Investigation of the Supercapacitive Behavior of Electroless Ni-B Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Electroless Ni-B and Ni Coatings
- Grinding with #600 and #800 SiC paper;
- Polishing;
- Five minutes of ultrasonic cleaning in acetone;
- Ten minutes of degreasing in NaOH (10 wt.%) solution;
- Thirty seconds of surface activation in cc. HCl (37 wt.%) solution.
2.2. Material Characterization
3. Primary Results
3.1. Composition and Specific Surface Area
3.2. Surface Morphology and Microstructure
3.3. Electrochemical Behavior
3.3.1. CV
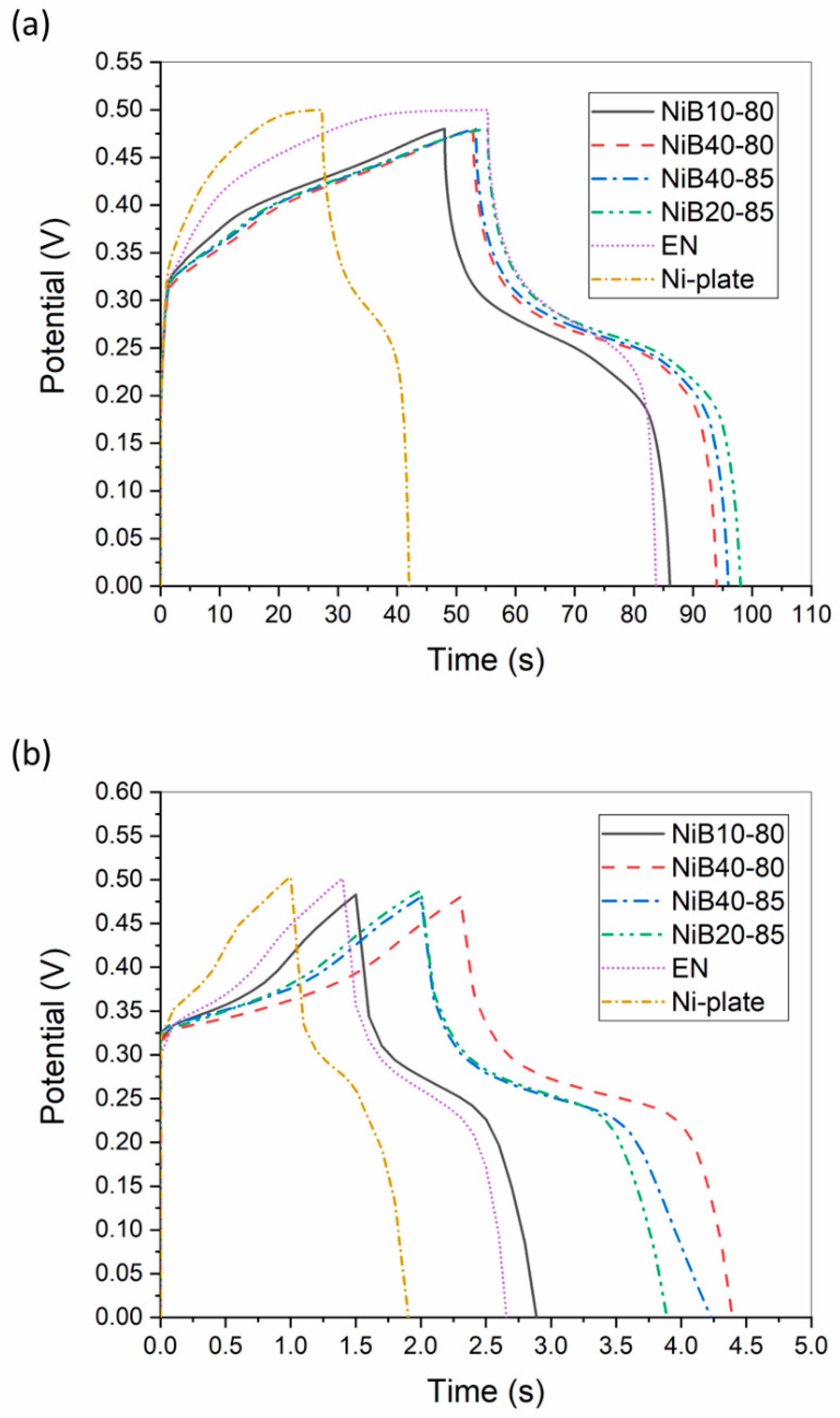
3.3.2. GCD Measurement
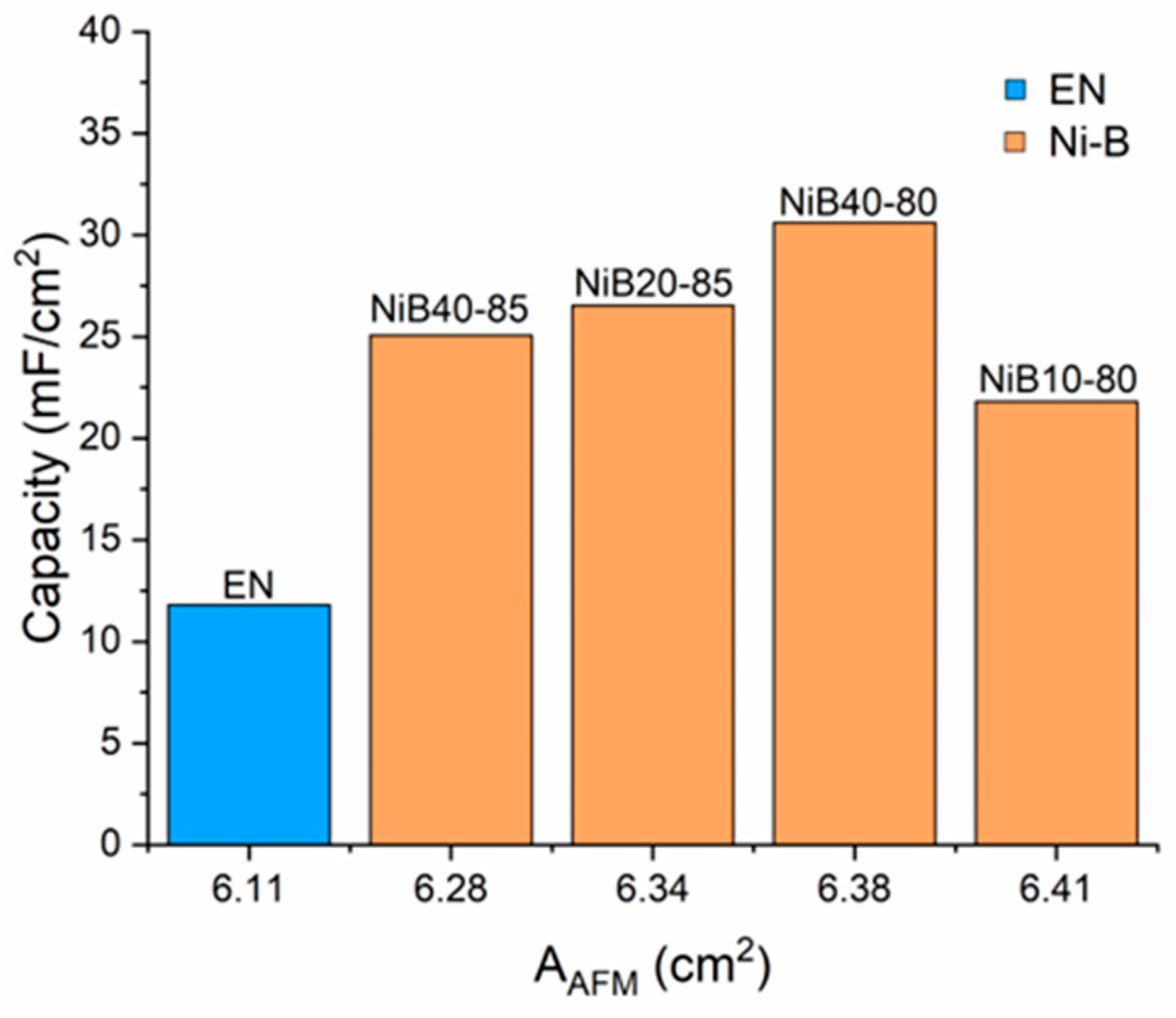
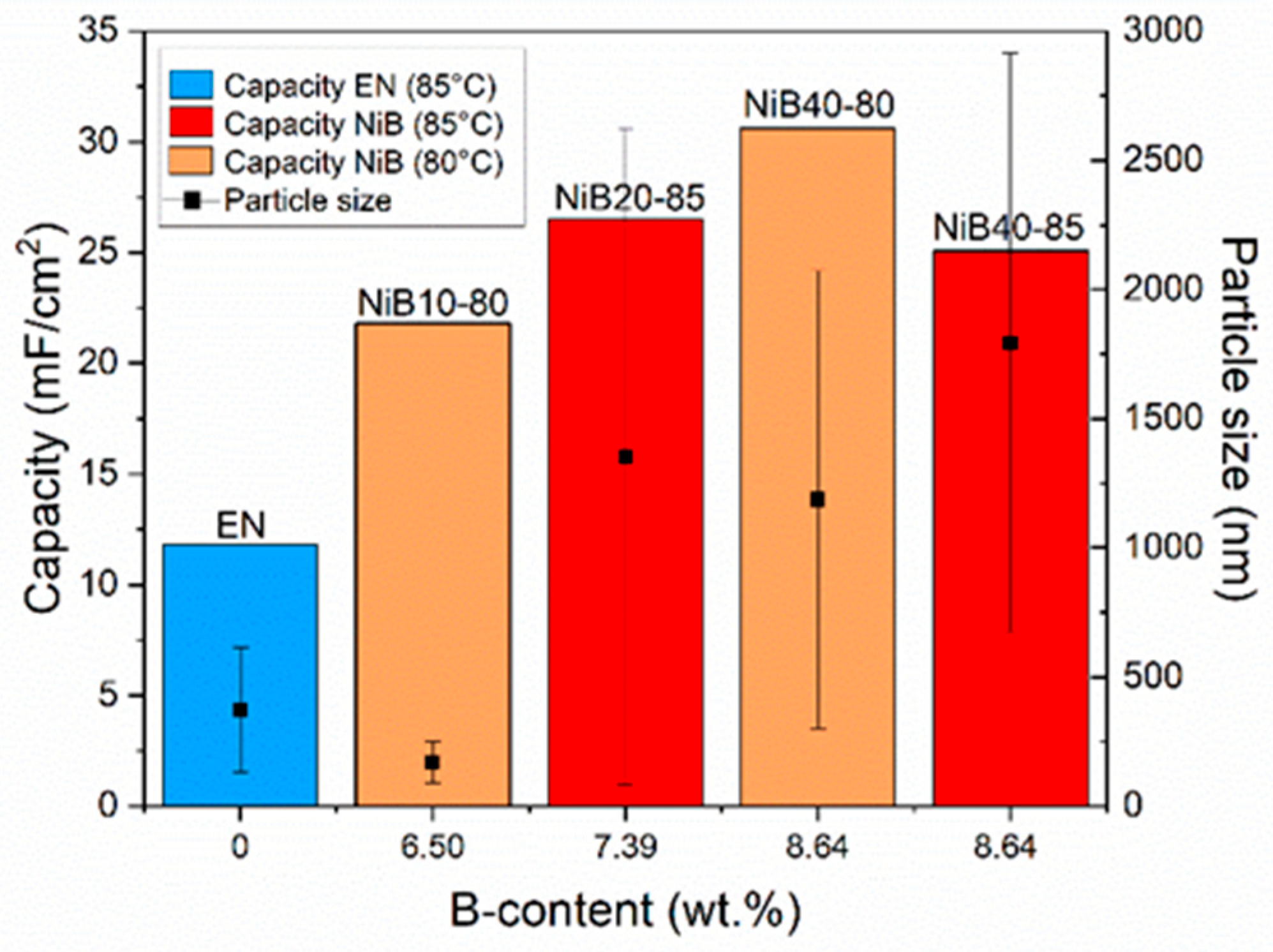
3.3.3. Specific Capacitance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wang, H.; Liu, G. Friction and Wear of Electroless Ni-P-CS Composite Coating. Metals 2023, 13, 315. [Google Scholar] [CrossRef]
- Czagány, M.; Baumli, P. Effect of Surfactants on the Behavior of the Ni-P Bath and on the Formation of Electroless Ni-P-TiC Composite Coatings. Surf. Coat. Technol. 2019, 361, 42–49. [Google Scholar] [CrossRef]
- Gul, H.; Algul, H.; Akyol, A.; Uysal, M.; Alp, A. Evaluation of Wear and Corrosion Behavior of Electroless Ni-B-P/CNT Composite Coatings on Aluminum Surfaces. Diam. Relat. Mater. 2023, 137, 110075. [Google Scholar] [CrossRef]
- Ma, B.; Wang, R. Tuning Interface to Improve Corrosion Resistance of Electroless Ni-P Coating on AZ31B Alloy. Metals 2018, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Takács, D.; Sziráki, L.; Török, T.I.; Sólyom, J.; Gácsi, Z.; Gál-Solymos, K. Effects of Pre-Treatments on the Corrosion Properties of Electroless Ni–P Layers Deposited on AlMg2 Alloy. Surf. Coat. Technol. 2007, 201, 4526–4535. [Google Scholar] [CrossRef]
- Gültekin, D.; Duru, E.; Akbulut, H. Improved Wear Behaviors of Lead-Free Electroless Ni B and Ni-B/CeO2 Composite Coatings. Surf. Coat. Technol. 2021, 422, 127525. [Google Scholar] [CrossRef]
- Gyökér, Z.; Gergely, G.; Koncz Horváth, D.; Bodnár, E.; Gácsi, Z. Role of Reinforcement Surface Treatment on the SnAg3Cu0.5 Microelectronic Joints. Appl. Surf. Sci. 2019, 475, 982–985. [Google Scholar] [CrossRef]
- Peeters, P.; Hoorn, G.V.D.; Daenen, T.; Kurowski, A.; Staikov, G. Properties of Electroless and Electroplated Ni–P and Its Application in Microgalvanics. Electrochim. Acta 2001, 47, 161–169. [Google Scholar] [CrossRef]
