Adsorption of Gold from Copper–Tartrate–ThiosulfateSolutions with Ion-Exchange Resins
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
- is the adsorption capacity of Au(S2O3)23− at time t,
- and are the gold concentrations at time 0 (initial) and t, respectively,
- is the volume of the solution,
- is the mass of the resin.
- qe and qt are the Au(S2O3)23− adsorption at equilibrium and time t,
- k1 and k2 are adsorption rate constants.
3. Results and Discussion
3.1. Adsorption Kinetics
3.2. Effect of Resin Dosage
3.3. Effect of the Composition of Solutions
3.3.1. Effect of Solution pH
3.3.2. Effect of Temperature
3.3.3. Effect of Copper Concentration
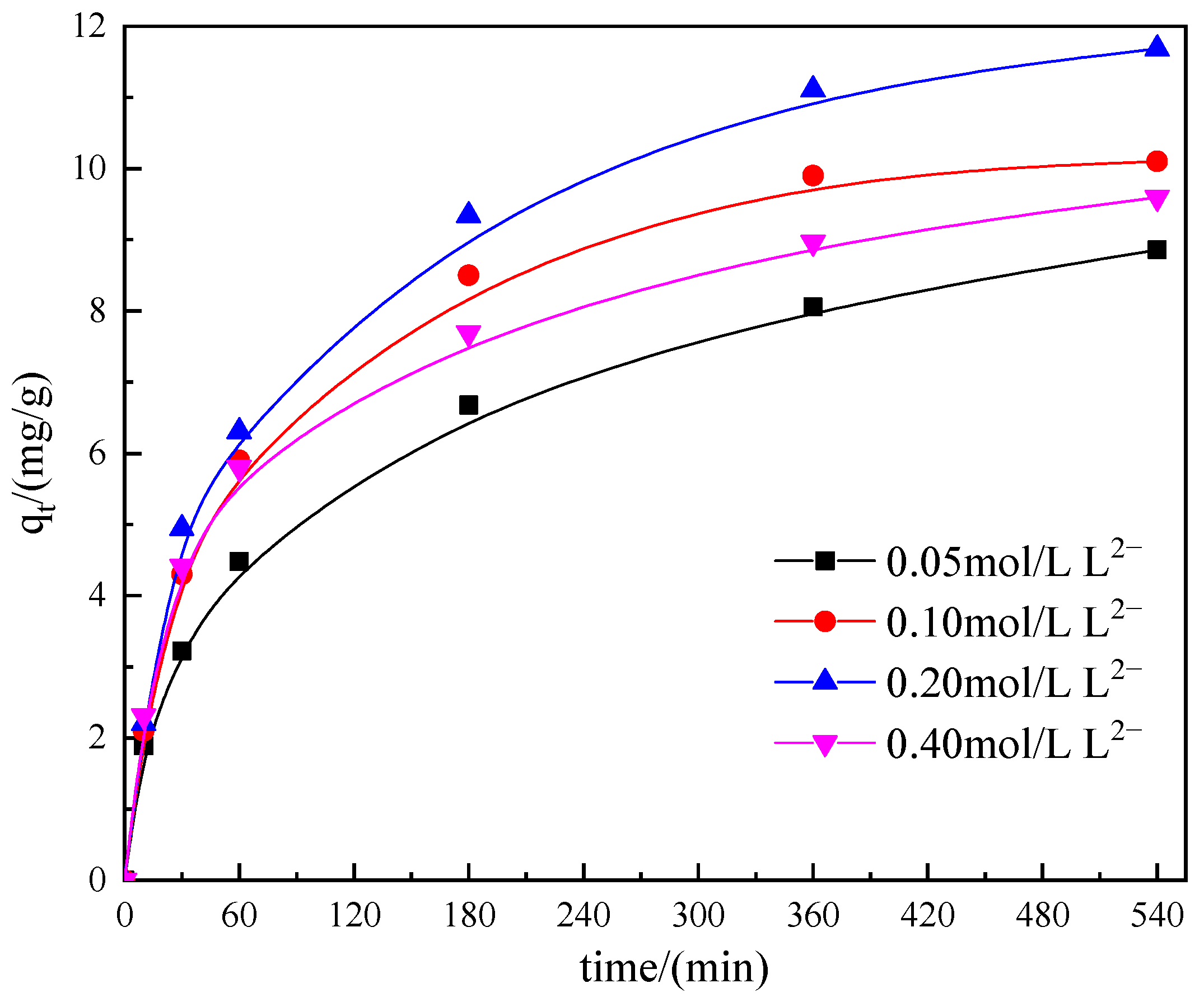
3.3.4. Effect of Thiosulfate Concentration
3.3.5. Effect of Tartrate Concentration
3.4. Characterization of the Resin
3.4.1. FT-IR Analysis
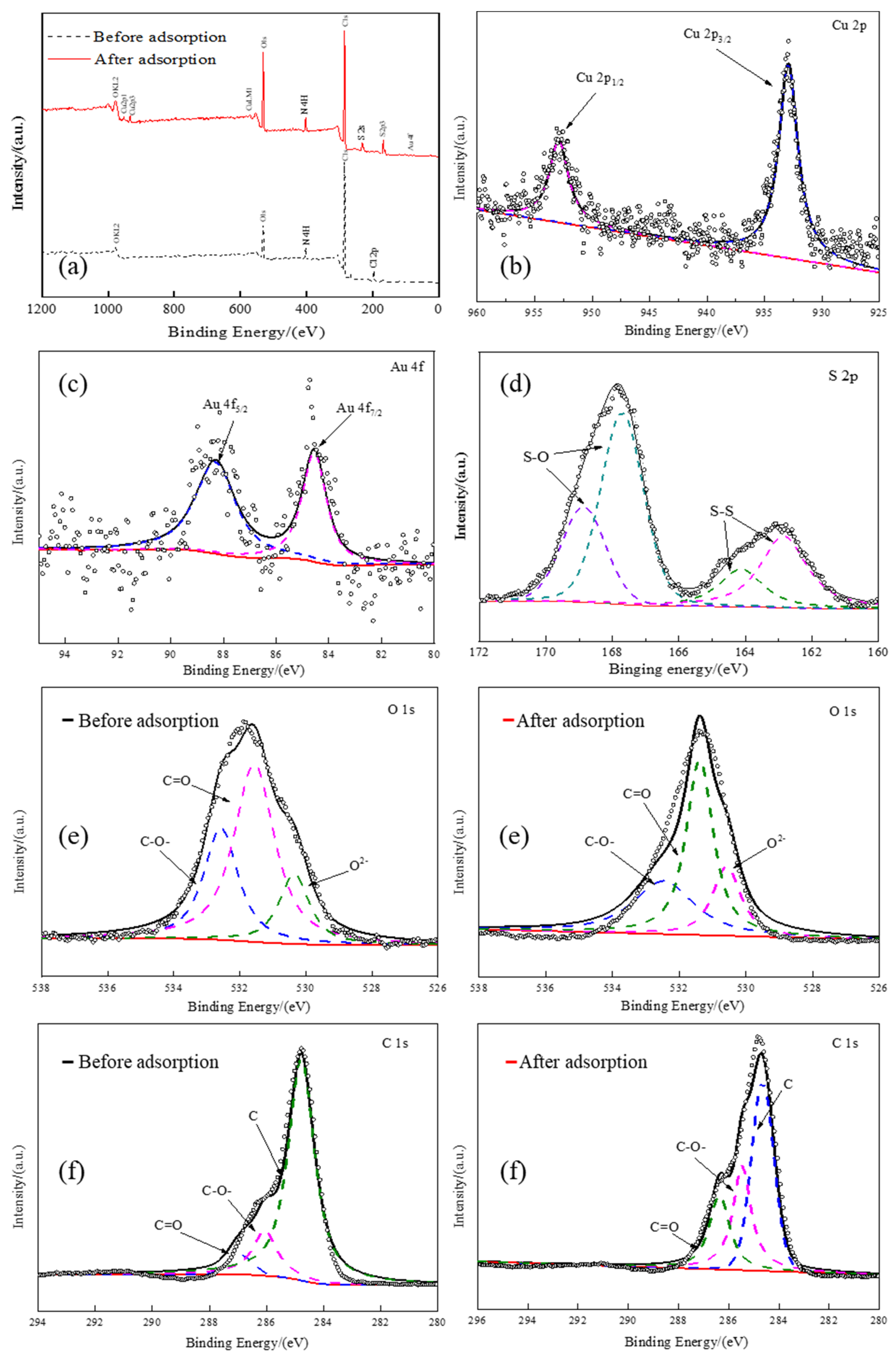
