, H.; Du, Y.; Zeng, Z.; Li, L.; Waldeck, D.H. Elemental Core Level Shift in High Entropy Alloy Nanoparticles via X-ray Photoelectron Spectroscopy Analysis and First-Principles Calculation. ACS Nano 2020, 14, 17704–17712. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal Shock Synthesis of High-Entropy-Alloy Nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef]
- Nam, S.; Song, Y.; Son, M.; Lee, K.-A.; Yoo, G.H.; Park, E.S.; Choi, H.; Lee, C.S. High-Temperature Mechanical and Thermochemical Properties of NbMoTaW Refractory High-Entropy Alloy Coatings Produced via Nano Particle Deposition System (NPDS). Int. J. Refract. Met. Hard Mater. 2021, 99, 105594. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, J.; Yi, G.; Shan, Y.; Geng, Y.; Wang, J.; Wang, W. Comparative Study of the Microstructure and Phase Evolution of FeCoCrNiAl High-Entropy Alloy-Matrix WC Nanocomposite Powders Prepared by Mechanical Alloying. J. Alloys Compd. 2023, 938, 168518. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Qin, Q.; Li, J.; Zhao, H.; Gao, X.; Su, X.; Chen, D. Influence of Cr and Al to FeCoNiCrxAl2-x Alloys Synthesised by Mechanochemistry. Mater. Sci. Technol. 2021, 37, 545–551. [Google Scholar] [CrossRef]
- Qiao, H.; Saray, M.T.; Wang, X.; Xu, S.; Chen, G.; Huang, Z.; Chen, C.; Zhong, G.; Dong, Q.; Hong, M.; et al. Scalable Synthesis of High Entropy Alloy Nanoparticles by Microwave Heating. ACS Nano 2021, 15, 14928–14937. [Google Scholar] [CrossRef]
- Tang, J.; Xu, J.L.; Ye, Z.G.; Li, X.B.; Luo, J.M. Microwave Sintered Porous CoCrFeNiMo High Entropy Alloy as an Efficient Electrocatalyst for Alkaline Oxygen Evolution Reaction. J. Mater. Sci. Technol. 2021, 79, 171–177. [Google Scholar] [CrossRef]
