Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
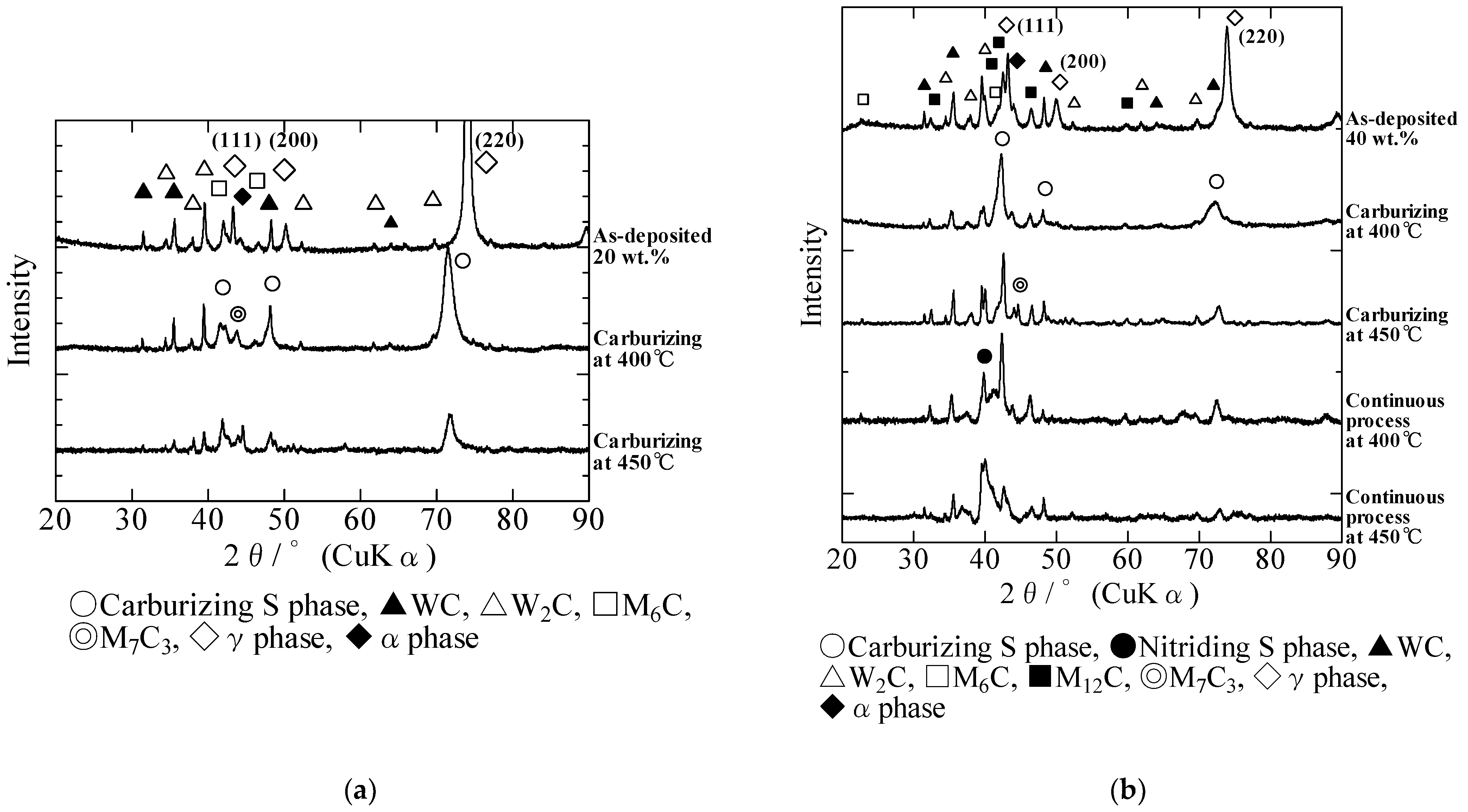
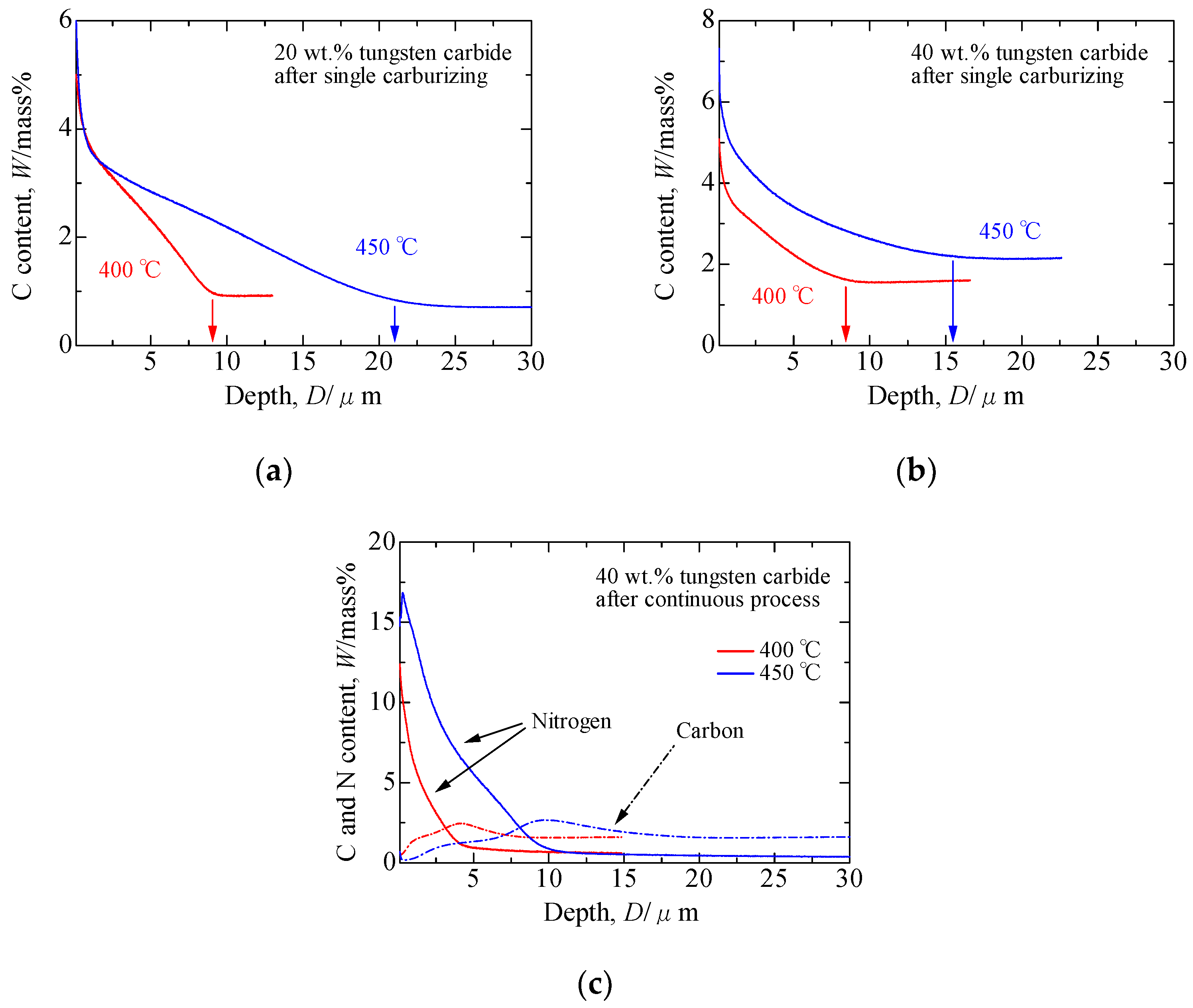
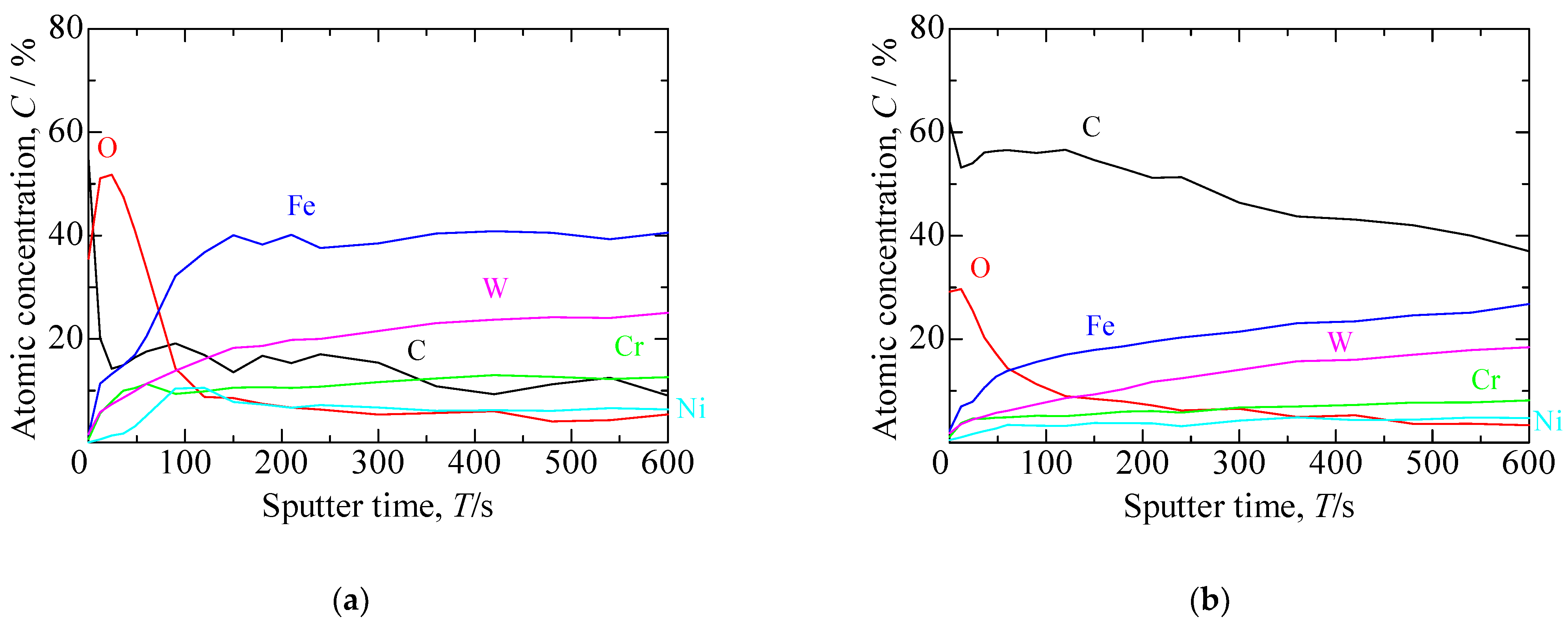
3.1. Formation Mechanism of the S Phase during a Single Carburizing Process
3.2. Formation Mechanism of the S Phase during a Continuous Plasma Nitriding after Carburizing Process
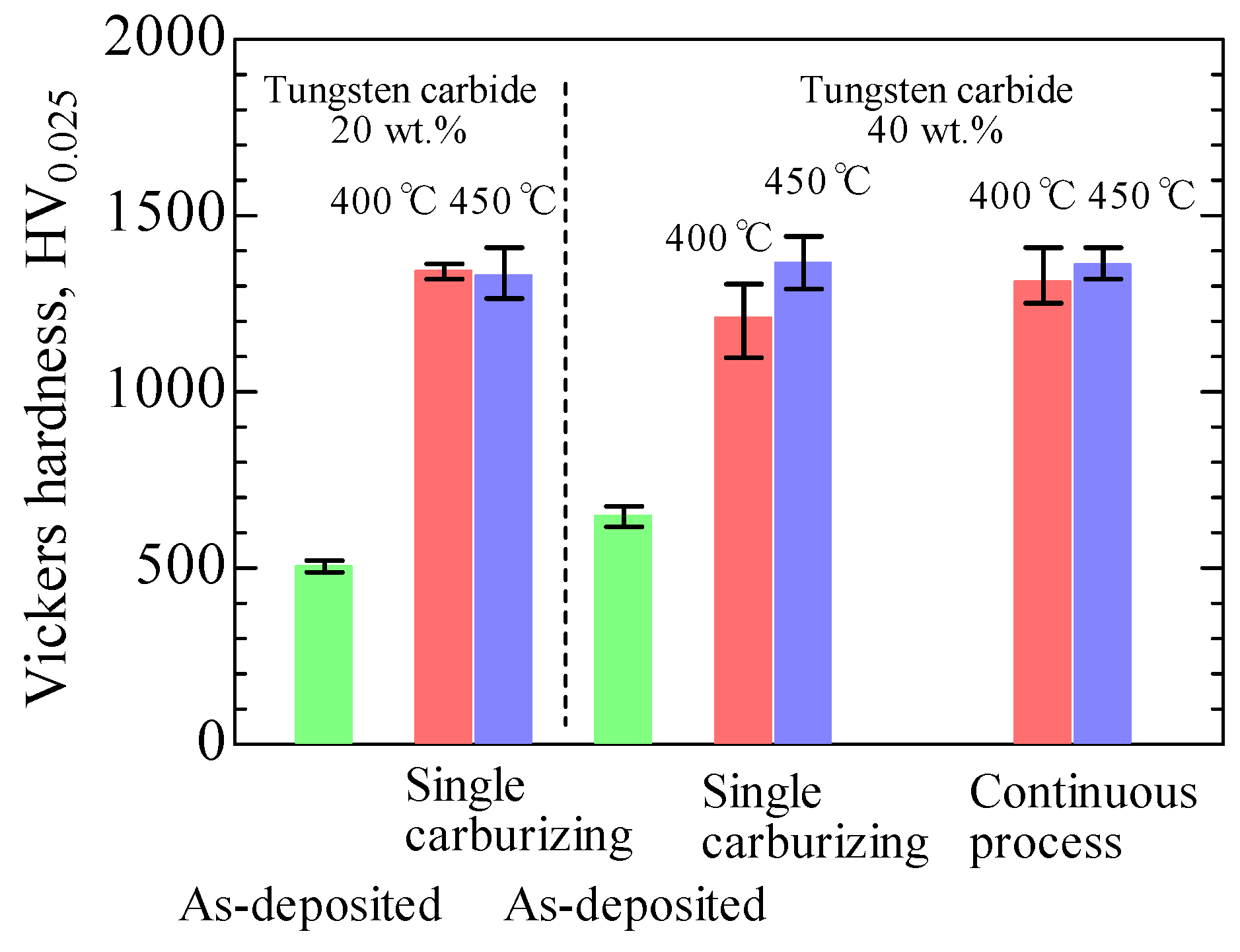
3.3. Hardness of the S Phase by a Single Carburizing Process and Continuous Nitriding after Carburizing Process
3.4. Anodic Polarization Measurement of the S Phase by a Single Carburizing Process and Continuous Nitriding after Carburizing Process
4. Conclusions
- (1)
- In the single carburizing process, the S phase was formed with the addition of diffused carbon to the solid-solution carbon originally present in the composite layers. The eutectic carbides in the as-deposited composite layers prevented carbon diffusion, and the S phase in the 40 wt.% composite layers, which included more eutectic carbides, was thinner than that in the 20 wt.% composite layers. However, the original solid-solution carbon was involved in the formation of the S phase. Therefore, the thickness of the S phase layer in the composites was higher than that of the S phase on the AISI 316L stainless-steel plates.
