Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Hydrothermal Coating
2.3. Microstructure Characterization
2.4. Corrosion Tests
3. Results and Discussion
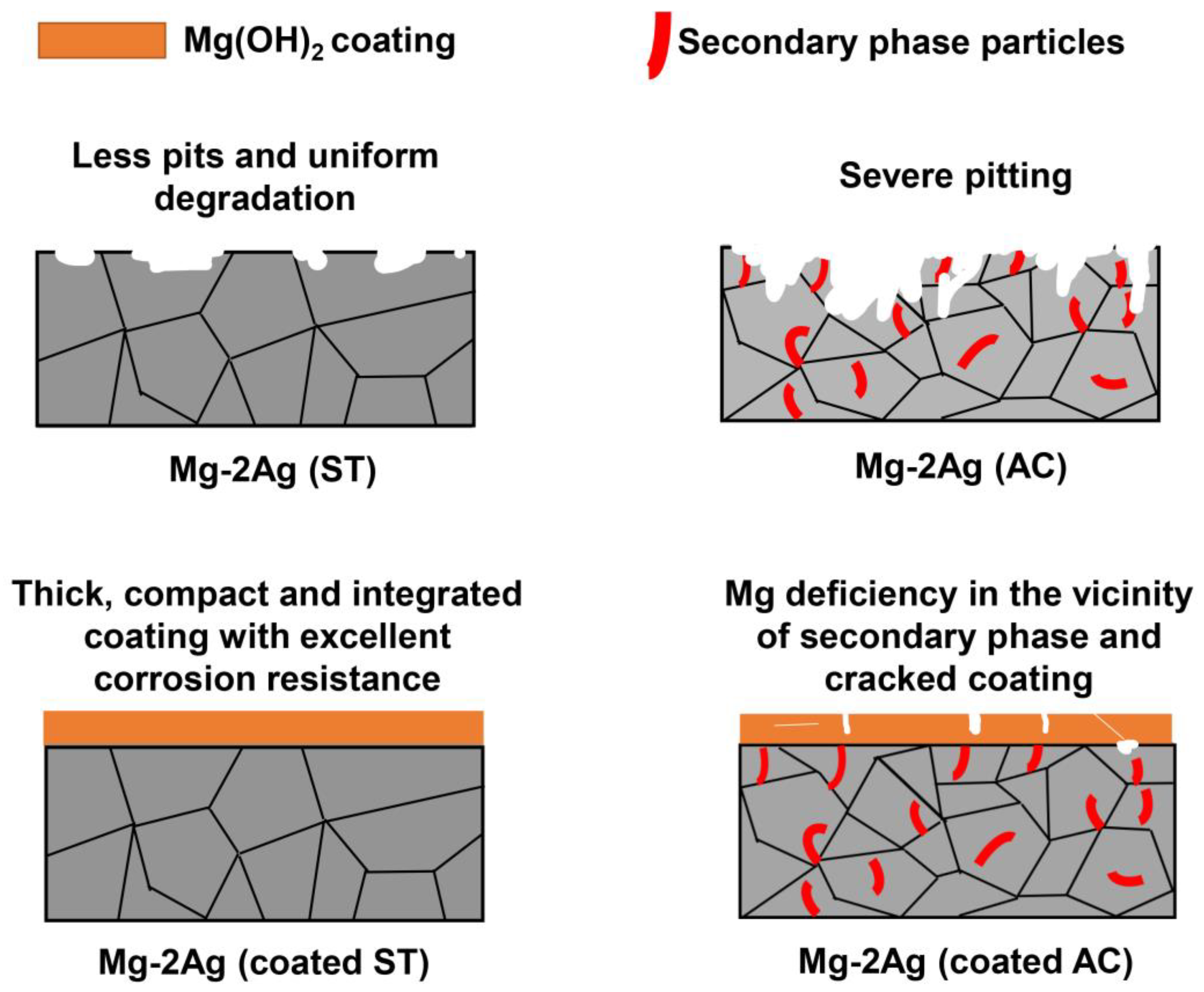
3.1. Microstructure Evolution
3.1.1. Substrate
3.1.2. Coating
3.2. Corrosion Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadi Zerankeshi, M.; Alizadeh, R.; Gerashi, E.; Asadollahi, M.; Langdon, T.G. Effects of Heat Treatment on the Corrosion Behavior and Mechanical Properties of Biodegradable Mg Alloys. J. Magnes. Alloys 2022, 10, 1737–1785. [Google Scholar] [CrossRef]
- Kumar, K.; Gill, R.S.; Batra, U. Challenges and Opportunities for Biodegradable Magnesium Alloy Implants. Mater. Technol. 2018, 33, 153–172. [Google Scholar] [CrossRef]
- Nie, Y.J.; Dai, J.W.; Zhang, X.B. Effect of Ag Addition on Microstructure, Mechanical and Corrosion Properties of Mg–Nd–Zn–Zr Alloy for Orthopedic Application. Acta Metall. Sin. (Engl. Lett.) 2023, 36, 295–309. [Google Scholar] [CrossRef]
- Lin, X.; Saijilafu; Wu, X.; Wu, K.; Chen, J.; Tan, L.; Witte, F.; Yang, H.; Mantovani, D.; Zhou, H.; et al. Biodegradable Mg-Based Alloys: Biological Implications and Restorative Opportunities. Int. Mater. Rev. 2022, 68, 365–403. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y.; et al. In Vitro and in Vivo Assessment of the Effect of Biodegradable Magnesium Alloys on Osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on Clinical Applications of Magnesium-Based Orthopaedic Implants: A Review from Clinical Translational Perspective. Biomaterials 2016, 112, 287–302. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Mohammadi-Zerankeshi, M.; Alizadeh, R. 3D-Printed PLA-Gr-Mg Composite Scaffolds for Bone Tissue Engineering Applications. J. Mater. Res. Technol. 2023, 22, 2440–2446. [Google Scholar] [CrossRef]
- Sabbaghian, M.; Mahmudi, R.; Shin, K.S. Microstructure, Texture, Mechanical Properties and Biodegradability of Extruded Mg–4Zn−xMn Alloys. Mater. Sci. Eng. A 2020, 792, 139828. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yang, K. Recent Advances on the Development of Biodegradable Magnesium Alloys: A Review. Mater. Technol. 2016, 31, 681–688. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical Properties, Corrosion Resistance and Biocompatibilities of Degradable Mg-RE Alloys: A Review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Mohammadi Zerankeshi, M.; Alizadeh, R. Ag-Incorporated Biodegradable Mg Alloys. Materialia 2022, 23, 101445. [Google Scholar] [CrossRef]
- Zohrevand, M.; Mohammadi-Zerankeshi, M.; Nobakht-Farin, F.; Alizadeh, R.; Mahmudi, R. Degradation Behavior of the As-Extruded and ECAP-Processed Mg–4Zn Alloy by Ca Addition and Hydrothermal Coating. J. Mater. Res. Technol. 2022, 20, 1204–1215. [Google Scholar] [CrossRef]