- Czagány, M.; Baumli, P.; Kaptay, G. The Influence of the Phosphorous Content and Heat Treatment on the Nano-Micro-Structure, Thickness and Micro-Hardness of Electroless Ni-P Coatings on Steel. Appl. Surf. Sci. 2017, 423, 160–169. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, Z.; Liu, X.; Xie, G.; Zhang, B. Electroless Nickel Plating on Alumina Ceramic Activated by Metallic Nickel as Electrocatalyst for Oxygen Evolution Reaction. Catal. Commun. 2021, 149, 106238. [Google Scholar] [CrossRef]
- He, J.; Song, L.; Yan, J.; Kang, N.; Zhang, Y.; Wang, W. Hydrogen Evolution Reaction Property in Alkaline Solution of Molybdenum Disulfide Modified by Surface Anchor of Nickel–Phosphorus Coating. Metals 2017, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Fundo, A.M.; Abrantes, L.M. The Electrocatalytic Behaviour of Electroless Ni–P Alloys. J. Electroanal. Chem. 2007, 600, 63–79. [Google Scholar] [CrossRef]
- Wang, D.; Kong, L.-B.; Liu, M.-C.; Zhang, W.-B.; Luo, Y.-C.; Kang, L. Amorphous Ni–P Materials for High Performance Pseudocapacitors. J. Power Sources 2015, 274, 1107–1113. [Google Scholar] [CrossRef]
- Lin, J.-D.; Chou, C.-T. The Influence of Acid Etching on the Electrochemical Supercapacitive Properties of Ni P Coatings. Surf. Coat. Technol. 2017, 325, 360–369. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Liu, Z.; Huang, Q.; Lu, B.; Wang, Y.; Wang, C.; Guo, L. Copper Oxide Nitrogen-Rich Porous Carbon Network Boosts High-Performance Supercapacitors. Metals 2023, 13, 981. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Adedoja, O.S.; Sadiku, E.R.; Hamam, Y. An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications. Polymers 2023, 15, 2272. [Google Scholar] [CrossRef]
- Bhojane, P. Recent Advances and Fundamentals of Pseudocapacitors: Materials, Mechanism, and Its Understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Nuilek, K.; Wongwiriyapan, W.; Sattayarut, V.; Simon, A.; Koncz-Horváth, D.; Ferenczi, T.; Kristály, F.; Baumli, P. Comparison of Acid Exfoliators in Carbon Nanosheets Synthesis from Stinging Nettle (Urtica Dioica) for Electrochemical Applications. Sci. Rep. 2020, 10, 17270. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of Carbon-Based Electrode Materials for Supercapacitor Energy Storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Cheng, J.P.; Zhang, J.; Liu, F. Recent Development of Metal Hydroxides as Electrode Material of Electrochemical Capacitors. RSC Adv. 2014, 4, 38893–38917. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive Materials for Electrochemical Capacitors: From Rational Synthesis to Capacitance Optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zheng, M.; Xue, H.; Pang, H. High Performance Electrochemical Capacitor Materials Focusing on Nickel Based Materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar] [CrossRef]
- Yus, J.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Sangiorgi, A.; Sangiorgi, N.; Gardini, D.; Sanson, A.; Galassi, C.; Caballero, A.; Morales, J.; et al. Semiconductor Water-Based Inks: Miniaturized NiO Pseudocapacitor Electrodes by Inkjet Printing. J. Eur. Ceram. Soc. 2019, 39, 2908–2914. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.S.; Wang, Z.; Madhavi, S.; Lou, X.W. (David) Green Synthesis of NiO Nanobelts with Exceptional Pseudo-Capacitive Properties. Adv. Energy Mater. 2012, 2, 1188–1192. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y.; Ghosh, K.; Jena, R.K. Review on Advances in Porous Nanostructured Nickel Oxides and Their Composite Electrodes for High-Performance Supercapacitors. J. Power Sources 2016, 308, 121–140. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Nickel Foam-Supported Porous Ni(OH)2/NiOOH Composite Film as Advanced Pseudocapacitor Material. Electrochim. Acta 2011, 56, 2627–2632. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel Hydroxides and Related Materials: A Review of Their Structures, Synthesis and Properties. Proc. R. Soc. A 2015, 471, 20140792. [Google Scholar] [CrossRef] [PubMed]
- Sudagar, J.; Lian, J.; Sha, W. Electroless Nickel, Alloy, Composite and Nano Coatings—A Critical Review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Hamid, Z.A.; Hassan, H.B.; Attyia, A.M. Influence of Deposition Temperature and Heat Treatment on the Performance of Electroless Ni–B Films. Surf. Coat. Technol. 2010, 205, 2348–2354. [Google Scholar] [CrossRef]
- Fang, J.; Chen, X.; Liu, B.; Yan, S.; Qiao, M.; Li, H.; He, H.; Fan, K. Liquid-Phase Chemoselective Hydrogenation of 2-Ethylanthraquinone over Chromium-Modified Nanosized Amorphous Ni–B Catalysts. J. Catal. 2005, 229, 97–104. [Google Scholar] [CrossRef]
