FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method
Abstract
:1. Introduction
2. Experimental Protocol
3. Results and Discussion
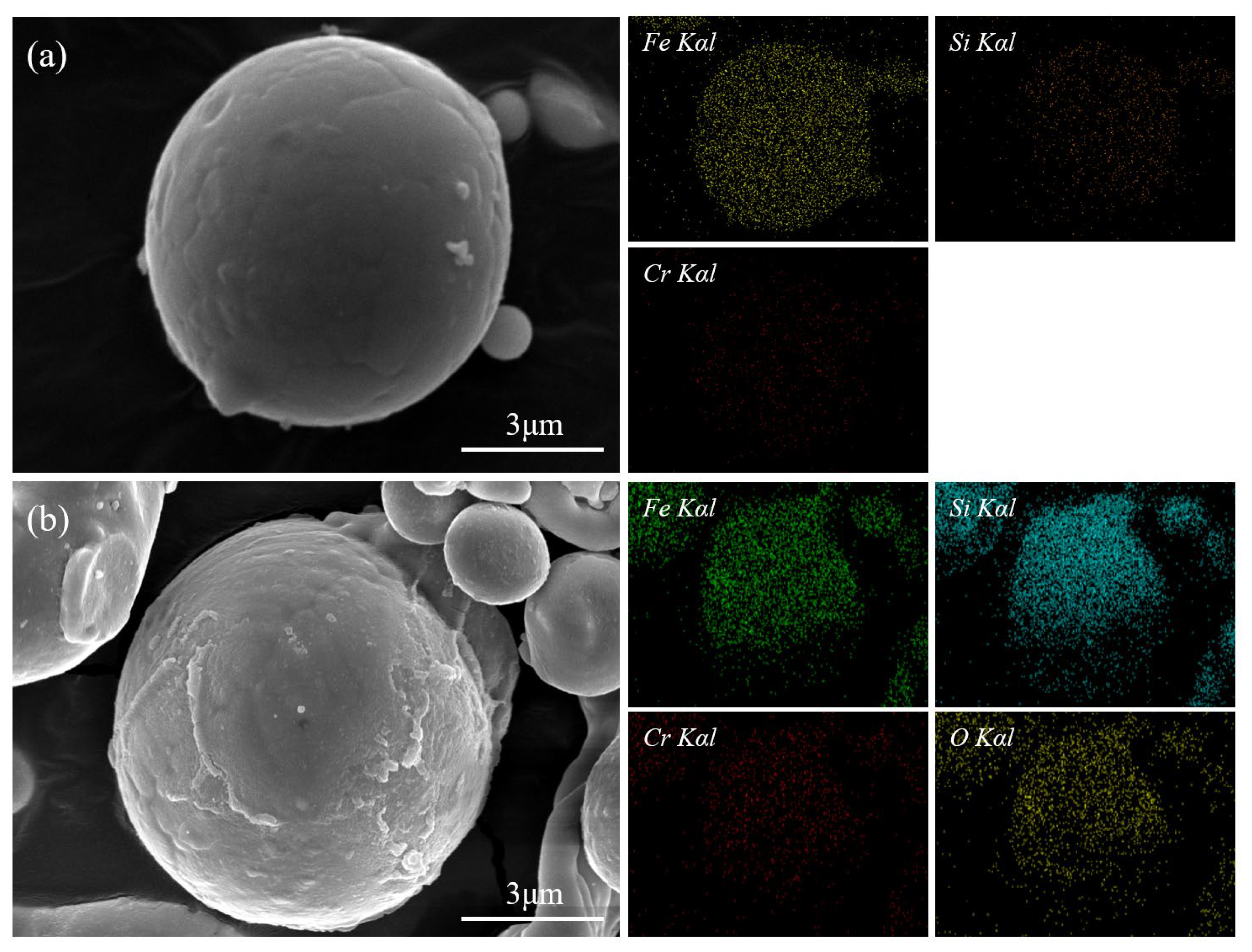
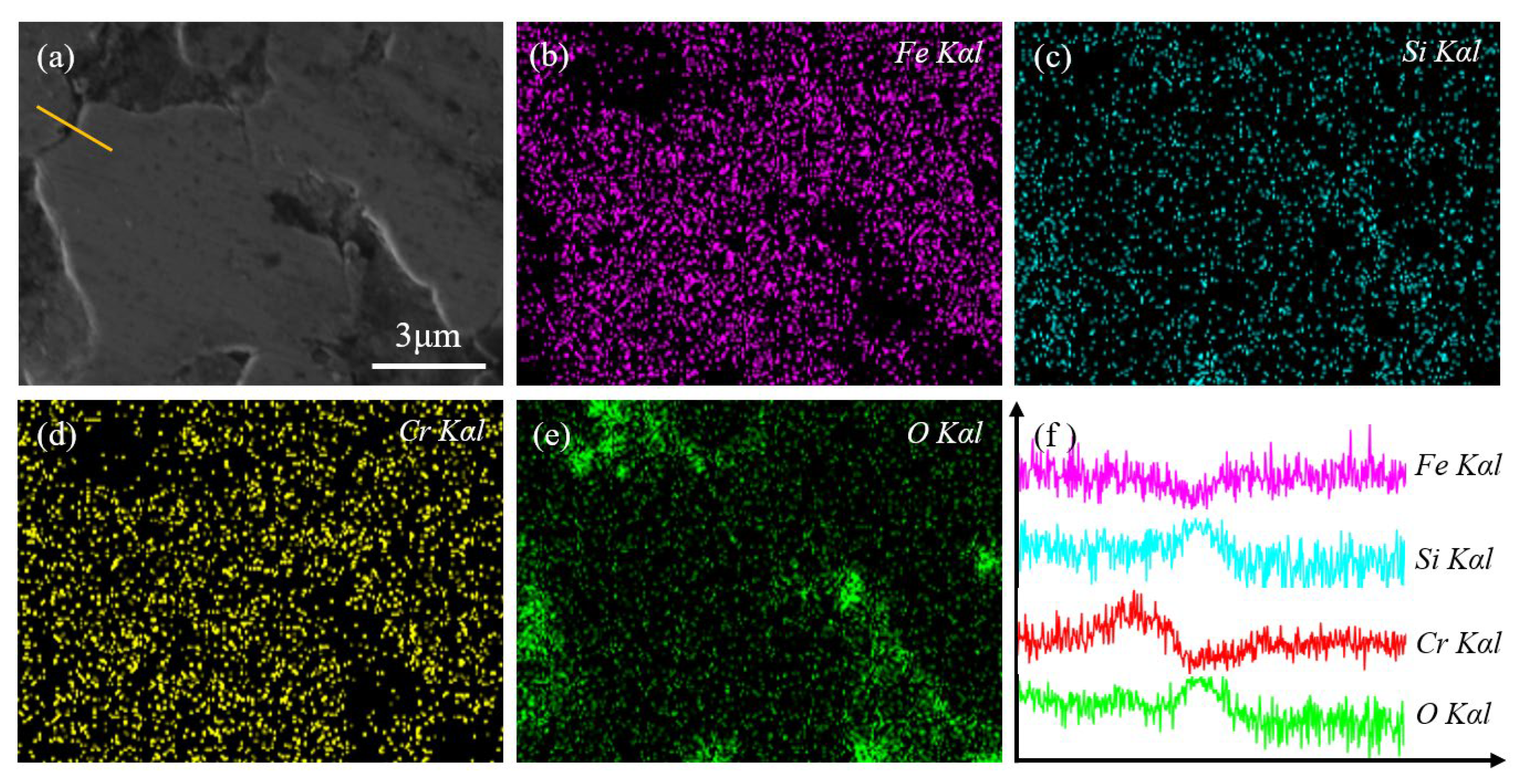
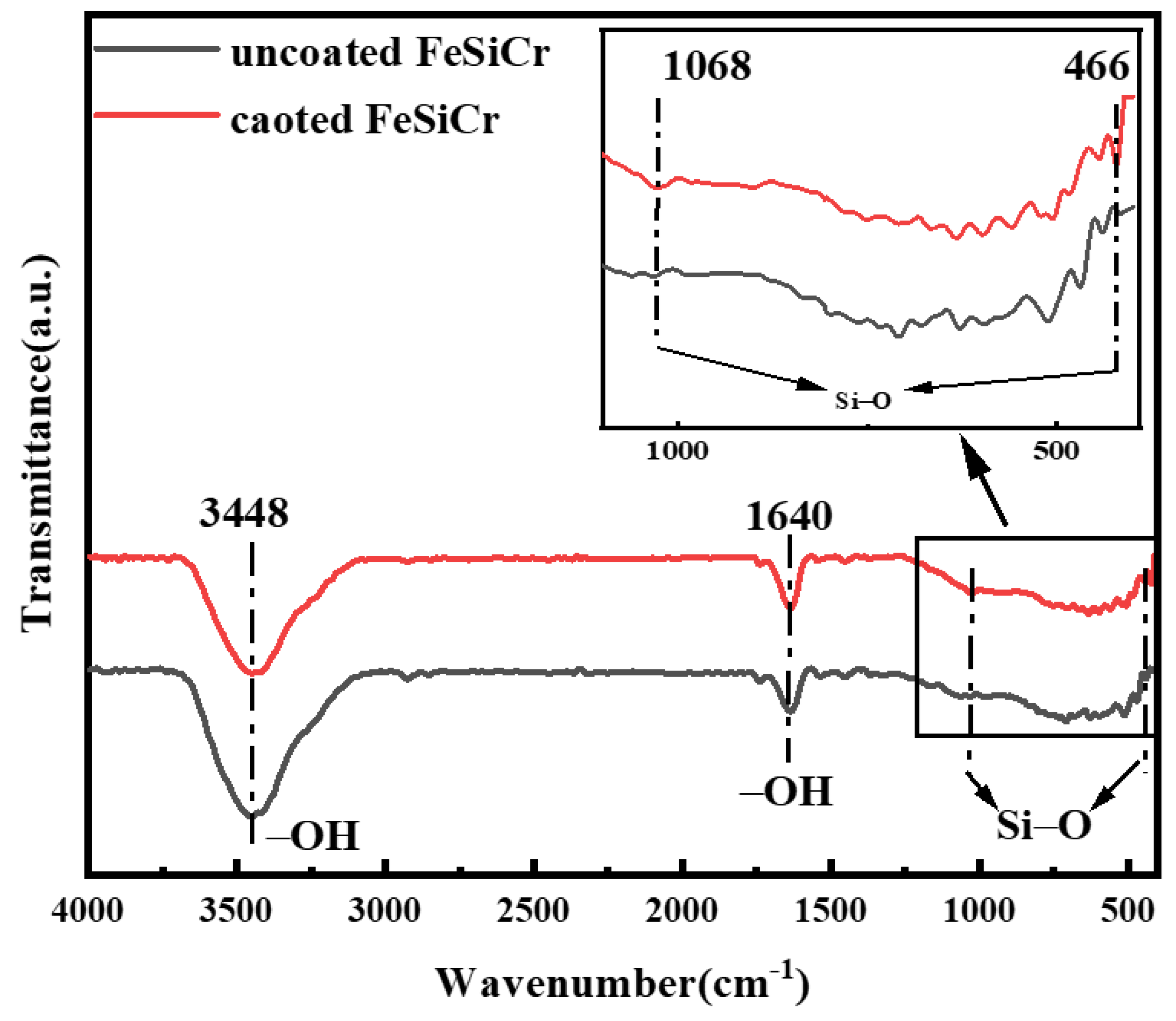
3.1. Structure Analysis
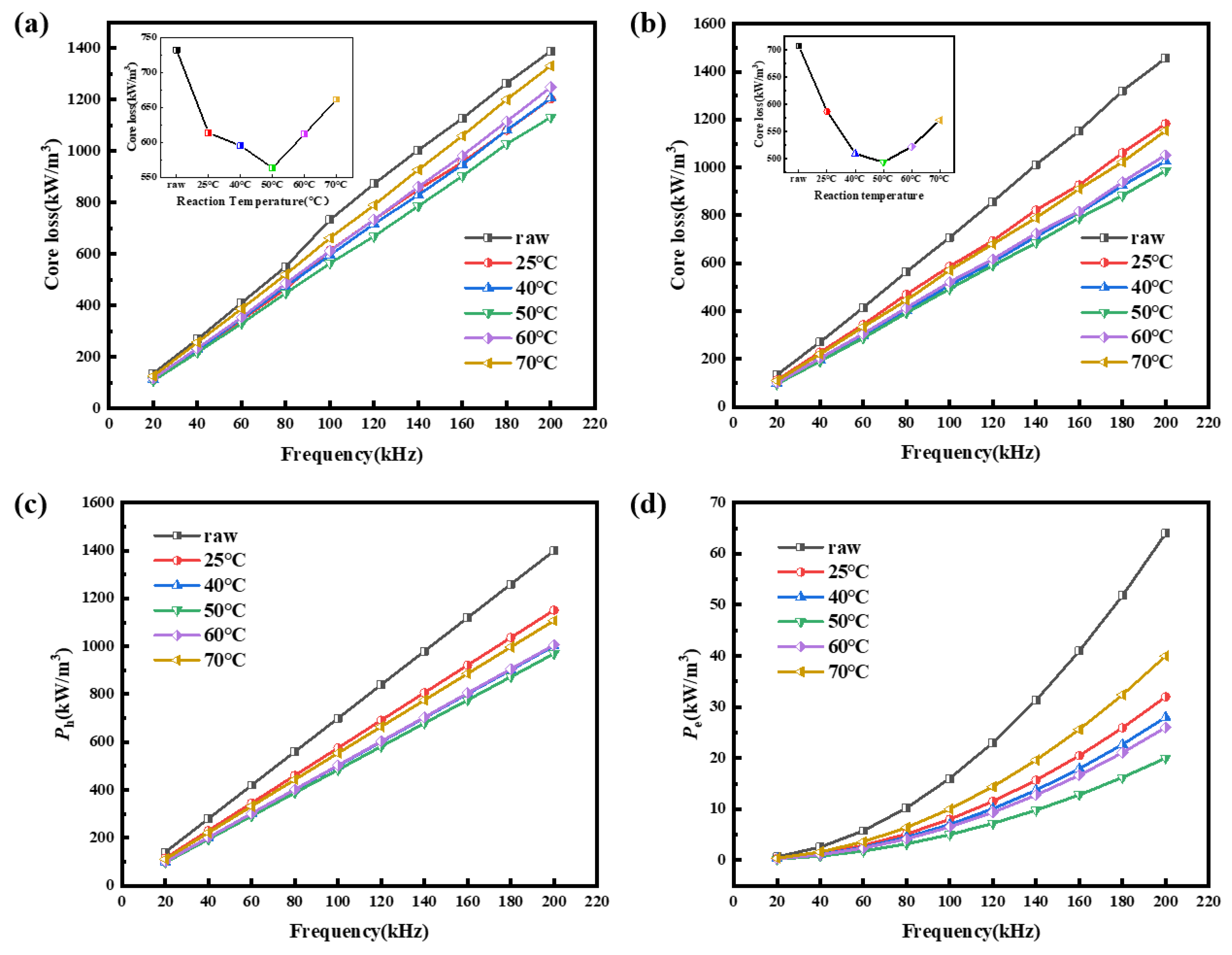
3.2. Effects of Reaction Temperature on Magnetic Properties
3.3. Effects of Ammonia Concentration on Magnetic Properties
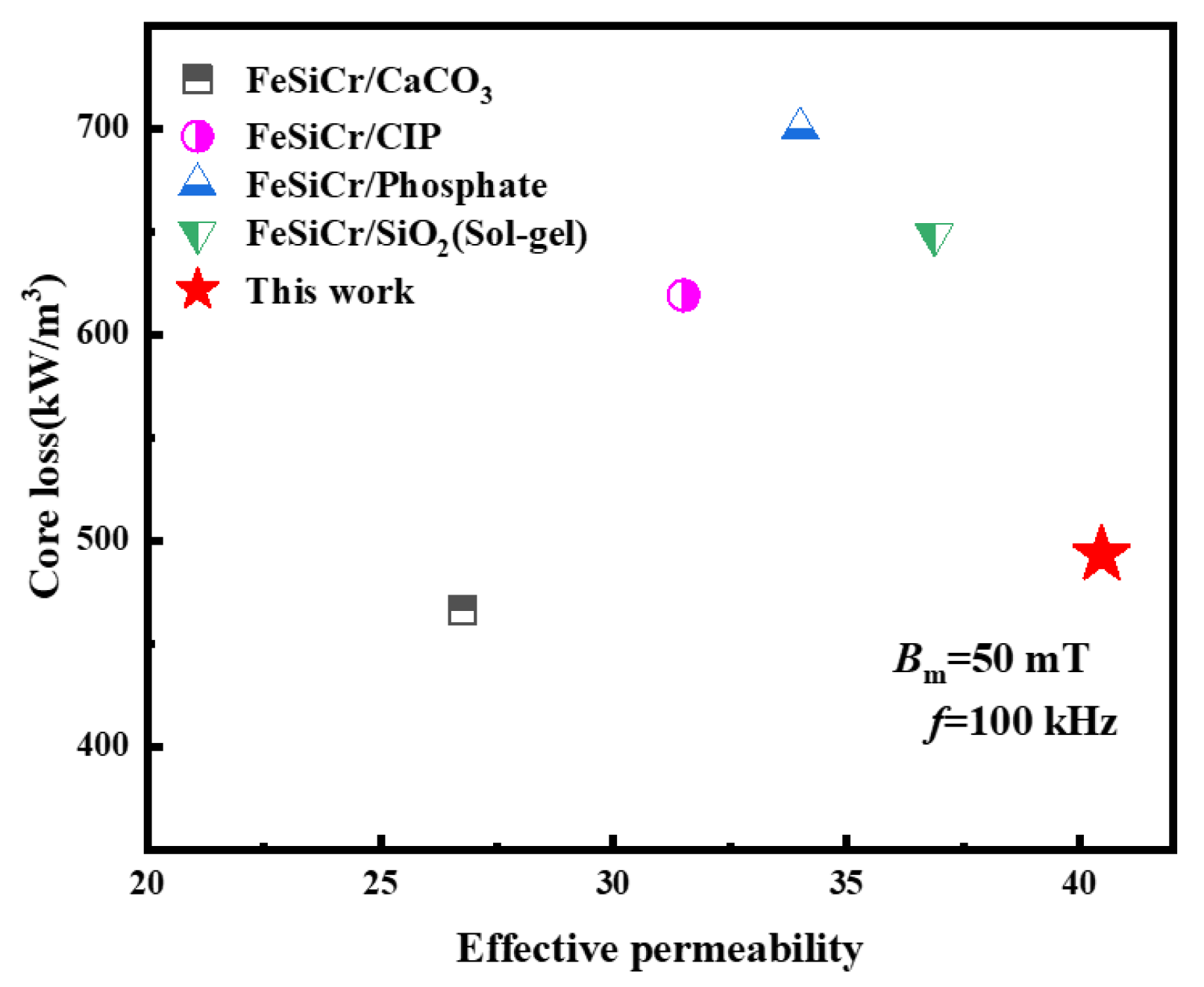
3.4. Comparison of Magnetic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Périgo, E.A.; Weidenfeller, B.; Kollár, P.; Füzer, J. Past, present, and future of soft magnetic composites. Appl. Phys. Rev. 2018, 5, 031301. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, S.; Mao, Y.; Xu, D.; Peng, Y.; Zhao, Y. Microstructure and properties of amorphous FeSiCrBC soft magnetic composites prepared by using HNO3 solution. J. Mater. Sci. Mater. Electron. 2023, 34, 862. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Dong, Y.; Zhang, R.; He, A.; Ling, Y.; Li, J.; Liu, X. Correlation Mechanism of Microstructure, Magnetic Domain, and Magnetic Softness of Fe-6.5 wt.%Si Composites with High Saturation. J. Supercond. Nov. Magn. 2023, 36, 733–743. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, X.; Li, J.; Ellis, T.; Wu, Q.; Xu, J.; Hong, B.; Wang, X.; Ge, H. Design, Preparation, and Magnetic Properties of Fe4N/Fe3N Soft Magnetic Composites Fabricated by Gas Nitridation. J. Supercond. Nov. Magn. 2023, 36, 923–929. [Google Scholar] [CrossRef]