- Feng, G.; Ning, F.; Song, J.; Shang, H.; Zhang, K.; Ding, Z.; Gao, P.; Chu, W.; Xia, D. Sub-2 Nm Ultrasmall High-Entropy Alloy Nanoparticles for Extremely Superior Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2021, 143, 17117–17127. [Google Scholar] [CrossRef]
- Wu, D.; Kusada, K.; Nanba, Y.; Koyama, M.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Seo, O.; Gueye, I.; Kim, J.; et al. Noble-Metal High-Entropy-Alloy Nanoparticles: Atomic-Level Insight into the Electronic Structure. J. Am. Chem. Soc. 2022, 144, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Jiang, Y.; Yang, H.; Wang, H.; Fang, Y.; Wang, L.; Xie, M.; Qiu, P.; Luo, W. Constructing Structurally Ordered High-Entropy Alloy Nanoparticles on Nitrogen-Rich Mesoporous Carbon Nanosheets for High-Performance Oxygen Reduction. Adv. Mater. 2022, 34, 2110128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Yang, X.; Zhang, J.; Sun, Z.; Chen, Y.; Liu, F. A Facile Synthesis of High Entropy Alloy Nanoparticle–Activated Carbon Nanocomposites for Synergetic Degradation of Methylene Blue. RSC Adv. 2021, 11, 24636–24646. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, H.; Morova, Y.; Asghari Alamdari, A.; Eroğlu, Z.; Sennaroğlu, A.; Guo, S.; Metin, O.; Motallebzadeh, A. Femtosecond Laser-Mediated Preparation of HfNbTaTiZr Refractory High-Entropy Alloy Nanoparticles for Photothermal Therapy Applications: Influence of Solvent and Fluence. Intermetallics 2023, 156, 107834. [Google Scholar] [CrossRef]
- Rawat, R.; Singh, B.K.; Tiwari, A.; Arun, N.; Pathak, A.P.; Shadangi, Y.; Mukhopadhyay, N.K.; Nelamarri, S.R.; Rao, S.V.; Tripathi, A. Formation of Cu-Ni Enriched Phases during Laser Processing of Non-Equiatomic AlSiCrMnFeNiCu High Entropy Alloy Nanoparticles. J. Alloys Compd. 2022, 927, 166905. [Google Scholar] [CrossRef]
