Erosion Mechanism of Carbon Brick in Hearth of 4000 m3 Industrial Blast Furnace
Abstract
:1. Introduction
2. Sampling and Analysis
2.1. General Situation of Blast Furnace
2.2. Introduction of Sampling
2.3. Analytical Methods
3. Results and Discussion
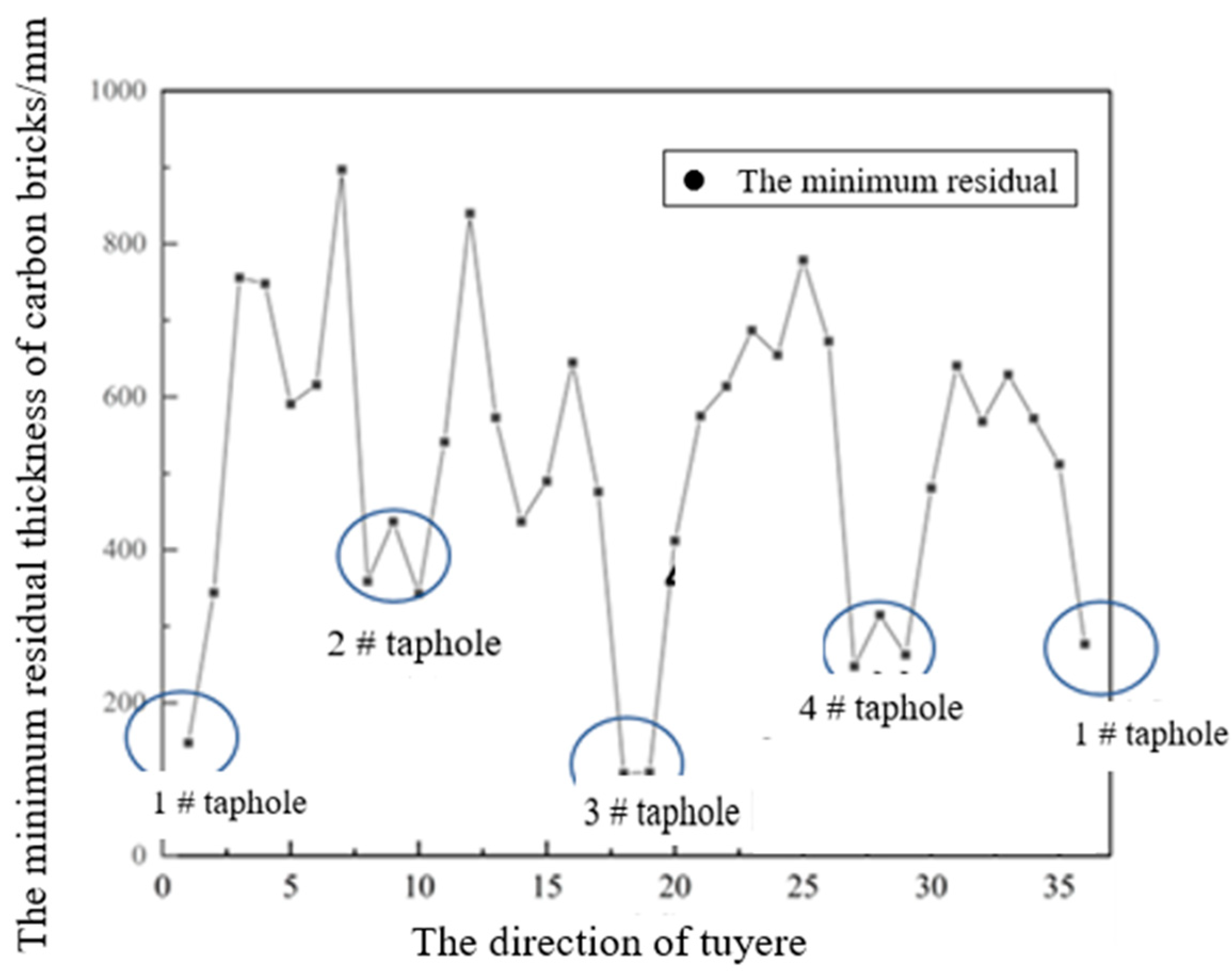
3.1. Investigation of the Carbon Bricks after Service
3.2. Calculation of Convective Heat Transfer Coefficient of Carbon Brick
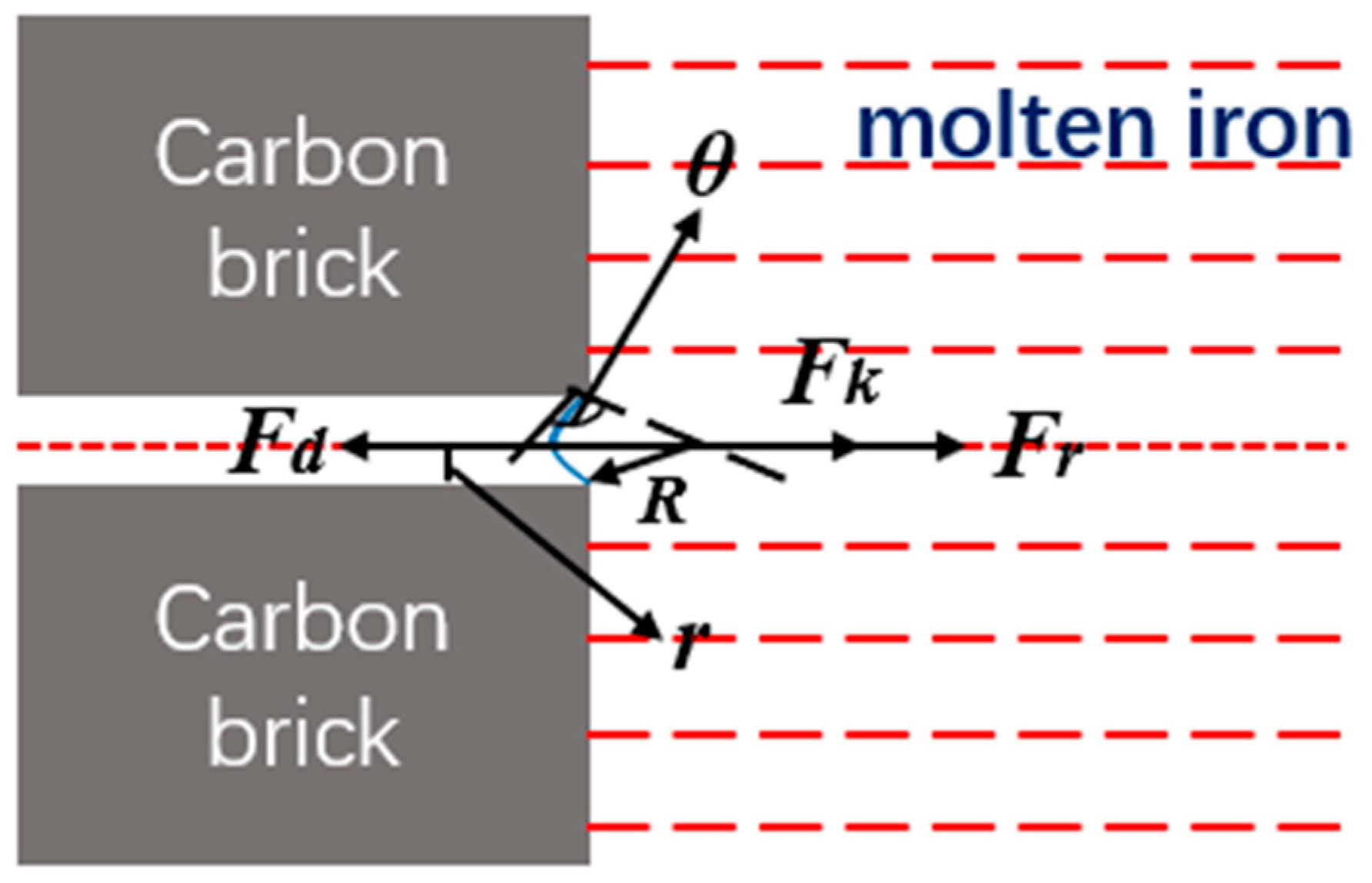
