Quantification of Hydrogen Flux from Atmospheric Corrosion of Steel Using the Scanning Kelvin Probe Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
2.2. EPT Measurements
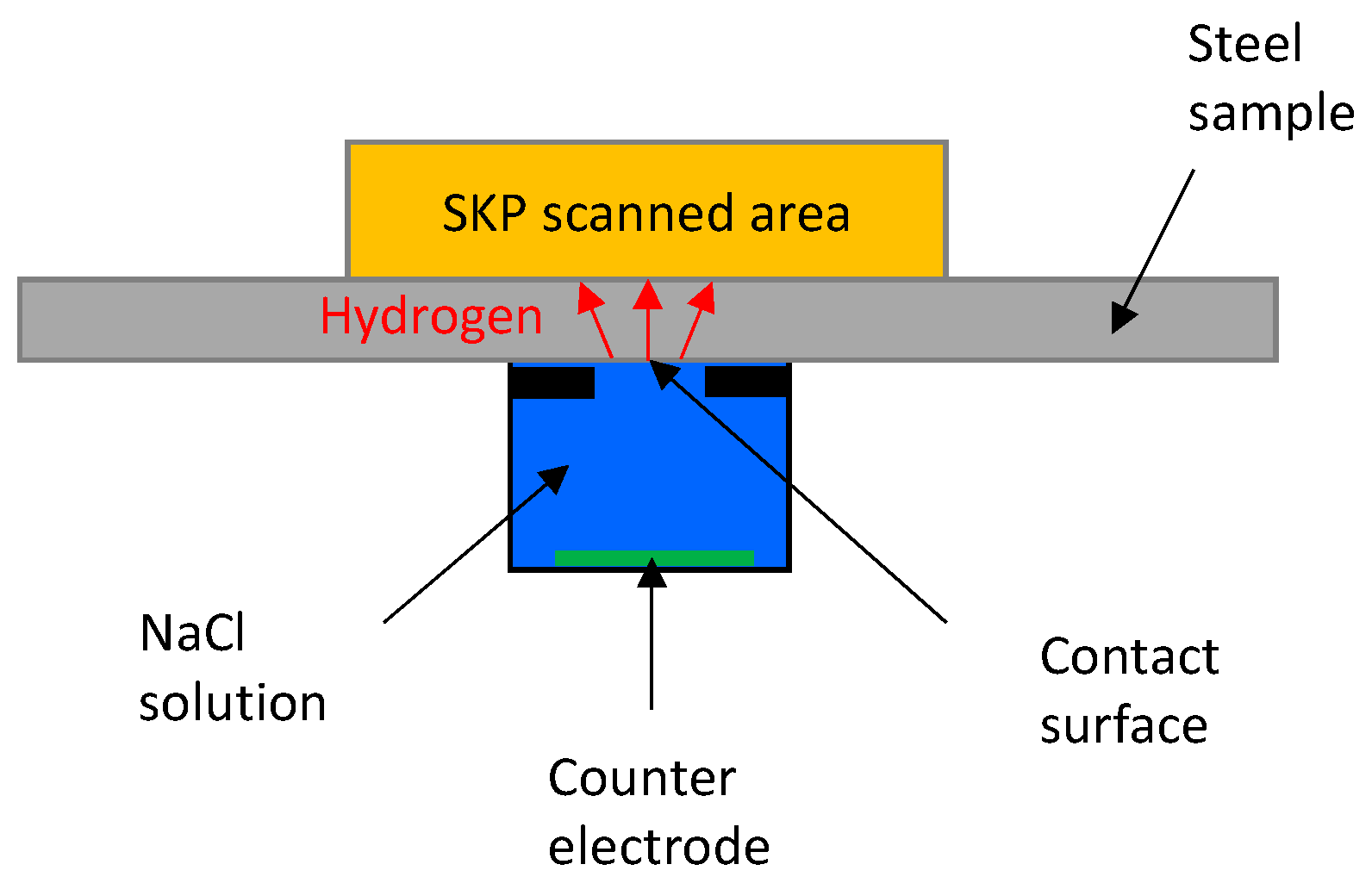
2.3. SKP Procedure
3. Results and Discussion
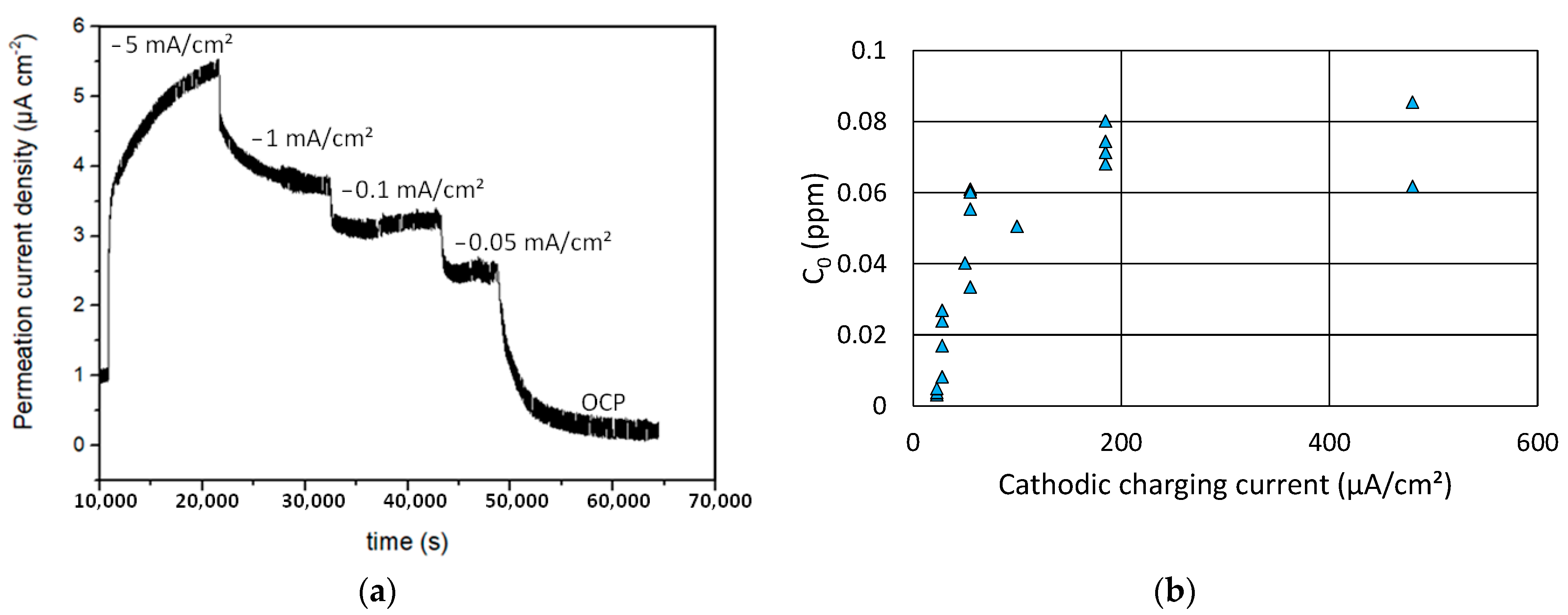
3.1. Hydrogen Activity under Cathodic Charging Conditions
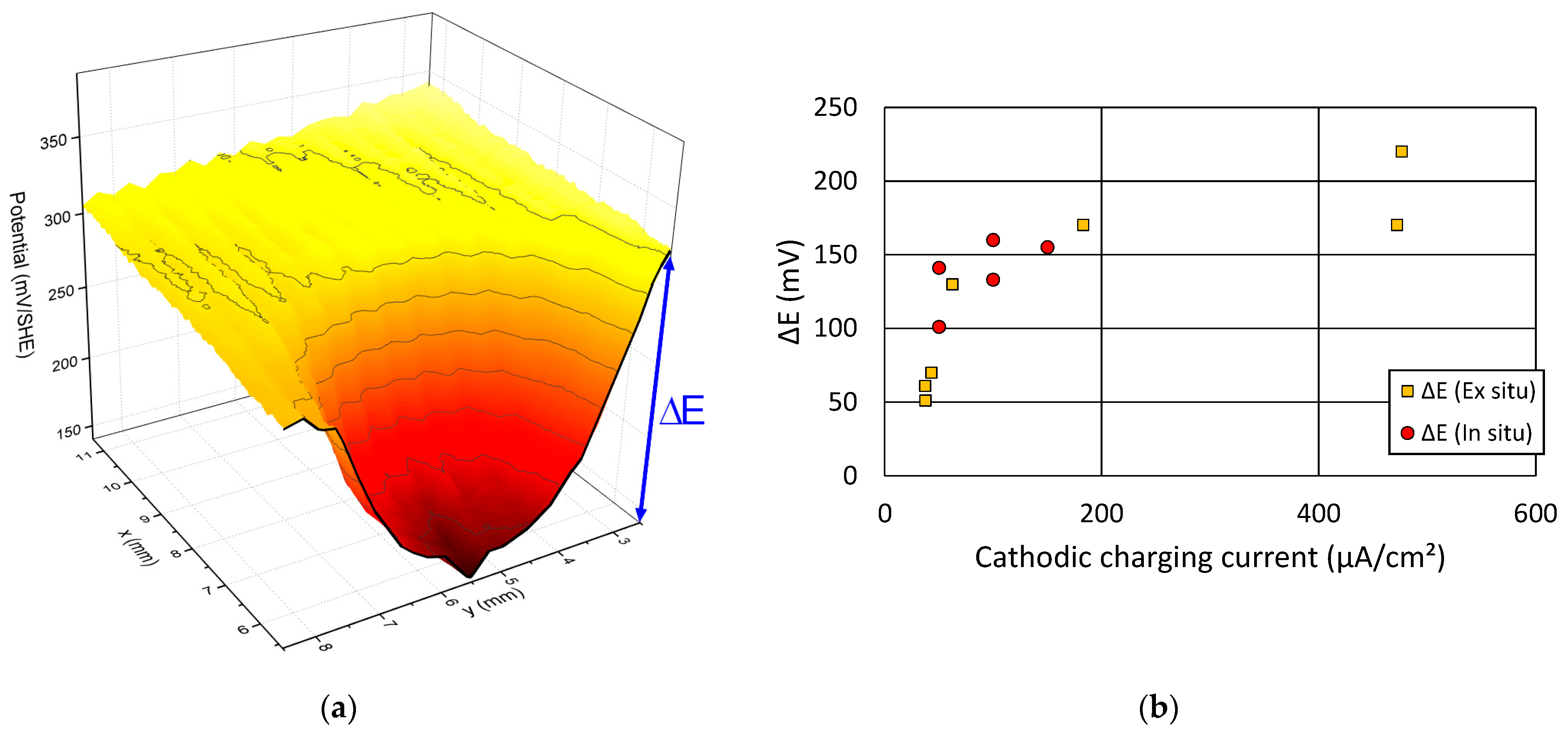
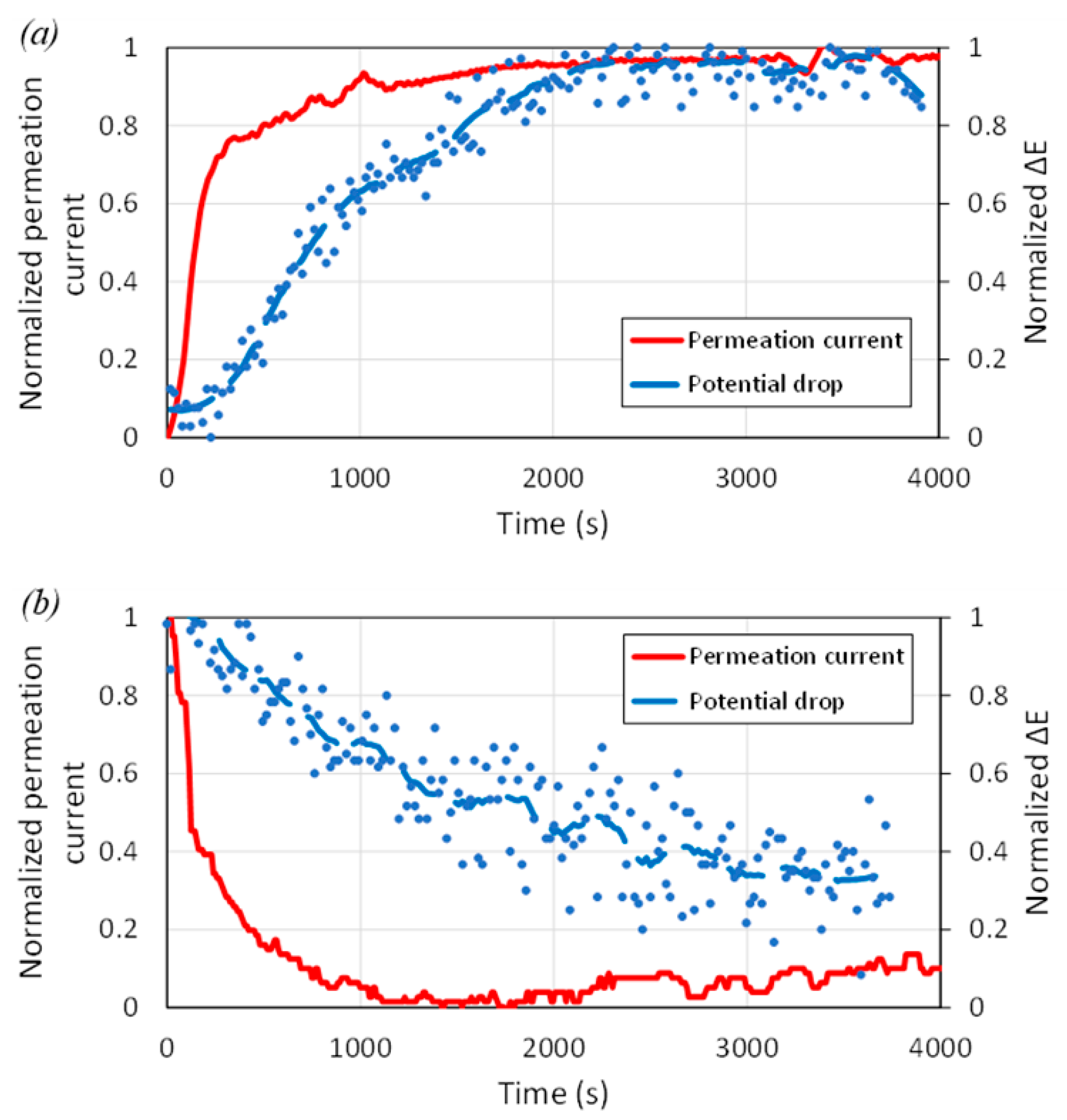
