Study on the Leaching Conditions of the Shihongtan Uranium Deposit
Abstract
:1. Introduction
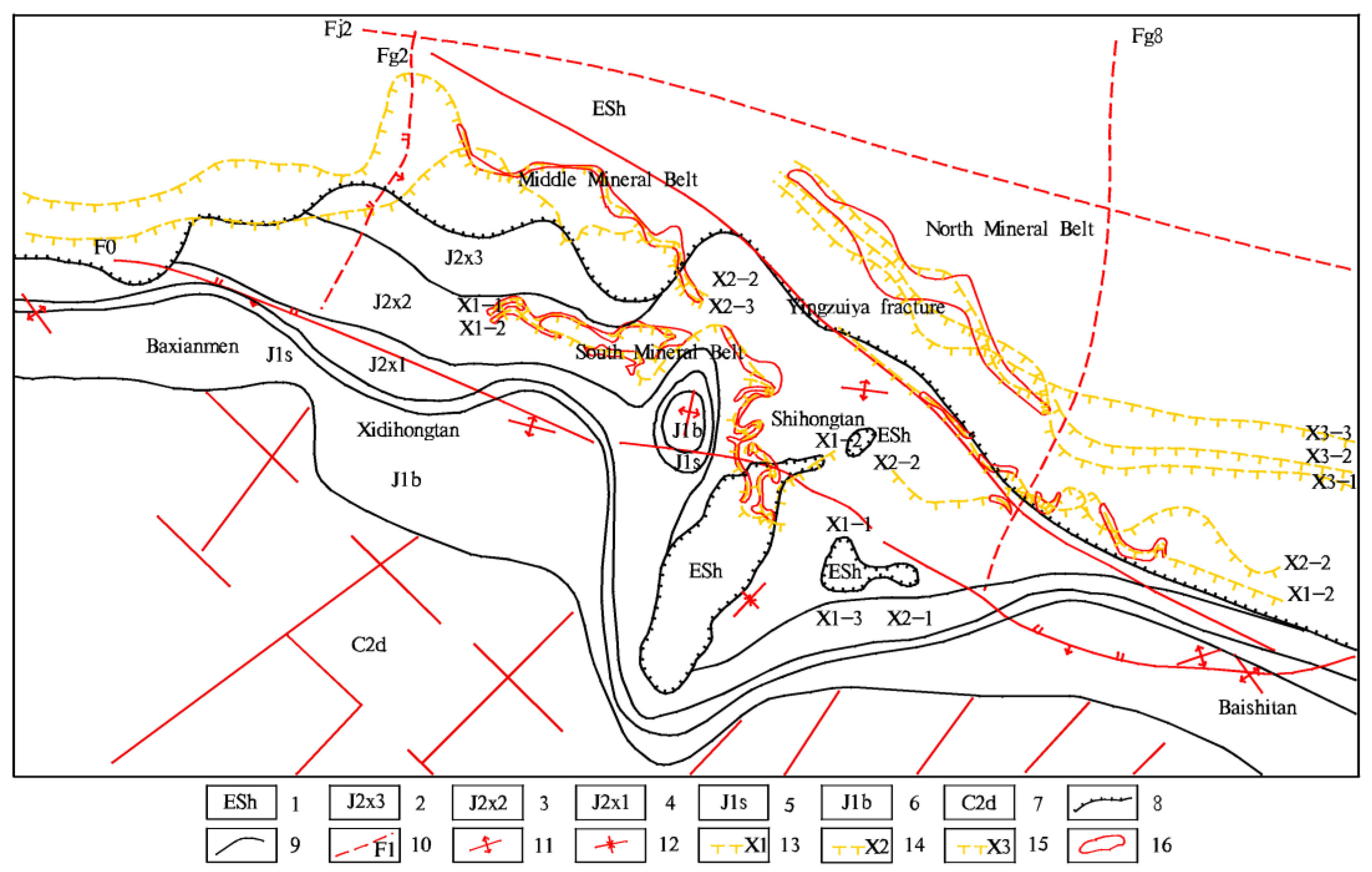
2. Tectonic Background and Regional Geological Setting
3. Material and Method
3.1. Material
3.1.1. Rock
3.1.2. Chemistry of Aquifer Groundwater
3.2. Method
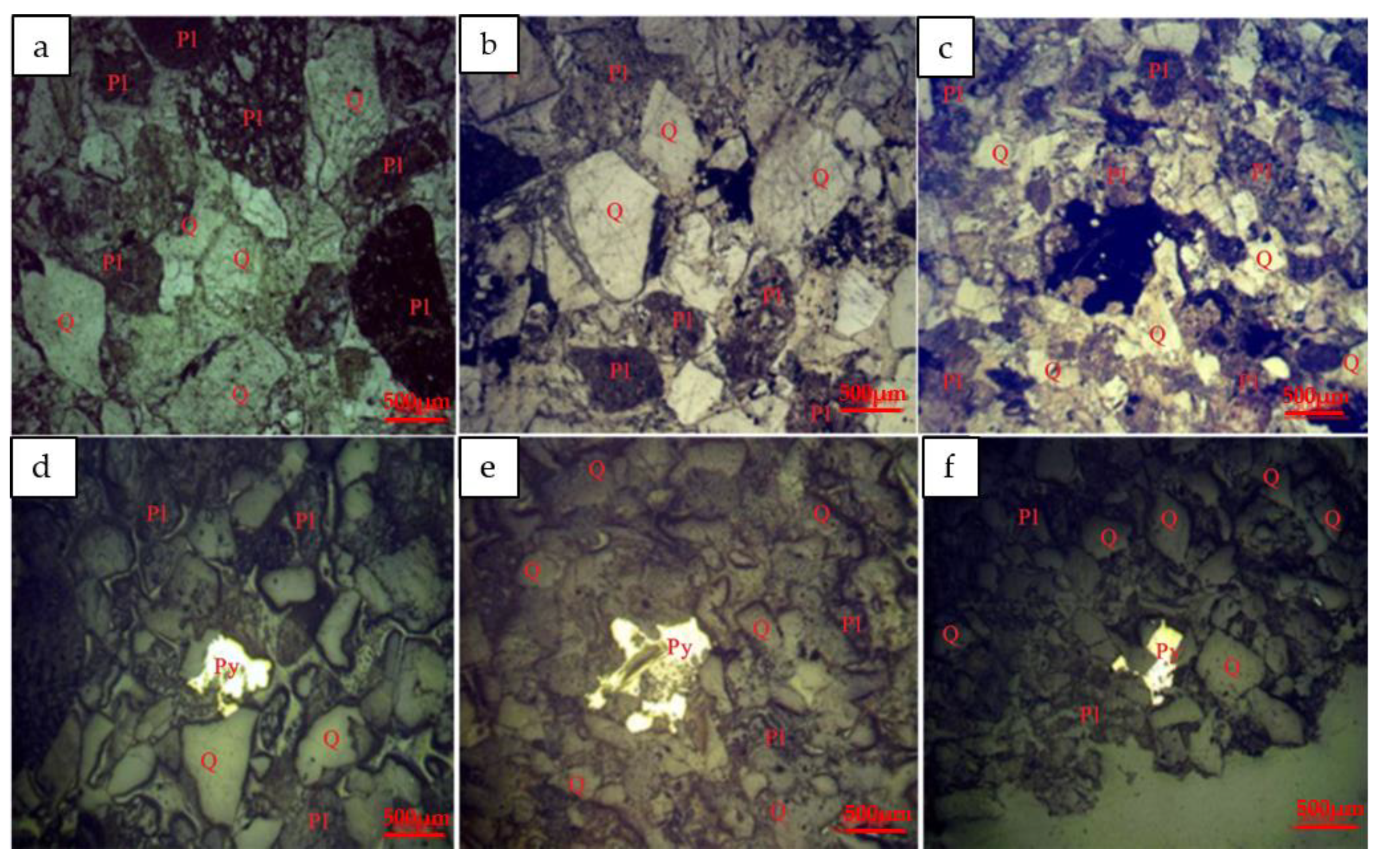
3.2.1. Mineralogy
3.2.2. Batch Test
- (1)
- Sample processing. Firstly, the mud skin was removed from the surface of the core sample to avoid its impact on the test. Secondly, the cores were broken and then screened according to the particle size of less than 0.25 mm, 0.25~0.5 mm, 0.5~1 mm, and greater than 1 mm. Thirdly, the rock samples of each particle size were thoroughly stirred to make them uniform. Finally, the samples were reconfigured according to the ore grading and reserved for leaching tests.
- (2)
- Preparation of solution. Using mineral water as the matrix, NH4HCO3 was added to prepare 750 mL of HCO3− extractant with concentrations of 500 mg/L, 650 mg/L, 800 mg/L, 950 mg/L, and 1100 mg/L, respectively. Ultrapure water was used as the HCO3− concentration 0 solution and it was placed in a sealed reactor.
- (3)
- Loading. The autoclave was charged with the sample, and then the bolts were tightened and evenly distributed on the flange to achieve reliable sealing without leakage.
- (4)
- Inject CO2 + O2. A gas mixture with a CO2:O2 ratio of 1:1 was injected and the pressure was adjusted to control the pH distribution of the leaching system to 6.2, 6.4, 6.6, and 6.8.
- (5)