3.3. Erosion of Molten Iron to Carbon Brick
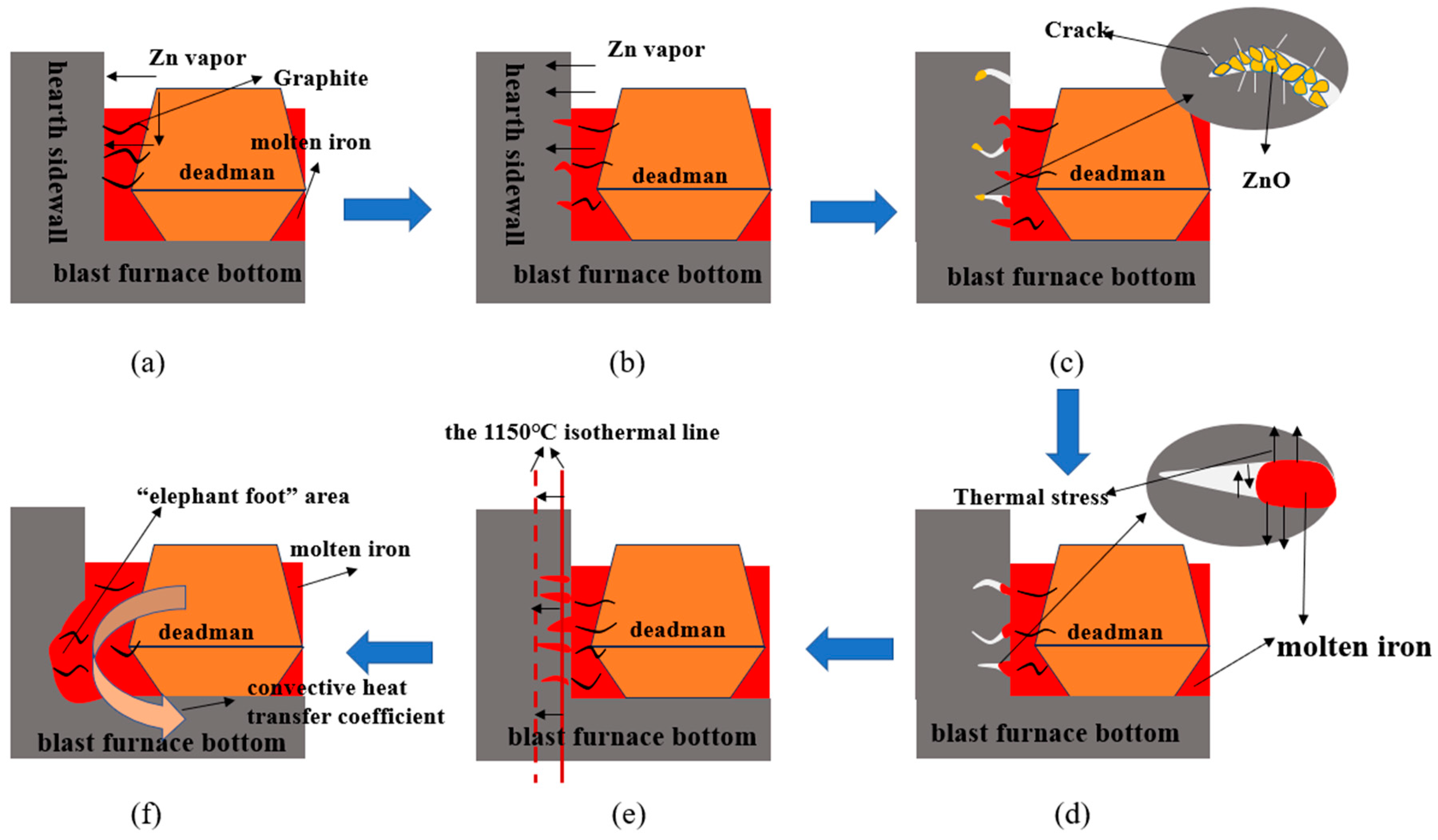
3.4. Analysis of Carbon Brick Erosion Mechanism
3.5. Suggestions for Operation and Construction of BF
- Since the molten iron in the BF is unsaturated in carbon, the carbon content in the carbon brick will dissolve with the molten iron. Therefore, it is possible to try to use ‘B’ carbon bricks [29] (carbon bricks with carbon content lower than ‘A’) instead of A carbon bricks in the hearth sidewall area.
- The molten iron may enter the depth of the carbon brick along the cracks and pores. Therefore, the sidewall of the hearth may be replaced by ultra-micro-porous carbon bricks.
- Because the convective heat transfer coefficient has a significant effect on the formation of “elephant foot” erosion, increasing the depth of the salamander so that the deadman does not contact the bottom of the BF will reduce its impact [22].