- Wang, D.; Kong, L.-B.; Liu, M.-C.; Luo, Y.-C.; Kang, L. An Approach to Preparing Ni-P with Different Phases for Use as Supercapacitor Electrode Materials. Chem. Eur. J. 2015, 21, 17897–17903. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Salunkhe, R.R.; Imura, M.; Yamauchi, Y. Phosphonate-Derived Nanoporous Metal Phosphates and Their Superior Energy Storage Application. ACS Appl. Mater. Interfaces 2016, 8, 9790–9797. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wu, M.; Wang, X.; Long, Y.; Lou, X. Direct Aqueous Solution Synthesis of an Ultra-Fine Amorphous Nickel–Boron Alloy with Superior Pseudocapacitive Performance for Advanced Asymmetric Supercapacitors. New J. Chem. 2017, 41, 7302–7311. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Increase of Boron Content in Electroless Nickel-Boron Coating by Modification of Plating Conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Agrawal, R.; Mukhopadhyay, A. Development of Ni-B Electroless Coating from Stabilizer Free Bath and Characterization of High Temperature Tribological Behaviour, Scratch and Corrosion Resistance. Surf. Topogr. Metrol. Prop. 2022, 10, 045028. [Google Scholar] [CrossRef]
- Agrawal, R.; Mukhopadhyay, A. Inclusion of W in Electroless Ni–B Coating Developed from a Stabilizer Free Bath and Investigation of Its Tribological Behaviour. J. Indian Chem. Soc. 2023, 100, 100966. [Google Scholar] [CrossRef]
- Yunacti, M.; Mégret, A.; Staia, M.H.; Montagne, A.; Vitry, V. Characterization of Electroless Nickel–Boron Deposit from Optimized Stabilizer-Free Bath. Coatings 2021, 11, 576. [Google Scholar] [CrossRef]
- Bulbul, F. The Effects of Deposition Parameters on Surface Morphology and Crystallographic Orientation of Electroless Ni-B Coatings. Met. Mater. Int. 2011, 17, 67–75. [Google Scholar] [CrossRef]
- Baskaran, I.; Sakthi Kumar, R.; Sankara Narayanan, T.S.N.; Stephen, A. Formation of Electroless Ni–B Coatings Using Low Temperature Bath and Evaluation of Their Characteristic Properties. Surf. Coat. Technol. 2006, 200, 6888–6894. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate Methods for Evaluating the Efficiency and Capacitive Behavior of Different Types of Supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Czagany, M.; Baumli, P. Effect of PH on the Characteristics of Electroless Ni-P Coatings. J. Min. Met. B Met. 2017, 53, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Barati, Q.; Hadavi, S.M.M. Electroless Ni-B and Composite Coatings: A Critical Review on Formation Mechanism, Properties, Applications and Future Trends. Surf. Interfaces 2020, 21, 100702. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, W.; Wang, X.; Zhao, Y.; Ma, P.; Jiang, W.; Song, G. The Microstructure and Tribological Behavior of Ultrasonic Electroless Ni-P Plating on TC4 Titanium Alloy with Heat-Treatment. Ferroelectrics 2022, 589, 1–11. [Google Scholar] [CrossRef]
- Duru, E.; Doğan, F.; Uysal, M.; Akbulut, H.; Aslan, S. Optimization of Ni-B Coating Bath and Effect of DMAB Concentration on Hardness and Wear. Surf. Interfaces 2021, 22, 100880. [Google Scholar] [CrossRef]
- Rao, Q.; Bi, G.; Lu, Q.; Wang, H.; Fan, X. Microstructure Evolution of Electroless Ni-B Film during Its Depositing Process. Appl. Surf. Sci. 2005, 240, 28–33. [Google Scholar] [CrossRef]
- Baskaran, I.; Narayanan, T.S.N.S.; Stephen, A. Effect of Accelerators and Stabilizers on the Formation and Characteristics of Electroless Ni–P Deposits. Mater. Chem. Phys. 2006, 99, 117–126. [Google Scholar] [CrossRef]
- Bonin, L.; Castro, C.C.; Vitry, V.; Hantson, A.-L.; Delaunois, F. Optimization of Electroless NiB Deposition without Stabilizer, Based on Surface Roughness and Plating Rate. J. Alloys Compd. 2018, 767, 276–284. [Google Scholar] [CrossRef]
- Pal, S.; Verma, N.; Jayaram, V.; Biswas, S.K.; Riddle, Y. Characterization of Phase Transformation Behaviour and Microstructural Development of Electroless Ni–B Coating. Mater. Sci. Eng. A 2011, 528, 8269–8276. [Google Scholar] [CrossRef]
- Vitry, V.; Kanta, A.-F.; Dille, J.; Delaunois, F. Structural State of Electroless Nickel–Boron Deposits (5 wt.% B): Characterization by XRD and TEM. Surf. Coat. Technol. 2012, 206, 3444–3449. [Google Scholar] [CrossRef]
- Kumar, P.S.; Nair, P.K. X-Ray Diffraction Studies on the Relative Proportion and Decomposition of Amorphous Phase in Electroless Ni-B Deposits. Nanostruct. Mater. 1994, 4, 183–198. [Google Scholar] [CrossRef]
- Haag, S.; Burgard, M.; Ernst, B. Pure Nickel Coating on a Mesoporous Alumina Membrane: Preparation by Electroless Plating and Characterization. Surf. Coat. Technol. 2006, 201, 2166–2173. [Google Scholar] [CrossRef]
