Influence of Temperature Regimes of Synthetic Iron Smelting on Casting Production Efficiency
Abstract
:1. Introduction
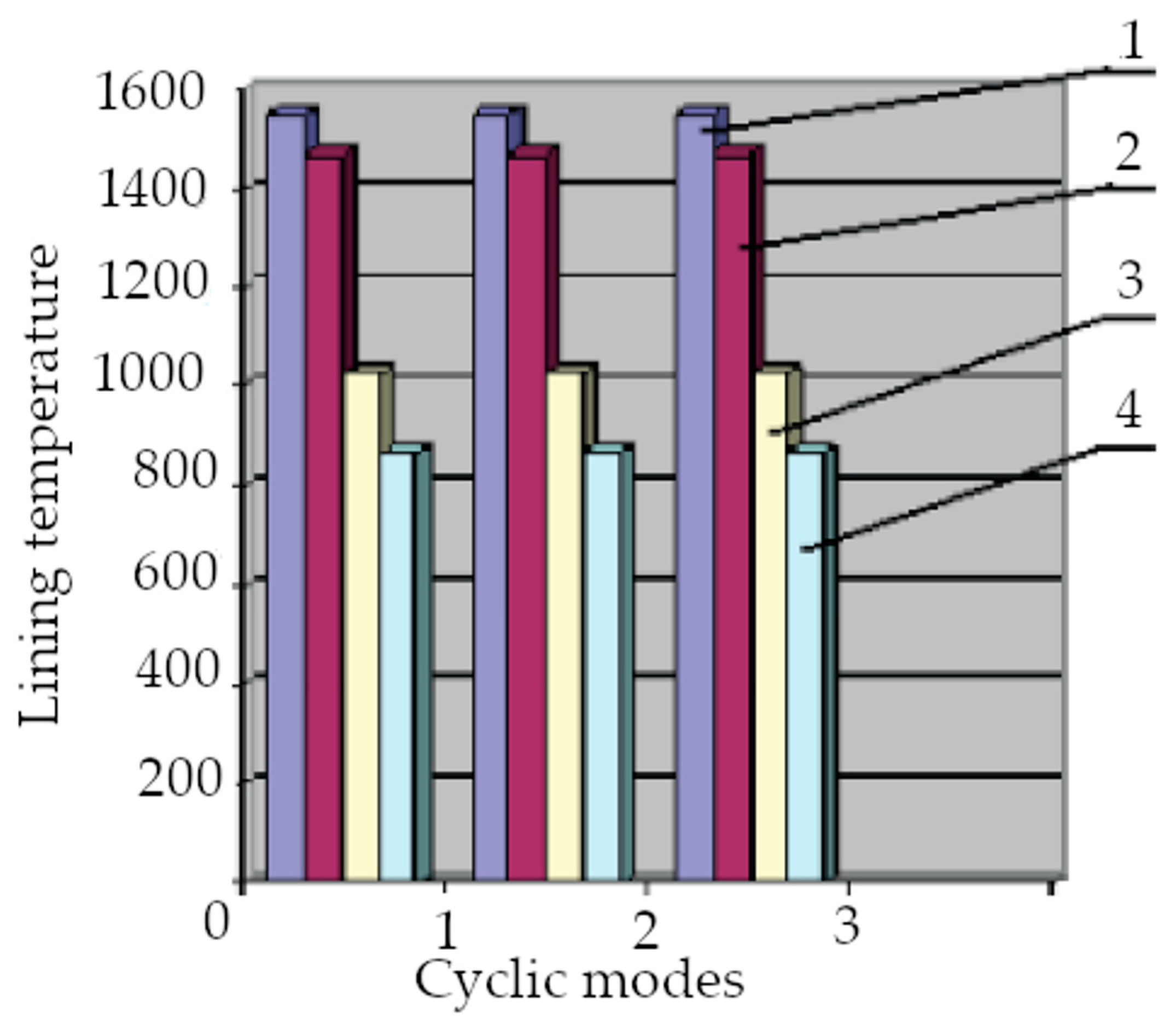
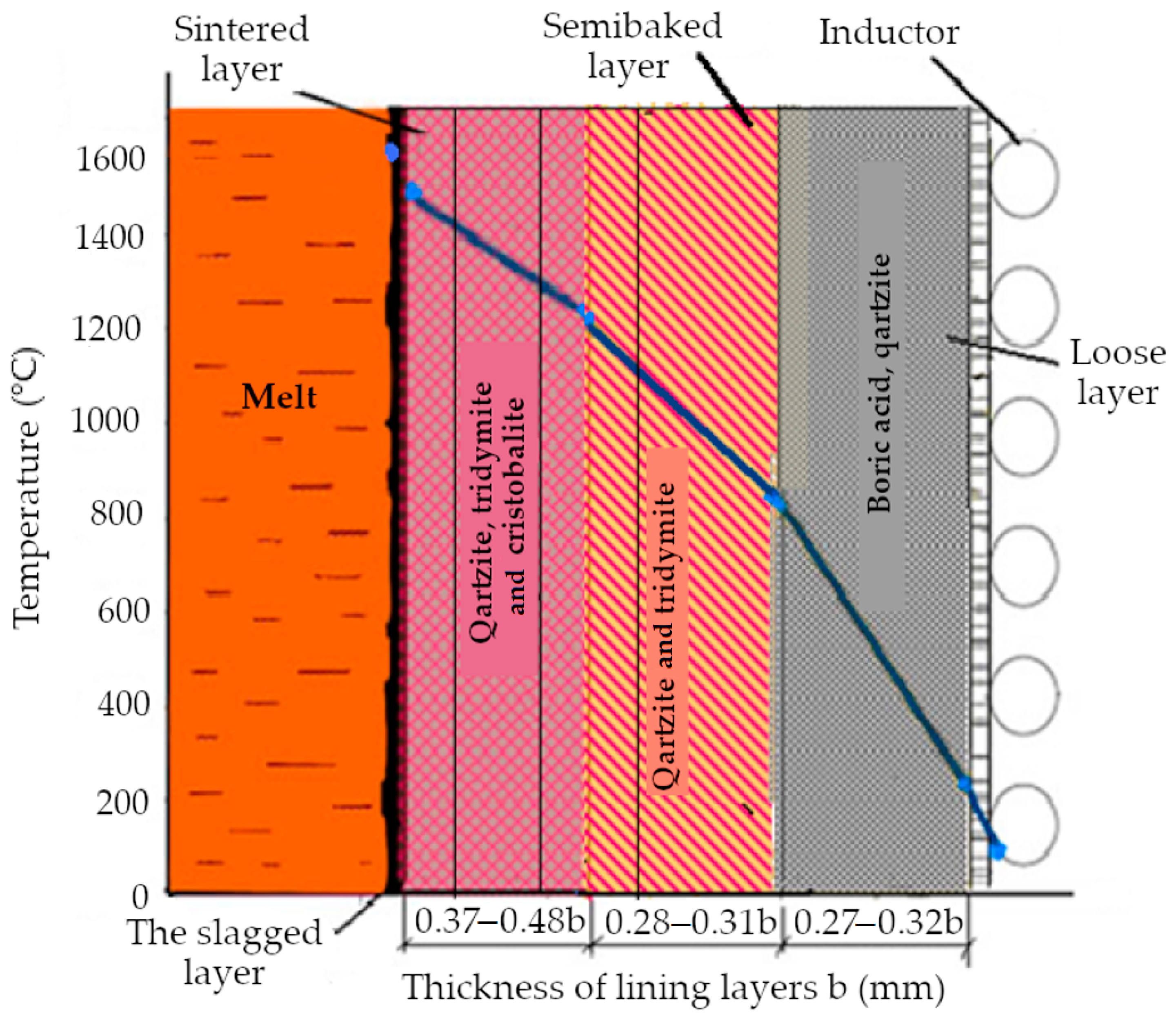
- The lining is held at 1550 °C at the end of the sintering process;
- The optimum temperature range required for the carburization melt is 1400–1470 °C;
- The temperature range of the finished melt prior to its discharge is 1350–1450 °C, depending on the brand of the cast iron;
- The furnace cooling temperature range after the first batch of metal, no refill, is 1000–1050 °C;
- The cooling temperature range of the lining when the metal is completely drained and loaded with a new batch is 800–900 °C.
- A total of 1400–1470 °C is the optimal melting point necessary for the carburizing of the melt;
- A total of 1350–1450 °C—the temperature of the first portion of the melt, depending on the brand of cast iron;
- A total of 1000–1050 °C—the temperature of the furnace cooling after the release of the second batch of metal, no refill;