- (2)
- In the continuous process, the first carburizing step induced carbon diffusion, and the diffused carbon and original solid-solution carbon were pushed inside by the diffused nitrogen during the second nitriding process. This phenomenon resulted in the formation of a dual layer S phase, viz., a nitriding S phase on the outside and a carburizing S phase inside. The thickness of the S phase produced by the continuous process was similar to that generated by the single carburizing process.
- (3)
- The average Vickers hardness of the S phase at the surface was 1210–1365 HV, and significant differences were not observed for different tungsten carbide compositions or plasma process conditions. On the other hand, the surface hardness profile of the S phase resulting from the continuous process at 450 °C was harder than that produced by the single carburizing process up to a depth of approximately 4 µm.
- (4)
- The anodic polarization curves, obtained in a NaCl solution, show that the corrosion current densities of the plasma-processed layers are slightly lower than those of the as-deposited layers except for the 20 wt.% layer carburized at 450 °C. The pitting and crevice corrosion occurred in the as-deposited layers. The surface morphologies of the S phase after the anodic polarization measurements exhibited slight discoloration. However, no sever corrosion was observed in the plasma-processed layers implying that the carburizing and nitriding S phase exhibited comparable corrosion resistance enhancements.
- (5)
- The results confirm that the single plasma carburizing process and continuous plasma process nitriding after carburizing at 400 and 450 °C improved the surface hardness and corrosion resistance of the composite layers. However, the metallurgical structure was very different from that of ordinary stainless-steel plates. These plasma treatments can be used as surface modification methods for additive manufacturing stainless-steel-based tungsten carbide composites that can withstand harsh environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gnaase, S.; Niggemeyer, D.; Lehnert, D.; Bödgerand, C.; Tröster, T. Comparative Study of the Influence of Heat Treatment and Additive Manufacturing Process (LMD & L-PBF) on the Mechanical Properties of Specimens Manufactured from 1.2709. Crystals 2023, 13, 157. [Google Scholar] [CrossRef]
- Kaligar, A.B.; Kumar, H.A.; Ali, A.; Abuzaid, W.; Egilmez, M.; Alkhader, M.; Abed, F.; Alnaser, A.S. Femtosecond Laser-Based Additive Manufacturing: Current Status and Perspectives. Quantum Beam Sci. 2022, 6, 5. [Google Scholar] [CrossRef]
- Ladani, L.; Sadeghilaridjani, M. Review of Powder Bed Fusion Additive Manufacturing for Metals. Metals 2021, 11, 1391. [Google Scholar] [CrossRef]
- Dass, A.; Moridi, A. State of the Art in Directed Energy Deposition: From Additive Manufacturing to Materials Design. Coatings 2019, 9, 418. [Google Scholar] [CrossRef]