- Çelik, İ.; Karakan, M.; Bülbül, F. Investigation of Structural and Tribological Properties of Electroless Ni–B Coated Pure Titanium. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2016, 230, 57–63. [Google Scholar] [CrossRef]
- Ekmekci, D.; Bülbül, F. Preparation and Characterization of Electroless Ni–B/Nano-SiO2, Al2O3, TiO2 and CuO Composite Coatings. Bull. Mater. Sci. 2015, 38, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Bhat, T.S.; Patil, P.S.; Rakhi, R.B. Recent Trends in Electrolytes for Supercapacitors. J. Energy Storage 2022, 50, 104222. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Ding, H.; Ni, C. Recent Progress in Nickel Based Materials for High Performance Pseudocapacitor Electrodes. J. Power Sources 2014, 267, 430–444. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ai, X.P.; Cao, Y.L.; Yang, H.X. Exceptional Electrochemical Activities of Amorphous Fe–B and Co–B Alloy Powders Used as High Capacity Anode Materials. Electrochem. Commun. 2004, 6, 780–784. [Google Scholar] [CrossRef]
- Lo, Y.L.; Hwang, B.J. In Situ Raman Studies on Cathodically Deposited Nickel Hydroxide Films and Electroless Ni-P Electrodes in 1 M KOH Solution. Langmuir 1998, 14, 944–950. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.L.; Ji, H.; Li, Y.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Nanoporous Ni(OH)2 Thin Film on 3D Ultrathin-Graphite Foam for Asymmetric Supercapacitor. ACS Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Tang, X.; Hu, W. High-Performance Three-Dimensional Nanoporous NiO Film as a Supercapacitor Electrode. J. Mater. Chem. 2012, 22, 11062. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Liu, Y.; Jia, B. Peak Potential Shift of Fast Cyclic Voltammograms Owing to Capacitance of Redox Reactions. J. Electroanal. Chem. 2020, 856, 113609. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Liu, X.; Huang, S.; Wang, T.; Wang, X.; Gu, C.; Tu, J.; Mao, S.X. Facile Synthesis of Ni-Coated Ni2P for Supercapacitor Applications. CrystEngComm 2013, 15, 7071. [Google Scholar] [CrossRef]
