A New Method for Preparing Titanium Aluminium Alloy Powder
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Introduction to AlCl3 and Selection of AlCl3-KCl Molten Salt Ratio
- (1)
- Physical properties of AlCl3
- (2)
- Basic Physical Properties of AlCl3-KCl Mixed Molten Salt
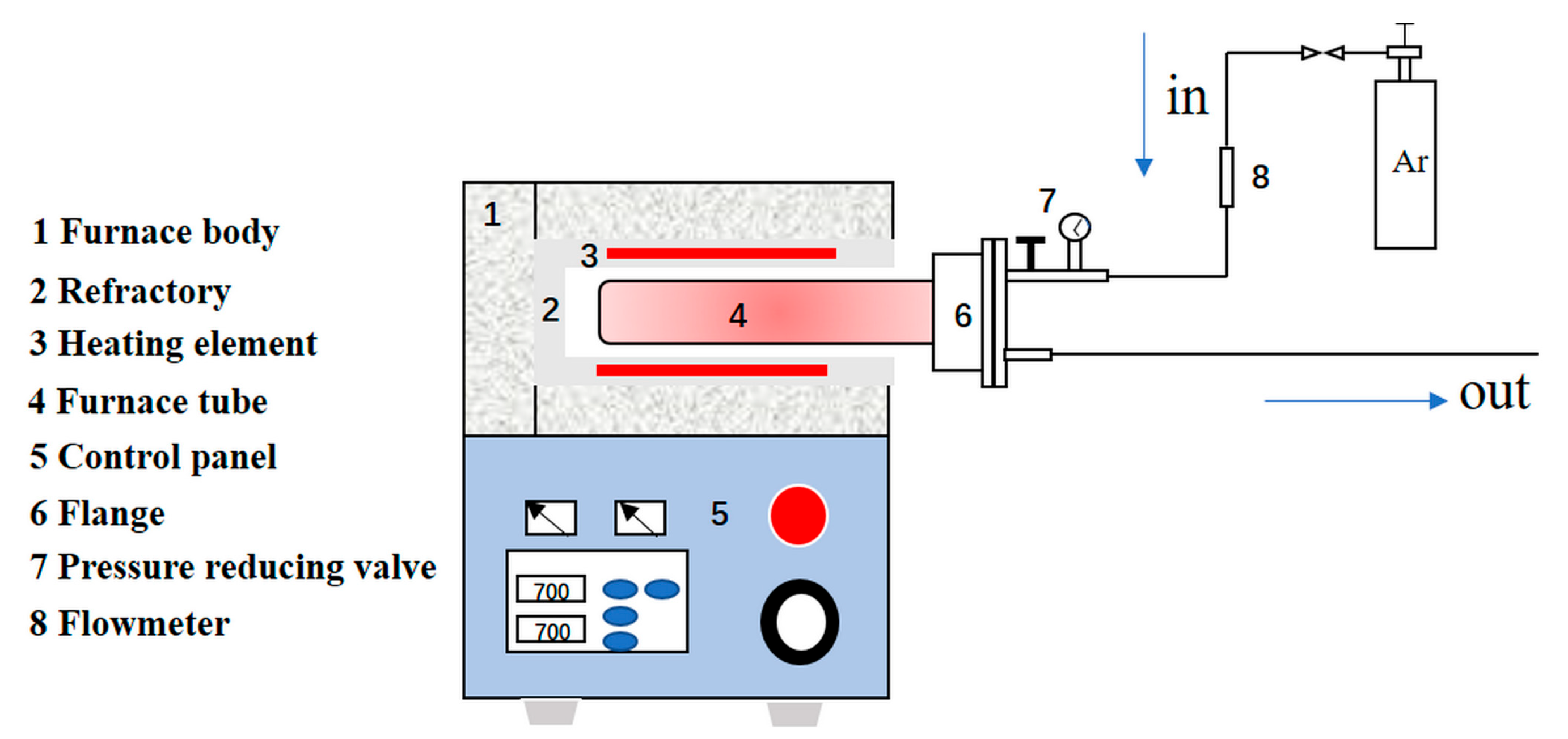
2.3. Methods
2.4. Analysis
3. Results and Discussion