- A total of 800–900 °C—the cooling temperature of the lining when the metal is completely drained and its new batch is loaded.
- A total of 1550–1600 °C—melting mode, carburizing, alloying, and modifying operation;
- A total of 1450–1470 °C—discharge of the first portion of the alloy;
- A total of 1000–1025 °C—the temperature of the furnace cooling after the release of the second batch of metal, no refill;
- A total 800–900 °C is the cooling temperature of the lining when the metal is completely drained and a new batch is loaded.
2. Problem Statement
- During moisture removal with different temperature modes;
- The temperature at the end of the sintering process of the lining;
- The temperature of the lining during loading of the next batch of bulk materials.
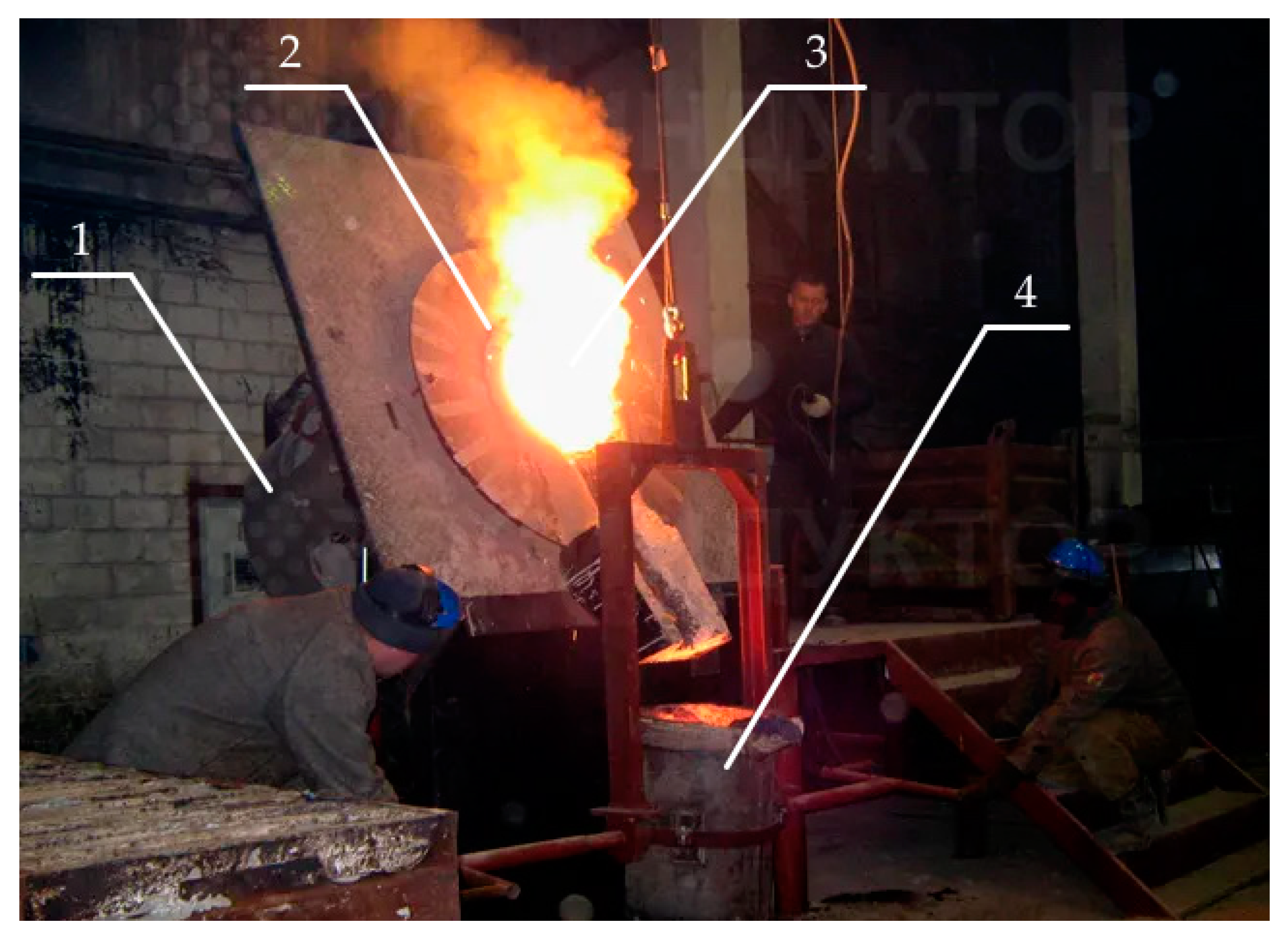
3. Materials and Methods
- The remainder was on grid No. 02, including 6–13;
- The remainder on grid No. 3.2 was not more than 5;
- Pass through grid No. 05, including 52–59;
- Netting No. 01, 33–32.