- Dong, X.; Cao, Y.; Wang, J.; Chan-Park, M.B.; Wang, L.; Huang, W.; Chen, P. Hybrid Structure of Zinc Oxide Nanorods and Three Dimensional Graphene Foam for Supercapacitor and Electrochemical Sensor Applications. RSC Adv. 2012, 2, 4364. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.; Gong, H. A High Energy Density Asymmetric Supercapacitor from Nano-Architectured Ni(OH)2/Carbon Nanotube Electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Liang, K.; Tang, X.; Wei, B.; Hu, W. Fabrication and Characterization of a Nanoporous NiO Film with High Specific Energy and Power via an Electrochemical Dealloying Approach. Mater. Res. Bull. 2013, 48, 3829–3833. [Google Scholar] [CrossRef]
- Lin, J.-D.; Chou, C.-T. The Influence of Phosphorus Content on the Microstructure and Specific Capacitance of Etched Electroless Ni-P Coatings. Surf. Coat. Technol. 2019, 368, 126–137. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Supercapacitor Studies on NiO Nanoflakes Synthesized Through a Microwave Route. ACS Appl. Mater. Interfaces 2013, 5, 2188–2196. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, B.; Du, J.; Chen, J.; Zhang, J.; Li, S. Porous Nickel Oxide Nanospindles with Huge Specific Capacitance and Long-Life Cycle. RSC Adv. 2012, 2, 2257. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Ravi, C.; Asokamani1, R. Electronic Structure, Bonding, and Ground-State Properties of AlB_{2}-Type Transition-Metal Diborides. Phys. Rev. B 2001, 63, 045115. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.-G.; Zhuo, K.; Cherevko, S.; Chung, C.-H. Formation of Nanoporous Nickel Oxides for Supercapacitors Prepared by Electrodeposition with Hydrogen Evolution Reaction and Electrochemical Dealloying. Korean J. Chem. Eng. 2012, 29, 1802–1805. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Xu, H.-T.; Liu, L.; Wang, Y. Correlation of the Structure and Applications of Dealloyed Nanoporous Metals in Catalysis and Energy Conversion/Storage. Nanoscale 2015, 7, 386–400. [Google Scholar] [CrossRef]
- Baskaran, I.; Sankara Narayanan, T.S.N.; Stephen, A. Corrosion Resistance of Electroless Ni–Low B Coatings. Trans. IMF 2009, 87, 221–224. [Google Scholar] [CrossRef]










| Element | Fe | Mn | Cr | Cu | Bi | Pb | V | Ni |
|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | 97.85 | 1.78 | 0.08 | 0.07 | 0.07 | 0.06 | 0.04 | 0.04 |
| Bath Composition | Concentration | |||
|---|---|---|---|---|
| NiB10-80 | NiB40-80 | NiB40-85 | NiB20-85 | |
| NiCl2 (g/L) | 10 | 40 | 40 | 20 |