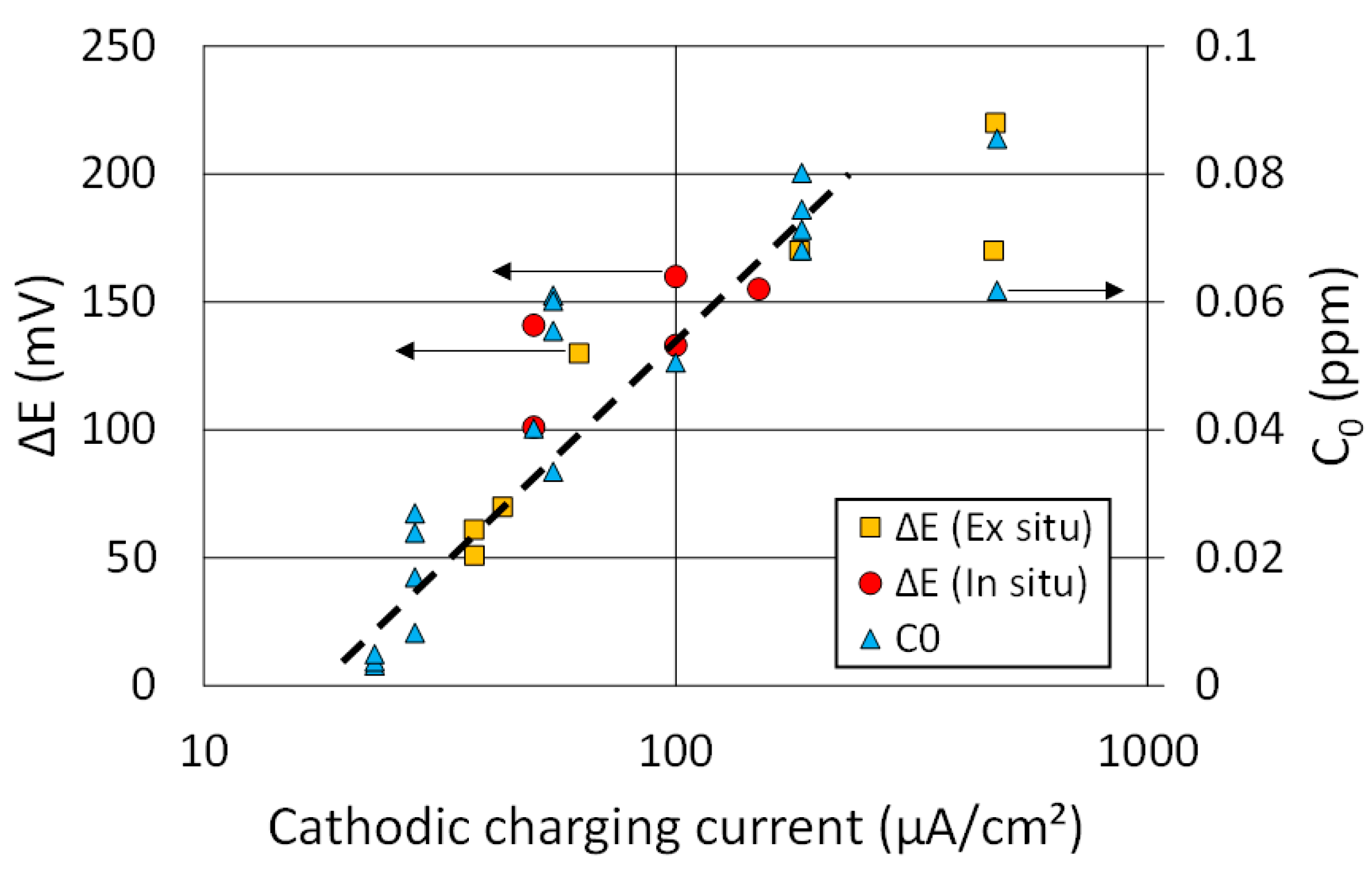
3.2. Calibration of SKP Potential Drop by EPT
3.3. Hydrogen from Corroding Droplet
4. Conclusions
- -
- A simple and fast calibration procedure was developed to quantify hydrogen activity using SKP detection on a thin steel membrane. The method is based on EPT transients and the measurement of SKP potential drop under similar cathodic charging conditions.
- -
- Both ex situ and in situ hydrogen-charging procedures can be used to calibrate the SKP method. The potential of steel is mainly influenced by hydrogen flux. However, it is recommended that ex situ measurement be performed quite soon after the cathodic charging, using a fast scan rate (large steps). For in situ, a similar approach to EPT tests could also be developed, using charging transients, but with extended stabilization time.
- -
- A linear relationship was found between the potential drop and hydrogen activity in the range of low charging currents and therefore low hydrogen activities. Some deviation from this linear relationship could be observed at higher charging current densities, because of the non-proportional increase of the hydrogen flux with increasing current for both EPT and SKP, due to the two different mechanisms.
- -
- The obtained relationship between potential drop and hydrogen activity was valid only for the investigated steel with the defined thickness, structure, and hydrogen traps. To investigate new steel, the calibration procedure must be repeated.
- -
- The calibrated SKP method shows a sensitivity even higher than 0.01 ppm for the quantification of the hydrogen activity. However, some saturation effect seemed to occur for higher charging current densities, at least under the applied test conditions.