- Waag, F.; Li, Y.; Ziefuß, A.R.; Bertin, E.; Kamp, M.; Duppel, V.; Marzun, G.; Kienle, L.; Barcikowski, S.; Gökce, B. Kinetically-Controlled Laser-Synthesis of Colloidal High-Entropy Alloy Nanoparticles. RSC Adv. 2019, 9, 18547–18558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, B.; Ke, X.; Xu, F.; Bozhilov, K.N.; Hu, L.; Shahbazian-Yassar, R.; Zachariah, M.R. Aerosol Synthesis of High Entropy Alloy Nanoparticles. Langmuir 2020, 36, 1985–1992. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, Q.; Yi, W.; Hao, X.; Li, J.; Zhang, B.; Huang, L.; Huang, Y.; Jiang, Y.; Xu, B.; et al. Facile and General Method to Synthesize Pt-Based High-Entropy-Alloy Nanoparticles. ACS Nano 2022, 16, 14017–14028. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Lv, J.; Jia, H.; Li, H.; Liu, W.; Xie, G.; Chen, Z.; Huang, Y.; Yuan, Q.; et al. Multi-Component Nanoporous Alloy/(Oxy)Hydroxide for Bifunctional Oxygen Electrocatalysis and Rechargeable Zn-Air Batteries. Appl. Catal. B Environ. 2020, 268, 118431. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, S. High-Entropy Materials for Catalysis: A New Frontier. Sci. Adv. 2021, 7, eabg1600. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2021, 18, 2104339. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, K.; Li, C.; Lin, X.; Liu, H.; Shi, W.; Qiu, H.-J.; Yuan, Y.; Yao, Y. High-Entropy Alloy Catalysts: From Bulk to Nano toward Highly Efficient Carbon and Nitrogen Catalysis. Carbon Energy 2022, 4, 731–761. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent Progress of Metal–Air Batteries—A Mini Review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Wen, Y.; Liu, P.; Xie, G.; Liu, X.; Sun, S. Nanoporous High-Entropy Alloys for Highly Stable and Efficient Catalysts. J. Mater. Chem. A 2019, 7, 6499–6506. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, X.; Hu, Y.; Tang, X.; Hu, K.; Madhav Reddy, K.; Lin, X.; Qiu, H.-J. A Fourteen-Component High-Entropy Alloy@oxide Bifunctional Electrocatalyst with a Record-Low Δ E of 0.61 V for Highly Reversible Zn–Air Batteries. Chem. Sci. 2022, 13, 12056–12064. [Google Scholar] [CrossRef]