- On-line sampling and monitoring. During the leaching process, all data in the autoclave were detected in real time by the detection and control system, and feedback was displayed in time by the pH display table, OPR display table, dissolved oxygen display table, and pressure display table. At the predetermined sampling time, the uranium concentration and bicarbonate concentration of the uranium mineral leaching solution were detected through the sampling port, and the sampling volume was measured.
- (6)
- When the test time reached 48 h, the oxygen outlet was closed and the exhaust port was opened. After the internal pressure of the reactor was restored to 1 atmospheric pressure (external pressure), the bolts of the cover were unscrewed in turn, the reactor cover was opened, the leaching supernatant and slag sample were removed, and the reactor was cleaned.
- (7)
- Solid–liquid separation. After the test, the supernatant was taken and placed in a polyethylene centrifuge bottle to analyze anions in the leaching solution. At the same time, the slag sample in the autoclave was placed in a centrifugal bottle, and the centrifugal speed was set at 45 r/min for 5 min to separate the solid and liquid. The chemical composition of centrifugal liquefaction components were detected.
- (8)
- Cleaning the slag. After centrifugation, the slag was washed. A total of 100 mL deionized water was added to each group of slag samples, and the samples were stirred evenly. The samples were put into a centrifuge for solid–liquid separation, and the centrifugal liquefaction components were detected. The cleaned slag sample was dried at a low temperature in a drier; then, the slag was weighed and mixed evenly, and 50 g was taken for conducting the major element analysis.