- Reducing the Zn of raw materials into the furnace will appropriately increase the temperature of the BF so that the zinc evaporates, moves upward, and generates ZnO, which is discharged with the furnace dust [7].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, R.; Zhang, J.; Li, Z.; Zhao, Y. Corrosion Behavior of Alumina Containing Refractory in Blast Furnace Hearth by CaO–SiO2–MgO–Al2O3–Cr2O3 Slags. ISIJ Int. 2019, 59, 1933. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Jiao, K.X.; Zhang, J.L.; Zhang, L.; Wang, C.; Gong, W.M.; Zheng, J.X.; Zhang, H.B. Dissection Investigation of Forming Process of Titanium Compounds Layer in the Blast Furnace Hearth: Ironmaking. ISIJ Int. 2020, 60, 2385. [Google Scholar] [CrossRef]
- Takatani, K.; Inada, T.; Takata, K. Mathematical Model for Transient Erosion Process of Blast Furnace Hearth. ISIJ Int. 2001, 41, 1139–1145. [Google Scholar] [CrossRef]
- Wang, C.; Cao, J.; Zhang, J.; Guo, Z.; Jiao, K.; Zhao, Y. Thermal conductivity of alumina-carbon composite brick and its related phase analysis in a dissected blast furnace. Met. Res. Technol. 2023, 120, 216. [Google Scholar] [CrossRef]
- Brulin, J.; Gasser, A.; Rekik, A.; Blond, E.; Roulet, F. Thermomechanical modelling of a blast furnace hearth. Constr. Build. Mater. 2022, 326, 126833. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, T.; Hu, D.; Xie, M.; Liu, B.; Yao, S. Consideration of long campaign based on analysis of 4038 m3 blast-furnace hearth core investigation. Met. Res. Technol. 2023, 120, 213. [Google Scholar] [CrossRef]
- Deng, Y.; Lyu, Q.; Zhang, J.; Jiao, K. Erosion of Carbon Brick by Zinc in Hearth of Blast Furnace. ISIJ Int. 2019, 60, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhang, J.-L.; Jiao, K.-X. Dissolution Mechanism of Carbon Brick into Molten Iron. ISIJ Int. 2018, 58, 815. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Zhang, J.; Liu, Z.; Kuang, S.; Liu, Y. Dissection Investigation of Ti(C,N) Behavior in Blast Furnace Hearth during Vanadium Titano-magnetite Smelting. ISIJ Int. 2017, 57, 48. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Fan, X.; Zhang, J.; Wang, K.; Zhao, Y. Corrosion behavior of alumina-carbon composite brick in typical blast furnace slag and iron. Ceram. Int. 2018, 44, 19981. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Jiao, K.; Zong, Y.; Wang, Z. Occurrence State and Behavior of Carbon Brick Brittle in a Large Dissected Blast Furnace Hearth. Steel Res. Int. 2021, 92, 2100273. [Google Scholar] [CrossRef]