- Dosoudil, R.; Franek, J.; Slama, J.; Usakova, M.; Gruskova, A. Electromagnetic Wave Absorption Performances of Metal Alloy/Spinel Ferrite/Polymer Composites. IEEE Trans. Magn. 2012, 48, 1524–1527. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Gong, W.; Feng, Z.; Gong, R. Electromagnetic properties of Fe-Si-Al/BaTiO3/Nd2Fe14B particulate composites at microwave frequencies. J. Appl. Phys. 2014, 115, 17C722. [Google Scholar] [CrossRef]
- Kim, I.G.; Song, K.H.; Park, B.O.; Choi, B.I.; Choi, H.J. Nano-sized Fe soft-magnetic particle and its magnetorheology. Colloid Polym. Sci. 2011, 289, 79–83. [Google Scholar] [CrossRef]
- Zhu, S.J.; Duan, F.; Feng, S.J.; Liu, X.S.; Kan, X.C.; Lv, Q.R.; Sun, W. Efficient inorganic-coated FeSiAl/WS2 soft magnetic composites with low magnetic loss. J. Alloys Compd. 2023, 936, 168190. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Shang, R.; Wang, K.; Wu, P.; Wang, Y.; Li, F.; Wang, T. Improved magnetic properties in amorphous FeSiBCr soft magnetic composites with easy-plane anisotropy for high-frequency applications. J. Phys. D Appl. Phys. 2023, 56, 065004. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, R.; Sun, H.; Zhang, J.; Wang, J. High density Fe-based soft magnetic composites with nice magnetic properties prepared by warm compaction. J. Alloys Compd. 2023, 947, 169460. [Google Scholar] [CrossRef]
- Garibaldi, M.; Ashcroft, I.; Lemke, J.N.; Simonelli, M.; Hague, R. Effect of annealing on the microstructure and magnetic properties of soft magnetic Fe-Si produced via laser additive manufacturing. Scr. Mater. 2018, 142, 121–125. [Google Scholar] [CrossRef]