- -
- Due to competition between reduction and oxidation processes on the detecting side of the steel membrane analyzed by SKP, the diffusion coefficient of hydrogen should not be calculated from the evolution of the potential drop, even using in situ experiments. The exact mechanism responsible for this deviation has not been clarified.
- -
- SKP can localize and quantify the hydrogen from a corroding steel membrane under mild atmospheric corrosion conditions. For bare steel, hydrogen is mainly produced at the anodic location of the atmospheric corrosion process due to local acidification.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mizuno, D.; Suzuki, S.; Fujita, S.; Hara, N. Corrosion monitoring and materials selection for automotive environments by using Atmospheric Corrosion Monitor (ACM) sensor. Corros. Sci. 2014, 83, 217–225. [Google Scholar] [CrossRef]
- LeBozec, N.; Blandin, N.; Thierry, D. Accelerated corrosion tests in the automotive industry: A comparison of the performance towards cosmetic corrosion. Mater. Corros. 2008, 59, 889–894. [Google Scholar] [CrossRef]
- Bednar, L. Automotive Body Component Field Corrosion Behavior in the De-Icing Salt Zone. In SAE Technical Paper; SAE International: Detroit, MI, USA, 1997; p. 971002. [Google Scholar]
- Ćwiek, J. Hydrogen degradation of high-strength steels. J. Achiev. Mater. Manuf. Eng. 2009, 37, 193–212. [Google Scholar]
- Nazarov, A.; Vucko, F.; Thierry, D. Scanning Kelvin Probe for detection of the hydrogen induced by atmospheric corrosion of ultra-high strength steel. Electrochim. Acta 2016, 216, 130–139. [Google Scholar] [CrossRef]
- Schimo-Aichhorn, G.; Traxler, I.; Muhr, A.; Commenda, C.; Rudomilova, D.; Schneeweiss, O.; Luckeneder, G.; Duchaczek, H.; Stellnberger, K.-H.; Faderl, J.; et al. Hydrogen Insertion into Complex-Phase High-Strength Steel during Atmospheric Corrosion at Low Relative Humidity. Metals 2022, 12, 624. [Google Scholar] [CrossRef]
- Nazarov, A.; Helbert, V.; Vucko, F. Scanning Kelvin Probe for Detection in Steel of Locations Enriched by Hydrogen and Prone to Cracking. Corros. Mater. Degrad. 2023, 4, 158–173. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Popov, B.N.; Lee, J.-W.; Djukic, M.B. Hydrogen Permeation and Hydrogen-Induced Cracking. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–162. [Google Scholar] [CrossRef]
- Lyu, A.; Lee, J.; Nam, J.-H.; Kim, M.; Lee, Y.-K. Hydrogen absorption and embrittlement of martensitic medium-Mn steels. Corr. Sci. 2023, 221, 111304. [Google Scholar] [CrossRef]
- Omura, T. Hydrogen Entry and its Effect on Delayed Fracture Susceptibility of High Strength Steel Bolts under Atmospheric Corrosion. ISIJ Int. 2012, 52, 267–273. [Google Scholar] [CrossRef]
- Ajito, S.; Tada, E.; Ooi, A.; Nishikata, A. Simultaneous Measurements of Corrosion Potential and Hydrogen Permeation Current in Atmospheric Corrosion of Steel. ISIJ Int. 2019, 59, 1659–1666. [Google Scholar] [CrossRef]
- Ootsuka, S.; Fujita, S.; Tada, E.; Nishikata, A.; Tsuru, T. Evaluation of hydrogen absorption into steel in automobile moving environments. Corros. Sci. 2015, 98, 430–437. [Google Scholar] [CrossRef]
- Akiyama, E.; Matsukado, K.; Wang, M.; Tsuzaki, K. Evaluation of hydrogen entry into high strength steel under atmospheric corrosion. Corros. Sci. 2010, 52, 2758–2765. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Zhou, Q.; Li, M. Hydrogen permeation behavior of QP1180 high strength steel in simulated coastal atmosphere. J. Mater. Res. Technol. 2022, 18, 2320–2330. [Google Scholar] [CrossRef]