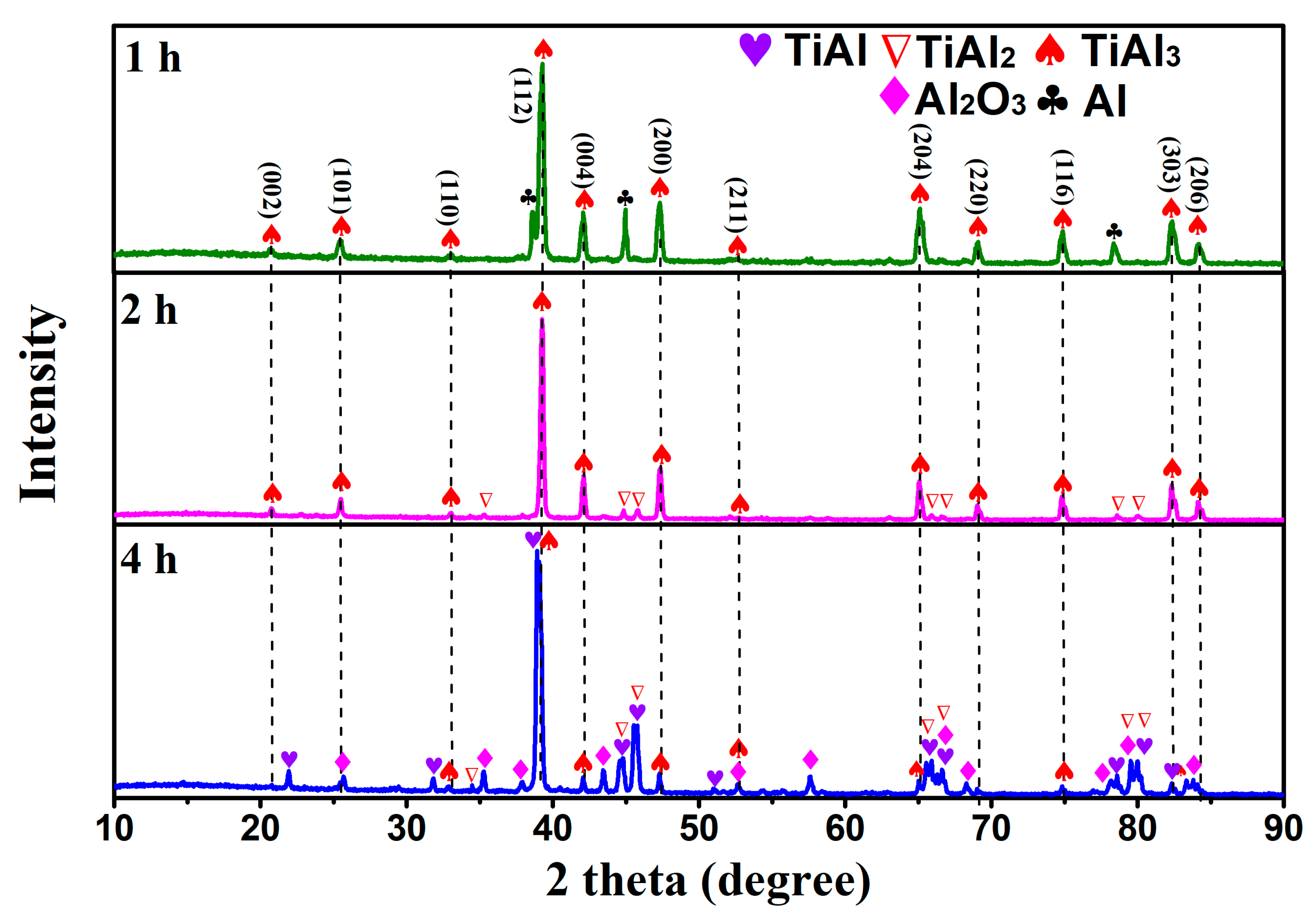
3.1. Phase Analysis of Reactants at Different Temperatures
3.2. Effect of Reduction Temperature
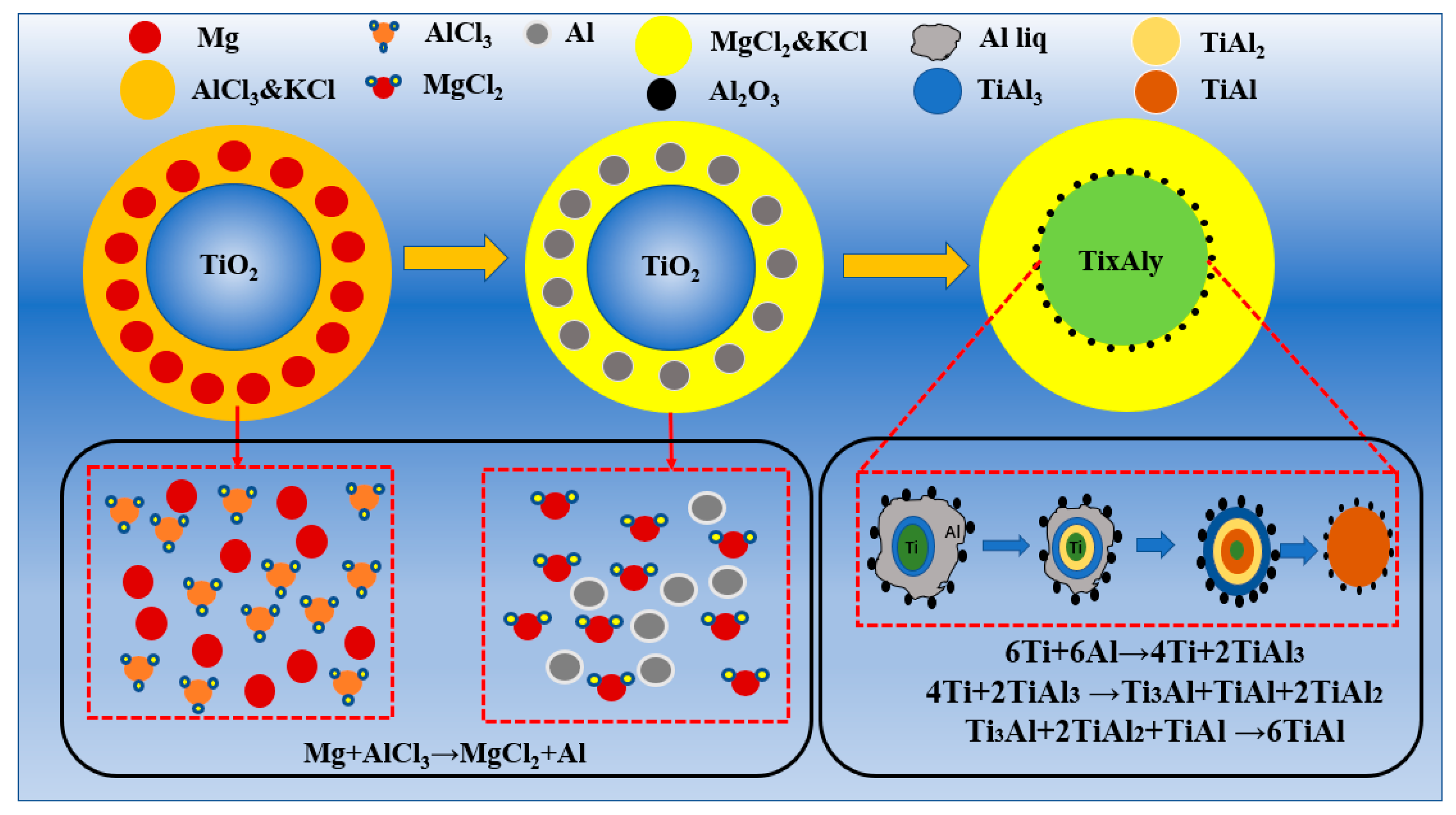
3.3. Analysis of the AlCl3&KCl-Molten-Salt-Assisted Magnesium Thermal Reduction Process
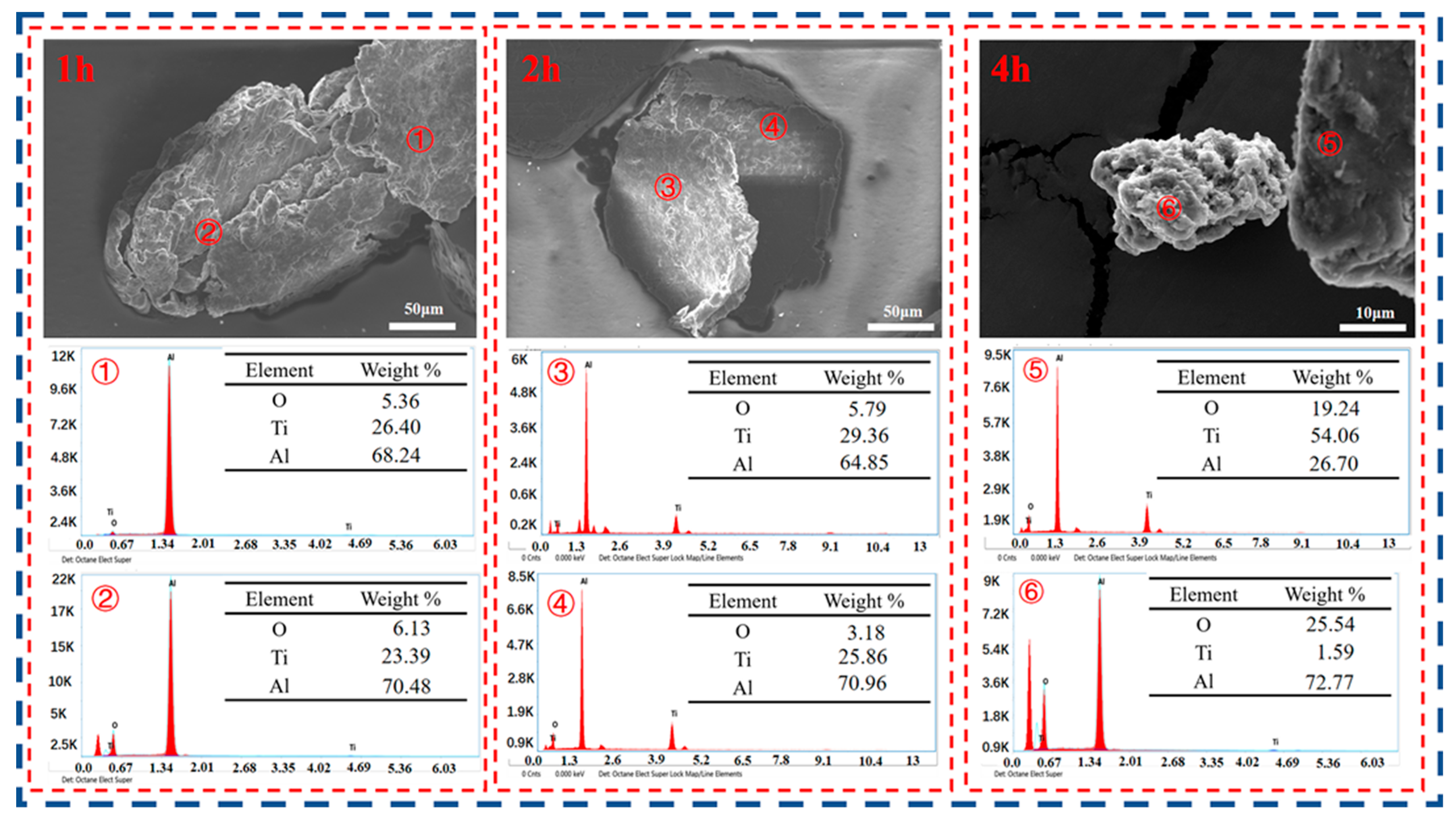
3.4. Product Analysis and Comparison
4. Conclusions
- (1)
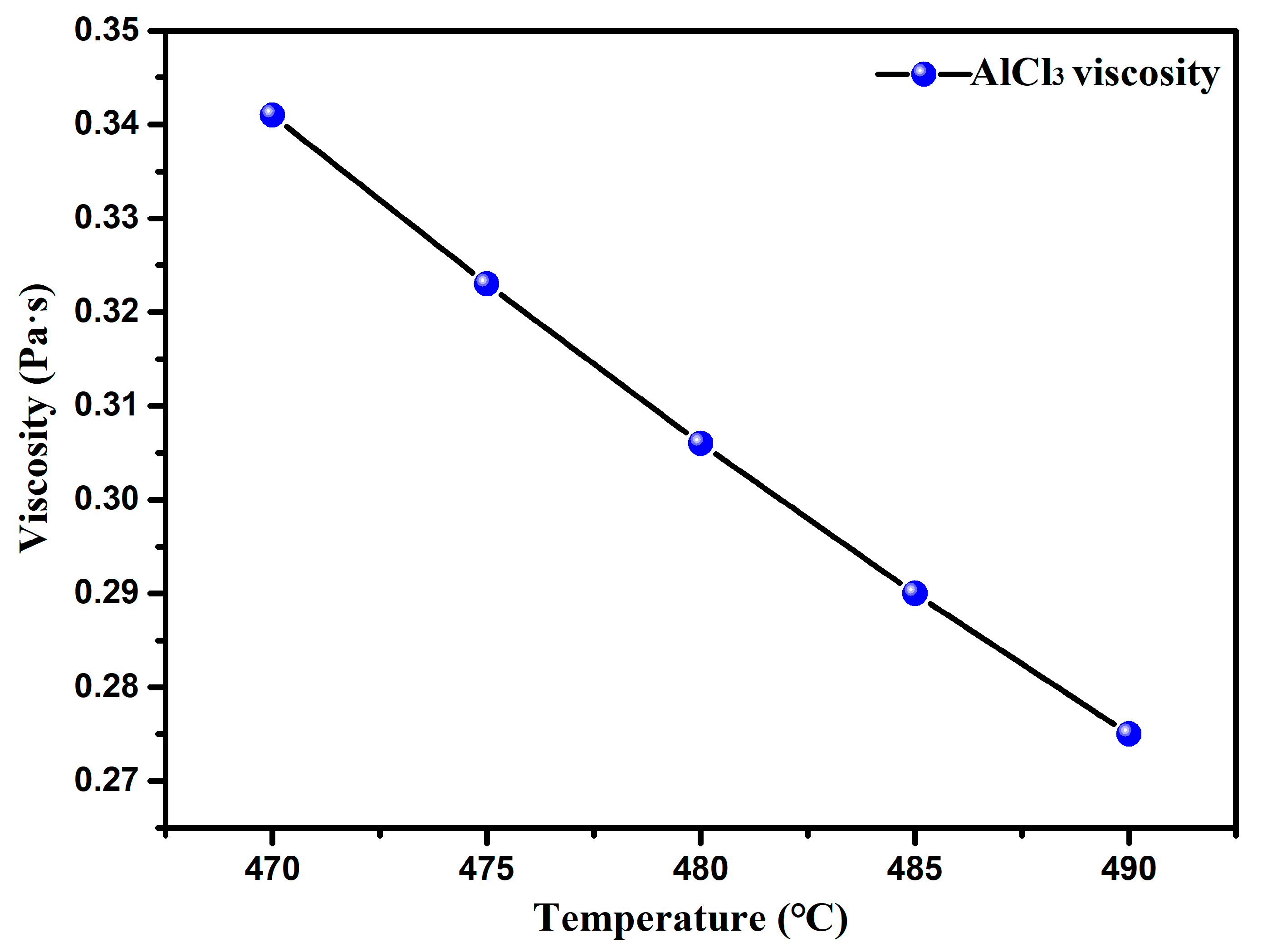
- Through the study of the basic physical properties of the AlCl3 and AlCl3&KCl, it was found that the density of the AlCl3 decreased from 1.3 g·cm−3 to 1 g·cm−3 in the temperature range of 460–560 °C, and the viscosity also decreased from 0.34 Pa·s to 0.27 Pa·s with the temperature. For AlCl3&KCl eutectic salts, when the KCl content is 20 wt% and 33.33 wt%, the density of the AlCl3&KCl decreases from 1.65 g·cm−3 to below 1.5 g·cm−3 within the range of 350–850 °C. However, when the KCl content is 50 wt% and 66.66 wt%, the temperature has little effect on the density change of the AlCl3&KCl, with a density of approximately 2 g·cm−3. To reduce the volatilization of the AlCl3, the experiment selected a mass ratio of 0.65 for the AlCl3/(AlCl3+KCl) as the selected eutectic salt ratio.