3.4.2. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.-N.; Wang, J.; Wang, W. Review of gold leaching in thiosulfate-based solutions. Trans. Nonferrous Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Guo, X.-Y.; Tian, Q.-H.; Zhang, L. A systematic review of gold extraction: Fundamentals, advancements, and challenges toward alternative lixiviants. J. Hazard. Mater. 2022, 440, 129778. [Google Scholar] [CrossRef]
- Aylmore, M.G. Alternative Lixiviants to Cyanide for Leaching Gold Ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 447–484. [Google Scholar] [CrossRef]
- Molleman, E.; Dreisinger, D. The treatment of copper–gold ores by ammonium thiosulfate leaching. Hydrometallurgy 2002, 66, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Yang, Y.B.; Li, Q.; Jiang, T.; Liu, S.Q.; Li, G.H. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate. Miner. Eng. 2016, 89, 138–147. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegliò, F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, T.; Xu, B.; Zhang, Y.; Li, Q.; Yang, Y.; He, Y. Thiosulphate leaching of gold in the Cu–NH3–S2O32−–H2O system: An updated thermodynamic analysis using predominance area and species distribution diagrams. Miner. Eng. 2020, 151, 106336. [Google Scholar] [CrossRef]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper(II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M. An electrochemical study of gold leaching in thiosulfate solutions containing copper and ammonia. Hydrometallurgy 2002, 65, 145–157. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M. Thiosulfate leaching kinetics of gold in the presence of copper and ammonia. Miner. Eng. 2000, 13, 1071–1081. [Google Scholar] [CrossRef]
- Gos, S.; Rubo, A. The Relevance of Alternative Lixiviants with Regard to Technical Aspects, Work Safety and Environmental Safety; Cyplus, Degussa AG: Hanau, Germany, 2001. [Google Scholar]
