Influence of Melt Infiltration Parameters on Structural and Mechanical Properties of Al-4.3wt.%Cu-EP Syntactic Foam
Abstract
1. Introduction
2. Materials and Methods
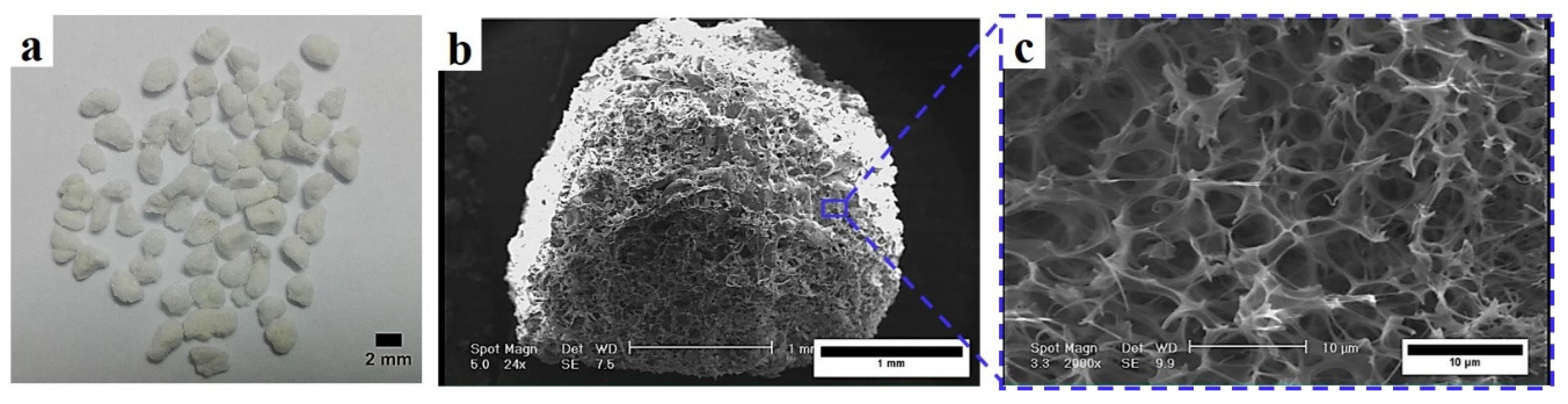
2.1. Raw Materials
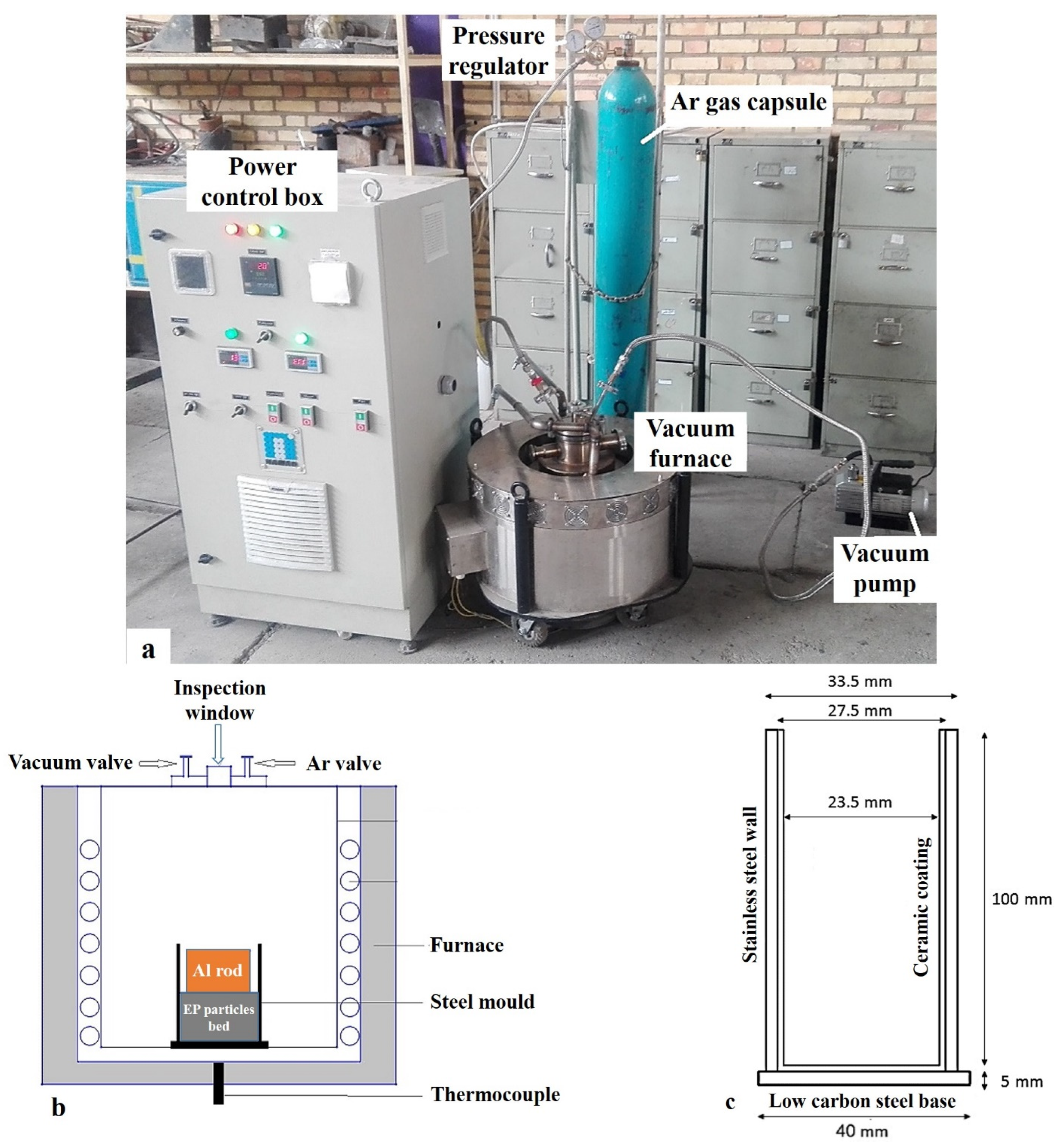
2.2. Fabrication Method
2.3. Characterization
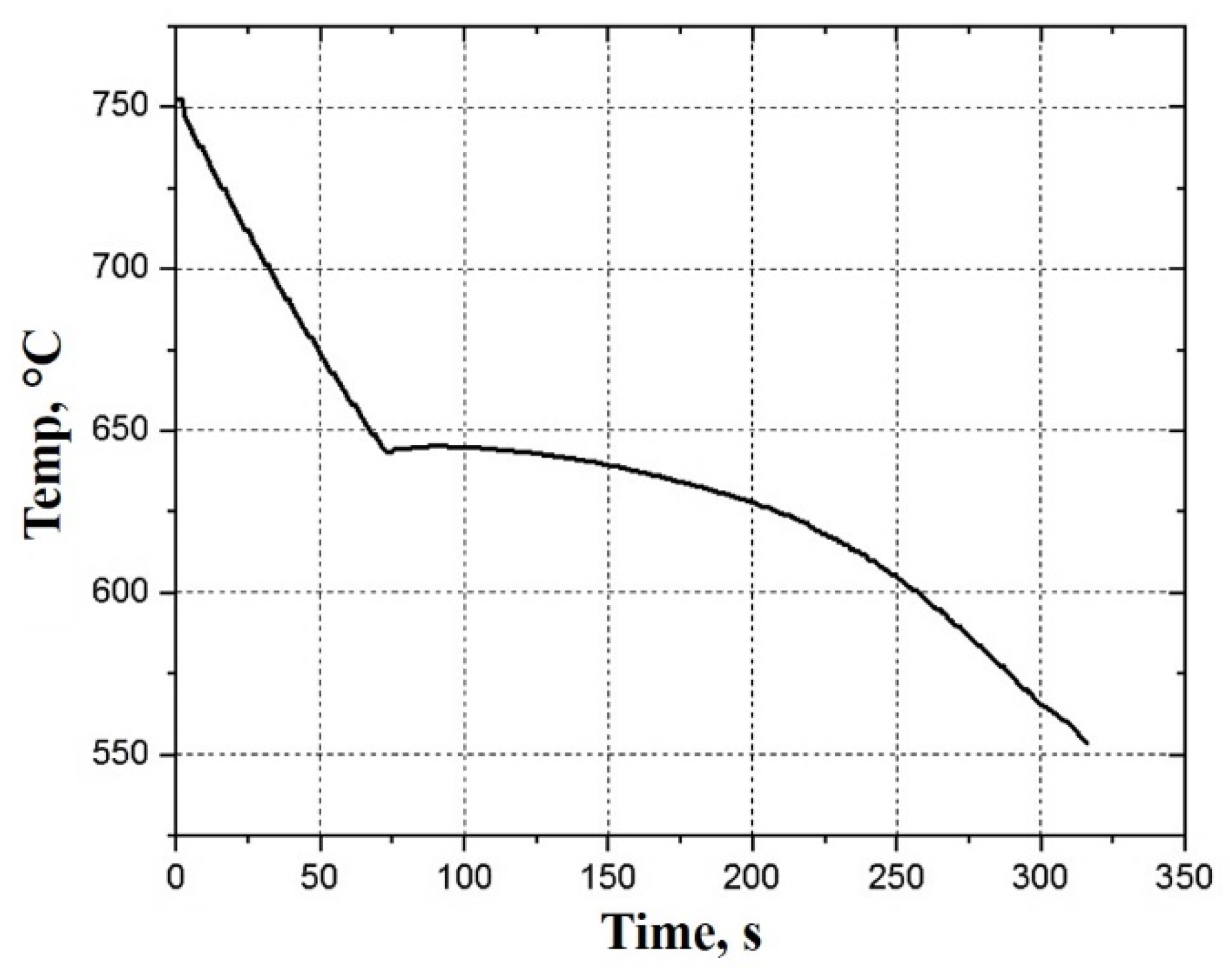
3. Results and Discussion
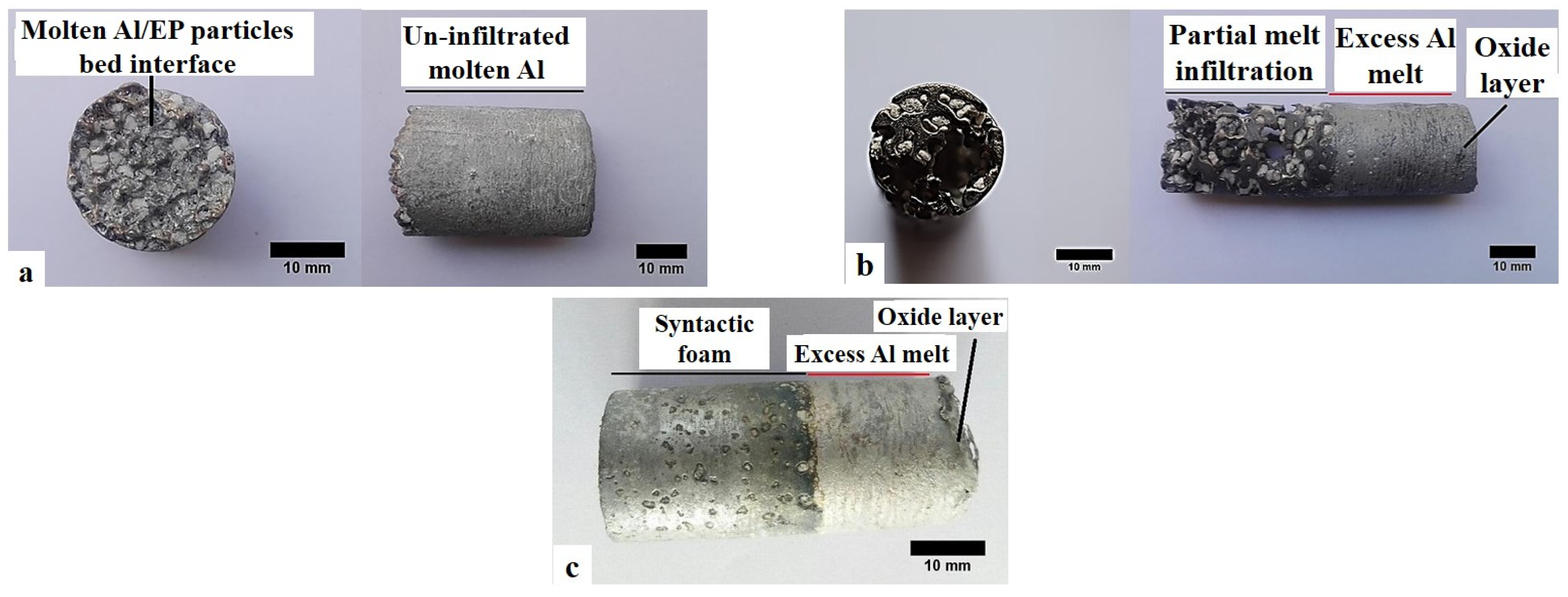
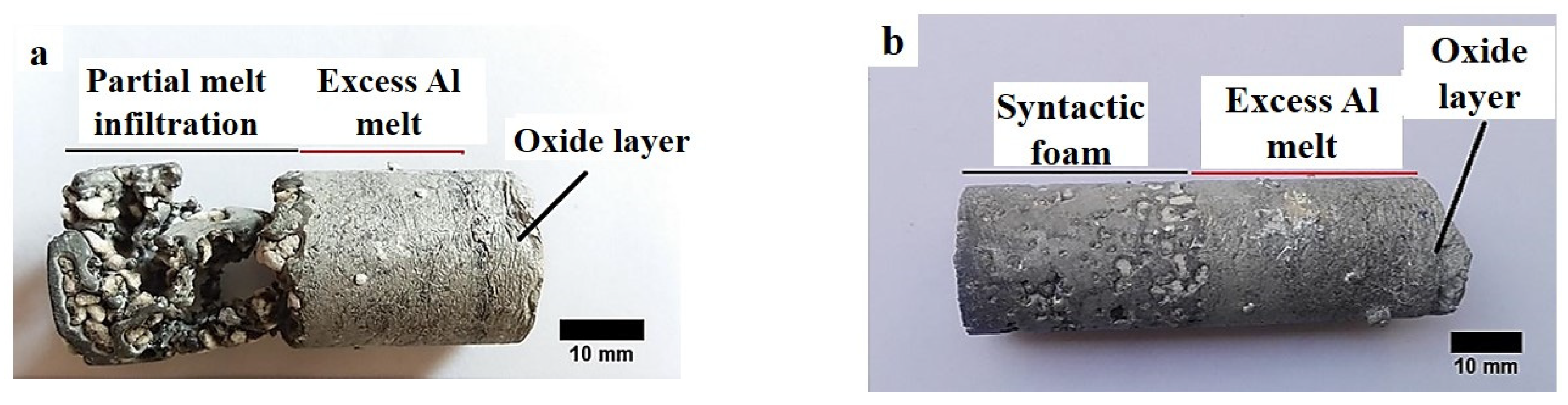
3.1. Infiltration of the EPPs Bed
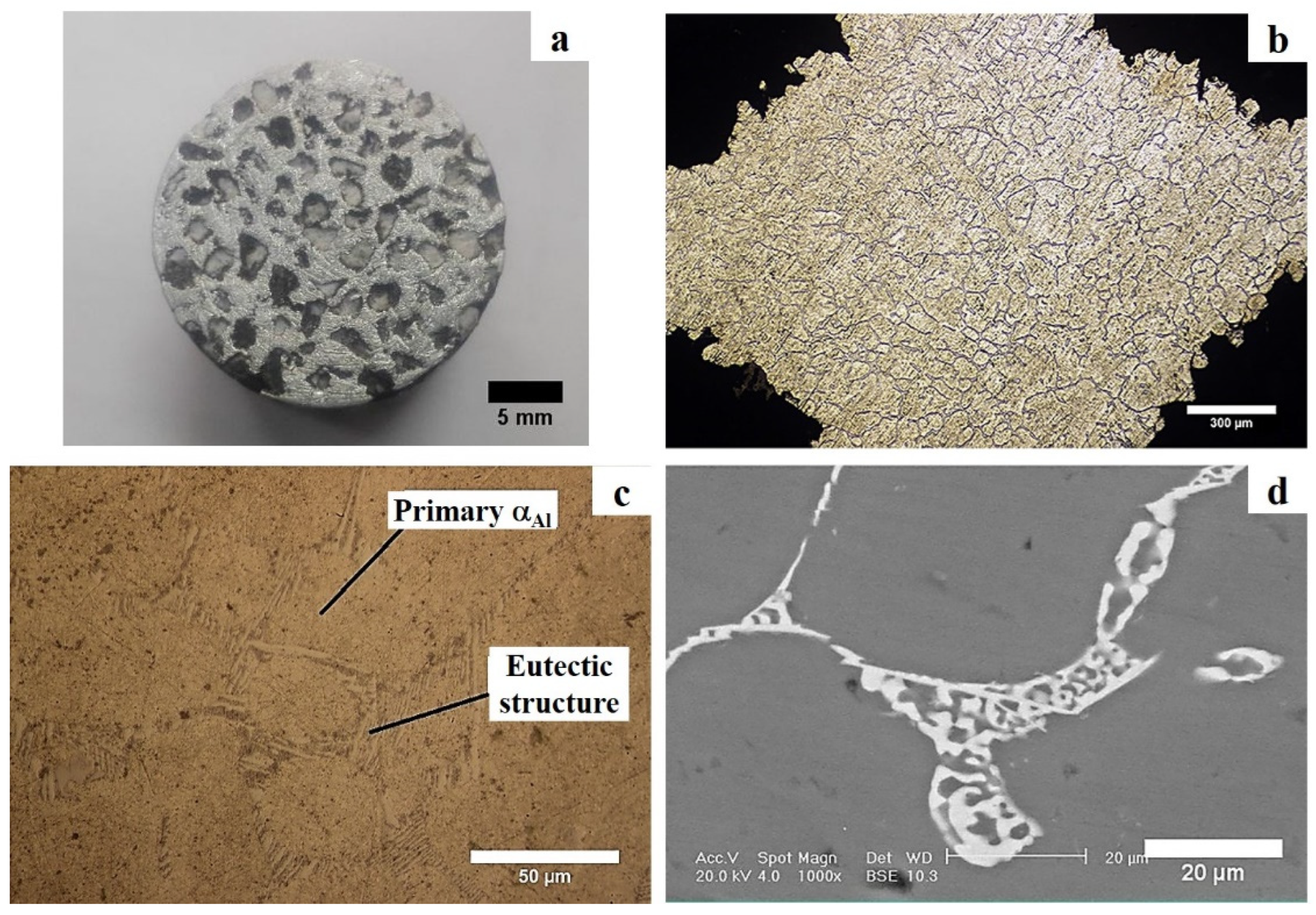
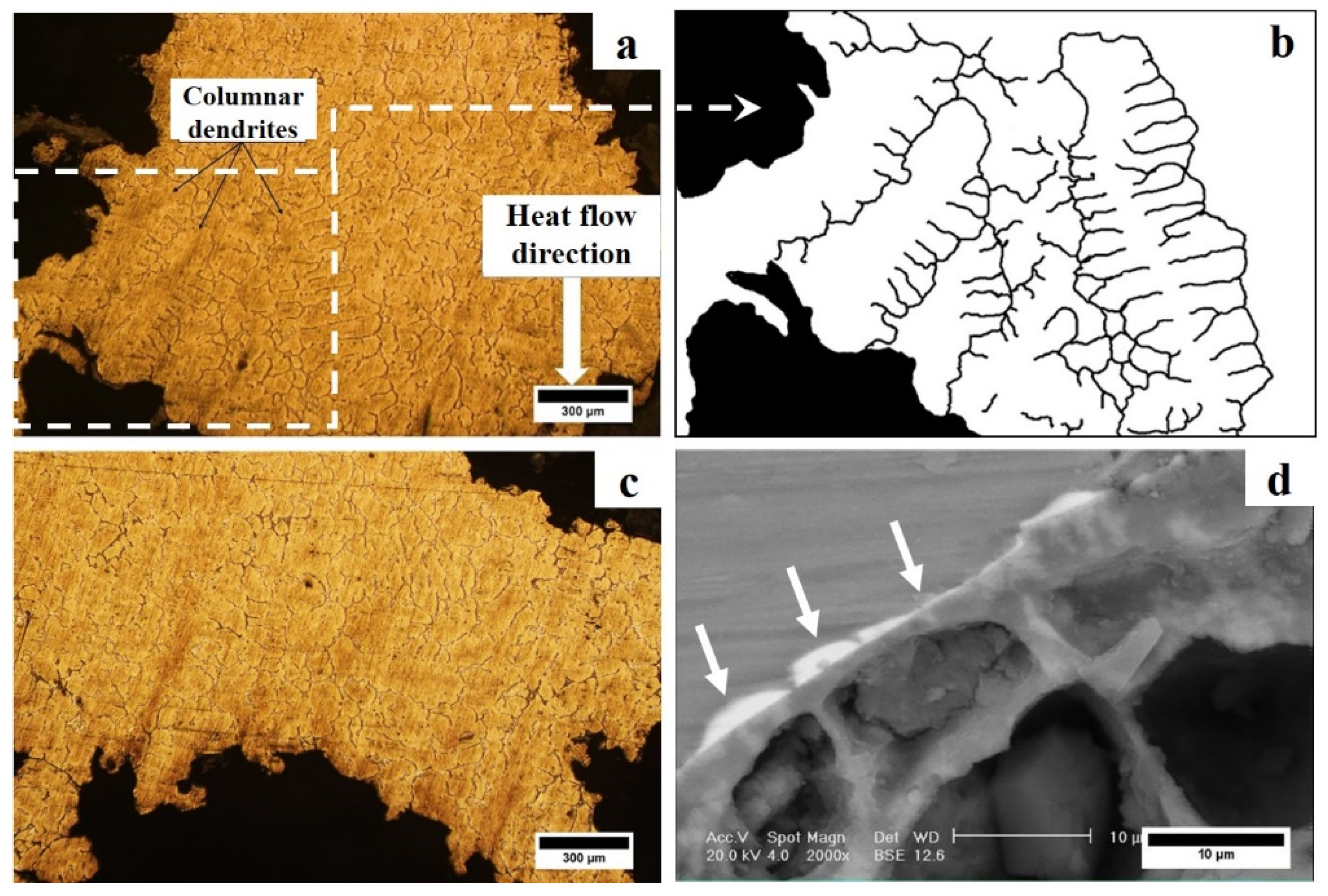
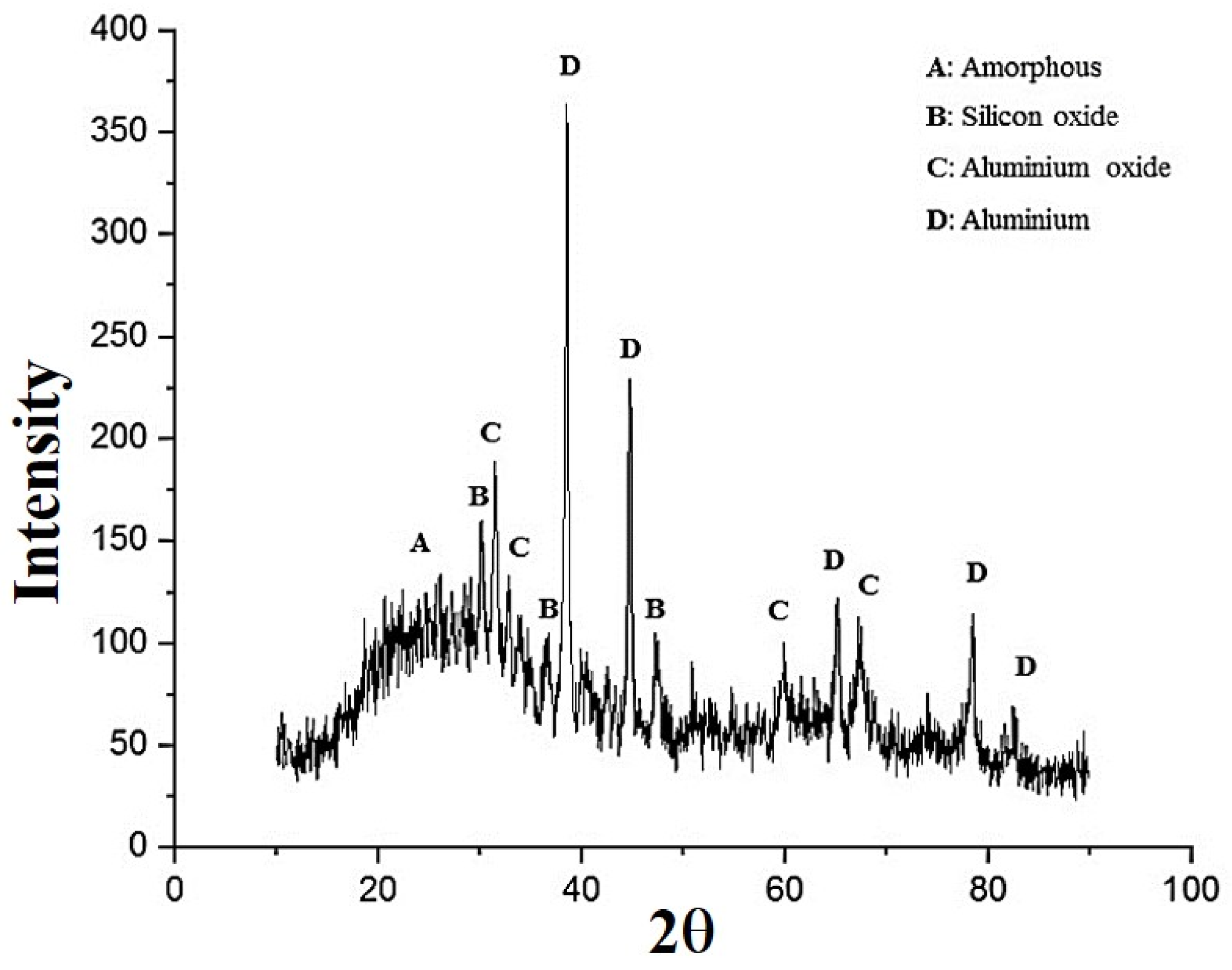
3.2. Microstructural Studies of the Optimum Sample
3.3. Heat Treatment
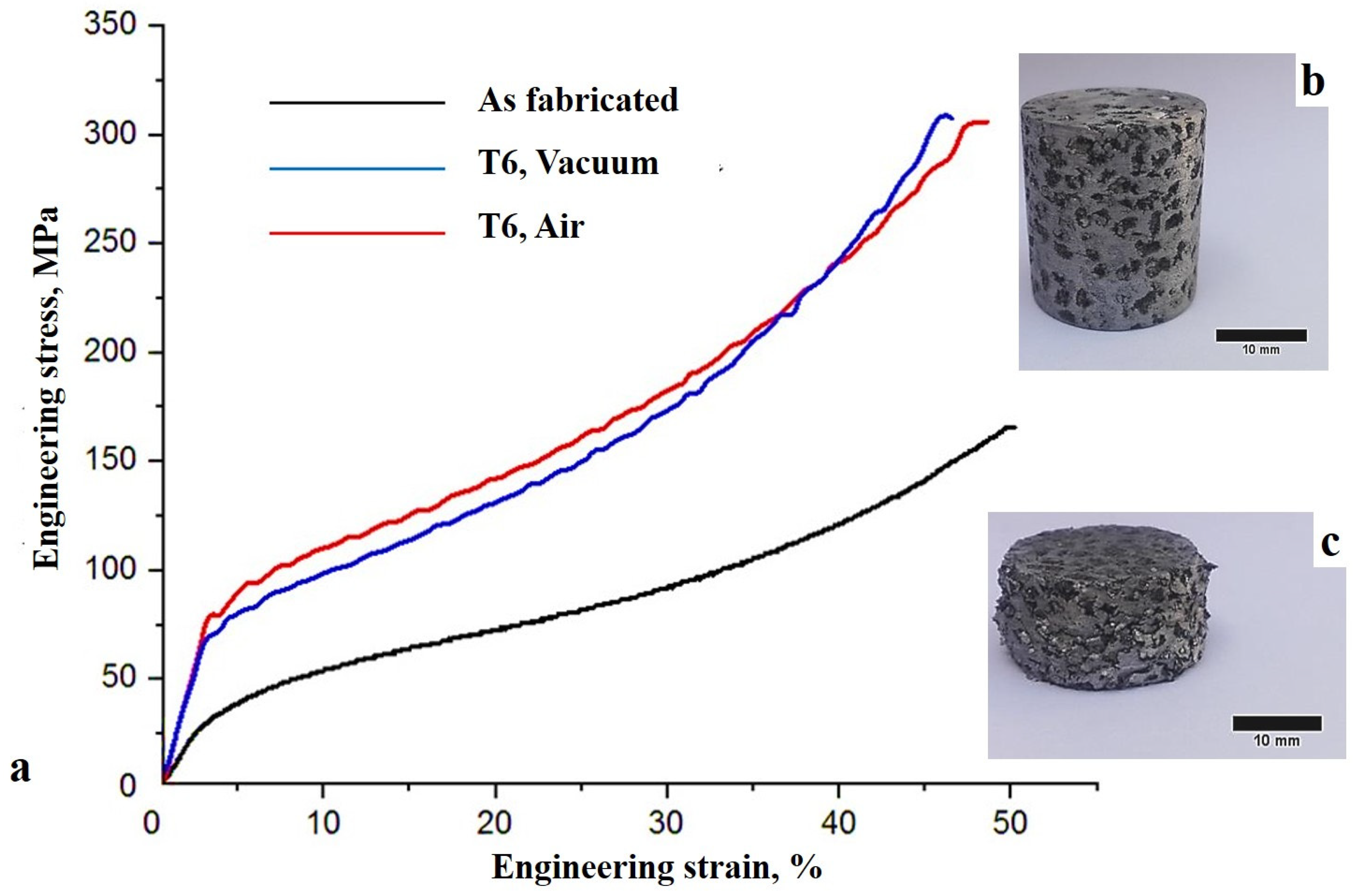
3.4. Mechanical Properties
4. Conclusions
- Al-4.3wt.%Cu-expanded perlite (EP) syntactic foams were successfully fabricated using a gas pressure infiltration method, and the effects of infiltration temperature and pressure were examined;
- The effects of the infiltration temperature on successful infiltration of the melt in the EP particles bed was found to be far greater than that of the infiltration pressure;
- Complete infiltration is achieved by preferential infiltration of the melt between the mould wall and the EP particles bed, complemented by radial melt infiltration toward the centre of the samples. The full melt infiltration is believed to be mainly affected by the more likely breakage of the aluminium oxide layer on the melt surface and lower melt viscosity at higher temperatures;