- (2)
- Using TiO2 as the raw material, magnesium thermal reduction experiments were conducted in a AlCl3&KCl molten salt medium, to clarify the reaction action and sequence of AlCl3 during the reaction process. During the magnesium thermal reduction process, Mg preferentially reacts with AlCl3 at 400–1000 °C to generate metal Al, which reacts with TiO2, and generates TiAl3 alloy powder at 750–950 °C.
- (3)
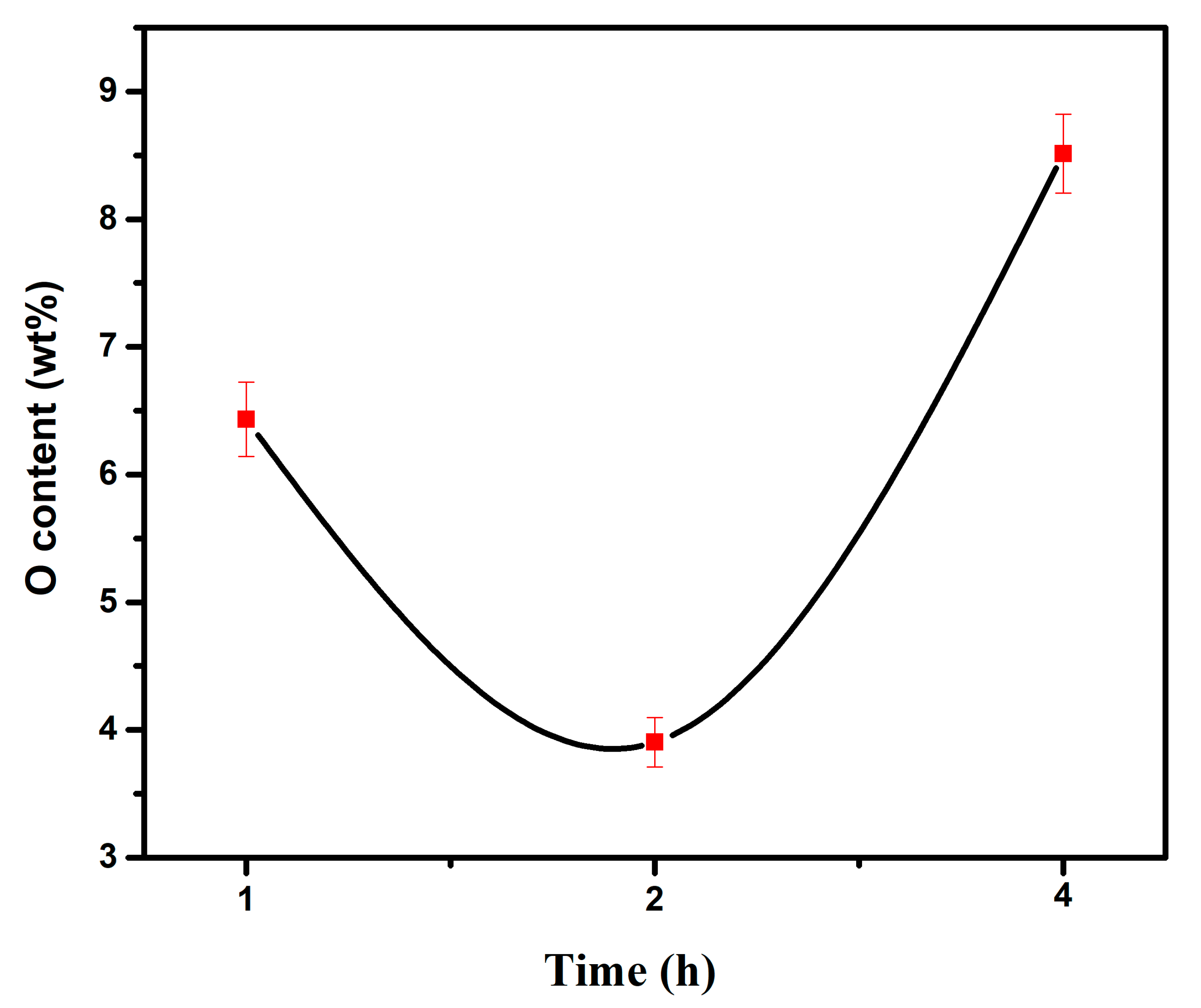
- When the experimental temperature is within the range of 750–950 °C, the products of the AlCl3&KCl molten-salt-assisted magnesium thermal reduction gradually form a TiAl alloy from the TiAl3 alloy at 750 °C to 950 °C. The oxygen content also increases from 4.23 wt% at 750 °C, to 11.23 wt% at 950 °C, and the final powder oxygen content also increases with the extension of the reduction time. After 4 h of reaction at 750 °C, the oxygen content reaches approximately 8.5 wt%, and the reaction temperature is at 950 °C; after 4 h of reaction time, it will cause an increase in, and adhesion of, the Al2O3 in the product. The optimal reaction time for this process is 2 h, and the reaction temperature is 750 °C. TiAl3 alloy powders with Ti, Al, and O contents of 28.41 wt%, 67.68 wt%, and 3.91 wt% can be obtained, respectively. The powder particle size is concentrated at around 450 μm.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tetsui, T. Impact Resistance of Commercially Applied TiAl Alloys and Simple-Composition TiAl Alloys at Various Temperatures. Metals 2022, 12, 2003. [Google Scholar]
- Wang, J.H.; Lu, Y.; Shao, X.H. First-Principles Calculation for the Influence of C and O on the Mechanical Properties of gamma-TiAl Alloy at High Temperature. Metals 2019, 9, 262. [Google Scholar]
- Yang, Y.; Liang, Y.F.; Li, C.; Lin, J. Microstructure and Mechanical Properties of TiAl Matrix Composites Reinforced by Carbides. Metals 2022, 12, 790. [Google Scholar]
- Mogale, N.F.; Matizamhuka, W.R. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. [Google Scholar]
- Wang, Z.H.; Sun, H.X.; Du, Y.L.; Yuan, J. Effects of Powder Preparation and Sintering Temperature on Properties of Spark Plasma Sintered Ti-48Al-2Cr-8Nb Alloy. Metals 2019, 9, 861. [Google Scholar]
- Dong, Z.C.; Feng, A.H.; Wang, H.; Qu, S.; Wang, H. Thermodynamic Study on Initial Oxidation Behavior of TiAl-Nb Alloys at High Temperature. Metals 2023, 13, 485. [Google Scholar]
- Zhu, X.P.; Zhu, C.L.; Lin, B.S.; Wang, Z. Research on Optimization Design of Cast Process for TiAl Case Casting. Metals 2022, 12, 1954. [Google Scholar]
- WilliamsI, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar]