| EDA (g/L) | 90 | 90 | 90 | 90 |
| NaOH (g/L) | 90 | 90 | 90 | 90 |
| NaBH4(g/L) | 0.8 | 0.8 | 0.8 | 1.2 |
| Thiourea (mg/L) | 1 | 1 | 1 | 1 |
| Conditions | ||||
| pH | >13 | |||
| T (°C) | 80 | 80 | 85 | 85 |
| Deposition time | 60 min | 60 min | 60 min | 60 min |
| Bath Composition | Concentration |
|---|---|
| Ni-acetate (g/L) | 15 |
| Na2-EDTA (g/L) | 5.95 |
| Lactic acid (g/L) | 13.5 |
| Hydrazine(g/L) | 20 |
| NaOH (g/L) | 7.5 |
| Conditions | |
| pH | 9.4 |
| T (°C) | 80 |
| Deposition time (min) | 60 |
| NiB10-80 | NiB40-80 | NiB40-85 | NiB20-85 | EN | |
|---|---|---|---|---|---|
| Mass (mg) | 8 ± 1 | 23 ± 1 | 23 ± 1 | 17 ± 1 | 24 ± 1 |
| B content (wt.%) | 6.50 | 8.64 | 8.64 | 7.39 | - |
| B content (at.%) | 27.42 | 33.95 | 33.95 | 30.25 | - |
| Asample (cm2) | 5.88 | 5.88 | 5.88 | 5.88 | 5.88 |
| AAFM/Aproj (%) | 109.11 | 108.55 | 106.90 | 107.90 | 104.05 |
| AAFM (cm2) | 6.41 | 6.38 | 6.28 | 6.34 | 6.12 |
| Cs (mF/cm2) | ||||||
|---|---|---|---|---|---|---|
| Ni plate | EN | NB10-80 | NB40-80 | NB40-85 | NB-20-85 | |
| CV (10 mV/s) | 11.65 | 11.81 | 21.80 | 30.61 | 25.07 | 26.53 |
| CV (100 mV/s) | 7.37 | 8.50 | 15.28 | 24.61 | 21.54 | 21.12 |
| GCD (1 mA/cm2) | 34.915 | 66.530 | 83.907 | 93.276 | 97.618 | 100.761 |
| GCD (5 mA/cm2) | 1.816 | 2.605 | 2.968 | 4.622 | 4.444 | 4.077 |
| Cs (F/g) | ||||||
|---|---|---|---|---|---|---|
| Ni plate | EN | NB10-80 | NB40-80 | NB40-85 | NB-20-85 | |
| CV (10 mV/s) | - | 3.01 | 16.02 | 7.82 | 6.41 | 9.75 |
| CV (100 mV/s) | - | 2.17 | 11.23 | 6.29 | 5.50 | 7.76 |
| GCD (0.5 A/g) | - | 3.087 | 16.992 | 6.342 | 5.859 | 8.349 |
| GCD (1 A/g) | - | 0.508 | 2.593 | 1.146 | 2.430 | 1.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czagany, M.; Hompoth, S.; Windisch, M.; Baumli, P. Investigation of the Supercapacitive Behavior of Electroless Ni-B Coatings. Metals 2023, 13, 1233. https://doi.org/10.3390/met13071233
Czagany M, Hompoth S, Windisch M, Baumli P. Investigation of the Supercapacitive Behavior of Electroless Ni-B Coatings. Metals. 2023; 13(7):1233. https://doi.org/10.3390/met13071233
Chicago/Turabian StyleCzagany, Mate, Szabolcs Hompoth, Márk Windisch, and Peter Baumli. 2023. "Investigation of the Supercapacitive Behavior of Electroless Ni-B Coatings" Metals 13, no. 7: 1233. https://doi.org/10.3390/met13071233