- 0.001–0.75 mm;
- 0.75–1.0 mm;
- 1.0–3.0 mm;
- 3.0 and more mm.
- The chemical composition of quartzite was determined, considering the presence of impurities;
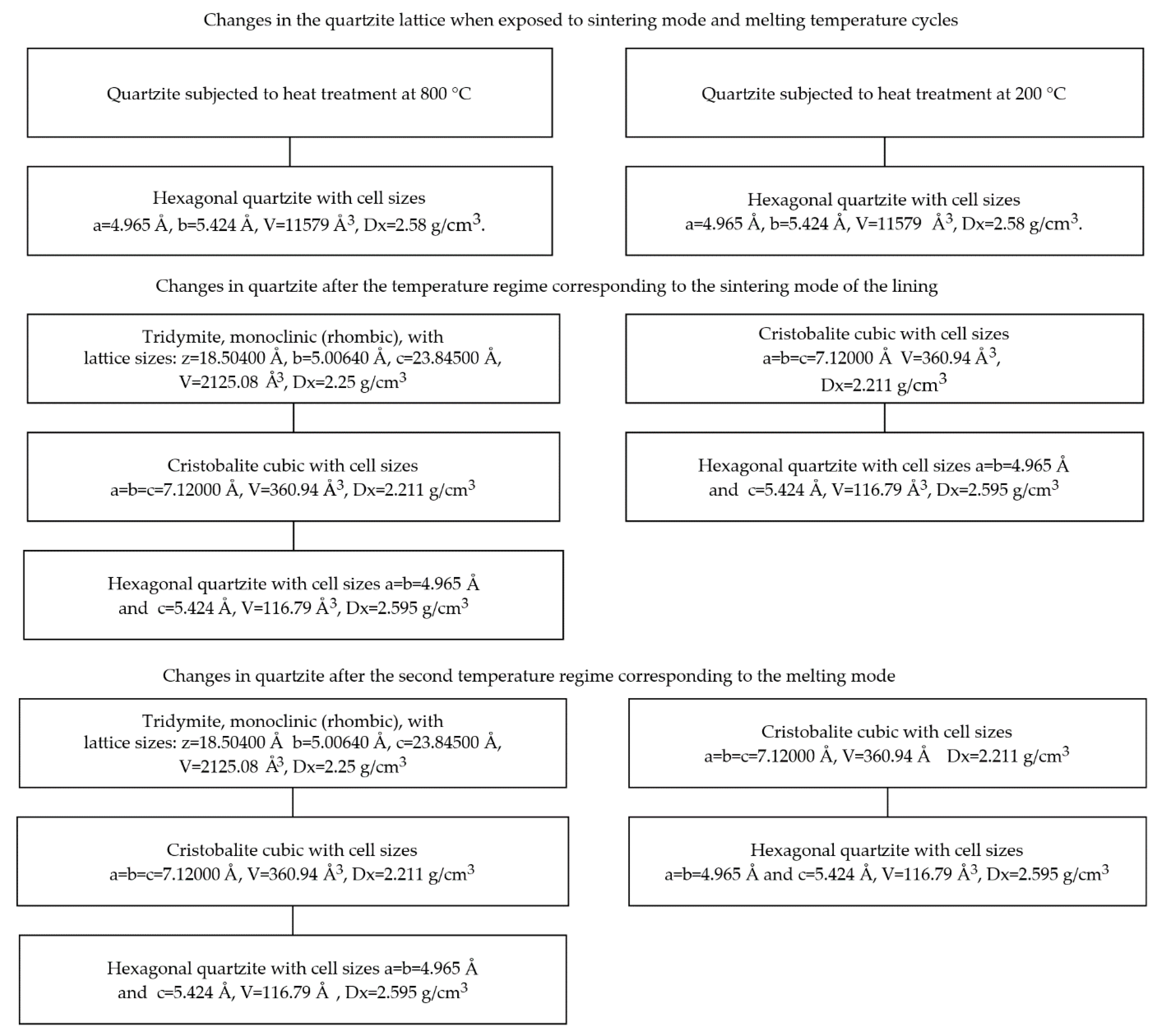
- The raw quartzite portion was heated to a temperature of 800 °C, given an exposure of 1 h, cooled to room temperature, and then subjected to subsequent heating to a temperature corresponding to the end of the process of sintering the lining (1550 °C) and subsequent temperatures, according to the melting temperature (twice) at each point to remove the card and determine the parameters of the crystal grille;
- The next batch of raw quartzite was heated to a temperature of 200 °C, given an exposure of 1 h, cooled to room temperature, and then the same heating mode was performed as described in point 2.
- The approximate temperatures for the removal of free moisture are 200 and 800 °C;
- The melting mode, carburizing, alloying, and modifying operation temperature range is 1550–1600 °C;
- The temperature drainage range of the first portion of the alloy is 1450–1470 °C;
- The second alloy discharge temperature is 1025 °C;
- The loading temperature range of fresh scrap metal is 800–900 °C.