4. Result
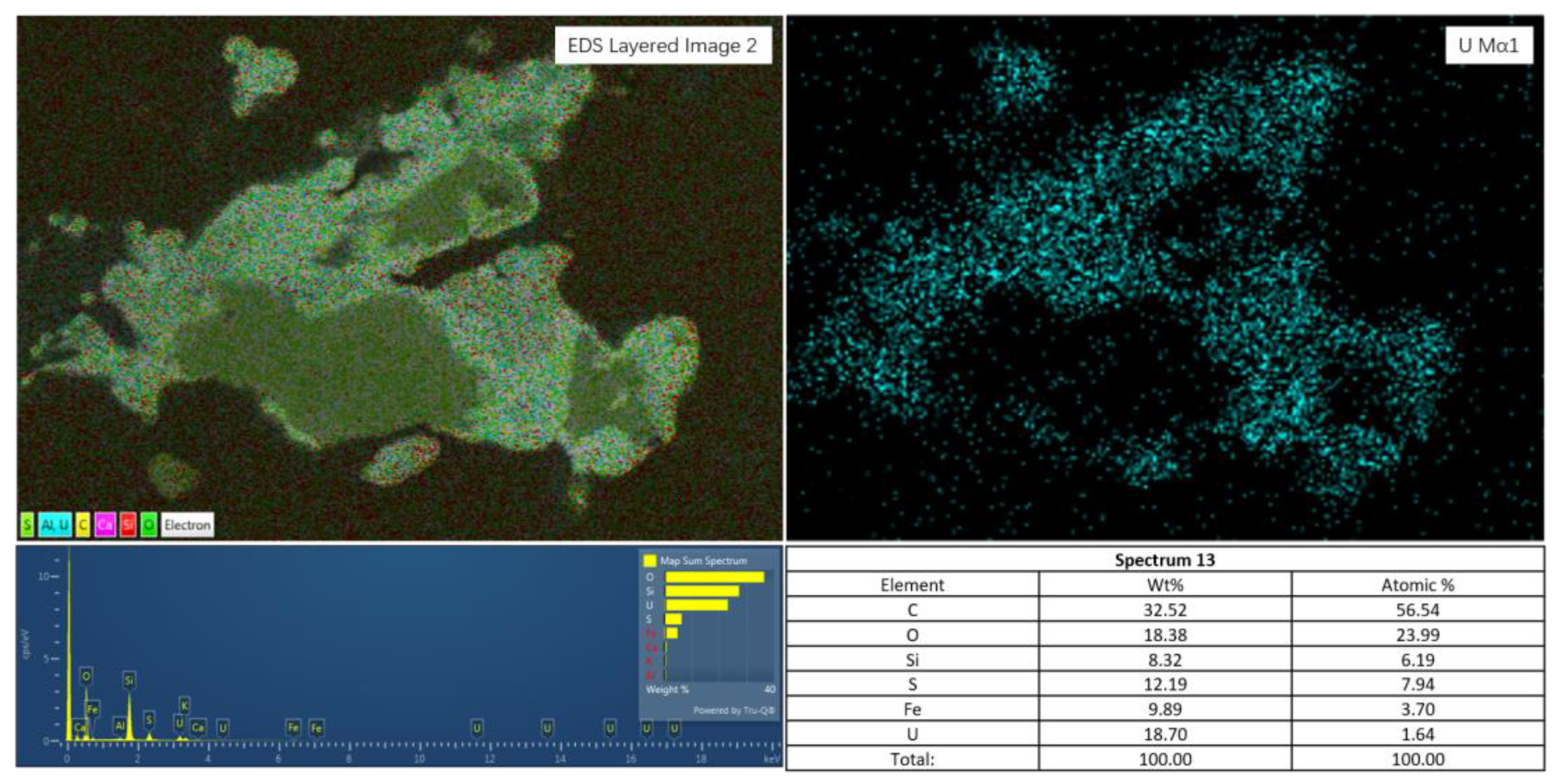
4.1. Form and Distribution of Uranium
4.2. Uranium
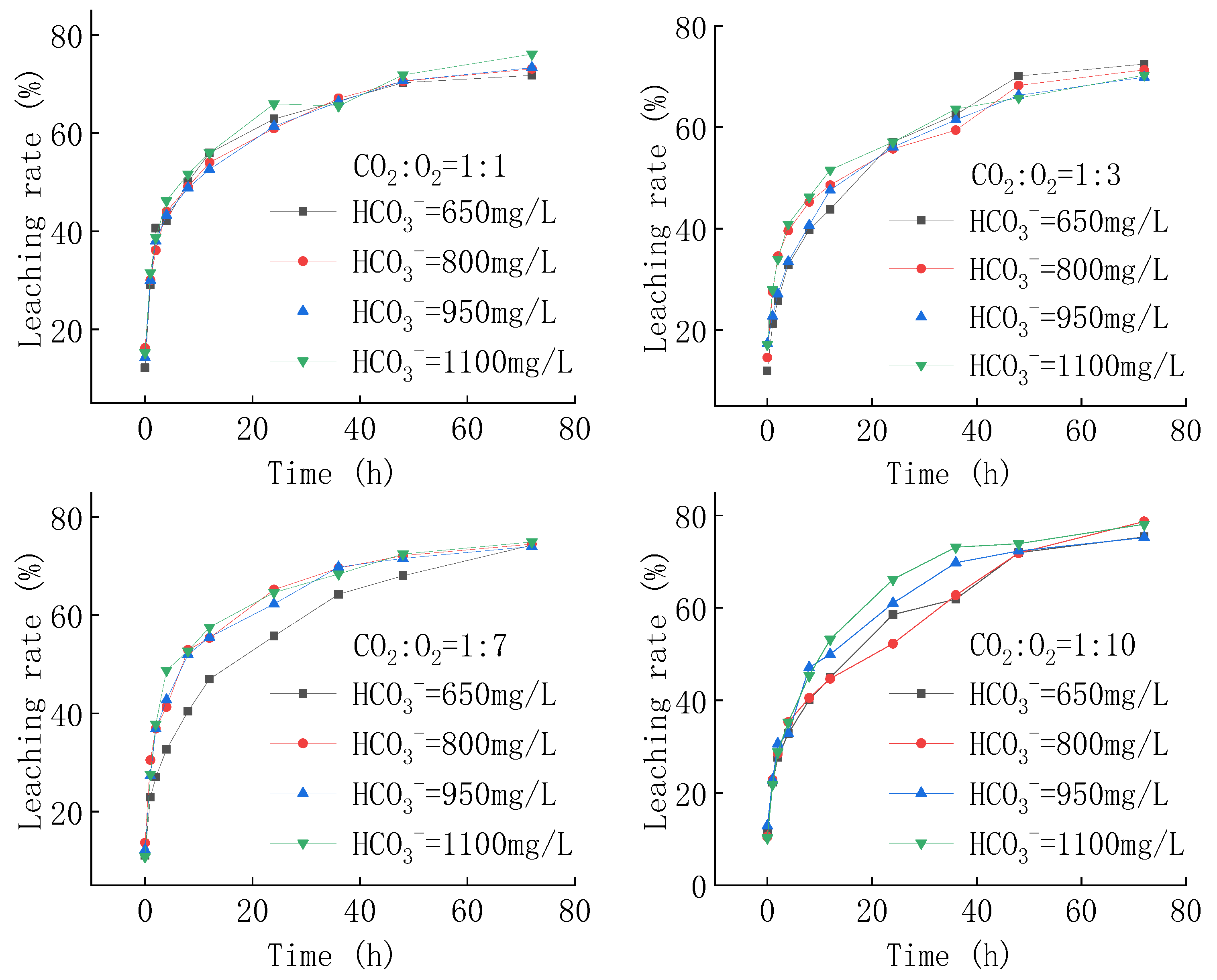
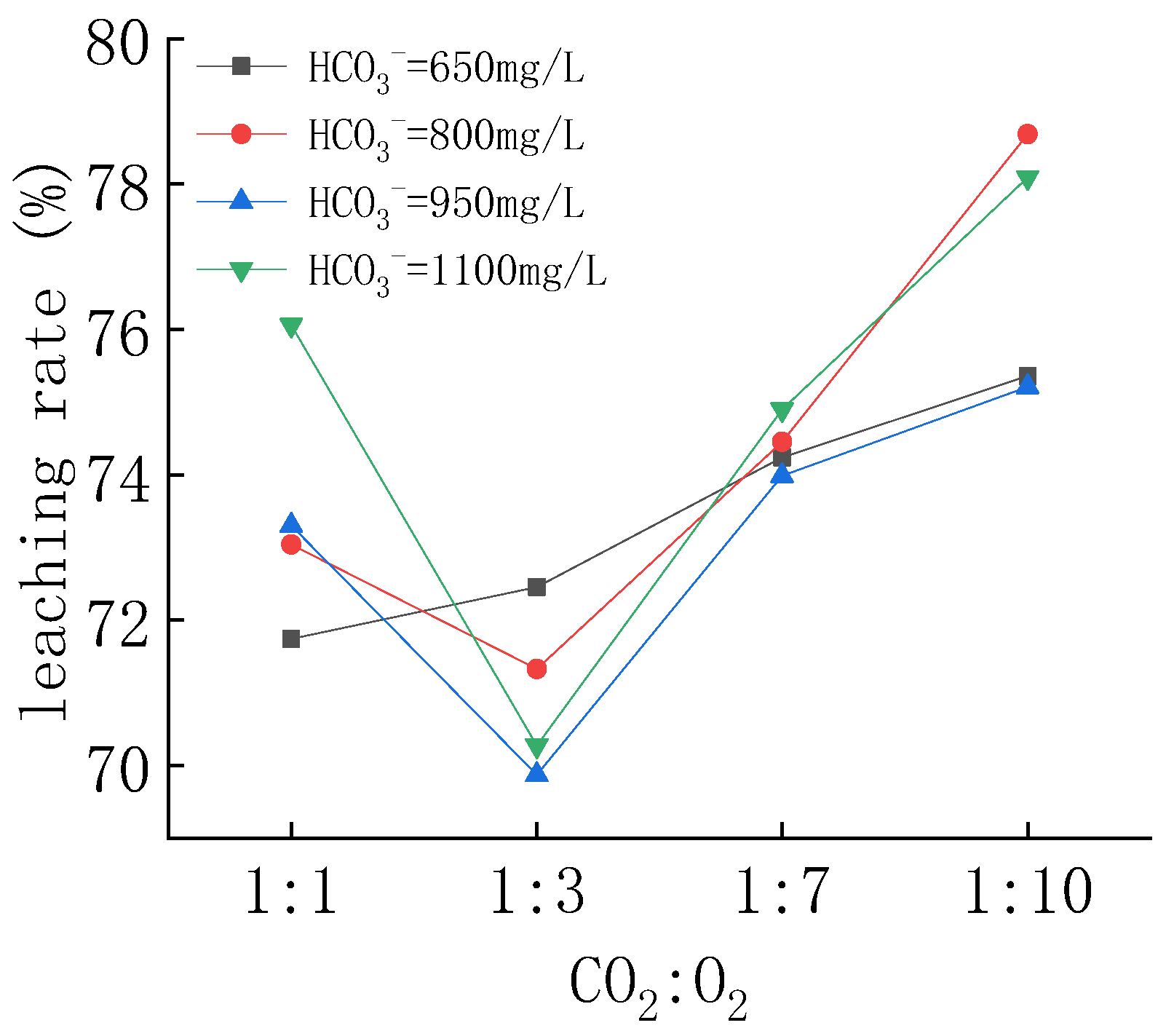
4.2.1. Uranium Concentration
4.2.2. Relationship between pH and HCO3−
4.2.3. Precipitation in U Leaching
5. Discussion
6. Result
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| t | CO2:O2 = 1:1 | CO2:O2 = 1:3 | CO2:O2 = 1:7 | CO2:O2 = 1:10 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | |
| 0 | 7.60458 | 10.11685 | 8.93803 | 9.5217 | 7.44197 | 9.08301 | 10.78159 | 10.64274 | 6.93894 | 8.52062 | 7.63175 | 6.73 | 6.967 | 6.56187 | 8.01769 | 6.37418 |
| 1 | 18.34184 | 18.90831 | 18.89222 | 19.84814 | 13.3461 | 17.29962 | 14.23818 | 17.54023 | 14.43997 | 19.21386 | 17.18365 | 17.38936 | 14.055 | 14.35018 | 14.29984 | 13.75811 |
| 2 | 25.79157 | 22.84245 | 24.04522 | 24.47714 | 16.30054 | 21.85135 | 17.0571 | 21.45476 | 17.08004 | 23.35944 | 23.35491 | 24.0103 | 17.523 | 18.02553 | 19.39315 | 18.26911 |
| 4 | 26.82705 | 28.01986 | 27.54211 | 29.42099 | 20.92645 | 25.16433 | 21.25599 | 26.00173 | 20.79791 | 26.25737 | 27.25434 | 31.21216 | 20.899 | 22.53461 | 20.84075 | 22.52334 |
| 8 | 32.09153 | 31.42265 | 31.25668 | 33.08043 | 25.57186 | 28.96378 | 26.06878 | 29.58387 | 26.00566 | 34.04085 | 33.45517 | 33.85589 | 25.784 | 26.03549 | 30.4218 | 29.2562 |
| 12 | 36.12264 | 34.77399 | 33.82721 | 36.0198 | 28.32943 | 31.24583 | 30.81595 | 33.23257 | 30.46729 | 35.7006 | 35.85647 | 37.16542 | 29.0455 | 28.8646 | 32.3488 | 34.63547 |
| 24 | 40.93494 | 39.61399 | 39.94124 | 42.96927 | 37.56604 | 36.21887 | 36.73767 | 37.06246 | 36.57258 | 42.55012 | 40.57395 | 42.07826 | 38.573 | 34.14263 | 40.0642 | 43.68072 |
| 36 | 43.52884 | 43.94837 | 43.4745 | 42.65271 | 41.42968 | 38.84805 | 40.54568 | 41.696 | 42.61494 | 45.66048 | 45.89132 | 44.75 | 40.951 | 41.60074 | 46.28897 | 48.614 |
| 48 | 46.23752 | 46.4745 | 46.5575 | 47.2405 | 46.9305 | 45.239 | 44.043 | 43.2655 | 45.35863 | 47.50854 | 47.17843 | 47.70715 | 48.216 | 48.197 | 48.13631 | 49.149 |
| 72 | 47.3419 | 48.33115 | 48.557 | 50.375 | 48.6845 | 47.521 | 46.6975 | 46.6055 | 49.95361 | 49.23819 | 48.98395 | 49.52528 | 50.745 | 53.2415 | 50.27 | 52.194 |
| t | CO2:O2 = 1:1 | CO2:O2 = 1:3 | CO2:O2 = 1:7 | CO2:O2 = 1:10 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | |
| 0 | 0.12187 | 0.16213 | 0.14324 | 0.15259 | 0.11926 | 0.14556 | 0.17278 | 0.17056 | 0.1112 | 0.13655 | 0.1223 | 0.10785 | 0.11165 | 0.10516 | 0.12849 | 0.12187 |
| 1 | 0.29096 | 0.30058 | 0.29999 | 0.31521 | 0.21224 | 0.27496 | 0.22722 | 0.27918 | 0.22933 | 0.30494 | 0.27273 | 0.27571 | 0.22327 | 0.22781 | 0.22742 | 0.29096 |
| 2 | 0.40621 | 0.36144 | 0.37971 | 0.38682 | 0.25795 | 0.34537 | 0.27082 | 0.33974 | 0.27017 | 0.36908 | 0.3682 | 0.37814 | 0.27692 | 0.28467 | 0.30621 | 0.40621 |