- Nie, W.; Yu, T.; Wang, Z.; Wei, X. High-performance core-shell-type FeSiCr@MnZn soft magnetic composites for high-frequency applications. J. Alloys Compd. 2021, 864, 158215. [Google Scholar] [CrossRef]
- Dong, B.; Qin, W.; Su, Y.; Wang, X. Magnetic properties of FeSiCr@MgO soft magnetic composites prepared by magnesium acetate pyrolysis for high-frequency applications. J. Magn. Magn. Mater. 2021, 539, 168350. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Chuang, K.-H.; Lee, W.-H. FeSiCr Alloy Powder to Carbonyl Iron Powder Mixing Ratio Effects on the Magnetic Properties of the Iron-Based Alloy Powder Cores Prepared Using Screen Printing. Materials 2021, 14, 1034. [Google Scholar] [CrossRef]
- Choi, Y.J.; Ahn, J.H.; Kim, D.H.; Kim, Y.R.; Lee, B.W. Core-loss reduction of Fe–Si–Cr crystalline alloy according to particle size in the high frequency band. Curr. Appl. Phys. 2022, 39, 324–330. [Google Scholar] [CrossRef]
- Ren, X.; Corcolle, R.; Daniel, L. Bounds and estimates on eddy current losses in soft magnetic composites. J. Appl. Phys. 2018, 123, 235109. [Google Scholar] [CrossRef] [Green Version]
- Simizu, S.; Ohodnicki, P.R.; McHenry, M.E. Metal Amorphous Nanocomposite Soft Magnetic Material-Enabled High Power Density, Rare Earth Free Rotational Machines. IEEE Trans. Magn. 2018, 54, 1–5. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, S.M.; Koh, J.H. Fe-Si-Cr/PTFE magnetic composite thick films on polyethylene terephthalate sheets for near field communications by aerosol deposition. J. Nanosci. Nanotechnol. 