- Singh, A.; Kapil, S.; Das, M. A comprehensive review of the methods and mechanisms for powder feedstock handling in directed energy deposition. Addit. Manuf. 2020, 35, 101388. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J. Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Ligabo, S.A.; Braga, V.; Ferreira, C.C.A.; Siqueira, R.H.M.; Lourenço, J.C.; Abdalla, A.J.; Lima, M.S.F. Microstructure and Corrosion Behavior of AISI 316 Steel Layers Deposited on AISI 347 Steel Substrate by Laser Metal Deposition. Metals 2022, 12, 2161. [Google Scholar] [CrossRef]
- Zinovieva, O.; Romanova, V.; Balokhonov, R. Effects of scanning pattern on the grain structure and elastic properties of additively manufactured 316L austenitic stainless steel. Mater. Sci. Eng. A 2022, 832, 142447. [Google Scholar] [CrossRef]
- Avanzini, A. Fatigue Behavior of Additively Manufactured Stainless Steel 316L. Materials 2023, 16, 65. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Moyle, M.; Primig, S. Additive manufacturing of steels: A review of achievements and challenges. J. Mater. Sci. 2021, 56, 64–107. [Google Scholar] [CrossRef]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Röttger, A.; Boes, J.; Theisen, W.; Thiele, M.; Esen, C.; Edelmann, A.; Hellmann, R. Microstructure and mechanical properties of 316L austenitic stainless steel processed by different SLM devices. J. Adv. Manuf. Technol. 2020, 108, 769–783. [Google Scholar] [CrossRef]
- Bassis, M.; Ron, T.; Leon, A.; Kotliar, A.; Kotliar, R.; Shirizly, A.; Aghion, E. The Influence of Intralayer Porosity and Phase Transition on Corrosion Fatigue of Additively Manufactured 316L Stainless Steel Obtained by Direct Energy Deposition Process. Materials 2022, 15, 5481. [Google Scholar] [CrossRef] [PubMed]
- Fetni, S.; Enrici, T.M.; Niccolini, T.; Tran, S.H.; Dedry, O.; Jardin, R.; Duchene, L.; Mertens, A.; Habraken, A.M. 2D thermal finite element analysis of laser cladding of 316L + WC composite layer coatings. Procedia Manuf. 2020, 50, 86–92. [Google Scholar] [CrossRef]
- Enrici, T.M.; Dedry, O.; Boschini, F.; Tchuindjang, J.T.; Mertens, A. Microstructural and thermal characterization of 316L + WC composite layer coatings obtained by laser cladding. Adv. Eng. Mater. 2020, 22, 2000291. [Google Scholar] [CrossRef]
- Fetni, S.; Enrici, T.M.; Niccolini, T. Thermal model for the directed energy deposition of composite layer coatings of 316L stainless steel enriched with tungsten carbides. Mater. Des. 2021, 204, 109661. [Google Scholar] [CrossRef]
- Benarji, K.; Kumar, Y.R.; Jinoop, A.N.; Paul, C.P.; Bindra, K.S. Effect of WC Composition on the Microstructure and Surface Properties of Laser Directed Energy Deposited SS 316-WC Composite layers. J. Mater. Eng. Perform. 2021, 30, 6732–6742. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Di, R.; Yuan, R.; Shi, C.; Lei, J. Effects of WC particles on microstructure and mechanical properties of 316L steel obtained by laser melting deposition. Ceram. Int. 2022, 48, 20388–20399. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Moskvina, V.; Maier, G.; Melnikov, E.; Astafurov, S.; Zagibalova, E.; Panchenko, M.; Reunova, K.; Nikolaev, A.; Ramazanov, K.; Astafurova, E. The effect of thin surface layer of nitrogen-expanded austenite on bulk γ-α’ phase transformation in low-temperature deformation of 316L stainless steel. Mater. Lett. 2021, 304, 130676. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Surface Modification of a Nickel-Free Austenitic Stainless Steel by Low-Temperature Nitriding. Metals 2021, 11, 1845. [Google Scholar] [CrossRef]