| 4 | 0.42194 | 0.44009 | 0.43284 | 0.46193 | 0.32822 | 0.3957 | 0.33462 | 0.40881 | 0.32665 | 0.4131 | 0.42744 | 0.48755 | 0.32821 | 0.35317 | 0.32821 | 0.42194 |
| 8 | 0.50045 | 0.49085 | 0.48824 | 0.51651 | 0.39751 | 0.45237 | 0.4064 | 0.46224 | 0.40432 | 0.52919 | 0.51992 | 0.52698 | 0.40107 | 0.40538 | 0.4711 | 0.50045 |
| 12 | 0.55946 | 0.5399 | 0.52586 | 0.55953 | 0.43787 | 0.48577 | 0.47588 | 0.51565 | 0.46963 | 0.55348 | 0.55507 | 0.57542 | 0.44881 | 0.44679 | 0.49931 | 0.55946 |
| 24 | 0.62856 | 0.6094 | 0.61365 | 0.65932 | 0.5705 | 0.55718 | 0.56091 | 0.57064 | 0.55729 | 0.65184 | 0.6228 | 0.64597 | 0.58561 | 0.52258 | 0.61009 | 0.62856 |
| 36 | 0.66508 | 0.67043 | 0.66341 | 0.65486 | 0.6249 | 0.5942 | 0.61453 | 0.63588 | 0.64238 | 0.69563 | 0.69768 | 0.68359 | 0.6191 | 0.6276 | 0.69774 | 0.66508 |
| 48 | 0.70247 | 0.7053 | 0.70596 | 0.71819 | 0.70083 | 0.68242 | 0.66281 | 0.65755 | 0.68025 | 0.72114 | 0.71545 | 0.72441 | 0.71938 | 0.71865 | 0.72324 | 0.70247 |
| 72 | 0.71741 | 0.73041 | 0.73301 | 0.76058 | 0.72456 | 0.71328 | 0.69871 | 0.70272 | 0.7424 | 0.74454 | 0.73987 | 0.749 | 0.75358 | 0.78688 | 0.7521 | 0.71741 |
| t | CO2:O2 = 1:1 | CO2:O2 = 1:3 | CO2:O2 = 1:7 | CO2:O2 = 1:10 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | |
| 0 | −0.481 | −0.4189 | −0.2072 | −0.2042 | −0.0229 | −0.0369 | 0.165 | 0.2484 | 0.1671 | 0.2438 | 0.3622 | 0.4832 | 0.4482 | 0.5511 | 0.5497 | 0.5713 |
| 1 | −0.4409 | −0.4215 | −0.1708 | −0.1611 | −0.0634 | −0.0446 | 0.2241 | 0.3131 | 0.292 | 0.3617 | 0.4353 | 0.4926 | 0.4804 | 0.6303 | 0.5835 | 0.6578 |
| 2 | −0.4284 | −0.3973 | −0.1539 | −0.1934 | −0.0462 | −0.0301 | 0.2513 | 0.3262 | 0.2576 | 0.3786 | 0.4643 | 0.5023 | 0.4616 | 0.6427 | 0.6275 | 0.6539 |
| 4 | −0.3781 | −0.4497 | −0.1607 | −0.101 | −0.0171 | −0.0221 | 0.1862 | 0.3163 | 0.289 | 0.3688 | 0.4478 | 0.5256 | 0.4279 | 0.631 | 0.5666 | 0.6658 |
| 8 | −0.3354 | −0.4229 | −0.1776 | −0.1331 | −0.1372 | 0.0073 | 0.0959 | 0.2382 | 0.2708 | 0.4027 | 0.4485 | 0.5267 | 0.4182 | 0.6486 | 0.498 | 0.652 |
| 12 | −0.298 | −0.3906 | −0.1655 | −0.1415 | −0.2494 | 0.0287 | 0.1347 | 0.2413 | 0.2454 | 0.3092 | 0.4919 | 0.5412 | 0.4072 | 0.6628 | 0.5642 | 0.6772 |
| 24 | −0.327 | −0.3423 | −0.1661 | −0.0173 | −0.2116 | 0.0392 | 0.0686 | 0.2127 | 0.1984 | 0.3617 | 0.4453 | 0.5215 | 0.4284 | 0.5727 | 0.5487 | 0.6004 |
| 36 | −0.2973 | −0.3214 | −0.1366 | −0.1177 | −0.165 | −0.0062 | 0.1128 | 0.275 | 0.3011 | 0.3071 | 0.4502 | 0.5623 | 0.3965 | 0.6036 | 0.6016 | 0.6781 |
| 48 | −0.2906 | −0.3249 | −0.1733 | −0.1034 | −0.1028 | −0.0073 | 0.1019 | 0.2694 | 0.2372 | 0.339 | 0.4595 | 0.5573 | 0.4167 | 0.5331 | 0.5474 | 0.6426 |
| 72 | −0.1911 | −0.3148 | −0.1338 | −0.063 | −0.0755 | 0.0238 | 0.1705 | 0.3263 | 0.2595 | 0.3456 | 0.4915 | 0.5723 | 0.4194 | 0.6047 | 0.6361 | 0.6265 |