2014, 14, 7915–7918. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, H.-I.; Wang, S.-K.; Chen, C.-C. Electromagnetic properties of FeSiCr alloy powders modified with amorphous SiO2. J. Magn. Magn. Mater. 2020, 514, 167151. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Lu, Z.; Cheng, C.; Gao, X. Magnetic properties of iron-based soft magnetic composites with SiO2 coating obtained by reverse microemulsion method. J. Magn. Magn. Mater. 2015, 381, 451–456. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.A.; Hu, W.; Luo, F.; Li, G.; Li, Y.; Liu, X.; Wang, J. Controllable SiO2 insulating layer and magnetic properties for intergranular insulating Fe-6.5 wt.%Si/SiO2 composites. Adv. Powder Technol. 2019, 30, 538–543. [Google Scholar] [CrossRef]
- Cheng, J.; Ni, X.; Zheng, H.; Li, B.; Zhang, X.; Zhang, D. Preparation of Fe (core)/SiO2 (shell) composite particles with improved oxidation-resistance. Mater. Res. Bull. 2006, 41, 1424–1429. [Google Scholar] [CrossRef]
- Lim, H.M.; Lee, J.; Jeong, J.-H.; Oh, S.-G.; Lee, S.-H. Comparative Study of Various Preparation Methods of Colloidal Silica. Engineering 2010, 2, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Liu, X.; Cheng, Y.; Li, Y.; Xu, G.; Cui, P. Size-controllable synthesis of monodispersed colloidal silica nanoparticles via hydrolysis of elemental silicon. J. Colloid Interface Sci. 2008, 326, 138–142. [Google Scholar] [CrossRef]