4. Results and Discussion
- Removing moisture using a temperature of 200 °C and then cooling;
- Ending the sintering process at 1550–1570 °C with an exposure of 2 h;
- Carrying out the first three to five melts with 1/3 of the capacity of the crucible and only then proceeding to the required melting mode.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scobolev, D. Encyclopaedia of Technologies. Evolution and Comparative Analysis of Resource Efficiency of Industrial Technologies; D.O., S., Ed.; Renome: SPb, Russia, 2019; ISBN 978-5-00125-250-4. [Google Scholar]
- Hopin, P.N. An Assessment of the Durability of Friction Couples with Solid Lubricant Coatings of Various Compositions Produced by Domestic and Foreign Manufacturers. Mach. Build. 2018, 1, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Konstantinos, I.V.; Charalambides, G. Market of High Purity Quartz Innovative Applications. Procedia Econ. Financ. 2015, 24, 734–742. [Google Scholar]
- Kukartsev, V.V.; Antamoshkin, O.A. Combined Method of Decision-Making on Reproduction of Fixed Production Assets. Probl. Mech. Eng. Autom. 2011, 1, 56–60. [Google Scholar]
- Onigbajumo, A.; Seidu, S.O.; Akinlabi, O.; Newton, I. Melting Time Prediction Model for Induction Furnace Melting Using Specific Thermal Consumption from Material Charge Approach. J. Miner. Mater. Charact. Eng. 2021, 9, 61–74. [Google Scholar]
- Futas, P.J.A.; Pribulova, A.; Junakova, A.; Petrik, J.; Pokusova, M. The Study of Synthetic Cast Iron Quality Made from Steel Scrap. In Proceedings of the 18th International Multidisciplinary Scientific GeoConference SGEM 2018, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 215–222. [Google Scholar]
- Fenner, C.N. The various forms of silica and their mutual relations. J. Wash. Acad. Sci. 1912, 2, 471–480. [Google Scholar]
- Zavertkin, A.S. Development of the Composition of the Lining of Induction Furnaces from Pervouralsk Quartzite and the Practice of Its Application. Proc. Kola Sci. Cent. Russ. Acad. Sci. 2015, 44, 532–534. [Google Scholar]
- Grinchuk, P.S. Mathematical Modeling of Thermal Modes of Operation of Electric Resistance Furnaces. Eng. Phys. J. 2010, 83, 28–37. [Google Scholar]
- Sassa, V.S. Lining of Induction Melting Furnaces and Mixers; Energoatomizdat: Moscow, Russia, 1983. [Google Scholar]
- Kostyukova, A.P. Institutional Method as the Basis for Monitoring the Induction Melting Furnace. Adv. Mod. Sci. 2017, 4, 19–23. [Google Scholar]
- Kukartsev, V.A. Application of Pervouralsky Quartzite in Acid Lining for Induction Iron-Smelting Furnaces. Liteyshchik Russ. 2012, 2, 35–37. [Google Scholar]
- Chisamera, M.; Stan, S. Enhanced Quality in Electric Melt Grey Cast Irons Iulian Riposan. ISIJ Int. 2017, 53, 1683–1695. [Google Scholar]
- Kukartsev, V.A.; Kukartsev, V.V.; Kukartsev, A.V. Cite as Effect of the Temperature Treatment of Quartzite on the Lining Resistance of Commercial-Frequency Induction Crucible Furnaces. Refract. Ind. Ceram. 2018, 59, 252–256. [Google Scholar] [CrossRef]
- Kosenko, N.F.; Smirnova, M.A. Mechanostimulated Polymorphic Quartz Transitions. Refract. Tech. Ceram. 2012, 7, 7–13. [Google Scholar]
- Potseluev, A.A.; Babkin, D.I.; Kotegov, V.I. Composition and Regularities of Gas Distribution in Quartz of the Kalgutinsky Rare Metal Deposit. Proc. Tomsk Polytech. Univ. 2005, 308, 36–42. [Google Scholar]
- Hongwu, Z.; Songjiang, G.; Jijun, W.; Dandan, W.; Kuixian, W.; Wenhui, M. Effect of Quartz Crystal Structure Transformations on the Removal of Iron Impurities. Hydrometallurgy 2021, 204, 105715. [Google Scholar]
- Fu, B.X.; Jacques, T.; Jacques, T. Fluid Inclusions in Coesitebearing Eclogites and Jadeite Quartzites at Schuanghe. China J. Metamorph. Geol. 2001, 19, 531–548. [Google Scholar] [CrossRef]
- Kinelovskii, S.A. Model of Polymorphic Transformation in a Shock Wave. 2. Silica. J. Appl. Mech. Tech. Phys. 2021, 62, 214–223. [Google Scholar] [CrossRef]