| t | CO2:O2 = 1:1 | CO2:O2 = 1:3 | CO2:O2 = 1:7 | CO2:O2 = 1:10 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | 650 | 800 | 900 | 1100 | |
| 0 | −0.1974 | −0.2548 | −0.1751 | −0.2517 | −0.2069 | −0.2137 | −0.1705 | −0.1698 | −0.2518 | −0.242 | −0.2061 | −0.1924 | −0.2067 | −0.1907 | −0.3127 | −0.3307 |
| 1 | −0.2016 | −0.2595 | −0.1688 | −0.2489 | −0.2093 | −0.2042 | −0.1685 | −0.1703 | −0.2177 | −0.2467 | −0.2019 | −0.1876 | −0.2012 | −0.1806 | −0.3065 | −0.2838 |
| 2 | −0.2123 | −0.2536 | −0.1636 | −0.2987 | −0.1998 | −0.2132 | −0.1689 | −0.1687 | −0.2224 | −0.2381 | −0.1877 | −0.1732 | −0.2102 | −0.1776 | −0.2803 | −0.2978 |
| 4 | −0.1917 | −0.2537 | −0.1707 | −0.2302 | −0.1979 | −0.2102 | −0.1681 | −0.1691 | −0.2118 | −0.2453 | −0.2049 | −0.1793 | −0.1946 | −0.1758 | −0.3032 | −0.2688 |
| 8 | −0.1966 | −0.269 | −0.1712 | −0.28 | −0.1873 | −0.2133 | −0.1973 | −0.2196 | −0.2167 | −0.2205 | −0.2011 | −0.1782 | −0.1968 | −0.176 | −0.298 | −0.2833 |
| 12 | −0.2056 | −0.2686 | −0.1673 | −0.2748 | −0.1862 | −0.2077 | −0.1693 | −0.2321 | −0.2082 | −0.2451 | −0.1812 | −0.1626 | −0.208 | −0.1817 | −0.2852 | −0.2729 |
| 24 | −0.2087 | −0.2682 | −0.1561 | −0.1713 | −0.1936 | −0.1898 | −0.1836 | −0.2421 | −0.1985 | −0.2204 | −0.1752 | −0.1573 | −0.1513 | −0.1779 | −0.2955 | −0.2918 |
| 36 | −0.1995 | −0.2529 | −0.1526 | −0.2639 | −0.1644 | −0.1833 | −0.167 | −0.1724 | −0.2067 | −0.2256 | −0.1693 | −0.1523 | −0.1844 | −0.1687 | −0.2866 | −0.2769 |
| 48 | −0.1994 | −0.2508 | −0.1866 | −0.2749 | −0.1439 | −0.1669 | −0.1567 | −0.181 | −0.1868 | −0.2255 | −0.1609 | −0.1482 | −0.1637 | −0.1857 | −0.2908 | −0.2566 |
| 72 | −0.1601 | −0.26 | −0.1544 | −0.1965 | −0.1313 | −0.1579 | −0.1562 | −0.1611 | −0.1814 | −0.1974 | −0.1587 | −0.1452 | −0.1571 | −0.1687 | −0.2909 | −0.2465 |
References
- Asghar, F.; Sun, Z.; Chen, G.; Zhou, Y.; Li, G.; Liu, H.; Zhao, K. Geochemical characteristics and uranium neutral leaching through a CO2 + O2 system—An example from Uranium Ore of the ELZPA Ore Deposit in Pakistan. Metals 2020, 10, 1616. [Google Scholar] [CrossRef]
- Asghar, F.; Zhou, Y.; Sun, Z.; Li, G.; Xu, L.; Zhao, K. CO2 + O2 neutral leaching experiment of uranium ore from Mengqiguer deposit in Xinjiang. Nonferrous Met. Extr. Metall. 2021, 8, 46–55. [Google Scholar]
- Cao, L.; Qiao, H.; Zhou, W.; Wu, Z. Analysis on affecting factors of metallogenic difference in the southern ore belt of Shihongtan uranium deposit. World Nucl. Geosci. 2018, 35, 77–83. [Google Scholar]
- Chen, J.; Zhu, P.; Xu, Q.; Wei, X.; Liu, W. Laboratory test of neutral leaching of a uranium ore in Inner Mongolia. Uranium Min. Metall. 2013, 32, 72–75+86. [Google Scholar]
- Cheng, W.; Miao, A.; Li, J.; Zhou, L.; Chang, J. Analysis of leachability for a sandstone uranium deposit with high acid consumption and sensitivities in inner Mongolia. Prog. Rep. China Nucl. Sci. Technol. 2013, 3, 156–162. [Google Scholar]