- Löffler, T.; Savan, A.; Meyer, H.; Meischein, M.; Strotkötter, V.; Ludwig, A.; Schuhmann, W. Design of Complex Solid-Solution Electrocatalysts by Correlating Configuration, Adsorption Energy Distribution Patterns, and Activity Curves. Angew. Chem. Int. Ed. 2020, 59, 5844–5850. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Li, T.; Wang, H.; Liu, Y.; Stein, H.S.; Mao, Y.; Gao, J.; Jiao, M.; Dong, Q.; et al. High-Throughput, Combinatorial Synthesis of Multimetallic Nanoclusters. Proc. Natl. Acad. Sci. USA 2020, 117, 6316–6322. [Google Scholar] [CrossRef]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative Production of Methanol from Industrial CO2. Renew. Energy 2020, 146, 1192–1203. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Bagger, A.; Rossmeisl, J. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Roy, D.; Mandal, S.C.; Pathak, B. Machine Learning-Driven High-Throughput Screening of Alloy-Based Catalysts for Selective CO2 Hydrogenation to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 56151–56163. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Mandal, S.C.; Pathak, B. Machine Learning Assisted Exploration of High Entropy Alloy-Based Catalysts for Selective CO2 Reduction to Methanol. J. Phys. Chem. Lett. 2022, 13, 5991–6002. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. Are Metal Chalcogenides, Nitrides, and Phosphides Oxygen Evolution Catalysts or Bifunctional Catalysts? ACS Energy Lett. 2017, 2, 1937–1938. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Gao, J.; Wen, Y.; Lv, J.; Li, H.; Xie, G.; Liu, X.; Sun, S. Noble Metal-Free Nanoporous High-Entropy Alloys as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Mater. Lett. 2019, 1, 526–533. [Google Scholar] [CrossRef]