- Isaev, V.A.; Kharakhan, M.L. On the Role of Gas-Liquid Inclusions in the Process of Crystallization of Natural Quartz. Min. Inf. Anal. Bull. 2005, 5, 25–33. [Google Scholar]
- Steinberg, M.V. Water and Hydrogen-Containing Groupings in Vein Quartz of Ural Deposits of Quartz Raw Materials. Lithosphere 2014, 3, 102–111. [Google Scholar]
- Shabelansky, A.H.; Bernabe, Y.; Mok, U.; Evans, J.B. Temperature Influence on Permeability of Sioux Quartzite Containing Mixtures of Water and Carbon Dioxide. Int. J. Rock Mech. Min. Sci. 2014, 70, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Panasyuk, G.P.; Kozerozhets, I.V.; Voroshilov, I.L.; Privalov, V.I.; Ivakin, Y.D.; Danchevskaya, M.N. Water Forms on the Surface and in the Bulk of Silicon Dioxide. Russ. J. Inorg. Chem. 2021, 66, 724–730. [Google Scholar] [CrossRef]
- Xin, C.; Zilong, Z.; Xueming, D. Water-Induced Variations in Dynamic Behavior and Failure Characteristics of Sandstone Subjected to Simulated Geo-Stress. Int. J. Rock Mech. Min. Sci. 2020, 130, 104339. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.; Schutzius, T. Surface Engineering for Phase Change Heat Transfer: A review. MRS Energy Sustain. 2014, 1, E4. [Google Scholar] [CrossRef] [Green Version]
- Kukartsev, V.A. Ncreasing the Resource of an Induction Crucible Furnace of Industrial Frequency at a Temperature above 1550 °C. Technol. Mech. Eng. 2014, 1, 5–6. [Google Scholar]
- Edalati, K. Influence of SiC and FeSi Addition on the Characteristics of Gray Cast Iron Melts Poured at Different Temperatures. Proc. Technol. 2005, 160, 183–188. [Google Scholar] [CrossRef]
- Zuno-Silva, J.; Bedolla-Jacuinde, A. Estudio a Nivel Laboratorio de La Degradación Atípica En Un Refractario Tipo SiO2 Utilizado En Hornos de Inducción Laboratory Scale Study of Uncommon Degradation SiO2 Refractories Used on Induction Furnaces. Rev. Electron. Nov. Sci. 2013, 6, 113–134. [Google Scholar]
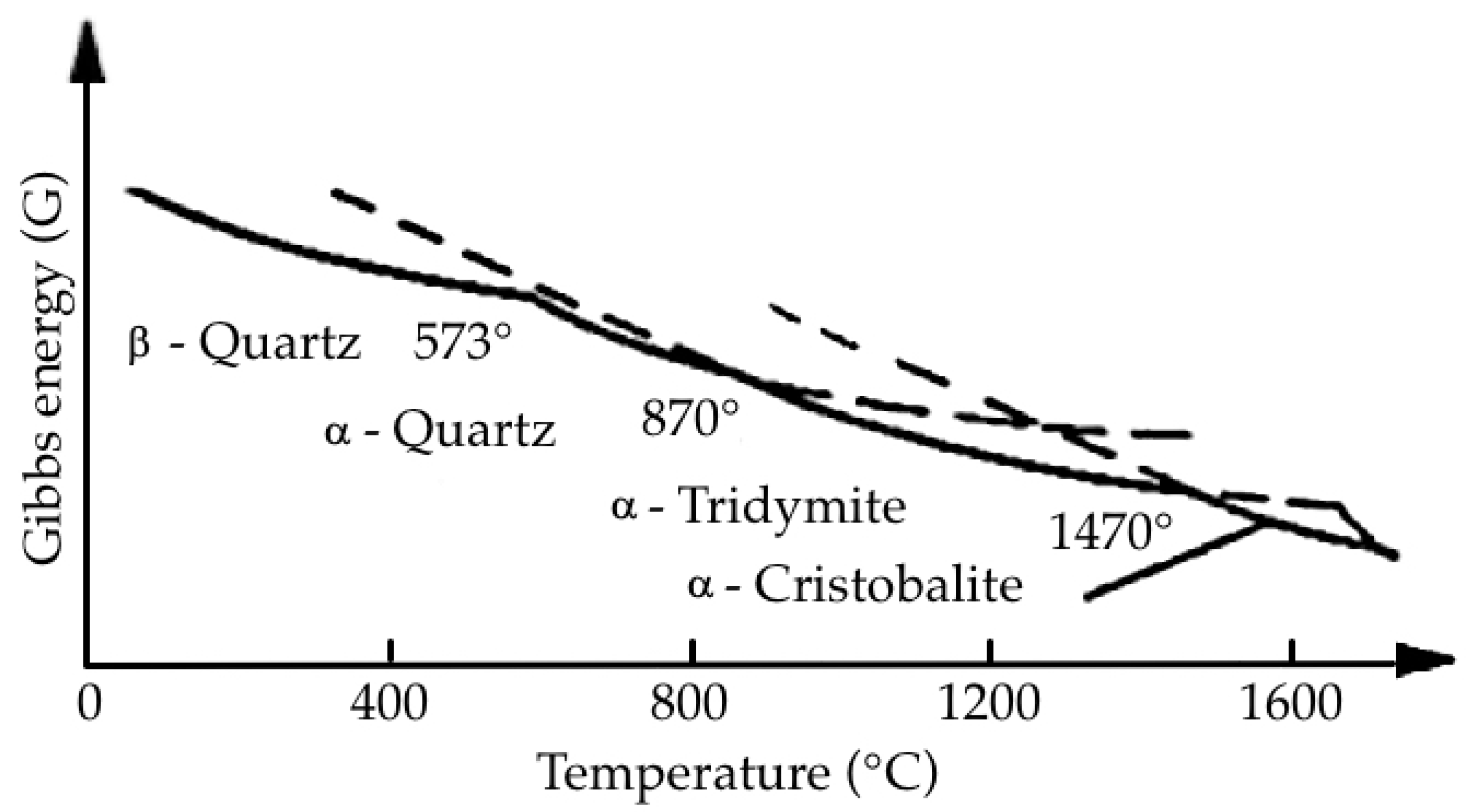
- Tgoupson, A.B.; Wennemer, M. Heat Capacities and Inversions in Tridymite, Cristobalite, and Tridymite-Cristobalite Mixedphases. Am. Mineral. 1979, 64, 1013–1026. [Google Scholar]
- Kaibicheva, M.N. Lining of Electric Furnaces; Metallurgy: Moscow, Russia, 1975. [Google Scholar]
- Masich, I.S.; Tyncheko, V.S.; Nelyub, V.A.; Bukhtoyarov, V.V.; Kurashkin, S.O.; Borodulin, A.S. Paired Patterns in Logical Analysis of Data for Decision Support in Recognition. Computation 2022, 10, 185. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Cherepanov, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kurochkin, S.O.; Tynchenko, V.V.; Sergienko, R.B.; Bashmur, K.A.; Boyko, A.A.; Bukhtoyarov, V.V. Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces. Metals 2022, 12, 1515. [Google Scholar] [CrossRef]
- Mass for the Printed Lining of Induction Furnaces. Available online: https://www.elibrary.ru/item.asp?id=39782161 (accessed on 21 February 2023).
- Mikhalev, A.S.; Tynchenko, V.S.; Nelyub, V.A.; Lugovaya, N.M.; Baranov, V.A.; Kukartsev, V.V.; Sergienko, R.B.; Kurashkin, S.O. The Orb-Weaving Spider Algorithm for Training of Recurrent Neural Networks. Symmetry Neurol. Int. 2022, 14, 2036. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Kukartsev, V.V.; Tynchenko, V.S.; Kurashkin, S.O.; Sergienko, R.B.; Tynchenko, S.V.; Panfilov, I.A.; Eremeeva, S.V.; Panfilova, T.A. Study of the Influence of the Thermal Capacity of the Lining of Acid Melting Furnaces on Their Efficiency. Metals 2023, 13, 337. [Google Scholar] [CrossRef]
- Eskin, D.G. Physical Metallurgy of Direct Chill Casting of Aluminum Alloys; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxfordshire, UK, 2008; Available online: https://www.taylorfrancis.com/books/mono/10.1201/9781420062823/physical-metallurgy-direct-chill-casting-aluminum-alloys-dmitry-eskin (accessed on 13 June 2023).