- Zhang, S.L.; Cui, N.; Sun, W.; Li, Q. Microstructural Characterization and Crack Propagation Behavior of a Novel beta-Solidifying TiAl Alloy. Metals 2021, 11, 1231. [Google Scholar]
- Yu, W.; Zhou, J.X.; Yin, Y.; Feng, X.; Nan, H.; Lin, J.; Ding, X.; Duan, W. Effects of Hot Isostatic Pressing and Heat Treatment on the Microstructure and Mechanical Properties of Cast TiAl Alloy. Metals 2021, 11, 1156. [Google Scholar]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar]
- Galati, M.; Gatto, M.L.; Bloise, N.; Fassina, L.; Saboori, A.; Visai, L.; Mengucci, P.; Iuliano, L. Electron Beam Powder Bed Fusion of Ti-48Al-2Cr-2Nb Open Porous Scaffold for Biomedical Applications: Process Parameters, Adhesion, and Proliferation of NIH-3T3 Cells. 3D Print. Addit. Manuf. 2022. [Google Scholar] [CrossRef]
- Xu, R.; Li, M.; Zhao, Y. A review of microstructure control and mechanical performance optimization of γ-TiAl alloys. J. Alloys Compd. 2023, 932, 167611. [Google Scholar]
- Subramanyam, R.B. Some recent innovations in the Kroll process of titanium sponge production. Bull. Mater. Sci. 1993, 16, 433–451. [Google Scholar]
- Wang, W.; Wu, F. Quantifying Heat Transfer Characteristics of the Kroll Reactor in Titanium Sponge Production. Front. Energy Res. 2021, 9, 759781. [Google Scholar]
- Wartman, F.S.; Baker, D.H.; Nettle, J.R.; Homme, V.E. Some Observations on the Kroll Process for Titanium. J. Electrochem. Soc. 1954, 101, 507–513. [Google Scholar]
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar]
- Kumaran, S.; Chantaiah, B.; Rao, T.S. Effect of niobium and aluminium additions in TiAl prealloyed powders during high-energy ball milling. Mater. Chem. Phys. 2008, 108, 97–101. [Google Scholar]
- Rao, K.P.; Du, Y.J. In situ formation of titanium silicides-reinforced TiAl-based composites. Mater. Sci. Eng. A 2000, 277, 46–56. [Google Scholar]
- Wang, G.X.; Mao, X. Preparation of TiAl/Mo and TiAl/NiAl composites by powder processing. J. Mater. Sci. 1997, 32, 6325–6329. [Google Scholar]
- Yan, M.; Yang, F.; Zhang, H.; Zhang, C.; Zhang, H.; Chen, C.; Guo, Z. Multiple intermetallic compounds reinforced Ti–48Al alloy with simple composition and high strength. Mater. Sci. Eng. A 2022, 858, 144152. [Google Scholar]
- Pilone, D.; Pulci, G.; Paglia, L.; Mondal, A.; Marra, F.; Felli, F.; Brotzu, A. Mechanical Behaviour of an Al2O3 Dispersion Strengthened γTiAl Alloy Produced by Centrifugal Casting. Metals 2020, 10, 1457. [Google Scholar]
- Shen, Y.; Jia, Q.; Zhang, X.; Liu, R.; Wang, Y.; Cui, Y.; Yang, R. Tensile Behavior of SiC Fiber-Reinforced γ-TiAl Composites Prepared by Suction Casting. Acta Metall. Sin. 2021, 34, 932–942. [Google Scholar]