- Atmospheric pressure and 750 °C and were found as the optimum processing conditions for full infiltration of the melt, and resulted in Al-4.3wt.%Cu-EP syntactic foams with an average density of 1.55 g/cm3 and EP volume percentage of 50.3%;
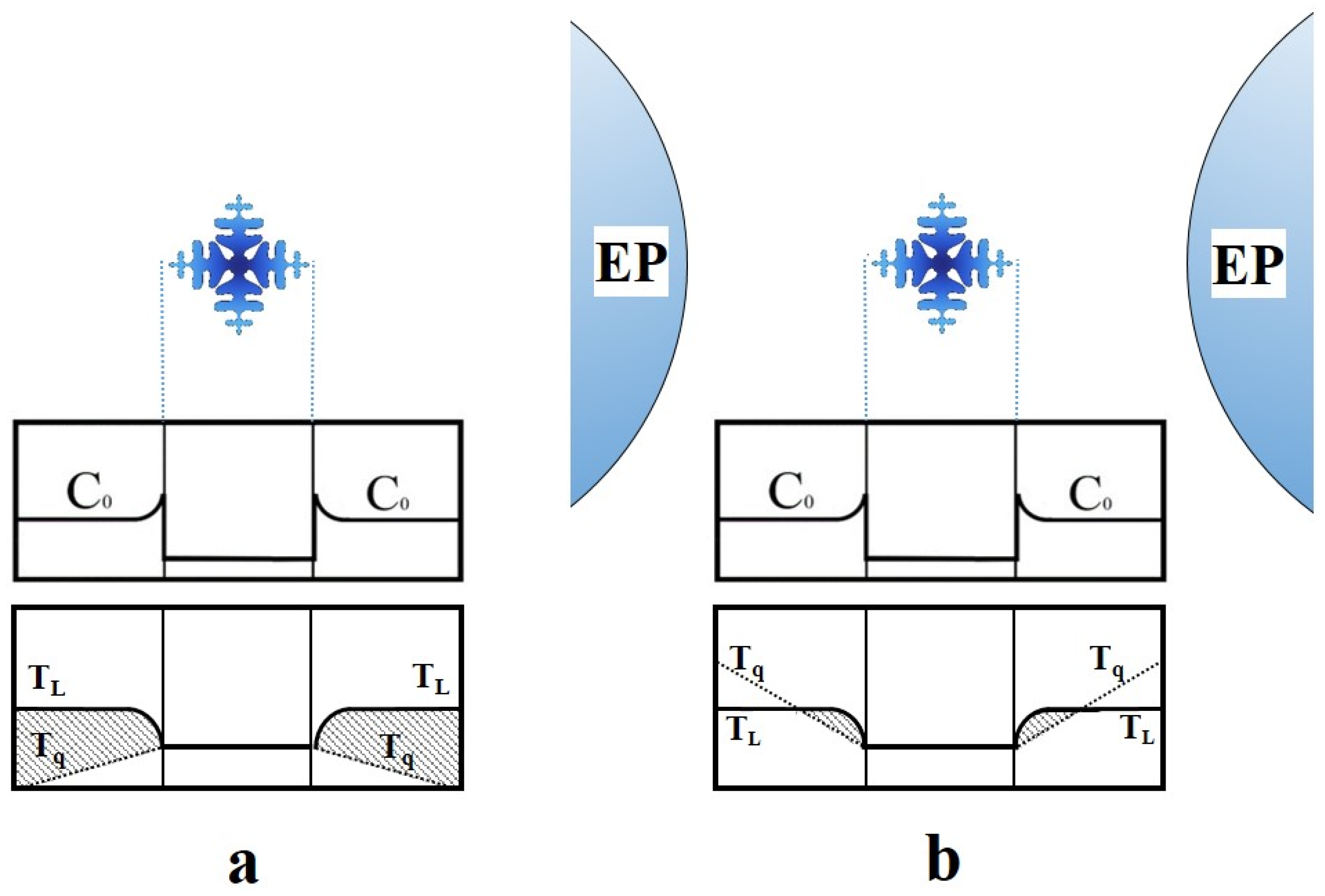
- The average dendrite cell spacing at the lower and upper part of the syntactic foams was about 41 and 64 μm, respectively, due to directional cooling of the samples;
- No evidence of extensive directional solidification of columnar grains was observed in the microstructures. The reasons were discussed in terms of the mechanical and thermal effects of EP particles on the growth of the nucleated primary α-aluminium phase;
- T6 heat treatment improved the average compressive tensile strength, plateau stress, and absorption capacity of the syntactic foams by 98–120%, 77–98%, and 75–100%, respectively;
- Similar densification strains of the as-fabricated and heat-treated syntactic foams (about 40%), and the uniform strain of all samples during the compression test suggested the uniform distribution of EP particles and metallic struts in the aluminium alloy matrix.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashby, M.F.; Evans, T.; Fleck, N.A.; Hutchinson, J.; Wadley, H.; Gibson, L. Metal Foams: A Design Guide; Butterworth-Heinemann: London, UK, 2000. [Google Scholar]