- Funch, C.V.; Somlo, K.; Christiansen, T.L.; Somers, M.A. Thermochemical post-processing of additively manufactured austenitic stainless steel. Surf. Coat. Technol. 2022, 441, 128495. [Google Scholar] [CrossRef]
- Lindner, T.; Kutschmann, P.; Löbel, M.; Lampke, T. Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding. Coatings 2018, 8, 348. [Google Scholar] [CrossRef]
- Adachi, S.; Egawa, M.; Yamaguchi, T.; Ueda, N. Low-temperature plasma nitriding for austenitic stainless steel layers with various nickel contents fabricated via direct laser metal deposition. Coatings 2020, 10, 365. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhang, X.; Li, W.; Ma, A.; Fan, K.; Xing, W. Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel. Materials 2022, 15, 6575. [Google Scholar] [CrossRef]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-S Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Effects of Surface Modification by Means of Low-Temperature Plasma Nitriding on Wetting and Corrosion Behavior of Austenitic Stainless Steel. Coatings 2020, 10, 98. [Google Scholar] [CrossRef]
- Yazıcı, M.; Çomaklı, O.; Yetim, T.; Yetim, A.F.; Çelik, A. The effect of plasma nitriding temperature on the electrochemical and semiconducting properties of thin passive films formed on 316 L stainless steel implant material in SBF solution. Surf. Coat. Technol. 2015, 261, 181–188. [Google Scholar] [CrossRef]
- Adachi, S.; Yamaguchi, T.; Ueda, N. Formation and Properties of Nitrocarburizing S-Phase on AISI 316L Stainless Steel-Based WC Composite Layers by Low-Temperature Plasma Nitriding. Metals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Savrai, R.A.; Skorynina, P.A. Structural-phase transformations and changes in the properties of AISI 321 stainless steel induced by liquid carburizing at low temperature. Surf. Coat. Technol. 2022, 443, 128613. [Google Scholar] [CrossRef]
- Liu, H.Y.; Che, H.L.; Gao, J.Y.; Li, G.B.; Lei, M.K. Low-pressure hollow cathode plasma source carburizing of AISI 304L austenitic stainless steel at low temperature. Surf. Coat. Technol. 2022, 442, 128548. [Google Scholar] [CrossRef]
- Montanari, R.; Lanzutti, A.; Richetta, M.; Tursunbaev, J.; Vaglio, E.; Varone, A.; Verona, C. Plasma Carburizing of Laser Powder Bed Fusion Manufactured 316 L Steel for Enhancing the Surface Hardness. Coatings 2022, 12, 258. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Zhang, S.; Feng, Y.; Peng, Y.; Gong, J. Deformation response of gradient low-temperature gaseous carburized case in austenitic stainless steel during cyclic nanoindentation. Mater. Today Commun. 2021, 28, 102714. [Google Scholar] [CrossRef]
- Maistro, G.; Kante, S.; Nyborg, L.; Cao, Y. Low-temperature carburized high-alloyed austenitic stainless steels in PEMFC cathodic environment. Surf. Interfaces 2021, 24, 101093. [Google Scholar] [CrossRef]
- Cheon, H.; Kim, K.S.; Kim, S.; Heo, S.B.; Lim, J.H.; Kim, J.H.; Yoon, S.Y. Effect of Deformation Structure of AISI 316L in Low-Temperature Vacuum Carburizing. Metals 2021, 11, 1762. [Google Scholar] [CrossRef]