- Du, Z.; Niu, X.; Su, X.; Guo, Z.; Cheng, Z.; Shen, H.; Zhang, W. CO2 + O2 in-situ leaching test of one uranium deposit in inner Mongolia. Uranium Min. Metall. 2013, 32, 1–4. [Google Scholar]
- Gao, B.; Du, Y.; Sun, Z. Analyzed the conditions of leaching uranium ins south area of Shihongtan uranium deposit. China Min. Mag. 2013, 22, 89–92. [Google Scholar]
- He, X.; Chen, L.; Xie, X.; Xiao, B.; Long, H.; Wen, C. Analysis of blockage formation reason during the acid in-situ leaching and chemical deblocking of 512 deposit. In Progress Report on China Nuclear Science & Technology, Proceedings of 2019 Academic Annual Meeting of Chinese Nuclear Society Volume 2 (Uranium Geology Volume 2), Baotou, China, 20–23 August 2019; China Atomic Energy Press: Beijing, China, 2019; pp. 346–352. [Google Scholar]
- Hu, P.; Li, X.; Chen, G.; Ren, J. Present situation and development of ISL radiation protection and regulation in China. Uranium Min. Metall. 2019, 38, 70–74. [Google Scholar]
- Ji, H.; Zhou, Y.; Sun, Z.; Yang, Y. Analysis of and discussion on CO2 + O2 in-situ leaching of uranium process at Mengqiguer deposit. Nonferrous Met. Extr. Metall. 2018, 3, 55–59. [Google Scholar]
- Jin, G.; Wu, Q.; Wang, T. Sedimentary system of the Second lithologic member of Xishanyao formation and its control on Uranium mineralization in the north belt of Shihongtan Uranium deposit, Turpan-Hami basin. Uranium Geol. 2021, 37, 1001–1012. [Google Scholar]
- Grancea, L.; Mihalasky, M.J.; Fairclough, M. Uranium Resources 2020, Production and Demand; International Atomic Energy Agency: Vienna, Austria, 2020. [Google Scholar]
- Li, J. Application of Artificial intelligence neural networks in lithology identification and porosity and permeability prediction-An example from Shihongtan uranium deposit. Northwestern Geol. 2010, 43, 6–7+32–37. [Google Scholar]
- Li, Y.; Pan, S.; Ning, S.; Shao, L.; Jing, Z.; Wang, Z. Coal measure metallogeny: Metallogenic system and implication for resource and environment. Sci. China Earth Sci. 2022, 65, 1211–1228. [Google Scholar]
- Liu, J.; Sun, Z.; Zhou, Y.; Li, X.; Shi, W. Hydrogeochemical conditions of in-situ leaching uranium exploration for Shihongtan deposit. Met. Mine 2010, 405, 77–79. [Google Scholar]
- Liu, S.; Liu, J.; Zhou, Y.; Yang, Y.; Wang, R.; Shi, W.; Liao, S. Study on characteristics of ore-containing layer blockage during in-situ leaching of uranium mining. Nonferrous Met. Extr. Metall. 2022, 8, 65–75. [Google Scholar]
- Liu, Z.; Yang, B.; Zhao, X.; Wang, T. Mineral ingredient and geochemical characteristics of uranium mineralization at Shihongtan uranium deposit, Turpan-Hami basin. Xinjiang Geol. 2018, 36, 257–261. [Google Scholar]