- Lim, H.M.; Shin, H.C.; Huh, S.H.; Lee, S.H. Effect of Catalyst on the Colloidal Silica Particle Growth in Direct Oxidation of Silicon. Solid State Phenom. 2007, 124–126, 667–670. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Zhao, H.; Wan, J.; Chen, K. Synthesis of magnetite–silica core–shell nanoparticles via direct silicon oxidation. J. Colloid Interface Sci. 2014, 432, 43–46. [Google Scholar] [CrossRef]
- Wu, C.; Huang, M.; Luo, D.; Jiang, Y.; Yan, M. SiO2 nanoparticles enhanced silicone resin as the matrix for Fe soft magnetic composites with improved magnetic, mechanical and thermal properties. J. Alloys Compd. 2018, 741, 35–43. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Wang, J.; Sun, H.; Chen, D.; Chen, W.; Liu, X. Industry-oriented Fe-based amorphous soft magnetic composites with SiO2-coated layer by one-pot high-efficient synthesis method. J. Magn. Magn. Mater. 2020, 509, 166924. [Google Scholar] [CrossRef]
- Olekšáková, D.; Kollár, P.; Jakubčin, M.; Füzer, J.; Tkáč, M.; Slovenský, P.; Bureš, R.; Fáberová, M. Energy loss separation in NiFeMo compacts with smoothed powders according to Landgraf’s and Bertotti’s theories. J. Mater. Sci. 2021, 56, 12835–12844. [Google Scholar] [CrossRef]
- Olekšáková, D.; Kollár, P.; Neslušan, M.; Jakubčin, M.; Füzer, J.; Bureš, R.; Fáberová, M. Impact of the Surface Irregularities of NiFeMo Compacted Powder Particles on Irreversible Magnetization Processes. Materials 2022, 15, 8937. [Google Scholar] [CrossRef]