- Molleja, J.G.; Nosei, L.; Ferrón, J.; Bemporad, E.; Lesage, J.; Chicot, D.; Feugeas, J. Characterization of expanded austenite developed on AISI 316L stainless steel by plasma carburization. Surf. Coat. Technol. 2010, 204, 3750–3759. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Surface hardness improvement of plasma-sprayed AISI 316L stainless steel coating by low-temperature plasma carburizing. Adv. Powder Technol. 2013, 24, 818–823. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Combined plasma carburizing and nitriding of sprayed AISI 316L steel coating for improved wear resistance. Surf. Coat. Technol. 2014, 259, 44–49. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Wear and corrosion properties of cold-sprayed AISI 316L coatings treated by combined plasma carburizing and nitriding at low temperature. Coatings 2018, 8, 456. [Google Scholar] [CrossRef]
- Azmi, F.; Basak, A.K.; Adenan, M.S.; Haruman, E.; Saedon, J.B. Responses of hybrid S phase layer to nanoscratching. Surf. Coat. Technol. 2022, 441, 128509. [Google Scholar] [CrossRef]
- Ullah, N.; Naeem, M.; Shafiq, M.; Mujahid, Z.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Zakaullah, M. Effect of methane concentration on surface properties of cathodic cage plasma nitrocarburized AISI-304. Appl. Phys. 2021, 127, 529. [Google Scholar] [CrossRef]
- Böcker, J.; Puth, A.; Dalke, A.; Röpcke, J.; Helden, J.P.H.; Biermann, H. Influence of the Active Screen Plasma Power during Afterglow Nitrocarburizing on the Surface Modification of AISI 316L. Coatings 2020, 10, 1112. [Google Scholar] [CrossRef]
- Wu, D.; Ge, Y.; Kahn, H.; Ernst, F.; Heuer, A.H. Diffusion profiles after nitrocarburizing austenitic stainless steel. Surf. Coat. Technol. 2015, 279, 180–185. [Google Scholar] [CrossRef]
- Scheuer, J.C.; Rodrigo, J.D.; Cardoso, P.; Sílvio, D.; Brunatto, F. Sequential low-temperature plasma-assisted thermochemical treatments of the AISI 420 martensitic stainless steel. Surf. Coat. Technol. 2021, 421, 127459. [Google Scholar] [CrossRef]
- Lindner, T.; Mehner, T.; Lampke, T. Surface modification of austenitic thermal-spray coatings by low-temperature nitrocarburizing. IOP Conf. Ser. Mater. Sci. Eng. 2016, 118, 012008. [Google Scholar] [CrossRef]
- Godec, M.; Donik, Č.; Kocijan, A.; Podgornik, B.; Skobir Balantič, D.A. Effect of post-treated low-temperature plasma nitriding on the wear and corrosion resistance of 316L stainless steel manufactured by laser powder-bed fusion. Addit. Manuf. 2020, 32, 101000–101008. [Google Scholar] [CrossRef]
- Kovács1, D.; Kemény, D.M. Effect of plasma nitriding of austenitic stainless steel produced by direct metal laser sintering. Acta Metall. Slovaca 2021, 27, 190–194. [Google Scholar] [CrossRef]
- Czerwiec, T.; He, H.; Weber, S.; Dong, C.; Michel, H. On the occurrence of dual diffusion layers during plasma-assisted nitriding of austenitic stainless steel. Surf. Coat. Technol. 2006, 200, 5289–5295. [Google Scholar] [CrossRef]
- Micheler, T. Influence of plasma nitriding on hydrogen environment embrittlement of 1.4301 austenitic stainless steel. Surf. Coat. Technol. 2008, 202, 1688–1695. [Google Scholar] [CrossRef]
- Ceschini, L.; Chiavari, C.; Lanzoni, E.; Martini, C. Low-temperature carburised AISI 316L austenitic stainless steel: Wear and corrosion behaviour. Mater. Des. 2012, 38, 154–160. [Google Scholar] [CrossRef]