- Mohammadi-Zerankeshi, M.; Alizadeh, R.; Labbaf, S. Improving Mechanical, Degradation and Biological Behavior of Biodegradable Mg–2Ag Alloy: Effects of Y Addition and Heat Treatment. J. Mater. Res. Technol. 2023, 22, 1677–1694. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, P.; Cao, X.Q.; Misra, R.D.K.; Wang, W.; Chen, K.Z. Tin-Induced Microstructural Changes and Associated Corrosion Resistance of Biodegradable Mg–Zn Alloy. Rare Met. 2022, 41, 883–888. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Huang, Q.; Li, Y.; Yang, Y. Enhancing Mechanical Properties and Degradation Performance of Mg−0.8wt.%Ca Alloy by Directional Solidification. Trans. Nonferrous Met. Soc. China 2023, 33, 409–421. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Liu, Z.; Schade, R.; Luthringer, B.; Hort, N.; Rothe, H.; Müller, S.; Liefeith, K.; Willumeit-Römer, R.; Feyerabend, F. Influence of the Microstructure and Silver Content on Degradation, Cytocompatibility, and Antibacterial Properties of Magnesium-Silver Alloys in Vitro. Oxid. Med. Cell. Longev. 2017, 2017, 8091265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Applications, O.; Dai, Y.; Liu, H.; Tang, Y.; Xu, X.; Long, H.; Yan, Y. A Potential Biodegradable Mg-Y-Ag Implant with Strengthened Antimicrobial Properties in Orthopedic Applications. Metals 2018, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Bian, M.; Huang, X.; Chino, Y. A Combined Experimental and Numerical Study on Room Temperature Formable Magnesium e Silver e Calcium Alloys. J. Alloys Compd. 2020, 834, 155017. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of Secondary Phase and Grain Size on the Corrosion of Biodegradable—Zn—Ca Alloys. Mater. Sci. Eng. C 2015, 48, 480–486. [Google Scholar] [CrossRef]
- Janbozorgi, M.; Karimi Taheri, K.; Karimi Taheri, A. Microstructural Evolution, Mechanical Properties, and Corrosion Resistance of a Heat-Treated Mg Alloy for the Bio-Medical Application. J. Magnes. Alloys 2019, 7, 80–89. [Google Scholar] [CrossRef]
- Bryła, K.; Horky, J.; Krystian, M.; Lityńska-Dobrzyńska, L.; Mingler, B. Microstructure, Mechanical Properties, and Degradation of Mg-Ag Alloy after Equal-Channel Angular Pressing. Mater. Sci. Eng. C 2020, 109, 110543. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.Z.; Qi, W.C.; Zeng, R.C.; Chen, X.B.; Gu, C.D.; Guan, S.K.; Zheng, Y.F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Zhang, Y.; Pan, F. Formation of a Hydrophobic and Corrosion Resistant Coating on Magnesium Alloy via a One-Step Hydrothermal Method. J. Colloid Interface Sci. 2017, 505, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, H.; Hou, P.; Ni, J.; Han, P.; Zhang, X. Enhanced Corrosion Resistance and Antibacterial Property of Zn Doped DCPD Coating on Biodegradable Mg. Mater. Lett. 2016, 180, 42–46. [Google Scholar] [CrossRef]