- Öchsner, A.; Murch, G.E.; De Lemos, M.J. Cellular and Porous Materials: Thermal Properties Simulation and Prediction; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Öechsner, A.; Augustin, C. Multifunctional Metallic Hollow Sphere Structures: Manufacturing, Properties and Application; Springer-Verlag: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Kádár, C.; Kubelka, P.; Szlancsik, A. On the Compressive Properties of Aluminum and Magnesium Syntactic Foams: Experiment and Simulation. Mater. Today Commun. 2023, 35, 106060. [Google Scholar] [CrossRef]
- Kemény, A.; Leveles, B.; Bubonyi, T.; Orbulov, I.N. Effect of Particle Size and Volume Ratio of Ceramic Hollow Spheres on the Mechanical Properties of Bimodal Composite Metal Foams. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106152. [Google Scholar] [CrossRef]
- Szlancsik, A.; Orbulov, I.N. Compressive Properties of Metal Matrix Syntactic Foams in Uni- and Triaxial Compression. Mater. Sci. Eng. A 2021, 827, 142081. [Google Scholar] [CrossRef]
- Kincses, D.B.; Károly, D.; Bukor, C. Production and Testing of Syntactic Metal Foams with Graded Filler Volume. Mater. Today Proc. 2021, 45, 4225–4228. [Google Scholar] [CrossRef]
- Bolat, C.; Bilge, G.; Gökşenli, A. An Investigation on the Effect of Heat Treatment on the Compression Behavior of Aluminum Matrix Syntactic Foam Fabricated by Sandwich Infiltration Casting. Mat. Res. 2021, 24, 175–182. [Google Scholar] [CrossRef]
- Samvatsar, K.; Dave, H. A Comprehensive Study on Using Fly Ash as Reinforcement Material in Aluminium and Magnesium Based Syntactic Foams. Mater. Today Proc. 2021, 47, 2384–2390. [Google Scholar] [CrossRef]
- Al-Sahlani, K.; Broxtermann, S.; Lell, D.; Fiedler, T. Effects of Particle Size on the Microstructure and Mechanical Properties of Expanded Glass-Metal Syntactic Foams. Mater. Sci. Eng. A 2018, 728, 80–87. [Google Scholar] [CrossRef]