- Kukartsev, V.; Mikhalev, A.; Stashkevich, A.; Moiseeva, K.; Kauts, I. Analysis of Data in Solving the Problem of Reducing the Accident Rate Through the Use of Special Means on Public Roads. In Proceedings of the 2022 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Toronto, ON, Canada, 1–4 June 2022; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2022; pp. 1–4. [Google Scholar]
- Repinskiy, O.D.; Konyukhov, V.Y.; Bovkun, A.S.; Schupletsov, A.F. Improving the competitiveness of Russian industry in the production of measuring and analytical equipment. J. Phys. Conf. Ser. 2021, 1728, 012032. [Google Scholar] [CrossRef]
- Novakovic, R.; Radic, S.M.; Ristic, M.M. Kinetics and Mechanism of Quartz-Tridymite Transformation. NTERCERAM Interceram 1986, 35, 29–30. [Google Scholar]
- Kireev, T.; Kukartsev, V.; Pilipenko, A.; Rukosueva, A.; Suetin, V. Analysis of the Influence of Factors on Flight Delays in the United States Using the Construction of a Mathematical Model and Regression Analysis. In Proceedings of the 2022 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Toronto, ON, Canada, 1–4 June 2022; Institute of Electrical and Electronics Engineers (IEEE): Toronto, ON, Canada, 2022; pp. 1–5. [Google Scholar]
- Breneman, R.C.; Halloran, J.W. Kinetics of Cristobalite Formation in Sintered Silica. J. Am. Ceram. Soc. 2014, 9, 2272–2278. [Google Scholar] [CrossRef] [Green Version]
- Shchiptsov, V.V.; Perepelitsyn, V.A.; Grishenkov, E.E.; Enenko, V.P. Pervoural’skii and Karel’skii Quartzites for the Lining of Crucible-Type Induction Furnaces. Refract. Ind. Ceram. 2003, 44, 67–74. [Google Scholar] [CrossRef]
- Kurashkin, S.; Bukhtoyarov, V.; Myrugin, A.; Bocharov, A.; Seregin, Y.; Rogova, D. Simulation Model of the Electron Beam Welding Process for the Formation of the Effective Technological Parameters during the Welding of Aerospace Structures. In Proceedings of the 2022 21st International Symposium Infoteh-Jahorina (Infoteh), East Sarajevo, Bosnia and Herzegovina, 16–18 March 2022; Institute of Electrical and Electronics Engineers Inc.: East Sarajevo, Bosnia and Herzegovina, 2022; pp. 1–5. [Google Scholar]
- Nikiforova, E.M.; Eromasov, R.G.; Simonova, N.S.; Vasilyeva, M.N.; Taskin, V.Y. Phase Transformations in the Siliceous Rocks-Mineralizer System. Mod. Probl. Sci. Educ. 2012, 1, 218–219. [Google Scholar]
- Rogova, D.; Kurashkin, S.; Tynchenko, V.; Shutkina, E.; Saidov, N.; Bocharov, A. Software System for Modeling Temperature Distribution During the Electron Beam Welding. In Proceedings of the 2022 IEEE International Conference on Design & Test of Integrated Micro & Nano-Systems (DTS), Cairo, Egypt, 6–9 June 2022; IEEE: Cairo, Egypt, 2022; pp. 1–6. [Google Scholar]