- Shokrollahi, H.; Janghorban, K. Soft magnetic composite materials (SMCs). J. Mater. Process. Technol. 2007, 189, 1–12. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Jiang, Z.; Fan, X.A.; Zhou, L.J.; Wang, W.L.; Xu, K. Facile synthesis of Fe-6.5 wt%Si/SiO2 soft magnetic composites as an efficient soft magnetic composite material at medium and high frequencies. J. Alloys Compd. 2018, 742, 90–98. [Google Scholar] [CrossRef]
- Chang, C.; Guo, J.; Li, Q.; Zhou, S.; Liu, M.; Dong, Y. Improvement of soft magnetic properties of FeSiBPNb amorphous powder cores by addition of FeSi powder. J. Alloys Compd. 2019, 788, 1177–1181. [Google Scholar] [CrossRef]
- Talaat, A.; Suraj, M.V.; Byerly, K.; Wang, A.; Wang, Y.; Lee, J.K.; Ohodnicki, P.R. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloys Compd. 2021, 870, 159500. [Google Scholar] [CrossRef]
- Kollár, P.; Birčáková, Z.; Füzer, J.; Bureš, R.; Fáberová, M. Power loss separation in Fe-based composite materials. J. Magn. Magn. Mater. 2013, 327, 146–150. [Google Scholar]
- Ammar, M.; Mazaleyrat, F.; Bonnet, J.P.; Audebert, P.; Brosseau, A.; Wang, G.; Champion, Y. Synthesis and characterization of core–shell structure silica-coated Fe29.5Ni70.5 nanoparticles. Nanotechnology 2007, 18, 285606. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fan, X.A.; Wu, Z.; Li, G. Regulation and control of insulated layers for intergranular insulated Fe/SiO2 soft magnetic composites. J. Mater. Sci. 2017, 52, 7091–7099. [Google Scholar] [CrossRef]
- Lu, X.; Liang, G.; Sun, Q.; Yang, C. High-frequency magnetic properties of FeNi3–SiO2 nanocomposite synthesized by a facile chemical method. J. Alloys Compd. 2011, 509, 5079–5083. [Google Scholar] [CrossRef]