- Wang, J.; Wang, R.; Pan, Y.; Liu, F.; Xu, Z. Thermodynamic analysis of gold leaching by copper-glycine-thiosulfate solutions using Eh-pH and species distribution diagrams. Miner. Eng. 2022, 179, 107438. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Pan, Y.; Wang, W. Leaching of Gold with Copper-citrate-thiosulfate Solutions. Miner. Process. Extr. Met. Rev. 2021, 43, 916–925. [Google Scholar] [CrossRef]
- Chen, J.N.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of Gold and Silver from a Complex Sulfide Concentrate in Copper-Tartrate-Thiosulfate Solutions. Metals 2022, 12, 1152. [Google Scholar] [CrossRef]
- Chen, J.N.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of a carbonaceous gold concentrate in copper-tartrate-thiosulfate solutions. Miner. Eng. 2022, 183, 107605. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Wang, W.; Bai, Y.L.; Fu, Y.; Dreisinger, D. Eco-friendly leaching of gold from a carbonaceous gold concentrate in copper-citrate-thiosulfate solutions. Hydrometallurgy 2020, 191, 105204. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, Y.B.; Li, Q. Recovery of Gold from Pregnant Thiosulfate Solutions by the Resin Adsorption Technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Zheng, Y.Q.; Li, Q.; Yang, Y.B.; Liu, X.L.; Jiang, T.; Lyu, X.J. Hexaamminecobalt(III) catalyzed thiosulfate leaching of gold from a concentrate calcine and gold recovery from its pregnant leach solution via resin adsorption. Miner. Eng. 2021, 171, 107079. [Google Scholar] [CrossRef]
- Zhang, H.G.; Dreisinger, D.B. The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy 2004, 72, 225–234. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.-T. Gold Recovery from Nickel Catalyzed Ammonium Thiosulfate Solution by Strongly Basic Anion Exchange Resin. Mater. Trans. 2003, 44, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Lin, Y.; Du, H.; Hu, J.; Yang, P.; Zhang, Y.; Yang, B. The first effective utilization of activated carbon in gold thiosulfate system: A more eco-friendly, easier method for gold recovery and material regeneration. Miner. Eng. 2020, 155, 106441. [Google Scholar] [CrossRef]