- Movahedi, N.; Vesenjak, M.; Krstulović-Opara, L.; Belova, I.V.; Murch, G.E.; Fiedler, T. Dynamic Compression of Functionally-Graded Metal Syntactic Foams. Compos. Struct. 2021, 261, 113308. [Google Scholar] [CrossRef]
- Movahedi, N.; Murch, G.E.; Belova, I.V.; Fiedler, T. Functionally Graded Metal Syntactic Foam: Fabrication and Mechanical Properties. Mater. Des. 2019, 168, 107652. [Google Scholar] [CrossRef]
- Borovinšek, M.; Taherishargh, M.; Vesenjak, M.; Ren, Z.; Fiedler, T. Geometrical Characterization of Perlite-Metal Syntactic Foam. Mater. Charact. 2016, 119, 209–215. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Szlancsik, A. On the Mechanical Properties of Aluminum Matrix Syntactic Foams. Adv. Eng. Mater. 2018, 20, 1700980. [Google Scholar] [CrossRef]
- Sánchez de la Muela, A.M.; García Cambronero, L.E.; Malheiros, L.F.; Ruiz-Román, J.M. New Aluminum Syntactic Foam: Synthesis and Mechanical Characterization. Materials 2022, 15, 5320. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, N.; Fiedler, T.; Taşdemirci, A.; Murch, G.E.; Belova, I.V.; Güden, M. Impact Loading of Functionally Graded Metal Syntactic Foams. Mater. Sci. Eng. A 2022, 839, 142831. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Ariff, Z.M.; Saiyid Hashim, S.F.; Alomayri, T.; Mahzan, S.; Kamarudin, K.A.; Dauda Muhammad, I. Syntactic Foams Formulations, Production Techniques, and Industry Applications: A Review. J. Mater. Res. Technol. 2020, 9, 10698–10718. [Google Scholar] [CrossRef]