- Li, W.; Xu, C.; Xie, A.; Chen, K.; Yang, Y.; Liu, L.; Zhu, S. Microstructure Study of Phase Transformation of Quartz in Potassium Silicate Glass at 900 °C and 1000 °C. Crystals 2021, 11, 1481. [Google Scholar] [CrossRef]
- Jin, S.L.; Gruber, D.; Harmuth, H.; Soudier, J.; Meunier, P.; Lemaistre, H. Optimisation of Monolithic Lining Concepts of Channel Induction Furnace. Int. J. Cast Met. Res. 2014, 27, 336–340. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Trunova, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kurashkin, S.O.; Tynchenko, Y.A.; Vivián, I.F.; Bashmur, K.A. Increasing the Efficiency of Synthetic Iron Production by the Use of New Kit Lining. Metals 2023, 13, 1184. [Google Scholar] [CrossRef]
- Ingham, J.P. Petrography of Geomaterials. Q. J. Eng. Geol. Hydrogeol. 2011, 44, 457–467. [Google Scholar] [CrossRef]
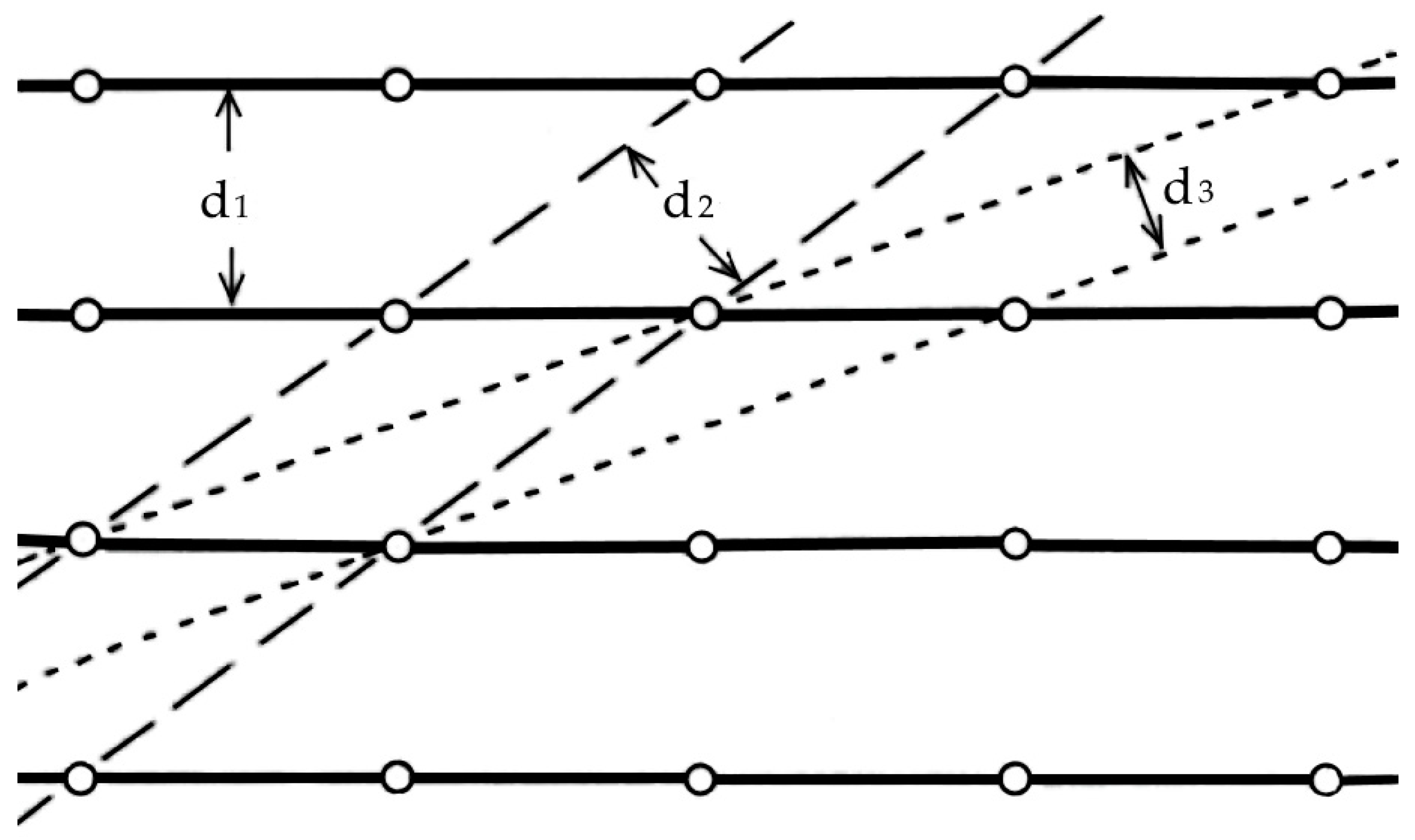
 —cells of the hexagonal quartzite characterized by card 00-012-0708 and
—cells of the hexagonal quartzite characterized by card 00-012-0708 and  —elementary cells of quartzite by card 001-083-2187 (remaining unspecified pixels and points are also red).
—elementary cells of quartzite by card 001-083-2187 (remaining unspecified pixels and points are also red).
 —cells of the hexagonal quartzite characterized by card 00-012-0708 and
—cells of the hexagonal quartzite characterized by card 00-012-0708 and  —elementary cells of quartzite by card 001-083-2187 (remaining unspecified pixels and points are also red).
—elementary cells of quartzite by card 001-083-2187 (remaining unspecified pixels and points are also red).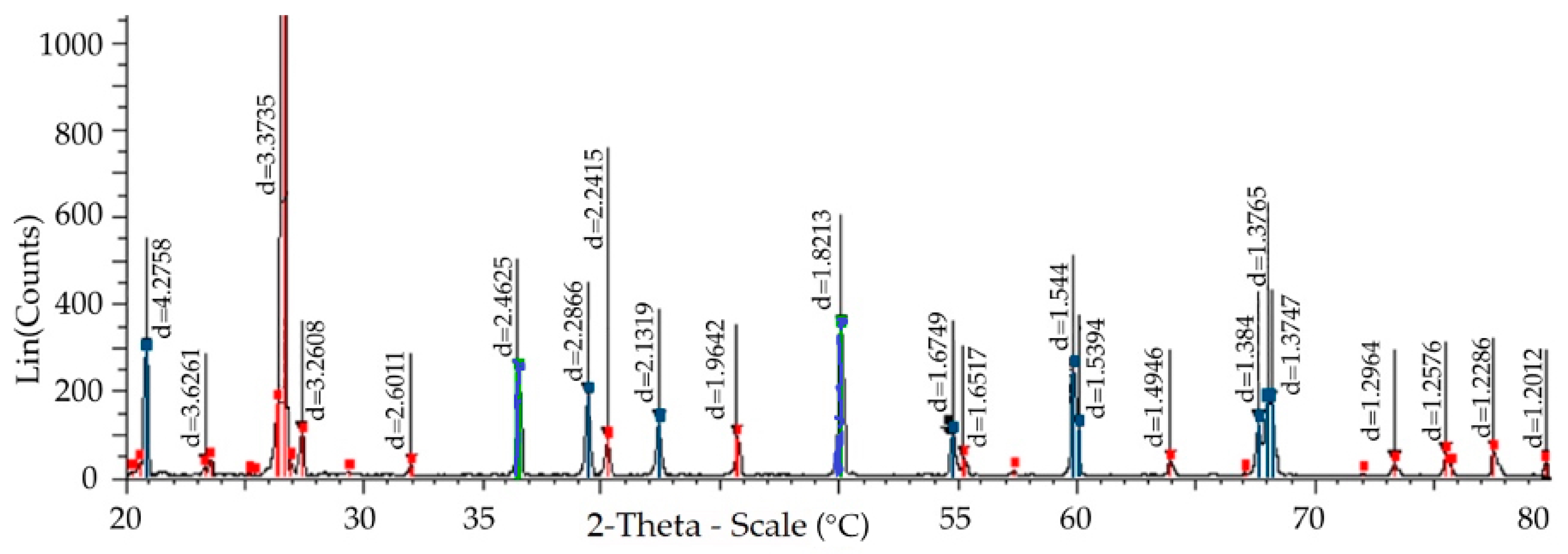
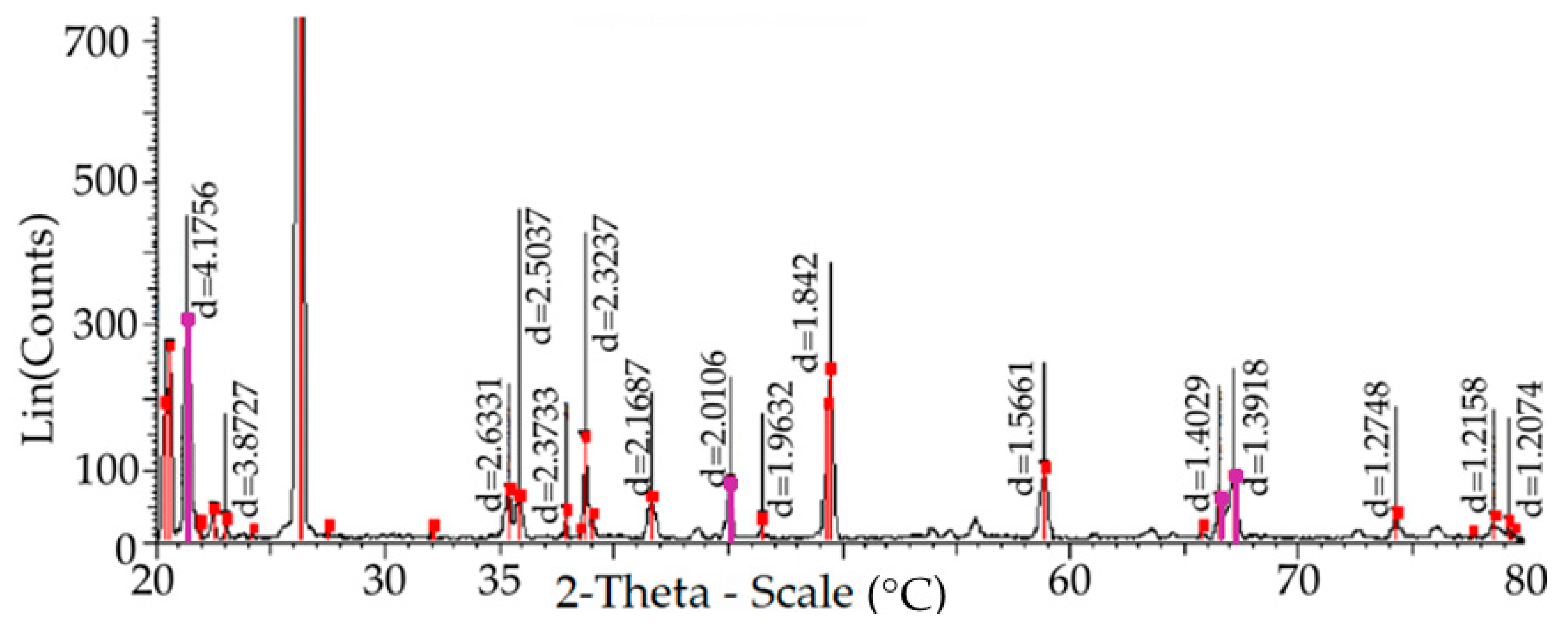
 —elementary cells of quartzite by card 01-085-0621;
—elementary cells of quartzite by card 01-085-0621;  —tridymite cells by card 00-018-1170; and
—tridymite cells by card 00-018-1170; and  —quartzite cells by card 01-01-071 (remaining peaks and points in red).
—quartzite cells by card 01-01-071 (remaining peaks and points in red).
 —elementary cells of quartzite by card 01-085-0621;
—elementary cells of quartzite by card 01-085-0621;  —tridymite cells by card 00-018-1170; and
—tridymite cells by card 00-018-1170; and  —quartzite cells by card 01-01-071 (remaining peaks and points in red).
—quartzite cells by card 01-01-071 (remaining peaks and points in red).
 —cells characterized by card 00-012-0708;
—cells characterized by card 00-012-0708;  —cells characterized by card 01-070-7344;
—cells characterized by card 01-070-7344;  —cells characterized by card 00-005-0490;
—cells characterized by card 00-005-0490;  —cells characterized by card 01-083-2187; and other cells—
—cells characterized by card 01-083-2187; and other cells— .