- Niu, Q.; Cao, L.; Sang, S.; Wang, W.; Yuan, W.; Chang, J.F.; Jia, X.; Zheng, W.; Zhang, Z.X. A small-scale experimental study of CO2 enhanced injectivity methods of the high-rank coal. Pet. Sci. 2021, 18, 1427–1440. [Google Scholar]
- Su, X.; Li, X.; Liu, N.; Wang, J.; Zhang, L. Application of the Environment Friendly Technology of In-Situ Leaching of Uranium. China Min. Mag. 2016, 25, 97–100. [Google Scholar]
- Sun, Z.; Asghar, F.; Zhao, K.; Zhou, Y.; Li, G.; Xu, L. Review and prospect of uranium mining and metallurgy in China. Nonferrous Met. Extr. Metall. 2021, 8, 1–8. [Google Scholar]
- Tan, X. Brief analysis of China’s nuclear energy development status and prospects. China Plant Eng. 2021, 483, 235–236. [Google Scholar]
- Wang, G.; Zhang, F.; He, D.; Li, J. Analysis of in-situ leaching feasibility in northern belt of Shihongtan uranium deposits in Xinjiang Turpan. J. East China Inst. Technol. Nat. Sci. Ed. 2015, 38, 58–63. [Google Scholar]
- Wu, Q.; Jin, G.; Yang, B.; Cao, L. Characteristics of clay minerals in the northern ore-belt sandstone of Shihongtan uranium deposit and its relation with uranium mineralization. Coal Geol. China 2021, 33, 55–61. [Google Scholar]
- Xu, L.; Yang, H.; Zhou, Y.; Li, G.; Sun, Z.; Shi, W.; Zhou, Z. Neutral leaching of uranium ore from a sandstone-type deposit. Nonferrous Met. Extr. Metall. 2020, 3, 38–44. [Google Scholar]
- Yan, J.; Zhang, B.; Liu, J.; Cao, J. Study on leaching effect of in-situ leaching uranium mine by CO2 + O2. Uranium Min. Metall. 2020, 39, 185–190. [Google Scholar]
- Yang, B.; Qiao, H.; Liu, Z.; Wu, Q. Calcareous sandstone distribution features and its impact on in-situ leaching of uranium exploitation in north ore belt of Shihongtan uranium deposit, Turpan-Hami basin. Coal Geol. China 2022, 34, 16–21. [Google Scholar]
- Yu, S. Study on Migration Behavior of Key Nuclides in Cavern Disposal Repository. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2021. [Google Scholar]
- Yuan, X.; Liu, J.; Zhou, Y.; He, T.; Chen, X.; Zahng, Y. Effect of CO2 + O2 In-situ Leaching of Uranium on Permeability of Ore-bearing Layer. Nonferrous Met. Extr. Metall. 2021, 5, 50–57. [Google Scholar]
- Zhang, J.; Li, Z.; Su, X.; Tan, Y.; Li, S.; Su, Y.; Li, J.; Deng, P.; Xu, L.; Pan, Z. Development strategy of nuclear energy mineral resources. Eng. Sci. 2019, 21, 113–118. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Fei, Z. CO2 + O2 Neutral in-situ leaching test of a uranium mineral deposit in Xinjiang. China Min. Mag. 2012, S1, 435–438. [Google Scholar]
- Zhang, X.; Nie, F.; Zhang, C.; Zhang, H.; Dong, F.; Dong, Y.; Lu, Y. Study on uranium occurrence state of sandstone-type uranium deposits in Mengqiguer deposit, Yili basin. Sci. Technol. Eng. 2015, 15, 18–23+47. [Google Scholar]
- Zhang, X.; Nie, F. Analysis of the provenance of the uranium-bearing target layer and its tectonic setting in the Shihongtan uranium deposit, Xinjiang: Evidence from the petrology and geochemistry. Sci. Technol. Eng. 2019, 19, 86–92. [Google Scholar]