- Bolat, C.; Akgün, I.C.; Gokşenli, A. On the Way to Real Applications: Aluminum Matrix Syntactic Foams. Eur. Mech. Sci. 2020, 4, 131–141. [Google Scholar] [CrossRef]
- Duarte, I.; Ferreira, J.M.F. Composite and Nanocomposite Metal Foams. Materials 2016, 9, 79. [Google Scholar] [CrossRef]
- S-de-la-Muela, A.M.; Cambronero, L.E.G.; Ruiz-Román, J.M. Molten Metal Infiltration Methods to Process Metal Matrix Syntactic Foams. Metals 2020, 10, 149. [Google Scholar] [CrossRef]
- Yang, Q.; Cheng, J.; Wei, Y.; Yu, B.; Miao, Z.; Gao, P. Innovative Compound Casting Technology and Mechanical Properties of Steel Matrix Syntactic Foams. J. Alloys Compd. 2021, 853, 156572. [Google Scholar] [CrossRef]
- Kemény, A.; Movahedi, N.; Fiedler, T.; Maróti, J.E.; Orbulov, I.N. The Influence of Infiltration Casting Technique on Properties of Metal Syntactic Foams and Their Foam-Filled Tube Structures. Mater. Sci. Eng. A 2022, 852, 143706. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. The Effect of Particle Shape on Mechanical Properties of Perlite/Metal Syntactic Foam. J. Alloys Compd. 2017, 693, 55–60. [Google Scholar] [CrossRef]
- Jamshidi-Alashti, R.; Kaskani, M.; Niroumand, B. Semisolid Melt Squeezing Procedure for Production of Open-Cell Al–Si Foams. Mater. Des. 2014, 56, 325–333. [Google Scholar] [CrossRef]