- Chen, J.N.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of Gold with Copper–Tartrate–Thiosulfate Solutions. Trans. Indian Inst. Met. 2023, 1–8. [Google Scholar] [CrossRef]
- Severino, F.; Brito, L.J.; Laine, J.; Fierro, J.L.G.; Agudo, A.L. Nature of Copper Active Sites in the Carbon Monoxide Oxidation on CuAl2O4and CuCr2O4Spinel Type Catalysts. J. Catal. 1998, 177, 82–95. [Google Scholar] [CrossRef]
- Hayez, V.; Franquet, A.; Hubin, A.; Terryn, H. XPS study of the atmospheric corrosion of copper alloys of archaeological interest. Surf. Interface Anal. 2004, 36, 876–879. [Google Scholar] [CrossRef]
- Deutsch, K.L.; Shanks, B.H. Active species of copper chromite catalyst in C–O hydrogenolysis of 5-methylfurfuryl alcohol. J. Catal. 2012, 285, 235–241. [Google Scholar] [CrossRef]
- Turner, N.H.; Murday, J.S.; Ramaker, D.E. Quantitative determination of surface composition of sulfur bearing anion mixtures by Auger electron spectroscopy. J. Vac. Sci. Technol. A. 1980, 17, 84–92. [Google Scholar] [CrossRef]
- Lindberg, B.J.; Hamrin, K.; Johansson, G.; Gelius, U.; Fahlman, A.; Nordling, C.; Siegbahn, K. Molecular Spectroscopy by Means of ESCA II. Sulfur compounds. Correlation of electron binding energy with structure. J. Electron. Spectrosc. 1970, 1, 286. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf. A 2001, 177, 23–45. [Google Scholar] [CrossRef]
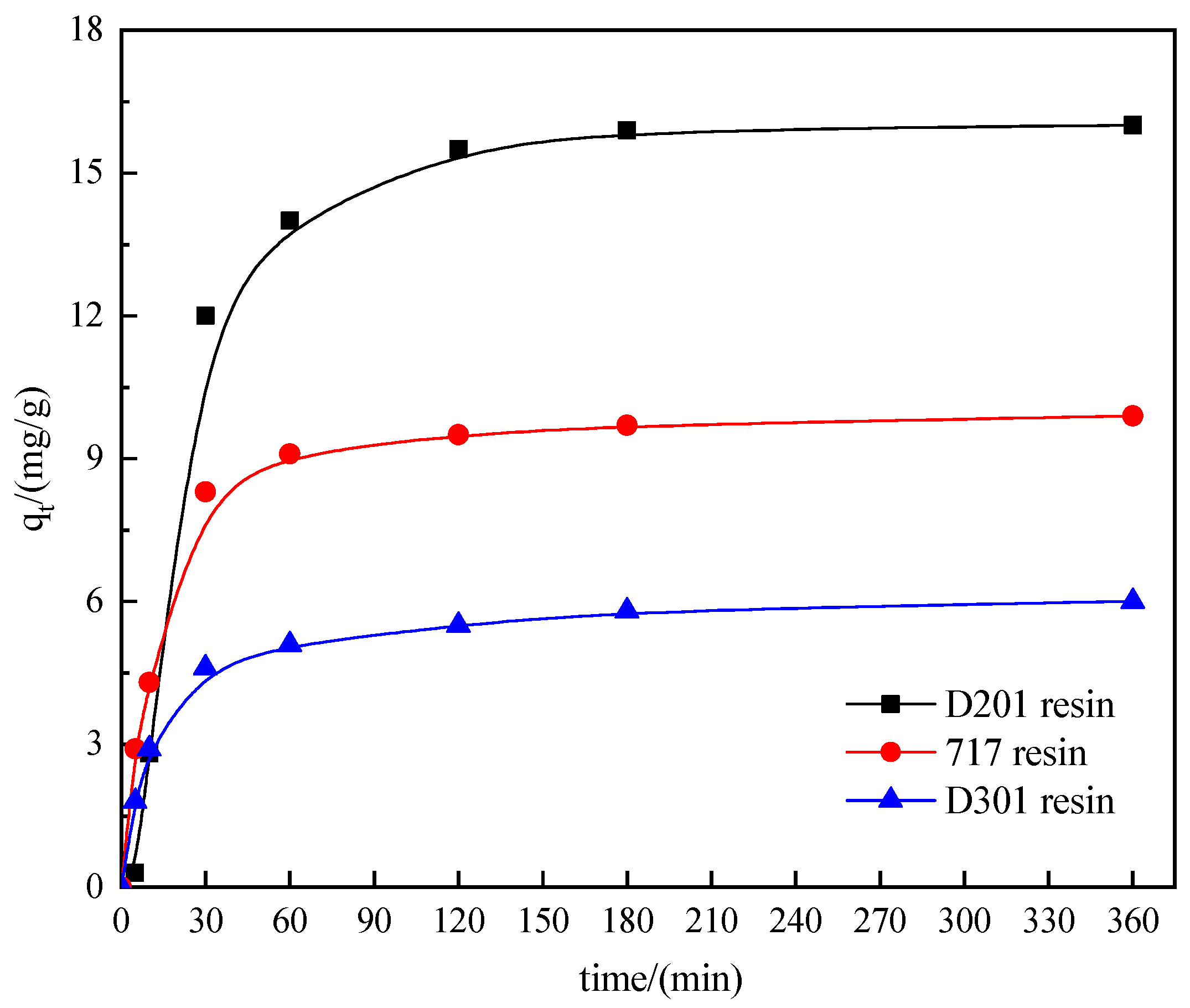



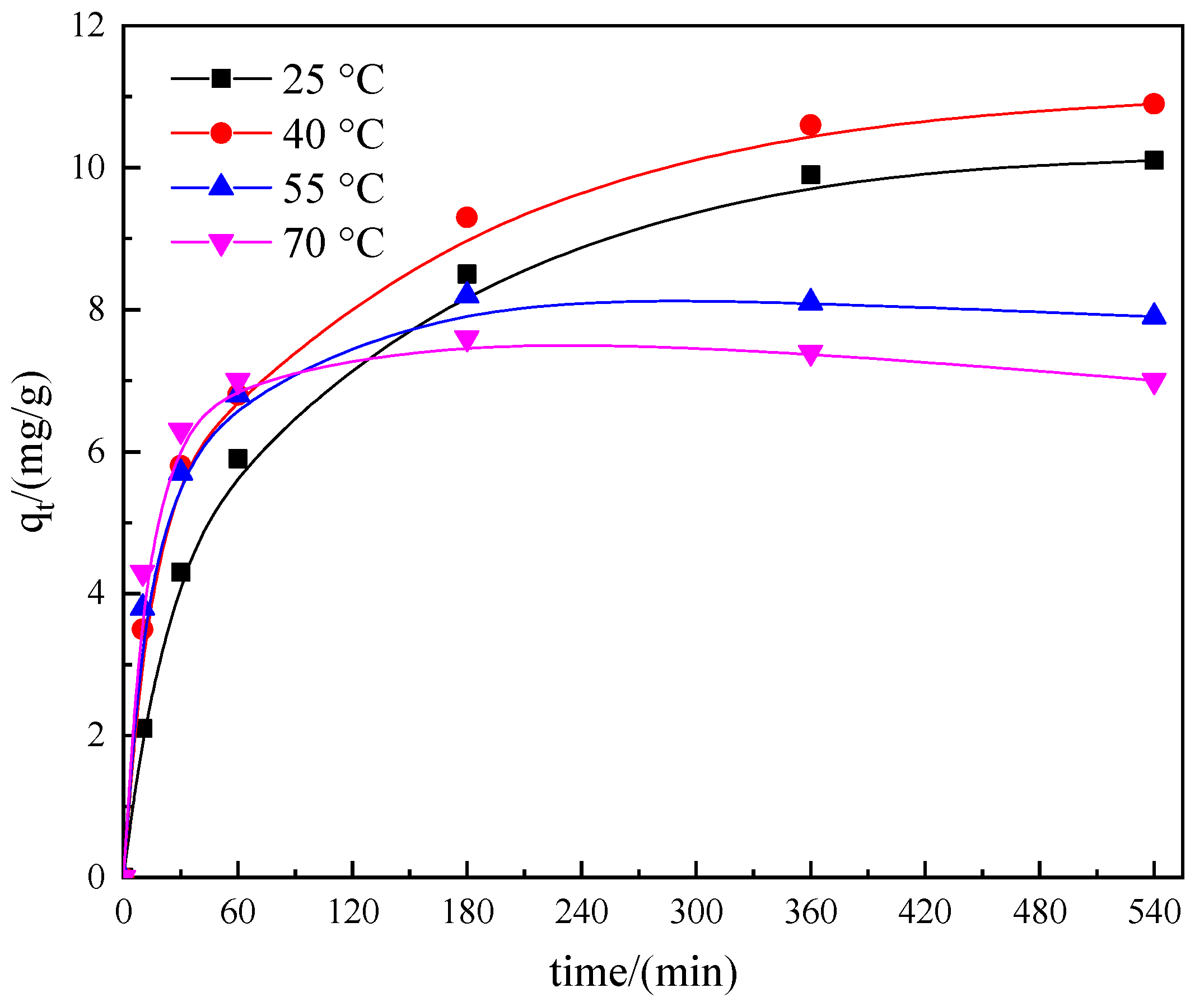
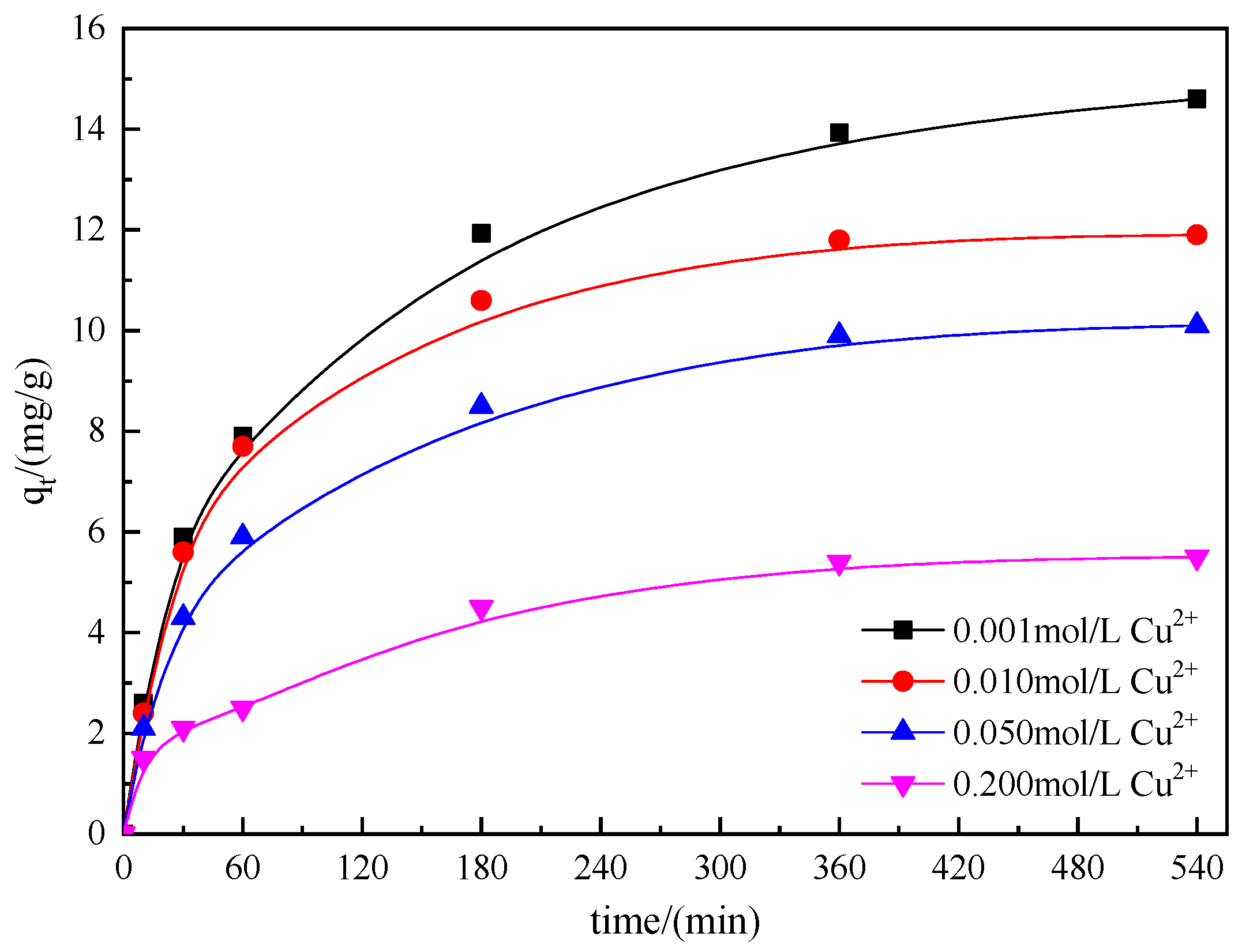
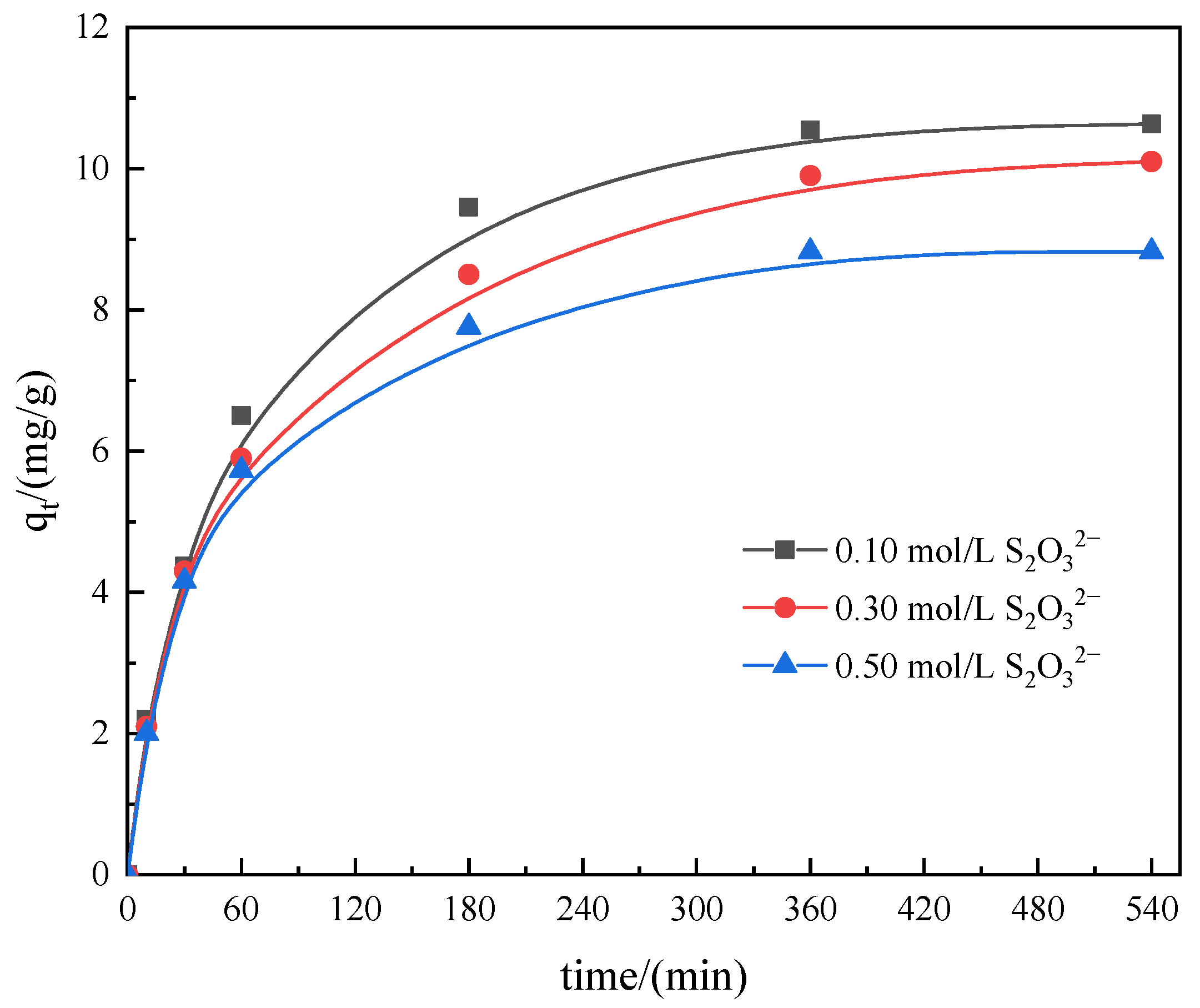

| Resin | D201 | 717 | D301 |
|---|---|---|---|
| CAS | 9050-97-9 | 9002-24-8 | 201615-38-5 |
| Type | Strongly alkaline | Strongly alkaline | Weakly alkaline |
| Functional group | [-N(CH3)2] | [-N(CH3)2] | [-N(CH3)2] |