- Tetsui, T. Selection of Additive Elements Focusing on Impact Resistance in Practical TiAl Cast Alloys. Metals 2022, 12, 544. [Google Scholar]
- Yu, Y.; Kou, H.; Wang, Y.; Wang, Y.; Jia, M.; Li, H.; Li, Y.; Wang, J.; Li, J. Controlling lamellar orientation of Ti-47.5Al-5Nb-2.5V-1Cr alloy by conventional casting. Scr. Mater. 2023, 223, 115080. [Google Scholar]
- Lapin, J.; Kamyshnykova, K.; Klimova, A. Comparative Study of Microstructure and Mechanical Properties of Two TiAl-Based Alloys Reinforced with Carbide Particles. Molecules 2020, 25, 3423. [Google Scholar]
- Gabrisch, H.; Stark, A.; Schimansky, F.-P.; Wang, L.; Schell, N.; Lorenz, U.; Pyczak, F. Investigation of carbides in Ti–45Al–5Nb–xC alloys (0 ≤ x ≤ 1) by transmission electron microscopy and high energy-XRD. Intermetallics 2013, 33, 44–53. [Google Scholar]
- Emiralioğlu, A.; Ünal, R. Additive manufacturing of gamma titanium aluminide alloys: A review. J. Mater. Sci. 2022, 57, 4441–4466. [Google Scholar]
- Liu, J.; Wang, M.; Zhang, P.; Chen, Y.; Wang, S.; Wu, T.; Xie, M.; Wang, L.; Wang, K. Texture refinement and mechanical improvement in beam oscillation superimposed laser welding of TiAl-based alloy. Mater. Charact. 2022, 188, 111892. [Google Scholar]
- Liu, Z.-Q.; Zhu, X.-O.; Yin, G.-L.; Zhou, Q. Direct bonding of bimetallic structure from Ti6Al4V to Ti48Al2Cr2Nb alloy by laser additive manufacturing. Mater. Sci. Technol. 2022, 38, 39–44. [Google Scholar]
- Soliman, H.A.; Elbestawi, M. Titanium aluminides processing by additive manufacturing—A review. Int. J. Adv. Manuf. Technol. 2022, 119, 5583–5614. [Google Scholar]
- Zhou, W.; Shen, C.; Hua, X.; Wang, L.; Zhang, Y.; Li, F.; Xin, J.; Ding, Y. The effect of vanadium on the microstructure and mechanical properties of TiAl alloy fabricated by twin-wire directed energy deposition-arc. Addit. Manuf. 2023, 62, 103382. [Google Scholar]
- Zhao, K.; Feng, N.; Wang, Y. Fabrication of Ti-Al intermetallics by a two-stage aluminothermic reduction process using Na2TiF6. Intermetallics 2017, 85, 156–162. [Google Scholar]
- Zhao, K.; Wang, Y.W.; Peng, J.P. Electrochemical preparation of titanium and titanium-copper alloys with K2Ti6O13 in KF-KCl melts. Rare Met. 2017, 36, 527–532. [Google Scholar]
- Song, Y.; Dou, Z.; Zhang, T.-A. Mechanisms of metal-slag separation behavior in thermite reduction for preparation of TiAl alloy. J. Mater. Eng. Perform. 2021, 30, 9315–9325. [Google Scholar]