- Shinotake, A.; Nakamura, H.; Yadoumaru, N.; Morizane, Y.; Meguro, M. Investigation of Blast-Furnace Hearth Sidewall Erosion by Core Sample Analysis and Consideration of Campaign Operation. ISIJ Int. 2003, 43, 321. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Liu, R.; Jiao, K.X.; Ma, H.X.; Guo, Z.Y.; Meng, S.; Song, M.B. Iron-Carbon Interface Phenomenon and Reaction Be-havior Analysis in Blast Furnace Hearth. ISIJ Int. 2023, 63, 253. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhai, Q.-J. Corrosion mechanism research and microstructure analysis of Baosteel No. 3 blast furnace hearth. J. Iron Steel Res. Int. 2017, 24, 1016. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zhang, J.; Yang, X.; Xu, J.; Zhang, G. Formation mechanism of the brittle layer in carbon brick of blast furnace hearth. Steel Res. Int. 2023. in Press. [Google Scholar] [CrossRef]
- Tian, R.Z. Zinc Alloy; Central South University Press: Changsha, China, 2010; p. 31. (In Chinese) [Google Scholar]
- Song, Q.J.; Ning, X.J.; Zhang, J.L.; Jiao, K.X.; Wang, C. Influences of zinc on the erosion of blast furnace hearth refractories. Mater. Sci. Technol. Conf. Exhib. 2017, 2017, 660–667. [Google Scholar]
- Jiao, K.X.; Zhang, J.L.; Zuo, H.B.; Liu, Z.J. Calculation Analysis of Zinc Dissolution Behaviors of the Slag-Iron in Blast Furnaces. J. Northeast. Univ. Nat. Sci. 2014, 35, 383. (In Chinese) [Google Scholar]
- Niu, Q.; Cheng, S.S.; Xu, W.X.; Niu, W.J. Microstructure and Phase of Carbon Brick and Protective Layer of a 2800 m3 Industrial Blast Furnace Heart. ISIJ Int. 2019, 59, 1776. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Zhang, J.; Jiao, K.; Zhang, J.; Wang, K. Distribution of harmful elements in dissected 125 m3 blast furnace. Can. Met. Q. 2019, 58, 400–409. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, J.G.; Liang, D.; Meng, S.; Jiao, K.X.; Zhang, J.L. Analysis on deadman behavior in hearth and cause of lon-gevity of large blast furnace. Iron Steel 2023, 1. (In Chinese) [Google Scholar] [CrossRef]
- Jiao, K.; Zhang, J.; Hou, Q.; Liu, Z.; Wang, G. Analysis of the Relationship between Productivity and Hearth Wall Temperature of a Commercial Blast Furnace and Model Prediction. Steel Res. Int. 2017, 88, 1600475. [Google Scholar] [CrossRef]
- Zhang, X.Z. Metallurgy Transmission Principle; Metallurgical Industry Press: Beijing, China, 1988; p. 169. (In Chinese) [Google Scholar]
- Jones, N.W. Kinetics of carbon dissolution in Fe-C alloy at 1550 °C. Ironmak. Steelmak. 1998, 25, 460. [Google Scholar]
- Kawai, Y.; Shiraishi, Y. Handbook of Physico-Chemical Properties at High Temperatures; The iron and steel institute of Japan: Tokyo, Japan, 1988. [Google Scholar]
- Jiao, K.; Zhang, J.; Zuo, H.; Xu, R.; Hong, J. Calculation and Analysis the Influence on the Cooling Water Velocity and Hot Metal Circulation to the Long Life BF. J. Mater. Sci. Eng. 2014, 5, 589. [Google Scholar] [CrossRef]