- Yamauchi, N.; Ueda, N.; Okamoto, A.; Sone, T.; Tsujikawa, M.; Oki, S. DLC Coating on Mg–Li Alloy. Surf. Coat. Technol. 2007, 201, 4913–4918. [Google Scholar] [CrossRef]
- Gao, Y.L.; Liu, Y.; Song, X.Y. Plasma-Sprayed Hydroxyapatite Coating for Improved Corrosion Resistance and Bioactivity of Magnesium Alloy. J. Therm. Spray Technol. 2018, 27, 1381–1387. [Google Scholar] [CrossRef]
- Minting, D.; Linlin, H.; Mengke, P.; Fenyan, H.; Qiang, G.; Yashao, C.; Peng, L. Preparation of Vancomycin-Loaded Alginate Hydrogel Coating on Magnesium Alloy with Enhanced Anticorrosion and Antibacterial Properties. Thin Solid Films 2020, 693, 137679. [Google Scholar] [CrossRef]
- Chang, L.; Wang, P.; Liu, W. Effect of Amino Acids on the Structure and Corrosion Resistance of Mg-Li Alloy Anodic Oxide Film. Adv. Mater. Res. 2011, 146–147, 785–788. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, R.; Li, L.; Wang, Y.; Luo, R.; Yang, L.; Wang, Y. Green Tea Polyphenol Induced Mg2+-Rich Multilayer Conversion Coating: Toward Enhanced Corrosion Resistance and Promoted In Situ Endothelialization of AZ31 for Potential Cardiovascular Applications. ACS Appl. Mater. Interfaces 2019, 11, 41165–41177. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Hu, Y.; Gao, F.; Gong, T.; Liu, T.; Li, X.; Pan, C. Biofunctionization of Biodegradable Magnesium Alloy to Improve the In Vitro Corrosion Resistance and Biocompatibility. Appl. Surf. Sci. 2018, 451, 20–31. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Bioactive Materials Layer-by-Layer Deposition of Bioactive Layers on Magnesium Alloy Stent Materials to Improve Corrosion Resistance and Biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Mensah-Darkwa, K.; Kumar, D. Corrosion Protective Conversion Coatings on Magnesium Disks Using a Hydrothermal Technique. J. Mater. Sci. Technol. 2014, 30, 47–53. [Google Scholar] [CrossRef]
- Song, D.; Lian, B.; Fu, Y.; Wang, G.; Qiao, Y.; Klu, E.E.; Gong, X.; Jiang, J. Dual-Layer Corrosion-Resistant Conversion Coatings on Mg-9li Alloy via Hydrothermal Synthesis in Deionized Water. Metals 2021, 11, 1396. [Google Scholar] [CrossRef]
- Song, D.; Guo, G.; Jiang, J.; Zhang, L.; Ma, A.; Ma, X.; Chen, J.; Cheng, Z. Hydrothermal Synthesis and Corrosion Behavior of the Protective Coating on Mg-2Zn-Mn-Ca-Ce Alloy. Prog. Nat. Sci. Mater. Int. 2016, 26, 590–599. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Zhang, L.; Ma, X.; Guo, G.; Zhang, F.; Jiang, J.; Ma, A. Decreasing Bio-Degradation Rate of the Hydrothermal-Synthesizing Coated Mg Alloy via Pre-Solid-Solution Treatment. Materials 2017, 10, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Su, Y.; Li, Q.; Liu, Y.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Researching a Highly Anti-Corrosion Superhydrophobic Film Fabricated on AZ91D Magnesium Alloy and Its Anti-Bacteria Adhesion Effect. Mater. Charact. 