| pH | 1–14 | 1–14 | 1–9 |
| Particle size (0.315–1.25 mm) | ≥95% | ≥95% | ≥95% |
| Kinetics Model | Concentration of Gold (mg/L) | R2 | qe (mg/g) | |
|---|---|---|---|---|
| Calculated | Experiment | |||
| Pseudo-first-order | 100 | 0.839 | 8.69 | 16.20 |
| Pseudo-second-order | 100 | 0.985 | 17.27 | 16.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Chen, J.; Xie, F.; Cao, Z.; Wang, W. Adsorption of Gold from Copper–Tartrate–ThiosulfateSolutions with Ion-Exchange Resins. Metals 2023, 13, 1443. https://doi.org/10.3390/met13081443
Fu Y, Chen J, Xie F, Cao Z, Wang W. Adsorption of Gold from Copper–Tartrate–ThiosulfateSolutions with Ion-Exchange Resins. Metals. 2023; 13(8):1443. https://doi.org/10.3390/met13081443
Chicago/Turabian StyleFu, Yan, Junnan Chen, Feng Xie, Zhichao Cao, and Wei Wang. 2023. "Adsorption of Gold from Copper–Tartrate–ThiosulfateSolutions with Ion-Exchange Resins" Metals 13, no. 8: 1443. https://doi.org/10.3390/met13081443