- Peng, Y.; Gong, J.; Chen, C.; Liu, Z.; Jiang, Y. Numerical Analysis of Stress Gradient and Traps Effects on Carbon Diffusion in AISI 316L during Low Temperature Gas Phase Carburization. Metals 2018, 8, 214. [Google Scholar] [CrossRef]
- Ernst, F.; Li, D.; Kahn, H.; Michal, G.M.; Heuer, A.H. The carbide M7C3 in low-temperature-carburized austenitic stainless steel. Acta Mater. 2011, 5, 2268–2276. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Low temperature gaseous nitriding and carburising of stainless steel. Surf. Eng. 2005, 21, 445–455. [Google Scholar] [CrossRef]
- Thaiwatthana, S.; Li, X.; Dong, H.B.; Bell, T. Comparison Studies on Properties of Nitrogen and Carbon S Phase on Low Temperature Plasma Alloyed AISI 316 Stainless Steel. Surf. Eng. 2002, 18, 433–437. [Google Scholar] [CrossRef]
- Sun, Y. Hybrid Plasma Surface Alloying of Austenitic Stainless Steels with Nitrogen and Carbon. Mater. Sci. Eng. A 2005, 404, 124–129. [Google Scholar] [CrossRef]
- Gu, X.; Michal, M.G.; Frank, E.; Kahn, H. Numerical Simulations of Carbon and Nitrogen Composition Depth Profiles in Nitrocarburized Austenitic Stainless Steels. Metall. Mater. Trans. A 2014, 45A, 4268–4279. [Google Scholar] [CrossRef]
- Buhagiar, J.; Spiteri, A.; Sacco, M.; Sinagra, E.; Dong, H. Augmentation of crevice corrosion resistance of medical grade 316LV stainless steel by plasma carburizing. Corros. Sci. 2012, 59, 169–178. [Google Scholar] [CrossRef]
- Borgioli, F. The Corrosion Behavior in Different Environments of Austenitic Stainless Steels Subjected to Thermochemical Surface Treatments at Low Temperatures: An Overview. Metals 2023, 13, 776. [Google Scholar] [CrossRef]



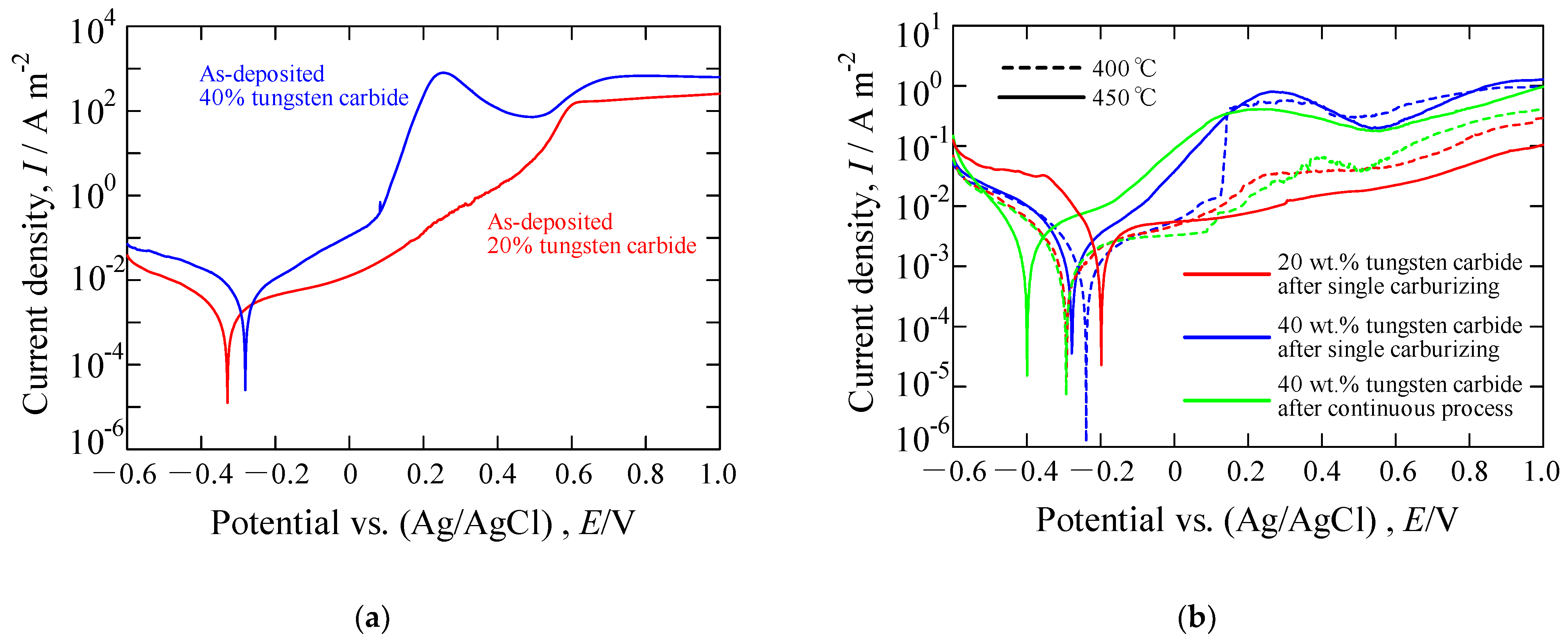
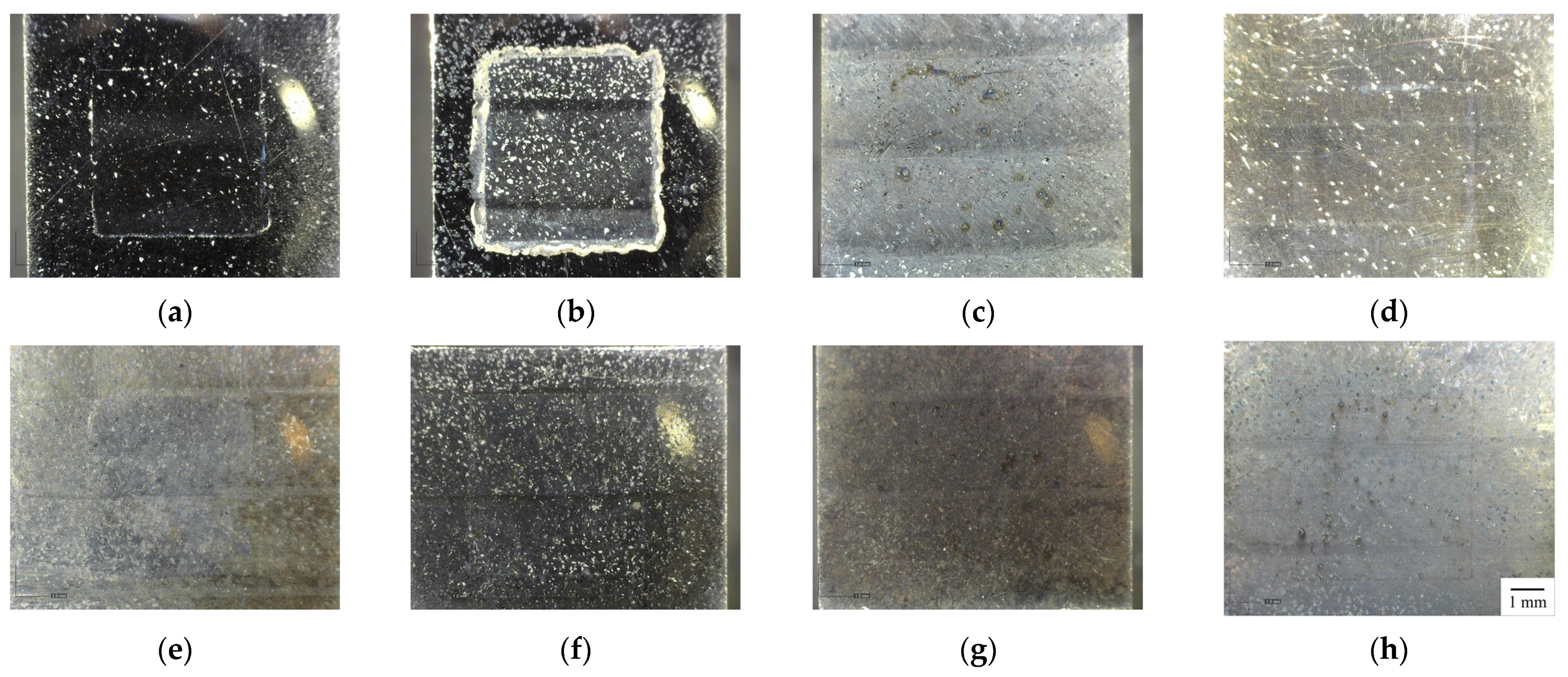
| Sample | Corrosion Potential | Corrosion Current Density |
|---|---|---|
| (V) | (A m−2) | |
| 20 wt.% as-deposited | −0.33 | 2.0 × 10−3 |
| 40 wt.% as-deposited | −0.28 | 4.4 × 10−3 |
| 20 wt.% carburized at 400 °C | −0.29 | 1.5 × 10−3 |
| 20 wt.% carburized at 450 °C | −0.20 | 4.1 × 10−3 |
| 40 wt.% carburized at 400 °C | −0.24 | 1.7 × 10−3 |
| 40 wt.% carburized at 450 °C | −0.28 | 1.8 × 10−3 |
| 40 wt.% continuously processed at 400 °C | −0.29 | 2.2 × 10−3 |
| 40 wt.% continuously processed at 450 °C | −0.40 | 3.8 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, S.; Yamaguchi, T.; Tanaka, K.; Nishimura, T.; Ueda, N. Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding. Metals 2023, 13, 1350. https://doi.org/10.3390/met13081350
Adachi S, Yamaguchi T, Tanaka K, Nishimura T, Ueda N. Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding. Metals. 2023; 13(8):1350. https://doi.org/10.3390/met13081350
Chicago/Turabian StyleAdachi, Shinichiro, Takuto Yamaguchi, Keigo Tanaka, Takashi Nishimura, and Nobuhiro Ueda. 2023. "Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding" Metals 13, no. 8: 1350. https://doi.org/10.3390/met13081350
APA StyleAdachi, S., Yamaguchi, T., Tanaka, K., Nishimura, T., & Ueda, N. (2023). Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding. Metals, 13(8), 1350. https://doi.org/10.3390/met13081350