- Fan, L.; Ji, Y.; Wang, G.; Chen, J.; Chen, K.; Liu, X.; Wen, Z. High Entropy Alloy Electrocatalytic Electrode toward Alkaline Glycerol Valorization Coupling with Acidic Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 7224–7235. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Zhao, H.; Qi, W.; Zhang, D.; Yu, Y.; Cai, W.; Li, S.; Lai, J.; Huang, B.; et al. Fast Site-to-Site Electron Transfer of High-Entropy Alloy Nanocatalyst Driving Redox Electrocatalysis. Nat. Commun. 2020, 11, 5437. [Google Scholar] [CrossRef]
- Xue, L.; Ding, Y.; Pradeep, K.G.; Case, R.; Castaneda, H.; Paredes, M. The Grain Size Effect on Corrosion Property of Al2Cr5Cu5Fe53Ni35 High-Entropy Alloy in Marine Environment. Corros. Sci. 2022, 208, 110625. [Google Scholar] [CrossRef]
- Drach, A. Utilization of Copper Alloys for Marine Applications. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2013. [Google Scholar]
- Johnson, L.A. Corrosion Behavior of Cold Sprayed Aluminum Oxide Reinforced Aluminum Coatings. Master’s Thesis, Naval Postgraduate School, Monterey, CA, USA, 2021. [Google Scholar]
- Romhanji, E.; Popović, M. Problems and Prospect of Al-Mg Alloys Application in Marine Constructions. Metalurgija 2006, 12, 297–307. [Google Scholar]
- Fahim, J.; Hadavi, S.M.M.; Ghayour, H.; Hassanzadeh Tabrizi, S.A. Cavitation Erosion Behavior of Super-Hydrophobic Coatings on Al5083 Marine Aluminum Alloy. Wear 2019, 424–425, 122–132. [Google Scholar] [CrossRef]
- Feng, S.; Guan, S.; Story, W.A.; Ren, J.; Zhang, S.; Te, A.; Gleason, M.A.; Heelan, J.; Walde, C.; Birt, A.; et al. Cold Spray Additive Manufacturing of CoCrFeNiMn High-Entropy Alloy: Process Development, Microstructure, and Mechanical Properties. J. Therm. Spray Tech. 2022, 31, 1222–1231. [Google Scholar] [CrossRef]