- Fan, X.A.; Wang, J.; Wu, Z.; Li, G. Core–shell structured FeSiAl/SiO2 particles and Fe3Si/Al2O3 soft magnetic composite cores with tunable insulating layer thicknesses. Mater. Sci. Eng. B 2015, 201, 79–86. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Y.; Chang, L.; Pan, Y.; Chi, Q.; Gong, M.; Li, J.; He, A.; Wang, X. High performance of Fe-based soft magnetic composites coated with novel nano-CaCO3/epoxy nanocomposites insulating layer. J. Solid State Chem. 2021, 304, 122634. [Google Scholar] [CrossRef]
- Gong, M.; Dong, Y.; Huang, J.; Chang, L.; Pan, Y.; Wang, F.; He, A.; Li, J.; Liu, X.; Wang, X. The enhanced magnetic properties of FeSiCr powder cores composited with carbonyl iron powder. J. Mater. Sci. Mater. Electron. 2021, 32, 8829–8836. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, S.; Zhang, G.; Dong, B.; Meng, L.; Li, Z.; Dong, Y.; Cao, X. The phosphating effect on the properties of FeSiCr alloy powder. J. Magn. Magn. Mater. 2022, 552, 168741. [Google Scholar] [CrossRef]



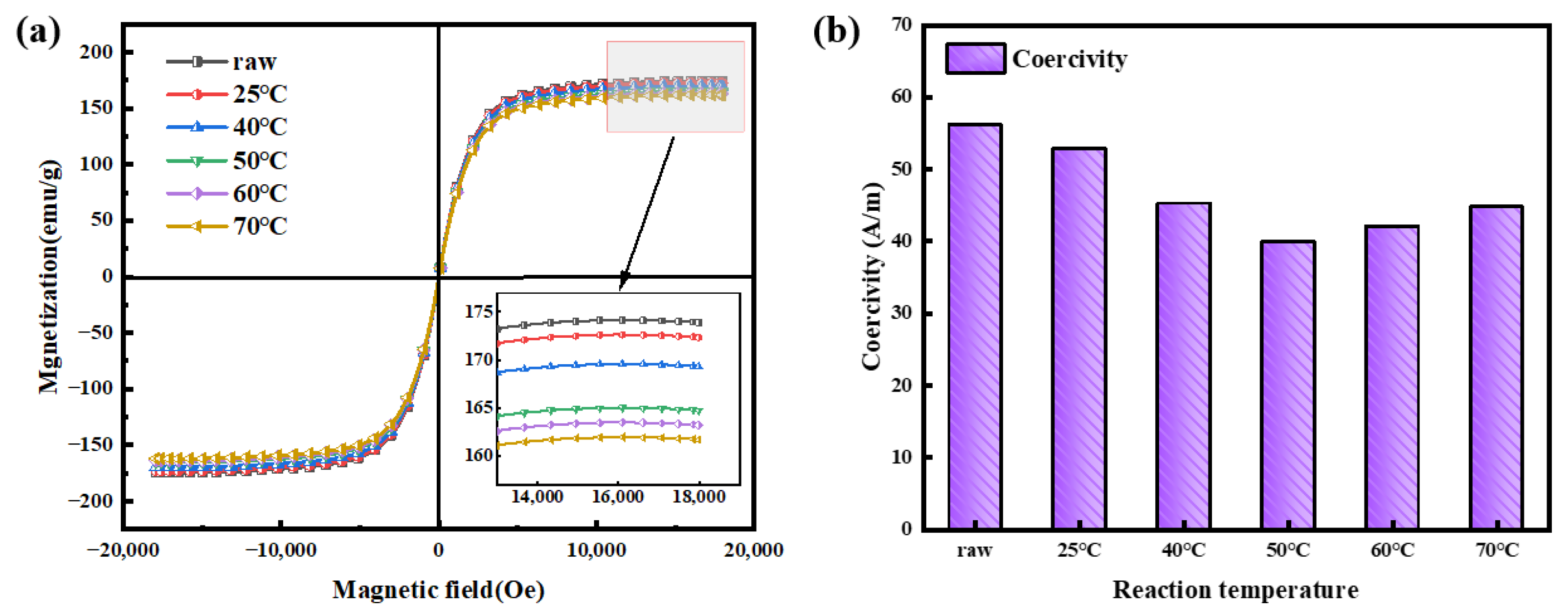
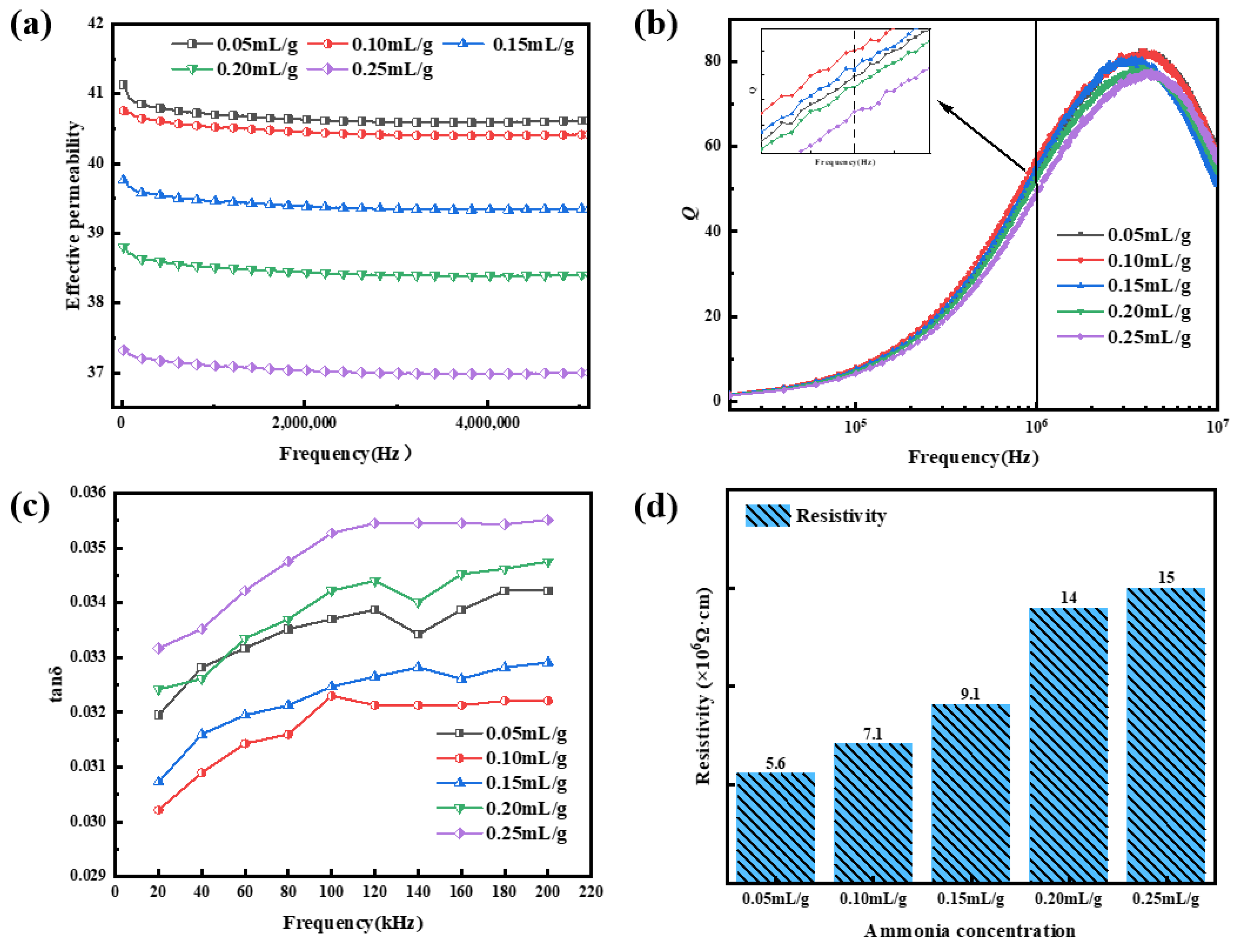
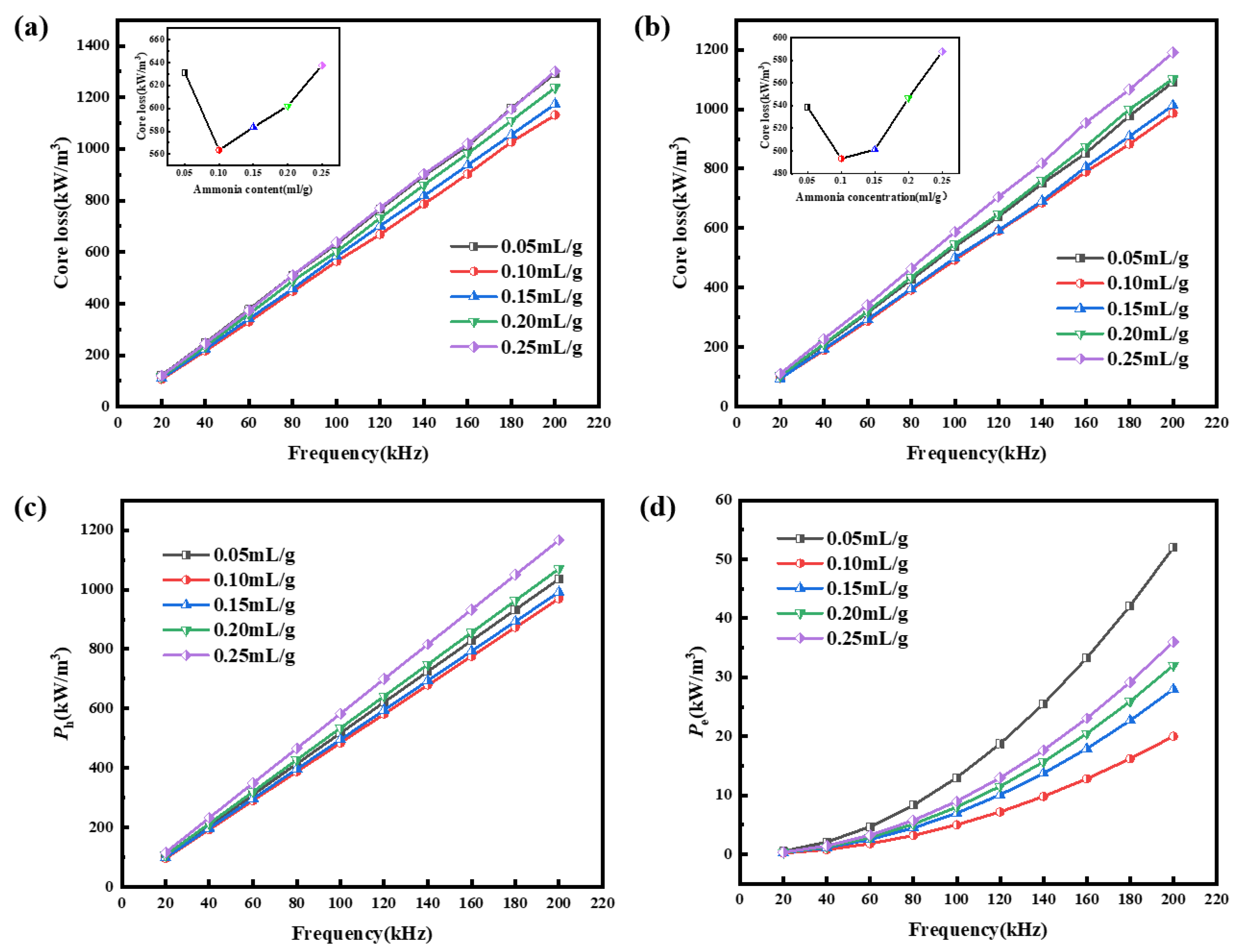
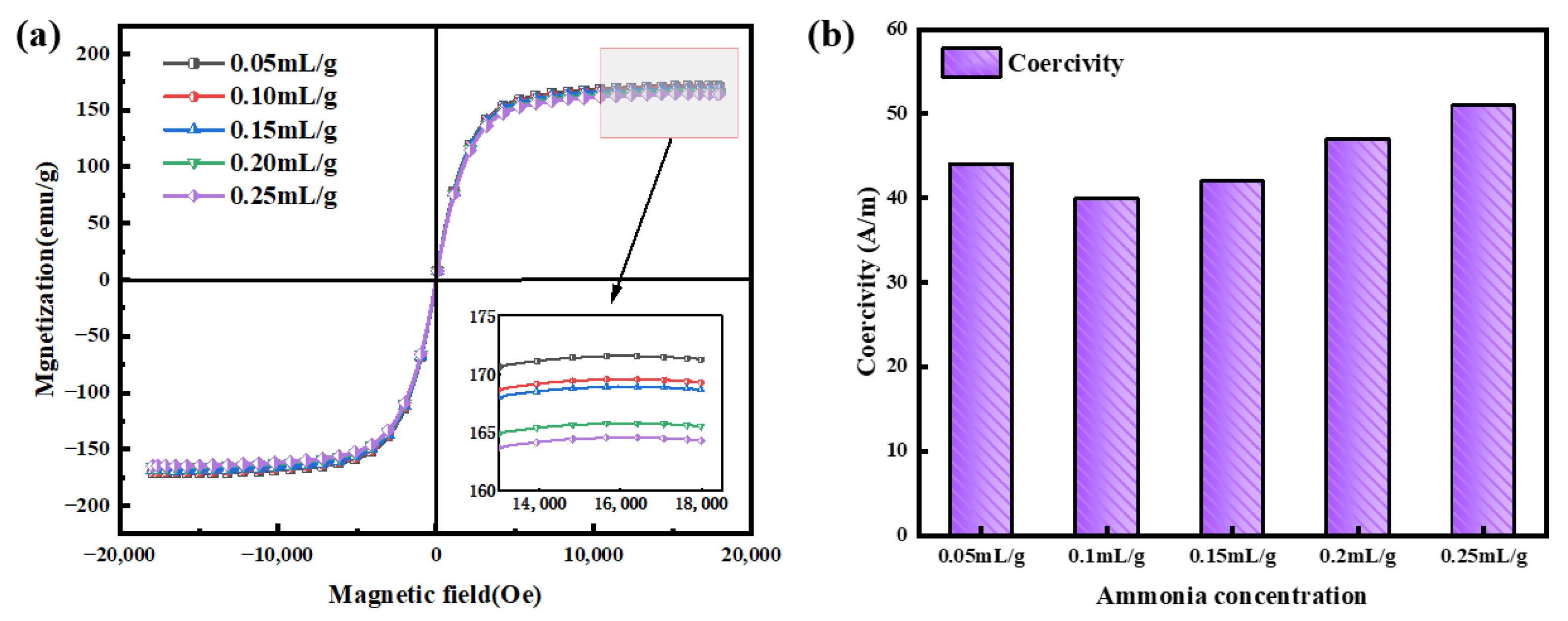
| Sample | ρ (Ω·cm) | μe (1 MHz) | Q (1 MHz) | Ps (kW/m3) @ 50 mT | |
|---|---|---|---|---|---|
| 100 kHz | 200 kHz | ||||
| raw | 1.5 × 105 | 40.73 | 46.70 | 707.4 | 1457 |
| 25 °C | 2.0 × 105 | 40.63 | 49.89 | 587.1 | 1183 |
| 40 °C | 5.0 × 106 | 40.59 | 54.09 | 509.4 | 1027 |