2015, 99, 200–209. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Q.K.; Liu, Y.Z.; Xiao, Z.; Xia, B.H.; Li, S.Q.; Zhang, F.; Cui, L.Y.; Zeng, R.C. Polyphosphate Assisted Hydrothermal Synthesis of Hydroxyapatite Coating on Mg Alloys: Enhanced Mechanical Properties and Corrosion Resistance. Surf. Coat. Technol. 2022, 432, 128033. [Google Scholar] [CrossRef]
- Ling, L.; Cai, S.; Li, Q.; Sun, J.; Bao, X.; Xu, G. Recent Advances in Hydrothermal Modification of Calcium Phosphorus Coating on Magnesium Alloy. J. Magnes. Alloys 2021, 10, 62–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, G.; Zhang, Y.; Zhao, Q. Applied Surface Science Growth and Characterization of Mg(OH)2 Film on Magnesium Alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Müller, W.; Schade, R.; Liefeith, K.; Kainer, K.; Willumeit, R. Antibacterial Biodegradable Mg-Ag Alloys. Eur. Cell. Mater. 2013, 25, 284–298, discussion 298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Q.; Zhang, Y.H.; Wu, G. Hydrothermal Synthesis of Protective Coating on Magnesium Alloy Using De-Ionized Water. Surf. Coat. Technol. 2012, 206, 2961–2966. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Liang, N.; Yang, F.; Jiang, J.; Sun, J.; Wu, G.; Ma, A.; Ma, X. Simultaneously Improving Corrosion Resistance and Mechanical Properties of a Magnesium Alloy via Equal-Channel Angular Pressing and Post Water Annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, Y.; Zhang, Y.; Yan, Y.; Ma, J.; Li, D.; Yu, K. Effects of Heat Treatment on Microstructure, Mechanical Properties, Corrosion Resistance and Cytotoxicity of ZM21 Magnesium Alloy as Biomaterials. J. Mater. Eng. Perform. 2019, 28, 33–43. [Google Scholar] [CrossRef]






| Solution | Chemical Composition (g/L) | |||
|---|---|---|---|---|
| NaCl | KCl | Na2HPO4 | KH2PO4 | |
| PBS | 8.00 | 1.15 | 0.20 | 0.20 |
| Alloy | Condition | Icorr (µA/cm2) | Ecorr (V) | Corrosion Rate (mm/y) |
|---|---|---|---|---|
| Mg-2Ag | AC | 84.52 ± 2.4 | −1.52 | 1.93 ± 0.11 |
| ST | 19.14 ± 1.6 | −1.44 | 0.44 ± 0.05 | |
| Coated Mg-2Ag | AC | 4.12 ± 1.6 | −0.34 | 0.09 ± 0.01 |
| ST | 1.84 ± 0.08 | −0.26 | 0.04 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi-Zerankeshi, M.; Zohrevand, M.; Alizadeh, R. Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy. Metals 2023, 13, 1260. https://doi.org/10.3390/met13071260
Mohammadi-Zerankeshi M, Zohrevand M, Alizadeh R. Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy. Metals. 2023; 13(7):1260. https://doi.org/10.3390/met13071260
Chicago/Turabian StyleMohammadi-Zerankeshi, Meysam, Mohammad Zohrevand, and Reza Alizadeh. 2023. "Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy" Metals 13, no. 7: 1260. https://doi.org/10.3390/met13071260