- Sova, A.; Doubenskaia, M.; Trofimov, E.; Samodurova, M. Deposition of High-Entropy Alloy Coating by Cold Spray Combined with Laser Melting: Feasibility Tests. J. Therm. Spray Tech. 2022, 31, 1112–1128. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn High Entropy Alloy (HEA) Coating via Cold Spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Wahl, M. Marine Epibiosis. I. Fouling and Antifouling: Some Basic Aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Zhu, S.; Qiao, Z.; Zhang, J.; Yang, J.; Liu, W. A Novel Cu-Doped High Entropy Alloy with Excellent Comprehensive Performances for Marine Application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]
- Tung, C.C.; Yeh, J.W.; Shun, T.; Chen, S.K.; Huang, Y.S. On the Elemental Effect of AlCoCrCuFeNi High-Entropy Alloy System. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, R.; Qin, G.; Li, X.; Su, Y.; Ding, H.; Guo, J.; Fu, H. Microstructure Evolution, Cu Segregation and Tensile Properties of CoCrFeNiCu High Entropy Alloy during Directional Solidification. J. Mater. Sci. Technol. 2020, 38, 19–27. [Google Scholar] [CrossRef]
- Ayyagari, A.; Barthelemy, C.; Gwalani, B.; Banerjee, R.; Scharf, T.W.; Mukherjee, S. Reciprocating Sliding Wear Behavior of High Entropy Alloys in Dry and Marine Environments. Mater. Chem. Phys. 2018, 210, 162–169. [Google Scholar] [CrossRef]
- Xue, L.; Ding, Y.; Pradeep, K.G.; Case, R.; Castaneda, H.; Paredes, M. Development of a Non-Equimolar AlCrCuFeNi High-Entropy Alloy and Its Corrosive Response to Marine Environment under Different Temperatures and Chloride Concentrations. J. Alloys Compd. 2022, 928, 167112. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Grewal, H.S. Enhanced Cavitation Erosion Resistance of a Friction Stir Processed High Entropy Alloy. Int. J. Miner. Metall. Mater. 