- Taherishargh, M.; Sulong, M.A.; Belova, I.V.; Murch, G.E.; Fiedler, T. On the Particle Size Effect in Expanded Perlite Aluminium Syntactic Foam. Mater. Des. 2015, 66, 294–303. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Kim, J.K.; Gupta, N.; Alaraj, S.; Daoud, A. Compressive Characteristics of A356/Fly Ash Cenosphere Composites Synthesized by Pressure Infiltration Technique. Compos. Part A Appl. Sci. Manuf. 2006, 37, 430–437. [Google Scholar] [CrossRef]
- Licitra, L.; Luong, D.D.; Strbik, O.M.; Gupta, N. Dynamic Properties of Alumina Hollow Particle Filled Aluminum Alloy A356 Matrix Syntactic Foams. Mater. Des. 2015, 66, 504–515. [Google Scholar] [CrossRef]
- Szlancsik, A.; Katona, B.; Bobor, K.; Májlinger, K.; Orbulov, I.N. Compressive Behaviour of Aluminium Matrix Syntactic Foams Reinforced by Iron Hollow Spheres. Mater. Des. 2015, 83, 230–237. [Google Scholar] [CrossRef]
- Movahedi, N.; Murch, G.E.; Belova, I.V.; Fiedler, T. Manufacturing and Compressive Properties of Sandwich Foam Tubes Containing Metal Syntactic Foam. Compos. Struct. 2023, 316, 117012. [Google Scholar] [CrossRef]
- Movahedi, N.; Murch, G.E.; Belova, I.V.; Fiedler, T. Manufacturing and Compressive Properties of Tube-Filled Metal Syntactic Foams. J. Alloys Compd. 2020, 822, 153465. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Wang, L.; Jiang, Y.; Wang, W.; Wu, G. Bending Behavior of Cenosphere Aluminum Matrix Syntactic Foam-Filled Circular Tubes. Eng. Struct. 2021, 243, 112650. [Google Scholar] [CrossRef]
- Salehi, M.; Mirbagheri, S.M.H.; Jafari Ramiani, A. Efficient Energy Absorption of Functionally-Graded Metallic Foam-Filled Tubes Under Impact Loading. Trans. Nonferr. Met. Soc. China 2021, 31, 92–110. [Google Scholar] [CrossRef]