- Zhao, K.; Zhang, Y.; Zhou, Y.; Li, G.; Sun, Z.; Liu, J.; Xu, L.; Yuan, X.; Hu, M. Interaction between gangue mineral and leaching agent in CO2 + O2 in-situ leaching. Nonferrous Met. Extr. Metall. 2023, 3, 68–87. [Google Scholar]
- Zhou, Y.; Li, G.; Xu, L.; Liu, J.; Sun, Z.; Shi, W. Uranium recovery from sandstone-type uranium deposit by acid in-situ leaching—An example from the Kujieertai. Hydrometallurgy 2020, 191, 105209. [Google Scholar] [CrossRef]
- Zhu, H.; Qiao, H.; Jia, H.; Xu, G. Discussion on distribution characteristics of calcareous sandstone in Shihongtan uranium deposit and its genesis. Uranium Geol. 2007, 5, 262–267+288. [Google Scholar]
- Liu, Y.; Luo, Y.; Li, X.; Wang, B.; Liu, C. Water rock reaction plugging mechanism of acid leaching uranium in Bayanwula uranium deposit. Nonferrous Met. Min. Sect. 2022, 5, 45–51. [Google Scholar]






| HCO3− | Cl− | SO42− | NO3− | Al3+ | Ca2+ | K+ | Na+ | Mg2+ | Fe2+ |
|---|---|---|---|---|---|---|---|---|---|
| 274.06 | 2564.01 | 2527.55 | 26.85 | <0.05 | 606.26 | 22.95 | 1979.92 | 300.04 | <0.1 |
| Subject | SiO2 | Al2O3 | K2O | CaO | Na2O | MgO | TiO2 | TFe2O3 | FeO | CO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R < 0.25 mm | 70.14 | 15.09 | 3.51 | 1.47 | 1.50 | 1.08 | 0.45 | 2.52 | 1.62 | 0.8 | 4.14 |
| 0.25~0.5 mm | 75.93 | 11.60 | 3.17 | 1.12 | 1.08 | 0.83 | 0.35 | 2.45 | 1.69 | 0.6 | 3.02 |
| 0.5~1 mm | 75.77 | 10.57 | 2.67 | 2.14 | 0.91 | 0.80 | 0.35 | 2.14 | 1.50 | 1.4 | 3.75 |
| R > 1 mm | 71.86 | 10.88 | 2.71 | 4.03 | 1.01 | 0.82 | 0.35 | 2.56 | 1.75 | 2.9 | 5.07 |
| Mixed sample | 73.29 | 11.75 | 2.96 | 2.25 | 1.07 | 0.89 | 0.38 | 2.65 | 1.92 | 1.5 | 4.05 |
| Subject | <0.25 mm | 0.25~0.5 mm | 0.5~1 mm | >1 mm | Mixed Sample |
|---|---|---|---|---|---|
| U (μg/g) | 415 | 303 | 302 | 307 | 312 |
| TFe2O3 (%) | 2.52 | 2.45 | 2.14 | 2.56 | 2.56 |
| FeO (%) | 1.62 | 1.69 | 1.50 | 1.75 | 1.68 |
| Sulfide S (%) | 0.03 | 0.05 | 0.08 | 0.20 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Liu, J.; Xu, L.; Zhou, Y.; Zhao, K.; Hu, M. Study on the Leaching Conditions of the Shihongtan Uranium Deposit. Metals 2023, 13, 1284. https://doi.org/10.3390/met13071284
Yuan X, Liu J, Xu L, Zhou Y, Zhao K, Hu M. Study on the Leaching Conditions of the Shihongtan Uranium Deposit. Metals. 2023; 13(7):1284. https://doi.org/10.3390/met13071284
Chicago/Turabian StyleYuan, Xin, Jinhui Liu, Lingling Xu, Yipeng Zhou, Kai Zhao, and Mintao Hu. 2023. "Study on the Leaching Conditions of the Shihongtan Uranium Deposit" Metals 13, no. 7: 1284. https://doi.org/10.3390/met13071284
APA StyleYuan, X., Liu, J., Xu, L., Zhou, Y., Zhao, K., & Hu, M. (2023). Study on the Leaching Conditions of the Shihongtan Uranium Deposit. Metals, 13(7), 1284. https://doi.org/10.3390/met13071284