2020, 27, 1353–1362. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally High Cavitation Erosion and Corrosion Resistance of a High Entropy Alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, S.; Li, Z.; Jiang, S.; Xie, Z.-H.; Munroe, P.; Lu, H. Remarkable Cavitation Erosion–Corrosion Resistance of CoCrFeNiTiMo High-Entropy Alloy Coatings. Corros. Sci. 2021, 190, 109663. [Google Scholar] [CrossRef]
- Cao, H.; Hou, G.; Fu, Z.; Ma, J.; An, Y.; Zhou, H.; Chen, J. Design of High-Entropy Alloy Coating for Cavitation Erosion Resistance by Different Energy-Induced Dynamic Cyclic Behaviors. ACS Appl. Mater. Interfaces 2023, 15, 3651–3663. [Google Scholar] [CrossRef] [PubMed]
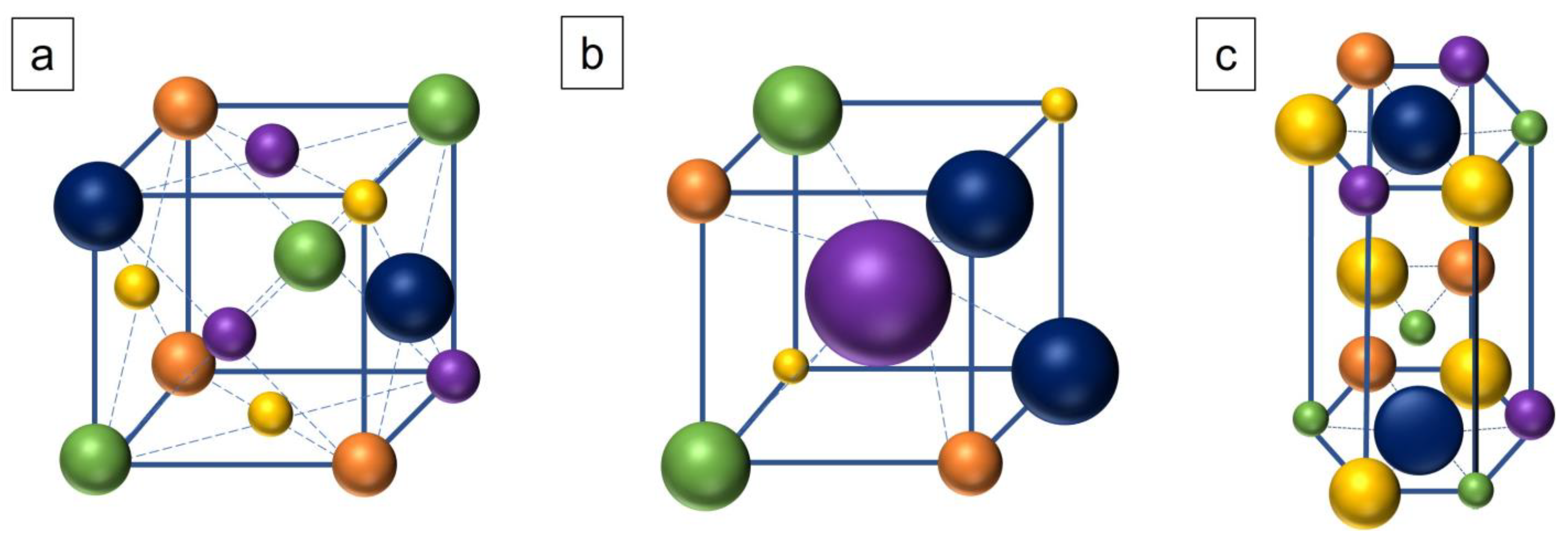


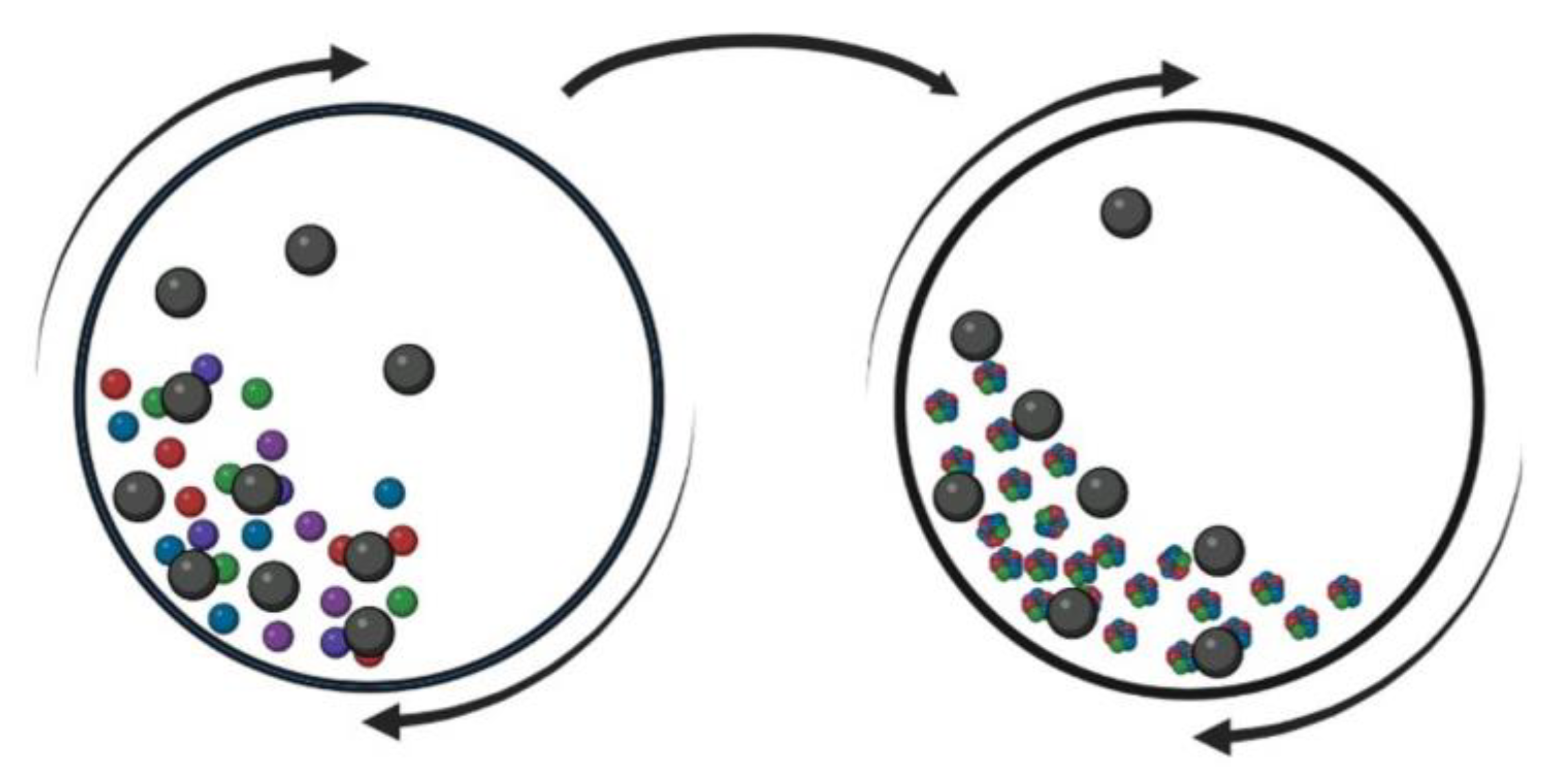
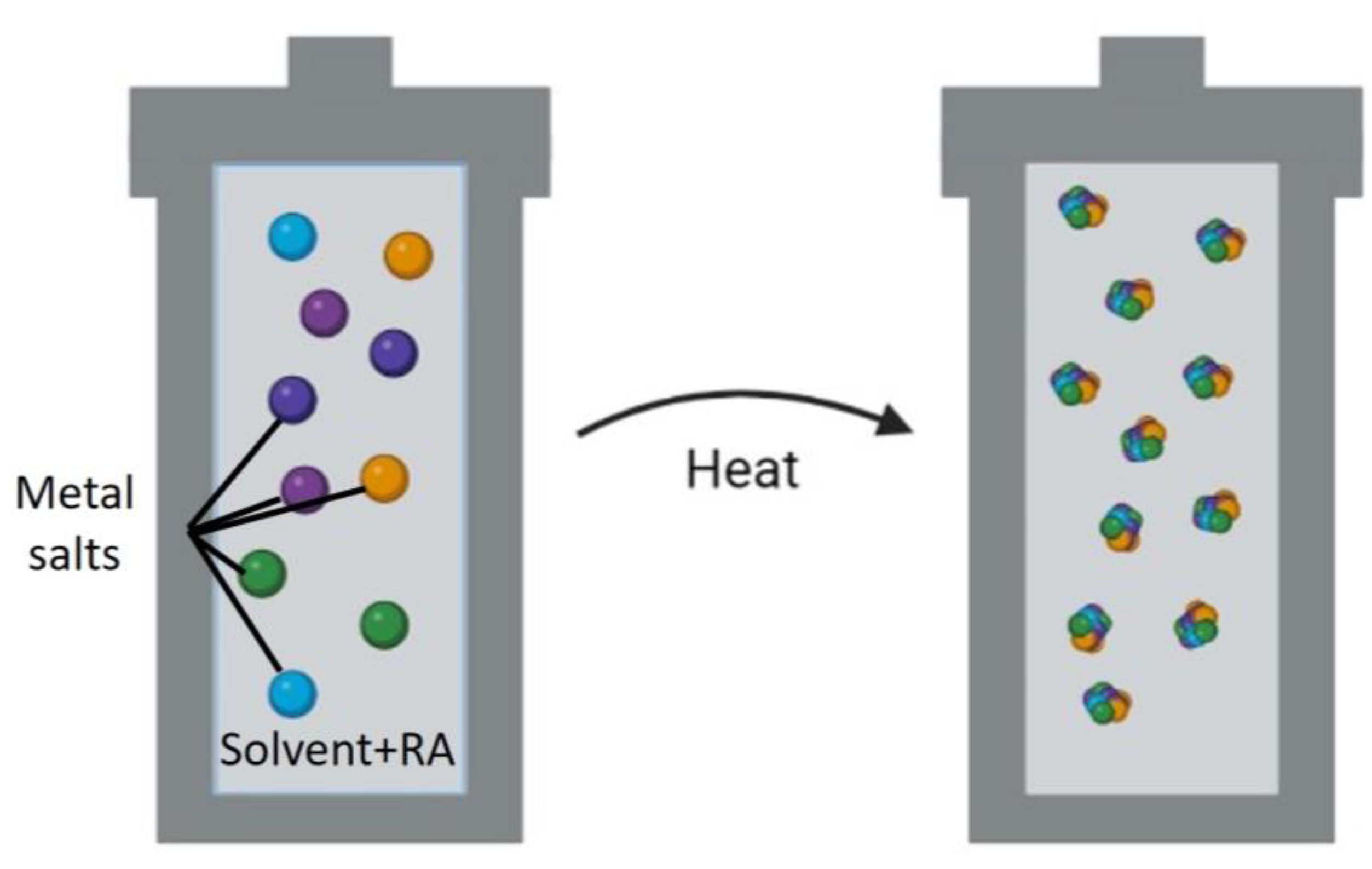

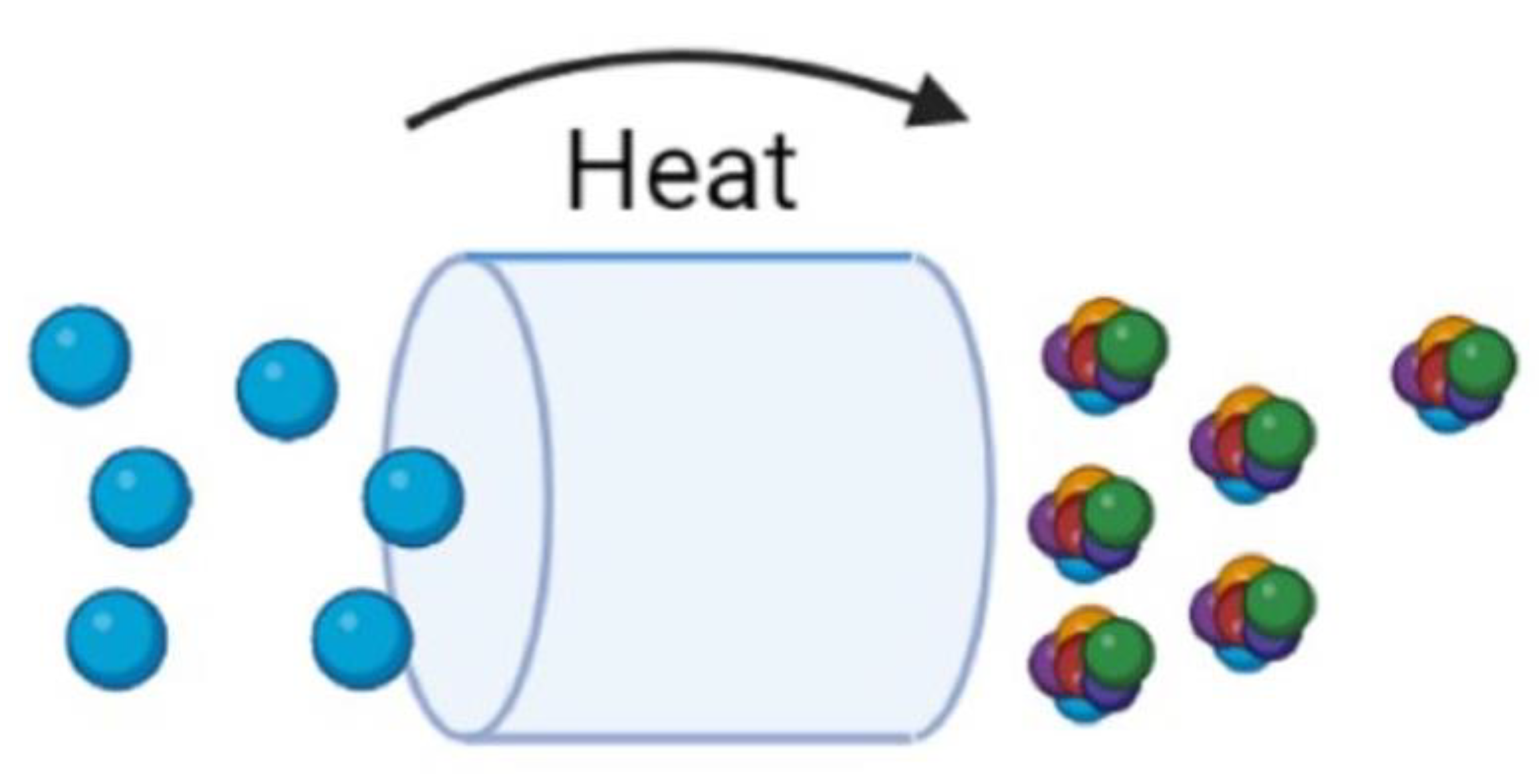

| Material | Ni | Cr | B | Si | Fe | P | C | S | Ti | Al | Zr | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNi-2 | Bal. | 7 | 3.12 | 4.59 | 3 | 0.02 | 0.06 | 0.02 | 0.05 | 0.05 | 0.10 | 0.6 |
| MBF601 | Bal. | 16 | 0.5 | 1.5 | 32 | 6 | 0 | 0 | 0 | 0 | 0 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bridges, D.; Fieser, D.; Santiago, J.J.; Hu, A. Novel Frontiers in High-Entropy Alloys. Metals 2023, 13, 1193. https://doi.org/10.3390/met13071193
Bridges D, Fieser D, Santiago JJ, Hu A. Novel Frontiers in High-Entropy Alloys. Metals. 2023; 13(7):1193. https://doi.org/10.3390/met13071193
Chicago/Turabian StyleBridges, Denzel, David Fieser, Jannira J. Santiago, and Anming Hu. 2023. "Novel Frontiers in High-Entropy Alloys" Metals 13, no. 7: 1193. https://doi.org/10.3390/met13071193
APA StyleBridges, D., Fieser, D., Santiago, J. J., & Hu, A. (2023). Novel Frontiers in High-Entropy Alloys. Metals, 13(7), 1193. https://doi.org/10.3390/met13071193