- Shah, A.W.; Ha, S.-H.; Siddique, J.A.; Kim, B.-H.; Yoon, Y.-O.; Lim, H.-K.; Kim, S.K. Microstructure Evolution and Mechanical Properties of Al–Cu–Mg Alloys with Si Addition. Materials 2023, 16, 2783. [Google Scholar] [CrossRef]
- Zamani, M.; Belov, I.; Sjölander, E.; Bjurenstedt, A.; Ghassemali, E.; Seifeddine, S. Study on Dissolution of Al2Cu in Al-4.3Cu and A205 Cast Alloys. Metals 2020, 10, 900. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, C.; Xu, C.; Chen, K. Microstructures and Tensile Fracture Behavior of 2219Wrought Al–Cu Alloys with Different Impurity of Fe. Metals 2021, 11, 174. [Google Scholar] [CrossRef]
- Bannaravuri, P.K.; Birru, A.K. Strengthening of Al-4.5%Cu Alloy with the Addition of Silicon Carbide and Bamboo Leaf Ash. Int. J. Struct. Integr. 2019, 10, 149–161. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Low-Density Expanded Perlite-Aluminium Syntactic Foam. Mater. Sci. Eng. A 2014, 604, 127–134. [Google Scholar] [CrossRef]
- Babaee, M.H.; Maleki, A.; Niroumand, B. A Novel Method to Improve Interfacial Bonding of Compound Squeeze Cast Al-Al Macrocomposite Bimetals: Simulation and Experimental Studies. Trans. Nonferr. Met. Soc. China 2019, 29, 1184–1199. [Google Scholar] [CrossRef]
- Chandler, H. Heat Treater’s Guide: Practices and Procedures for Nonferrous Alloys; ASM International: New York, NY, USA, 1996. [Google Scholar]
- ASM. ASM Handbook: Volume 4: Heat Treating; ASM International: New York, NY, USA, 1991. [Google Scholar]
- ASTM E112-13(2021); Standard Test Methods for Determining Average Grain Size. ASTM International: Conshohocken, PA, USA, 2021.