- Han, X.; Sakairi, M. The promotion effect of aluminium ion on hydrogen entry into steel during atmospheric corrosion. Electrochim. Acta 2023, 458, 142505. [Google Scholar] [CrossRef]
- Ootsuka, S.; Tada, E.; Ooi, A.; Nishikata, A. Effect of Environmental Factors on Hydrogen Absorption into Steel Sheet under a Wet-dry Cyclic Corrosion Condition. ISIJ Int. 2021, 61, 1229–1235. [Google Scholar] [CrossRef]
- Ma, Z.; Xiong, X.; Chen, L.; Su, Y. Quantitative calibration of the relationship between Volta potential measured by scanning Kelvin probe force microscope (SKPFM) and hydrogen concentration. Electrochim. Acta 2021, 366, 137422. [Google Scholar] [CrossRef]
- Gruenewald, P.; Hautz, N.; Motz, C. Implementation of an experimental setup to qualitatively detect hydrogen permeation along grain boundaries in nickel using Scanning Kelvin Probe Force Microscopy under varying atmospheres. Int. J. Hydrogen Energy 2022, 47, 15922–15932. [Google Scholar] [CrossRef]
- Rudomilova, D.; Prošek, T.; Ström, M. Hydrogen Entry into Steel Under Corrosion Products. Corrosion 2021, 77, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Determination of the critical hydrogen concentration for delayed fracture of high strength steel by constant load test and numerical calculation. Corros. Sci. 2006, 48, 2189–2202. [Google Scholar] [CrossRef]
- Bergmann, C.; Mraczek, K.; Kröger, B.; Sturel, T.; Jürgensen, J.; Yagodzinskyy, Y.; Guo, X.; Vucko, F.; Kuhlmann, M.; Veith, S.; et al. Hydrogen Embrittlement Resistance Evaluation of Advanced High Strength Steels in Automotive Applications; SteelyHydrogen: Ghent, Belgium, 2018. [Google Scholar]
- Akiyama, E.; Wang, M.; Li, S.; Zhang, Z.; Kimura, Y.; Uno, N.; Tsuzaki, K. Studies of evaluation of hydrogen embrittlement property of high-strength steels with consideration of the effect of atmospheric corrosion. Met. Mater. Trans. A 2012, 44, 1290–1300. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, M.-G.; Yoon, S.-C.; Vucko, F.; Lee, C.-W.; Theirry, D.; Kim, S.-J. Diffusible hydrogen behavior and delayed fracture of cold rolled martensitic steel in consideration of automotive manufacturing process and vehicle service environment. J. Mater. Res. Technol. 2020, 9, 13483–13501. [Google Scholar] [CrossRef]
- Rudomilova, D.; Prošek, T.; Traxler, I.; Faderl, J.; Luckeneder, G.; Schimo-Aichhorn, G.; Muhr, A. Critical Assessment of the Effect of Atmospheric Corrosion Induced Hydrogen on Mechanical Properties of Advanced High Strength Steel. Metals 2020, 11, 44. [Google Scholar] [CrossRef]
- Koul, M.G.; Sheetz, A.; Ault, P.; Repp, J.; Whitfield, A. Effect of Zn-Rich Coatings on the Corrosion and Cracking Resistance of High-Strength Armor Steel. Corrosion 2014, 70, 337–350. [Google Scholar] [CrossRef]
- Nazarov, A.; Thierry, D. Corrosion and hydrogen entry into high strength steel. In situ SKP imaging of the hydrogen flux. In Proceedings of the 65th Annual Meeting of the International Society of Electrochemistry, Lausanne, Switzerland, 31 August–5 September 2014. [Google Scholar]
- Schimo, G.; Burgstaller, W.; Hassel, A.W. Influence of atmospheric oxygen on hydrogen detection on Pd using Kelvin probe technique. J. Solid State Electrochem. 2018, 22, 495–504. [Google Scholar] [CrossRef]
- Burgstaller, W.; Schimo, G.; Hassel, A.W. Challenges in hydrogen quantification using Kelvin probe technique at different levels of relative humidity. J. Solid State Electrochem. 2017, 21, 1785–1796. [Google Scholar] [CrossRef]