- Song, Y.; Dou, Z.; Liu, Y.; Zhang, T.-A. Study on the Preparation Process of TiAl Alloy by Self-Propagating Metallurgy. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Janz, G.J.; Tomkins, R.P.T.; Allen, C.B.; Downey, J.R.; Garner, G.L.; Krebs, U.; Singer, S.K. Molten salts: Volume 4, part 2, chlorides and mixtures—Electrical conductance, density, viscosity, and surface tension data. J. Phys. Chem. Ref. Data 1975, 4, 871–1178. [Google Scholar]
- Školáková, A.; Leitner, J.; Salvetr, P.; Novák, P.; Deduytsche, D.; Kopeček, J.; Detavernier, C.; Vojtěch, D. Kinetic and thermodynamic description of intermediary phase formation in Ti-Al system during reactive sintering. Mater. Chem. Phys. 2019, 230, 122–130. [Google Scholar] [CrossRef]
- Song, Y.L.; Dou, Z.H.; Zhang, T.A.; Liu, Y. Research Progress on the Extractive Metallurgy of Titanium and Its Alloys. Miner. Process. Extr. Metall. Rev. 2020, 42, 535–551. [Google Scholar]
- Choi, K.; Choi, H.; Sohn, I. Understanding the magnesiothermic reduction mechanism of TiO2 to produce Ti. Metall. Mater. Trans. B 2017, 48, 922–932. [Google Scholar]
- Zhao, K.; Wang, Y.W.; Feng, N.X. Cleaner production of Ti powder by a two-stage aluminothermic reduction process. JOM 2017, 69, 1795–1800. [Google Scholar]


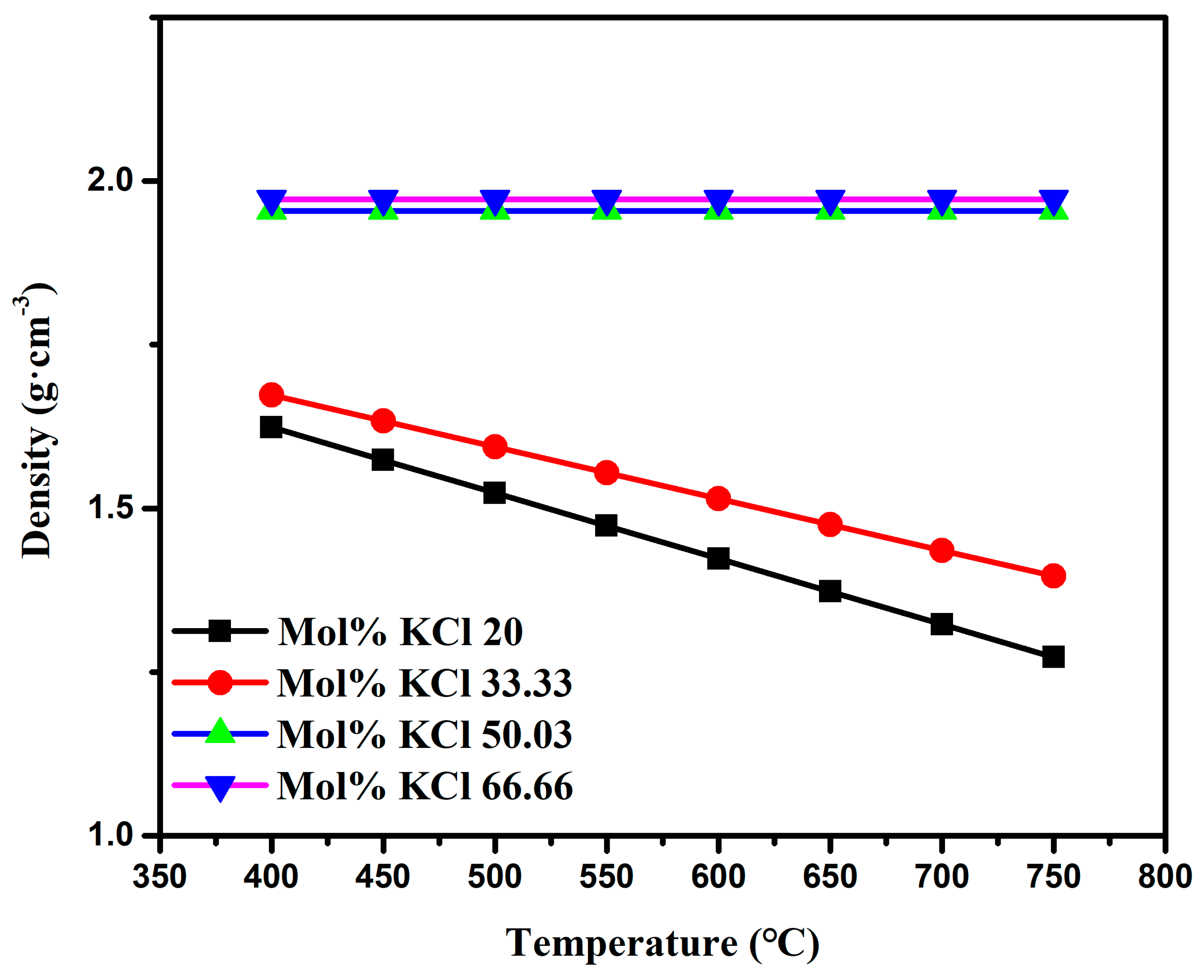






| Mol % KCl | a | b·10−3 | Standard Error |
|---|---|---|---|
| 20.00 | 2.0252 | −1.0038 | 0.11% |
| 33.33 | 1.9889 | −0.7901 | 0.14% |
| 50.03 | 1.9556 | −0.6622 | 0.05% |
| 66.66 | 1.9734 | −0.6101 | 0.02% |
| Ti | Al | O |
|---|---|---|
| 28.41 | 67.68 | 3.91 |
| Method | Features | Advantages | Disadvantages |
|---|---|---|---|
| Perform reduction process (PRP) [39] | Ca as a reducing agent | Complete reaction with a high recovery rate | Unable to apply on a large scale |
| SHS process [40] | Spontaneous reaction through the heating agent | Efficiency, high energy consumption, and low consumption | Uncontrollable reaction process |
| Two-stage thermite process [41] | Reduction using Na2TiF6 as a raw material | Short process and low energy consumption | Multiple reactions |
| AlCl3&KCl-molten-salt assisted magnesium thermal reduction | Low temperature, AlCl3&KCl as the reaction medium | Low energy consumption, controllable reaction | AlCl3 volatilization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Cui, Y.; Zhong, D.; Qiu, G.; Lv, X. A New Method for Preparing Titanium Aluminium Alloy Powder. Metals 2023, 13, 1436. https://doi.org/10.3390/met13081436
Kang J, Cui Y, Zhong D, Qiu G, Lv X. A New Method for Preparing Titanium Aluminium Alloy Powder. Metals. 2023; 13(8):1436. https://doi.org/10.3390/met13081436
Chicago/Turabian StyleKang, Jialong, Yaoran Cui, Dapeng Zhong, Guibao Qiu, and Xuewei Lv. 2023. "A New Method for Preparing Titanium Aluminium Alloy Powder" Metals 13, no. 8: 1436. https://doi.org/10.3390/met13081436