- ISO 13314:2011; Mechanical Testing of Metals—Ductility Testing—Compression Test for Porous and Cellular Metals. The International Organization for Standardization: Geneva, Switzerland, 2011.
- Sochi, T. Non-Newtonian Flow in Porous Media. Polymer 2010, 51, 5007–5023. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.N.; Larachi, F. Reactions, Hydrodynamics and Mass Transfer in Micro-Packed Beds—Overview and New Mass Transfer Data. Chem. Eng. Process. 2016, 110, 80–96. [Google Scholar] [CrossRef]
- Renken, A.; Kiwi-Minsker, L. Advances in Catalysis; Diéguez, M., Núñez, R., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 53, Chapter 2. [Google Scholar]
- Orbulov, I.N. Metal Matrix Syntactic Foams Produced by Pressure Infiltration—The Effect of Infiltration Parameters. Mater. Sci. Eng. A 2013, 583, 11–19. [Google Scholar] [CrossRef]
- Campbell, J. Complete Casting Handbook: Metal Casting Processes, Metallurgy, Techniques and Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Hagart-Alexander, C. Instrumentation Reference Book, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 269–326. [Google Scholar]
- Drotning, W.D. Thermal Expansion of Molten Tin, Lead, and Aluminum to 1300/sup 0/K. High Temp. Sci. 1979, 11, 265–276. Available online: https://www.osti.gov/biblio/5186228 (accessed on 15 June 2023).
- Huntz, A.M.; Maréchal, L.; Lesage, B.; Molins, R. Thermal Expansion Coefficient of Alumina Films Developed by Oxidation of a FeCrAl Alloy determined by a deflection technique. Appl. Surf. Sci. 2006, 252, 7781–7787. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Castro, J.A.D.; Ferreira, L.d.O. Predicting Secondary-Dendrite Arm Spacing of the Al-4.5wt% Cu Alloy During Unidirectional Solidification. Mater. Res. 2017, 20, 68–75. [Google Scholar] [CrossRef]
- Grosse, C.U. Advances in Construction Materials; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Pfundstein, M.; Gellert, R.; Spitzner, M.; Rudolphi, A. Insulating Materials: Principles, Materials, Applications; Birkhäuser Architecture: Basel, Switzerland, 2008. [Google Scholar]
- Kurz, W.; Fisher, D.J.; Rappaz, M. Fundamentals of Solidification, 5th ed.; Trans Tech Publications: Baech, Switzerland, 2023. [Google Scholar]
- Somiya, S. Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Liu, W.; Köster, U. Fabrication of Ceramic/Metal Composites by Reduction of Glass SiO2 Preforms in Molten Metals and Alloys. J. Mater. Res. Lett. 1996, 15, 2188–2191. [Google Scholar] [CrossRef]
- Balch, D.K.; Dunand, D.C. Load Partitioning in Aluminum Syntactic Foams Containing Ceramic Microspheres. Acta Mater. 2006, 54, 1501–1511. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Guo, R.Q.; Iksan, H.; Borchelt, E.J.; Asthana, R. Pressure Infiltration Technique for Synthesis of Aluminum-Fly Ash Particulate Composite. Mater. Sci. Eng. A 1998, 244, 22–30. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Ginsztler, J. Compressive characteristics of metal matrix syntactic foams. Compos. Part A Appl. Sci. Manuf. 2012, 43, 553–561. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Szlancsik, A.; Kemény, A.; Kincses, D. Compressive Mechanical Properties of Low-Cost Aluminium Matrix Syntactic Foams. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105923. [Google Scholar] [CrossRef]





| Al | Cu | Fe | Si | Mg | Other Elements |
|---|---|---|---|---|---|
| Bal. | 4.34 | 0.27 | 0.31 | 0.07 | 0.55 |
| Designation | Pressure, Bar | Temp., °C |
|---|---|---|
| F0.8-700 | 0.8 | 700 |
| F0.8-725 | 725 | |
| F0.8-750 | 750 | |
| F1.5-700 | 1.5 | 700 |
| F1.5-725 | 725 | |
| F1.5-750 | 750 | |
| F2.0-700 | 2.0 | 700 |
| F2.0-725 | 725 | |
| F2.0-750 | 750 |
| As-Fabricated | T6 in Air (Change, %) | T6 in Vacuum (Change, %) | |
|---|---|---|---|
| Tensile strength, MPa | 36.3 ± 0.8 | 80.0 ± 1.0 (120%) | 72.0 ± 1.0 (98%) |
| Plateau stress, MPa | 92.1 ± 0.9 | 182.0 ± 1.0 (98%) | 163 ± 2.0 (77%) |
| Densification strain, % | 39.6 ± 0.2 | 39.0 ± 0.2 (−1.5%) | 39.8 ± 0.1 (0.5%) |
| Absorbed energy, MJ/m3 | 27.8 ± 0.3 | 55.6 ± 0.6 (100%) | 48.6 ± 0.3 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niroumand, B.; Jazini Dorcheh, A. Influence of Melt Infiltration Parameters on Structural and Mechanical Properties of Al-4.3wt.%Cu-EP Syntactic Foam. Metals 2023, 13, 1345. https://doi.org/10.3390/met13081345
Niroumand B, Jazini Dorcheh A. Influence of Melt Infiltration Parameters on Structural and Mechanical Properties of Al-4.3wt.%Cu-EP Syntactic Foam. Metals. 2023; 13(8):1345. https://doi.org/10.3390/met13081345
Chicago/Turabian StyleNiroumand, Behzad, and Amir Jazini Dorcheh. 2023. "Influence of Melt Infiltration Parameters on Structural and Mechanical Properties of Al-4.3wt.%Cu-EP Syntactic Foam" Metals 13, no. 8: 1345. https://doi.org/10.3390/met13081345
APA StyleNiroumand, B., & Jazini Dorcheh, A. (2023). Influence of Melt Infiltration Parameters on Structural and Mechanical Properties of Al-4.3wt.%Cu-EP Syntactic Foam. Metals, 13(8), 1345. https://doi.org/10.3390/met13081345