- Schaller, R.F.; Scully, J.R. Measurement of effective hydrogen diffusivity using the Scanning Kelvin Probe. Electrochem. Commun. 2014, 40, 42–44. [Google Scholar] [CrossRef]
- Evers, S.; Rohwerder, M. The hydrogen electrode in the dry: A Kelvin probe approach to measuring hydrogen in metals. Electrochem. Commun. 2012, 24, 85–88. [Google Scholar] [CrossRef]
- Nazarov, A.; Vucko, F.; Thierry, D. Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain. Corros. Mater. Degrad. 2020, 1, 187–197. [Google Scholar] [CrossRef]
- Vucko, F.; Ootsuka, S.; Rioual, S.; Diler, E.; Nazarov, A.; Thierry, D. Hydrogen detection in high strength dual phase steel using scanning Kelvin probe technique and XPS analyses. Corros. Sci. 2022, 197, 110072. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Newman, R.C. Surface oxide reduction by hydrogen permeation through iron foil detected using a scanning Kelvin probe. Electrochem. Commun. 2013, 27, 144–147. [Google Scholar] [CrossRef]
- Zakroczymski, T. Electrochemical determination of hydrogen in metals. J. Electroanal. Chem. 1999, 475, 82–88. [Google Scholar] [CrossRef]
- Manolatos, P.; Jerome, M. A thin palladium coating on iron for hydrogen permeation studies. Electrochim. Acta 1996, 41, 359–365. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The Adsorption and Diffusion of Electrolytic Hydrogen in Palladium. Proc. R. Soc. A Math. Phys. Eng. Sci. 1962, 270, 90–102. [Google Scholar]
- Zakroczymski, T. Adaptation of the electrochemical permeation technique for studying entry, transport and trapping of hydrogen in metals. Electrochim. Acta 2006, 51, 2261–2266. [Google Scholar] [CrossRef]
- Akiyama, E.; Li, S. Electrochemical Hydrogen Permeation Tests under Conventional Potentiostatic Hydrogen Charging Conditions Conventionally Used for Hydrogen Embrittlement Study. ECS Trans. 2017, 75, 23–31. [Google Scholar] [CrossRef]
- Akiyama, E.; Li, S. Electrochemical hydrogen permeation tests under galvanostatic hydrogen charging conditions conventionally used for hydrogen embrittlement study. Corros. Rev. 2016, 34, 103–112. [Google Scholar] [CrossRef]
- Henriksen, J.F. The distribution of NaCl on Fe during atmospheric corrosion. Corros. Sci. 1969, 9, 573–576. [Google Scholar] [CrossRef]
- Cho, S.W.; Ko, S.J.; Yoo, J.S.; Yoo, Y.H.; Song, Y.K.; Kim, J.G. Effect of Cr on aqueous and atmospheric corrosion of automotive carbon steel. Materials 2021, 14, 2444. [Google Scholar] [CrossRef]

| Fe | C | Mn | P | S |
|---|---|---|---|---|
| Bal. | <0.08 | <0.4 | <0.03 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vucko, F.; Helbert, V.S.; Nazarov, A. Quantification of Hydrogen Flux from Atmospheric Corrosion of Steel Using the Scanning Kelvin Probe Technique. Metals 2023, 13, 1427. https://doi.org/10.3390/met13081427
Vucko F, Helbert VS, Nazarov A. Quantification of Hydrogen Flux from Atmospheric Corrosion of Steel Using the Scanning Kelvin Probe Technique. Metals. 2023; 13(8):1427. https://doi.org/10.3390/met13081427
Chicago/Turabian StyleVucko, Flavien, Varvara Shubina Helbert, and Andrei Nazarov. 2023. "Quantification of Hydrogen Flux from Atmospheric Corrosion of Steel Using the Scanning Kelvin Probe Technique" Metals 13, no. 8: 1427. https://doi.org/10.3390/met13081427
APA StyleVucko, F., Helbert, V. S., & Nazarov, A. (2023). Quantification of Hydrogen Flux from Atmospheric Corrosion of Steel Using the Scanning Kelvin Probe Technique. Metals, 13(8), 1427. https://doi.org/10.3390/met13081427