| 50 °C | 7.1 × 106 | 40.46 | 57.06 | 493.3 | 987 |
| 60 °C | 4.3 × 107 | 36.59 | 52.15 | 522.1 | 1052 |
| 70 °C | 4.4 × 107 | 33.66 | 51.11 | 571.1 | 1154 |
| Sample | ρ (Ω·cm) | μe (1 MHz) | Q (1 MHz) | Ps (kW/m3) @ 50 mT | |
|---|---|---|---|---|---|
| 100 kHz | 200 kHz | ||||
| 0.05 mL/g | 5.6 × 106 | 40.55 | 53.71 | 538.8 | 1091 |
| 0.10 mL/g | 7.1 × 106 | 40.46 | 57.06 | 493.3 | 987 |
| 0.15 mL/g | 9.0 × 106 | 39.45 | 54.58 | 501.2 | 1013 |
| 0.20 mL/g | 1.4 × 107 | 37.10 | 52.38 | 546.7 | 1102 |
| 0.25 mL/g | 1.5 × 107 | 35.50 | 49.57 | 587.8 | 1190 |
| Sample | ρ (Ω·cm) | μe (1 MHz) | Q (1 MHz) | Ps (kW/m3) @ 50 mT | ||
|---|---|---|---|---|---|---|
| 100 kHz | 200 kHz | 300 kHz | ||||
| Sol-gel | 8.3 × 107 | 33.96 | 60.07 | 648.2 | 1270 | 2022 |
| This work | 7.1 × 106 | 40.46 | 57.07 | 493.3 | 987 | 1650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yu, H.; Han, G.; Liu, Z. FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method. Metals 2023, 13, 1444. https://doi.org/10.3390/met13081444
Li C, Yu H, Han G, Liu Z. FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method. Metals. 2023; 13(8):1444. https://doi.org/10.3390/met13081444
Chicago/Turabian StyleLi, Chunlong, Hongya Yu, Guangze Han, and Zhongwu Liu. 2023. "FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method" Metals 13, no. 8: 1444. https://doi.org/10.3390/met13081444
APA StyleLi, C., Yu, H., Han, G., & Liu, Z. (2023). FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method. Metals, 13(8), 1444. https://doi.org/10.3390/met13081444