- Meng, S.; Jiao, K.; Zhang, J.; Zong, Y.; Zhang, L.; Ma, H.; Guo, Z. Dissection study of the deadman in a commercial blast furnace hearth. Fuel Process. Technol. 2021, 221, 106916. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Jiao, K.; Zou, Z.; Zhao, Y. Observation of Deadman Samples in a Dissected Blast Furnace Hearth. ISIJ Int. 2019, 59, 1991. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, J.; Chen, W.; Li, X.; Jiao, K.; Pang, Z.; Wang, Z.; Wang, T.; Liu, Z. Comparative Analysis on the Corrosion Resistance to Molten Iron of Four Kinds of Carbon Bricks Used in Blast Furnace Hearth. Metals 2022, 12, 871. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, R.; Jiao, K.; Chen, Y. Wetting behavior and mechanism between hot metal and carbon brick. J. Eur. Ceram. Soc. 2021, 41, 5740. [Google Scholar] [CrossRef]
- Nakashima, K.; Mori, K. Interfacial Properties of Liquid Iron Alloys and Liquid Slags Relating to Iron- and Steel-making Processes. ISIJ Int. 1992, 32, 11. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.; Cramb, A.W. Dynamic and equilibrium interfacial phenomena in liquid steel-slag systems. Met. Mater. Trans. B 2000, 31, 957–971. [Google Scholar] [CrossRef]

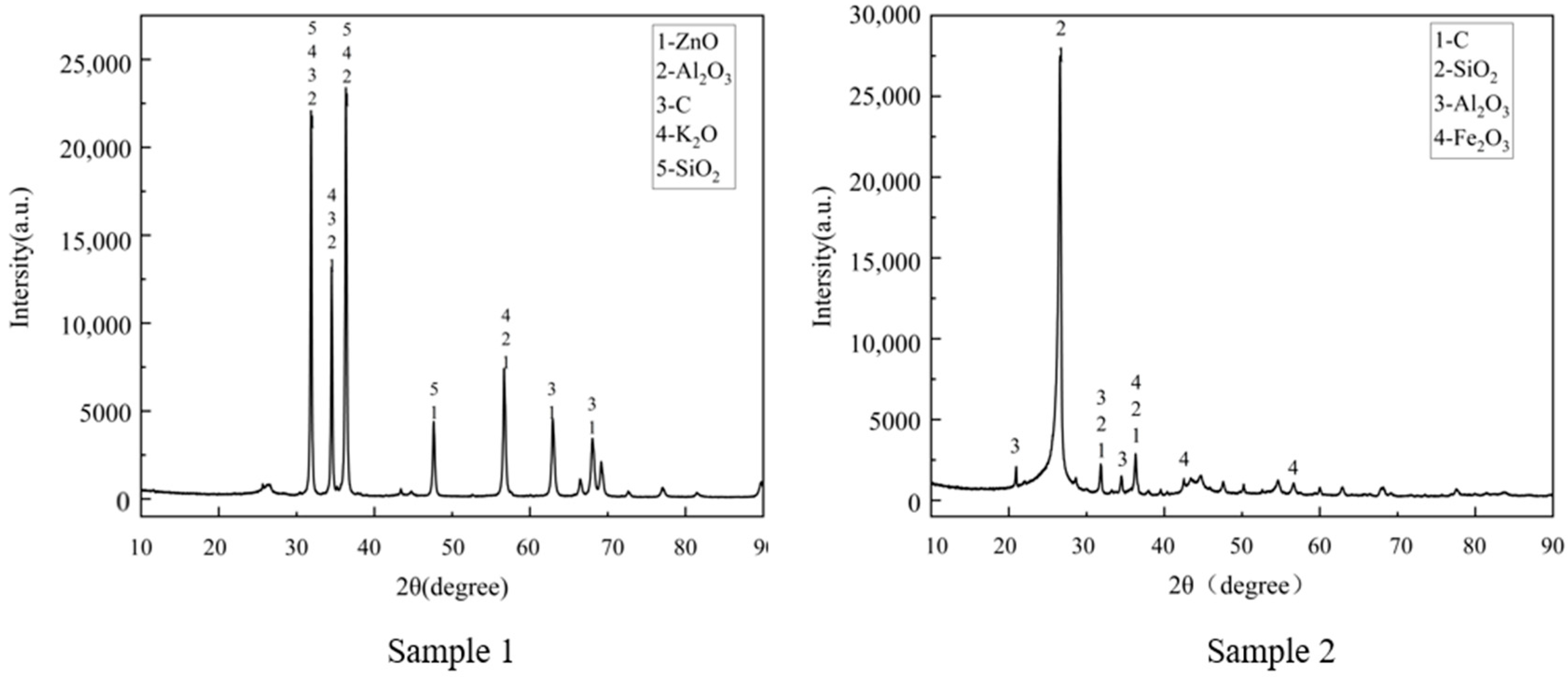



| Parameter | C | ZnO | SiO2 | K2O | Al2O3 | Fe2O3 | Na2O | Cl |
|---|---|---|---|---|---|---|---|---|
| Sample 1 | 22.25 | 69.69 | 2.14 | 1.71 | 0.76 | 1.69 | 0.27 | 0.3 |
| Sample 2 | 83.84 | 3.34 | 6.24 | 0.24 | 1.29 | 3.03 | 0.12 | 0.05 |
| Parameter | Unit | Value | Parameter | Unit | Value |
|---|---|---|---|---|---|
| μ | Pa·s | 6.0 × 10−3 | L | m | 3 |
| η | t/(d·m3) | 2.26 | θ | ° | 10 |
| ρ | Kg/(m3) | 6680 | α | ° | 45 |
| Cp | J/(kg·K) | 610 | d1 | m | 13.5 |
| W/(m·K) | 6.88 | T d2 | m | 10.5 | |
| V | m3 | 4000 | L0 | m | 1.6 |
| R | J/(mol·K) | 8.314 | ∆h | m | 0.71 |
| ε | - | 0.3 | v | m | 2.76 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, J.; Wang, C.; Deng, Y.; Zhang, G.; Song, M. Erosion Mechanism of Carbon Brick in Hearth of 4000 m3 Industrial Blast Furnace. Metals 2023, 13, 1371. https://doi.org/10.3390/met13081371
Cao J, Zhang J, Wang C, Deng Y, Zhang G, Song M. Erosion Mechanism of Carbon Brick in Hearth of 4000 m3 Industrial Blast Furnace. Metals. 2023; 13(8):1371. https://doi.org/10.3390/met13081371
Chicago/Turabian StyleCao, Jian, Jianliang Zhang, Cui Wang, Yong Deng, Guohua Zhang, and Mingbo Song. 2023. "Erosion Mechanism of Carbon Brick in Hearth of 4000 m3 Industrial Blast Furnace" Metals 13, no. 8: 1371. https://doi.org/10.3390/met13081371
APA StyleCao, J., Zhang, J., Wang, C., Deng, Y., Zhang, G., & Song, M. (2023). Erosion Mechanism of Carbon Brick in Hearth of 4000 m3 Industrial Blast Furnace. Metals, 13(8), 1371. https://doi.org/10.3390/met13081371