.
 —cells characterized by card 00-012-0708;
—cells characterized by card 00-012-0708;  —cells characterized by card 01-070-7344;
—cells characterized by card 01-070-7344;  —cells characterized by card 00-005-0490;
—cells characterized by card 00-005-0490;  —cells characterized by card 01-083-2187; and other cells—
—cells characterized by card 01-083-2187; and other cells— .
.
 —cards 00-011-0695 and 00-002-0278, and of quartzite, where
—cards 00-011-0695 and 00-002-0278, and of quartzite, where  —for the rest (card 01-071-0911). Remaining peaks and points are also red (
—for the rest (card 01-071-0911). Remaining peaks and points are also red ( ).
).
 —cards 00-011-0695 and 00-002-0278, and of quartzite, where
—cards 00-011-0695 and 00-002-0278, and of quartzite, where  —for the rest (card 01-071-0911). Remaining peaks and points are also red (
—for the rest (card 01-071-0911). Remaining peaks and points are also red ( ).
).
| Chemical Composition of PKMVI-1 Quartzite | Maintenance (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | TiO2 | Fe2O3 | P2O5 | MnO | Na2O | K2O | |
| According to TU | 97.5 | 1.1 | - | - | - | 0.6 | - | - | - | - |
| Spectrometer of dried at 200 °C | 96.64 | 0.982 | 0.089 | 0.025 | 0.205 | 0.93 | 0.014 | 0.031 | 0.105 | 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukartsev, V.A.; Trunova, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kurashkin, S.O.; Bashmur, K.A.; Tynchenko, Y.A.; Sergienko, R.B.; Tynchenko, S.V. Influence of Temperature Regimes of Synthetic Iron Smelting on Casting Production Efficiency. Metals 2023, 13, 1234. https://doi.org/10.3390/met13071234
Kukartsev VA, Trunova AI, Kukartsev VV, Tynchenko VS, Kurashkin SO, Bashmur KA, Tynchenko YA, Sergienko RB, Tynchenko SV. Influence of Temperature Regimes of Synthetic Iron Smelting on Casting Production Efficiency. Metals. 2023; 13(7):1234. https://doi.org/10.3390/met13071234
Chicago/Turabian StyleKukartsev, Viktor Alekseevich, Alina Igorevna Trunova, Vladislav Viktorovich Kukartsev, Vadim Sergeevich Tynchenko, Sergei Olegovich Kurashkin, Kirill Aleksandrovich Bashmur, Yadviga Aleksandrovna Tynchenko, Roman Borisovich Sergienko, and Sergei Vasilievich Tynchenko. 2023. "Influence of Temperature Regimes of Synthetic Iron Smelting on Casting Production Efficiency" Metals 13, no. 7: 1234. https://doi.org/10.3390/met13071234
APA StyleKukartsev, V. A., Trunova, A. I., Kukartsev, V. V., Tynchenko, V. S., Kurashkin, S. O., Bashmur, K. A., Tynchenko, Y. A., Sergienko, R. B., & Tynchenko, S. V. (2023). Influence of Temperature Regimes of Synthetic Iron Smelting on Casting Production Efficiency. Metals, 13(7), 1234. https://doi.org/10.3390/met13071234







