In Situ Study the Grooving Effect Induced by Ag Particles on Rapid Growth of Cu6Sn5 Grain at Sn-xAg/Cu Soldering Interface during the Heat Preservation Stage
Abstract
:1. Introduce
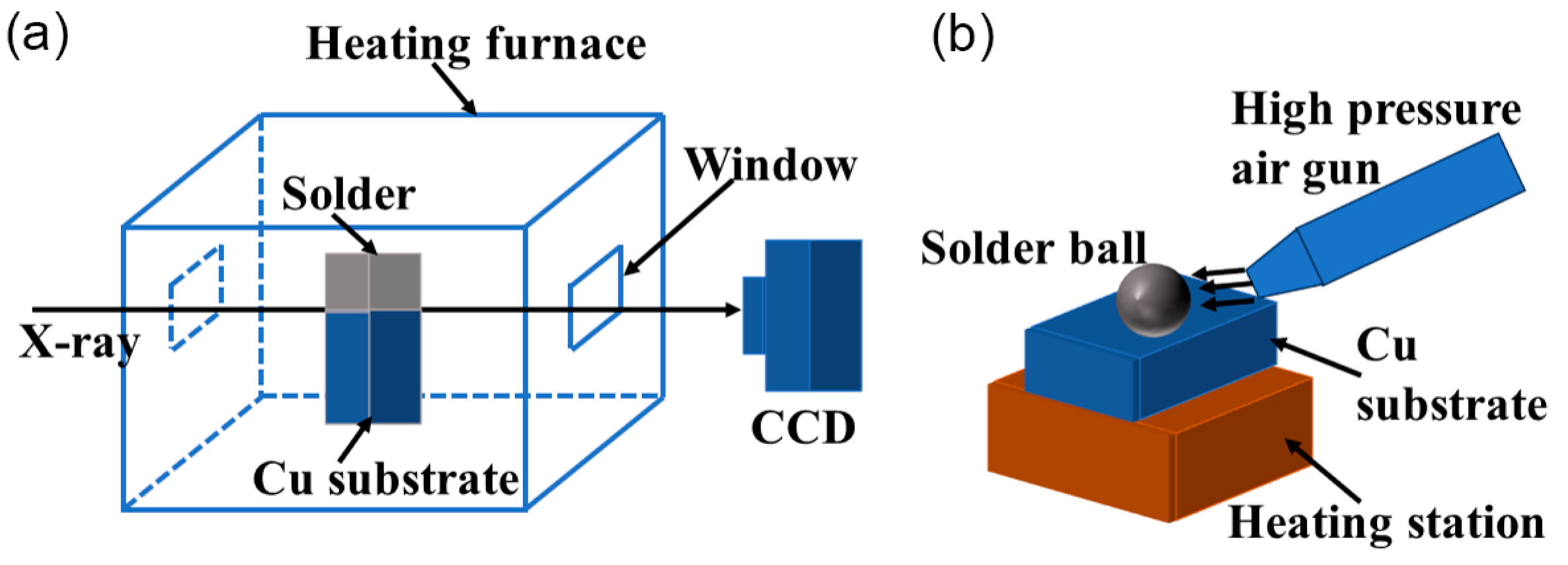
2. Experiment and Methods
3. Results and Discussion
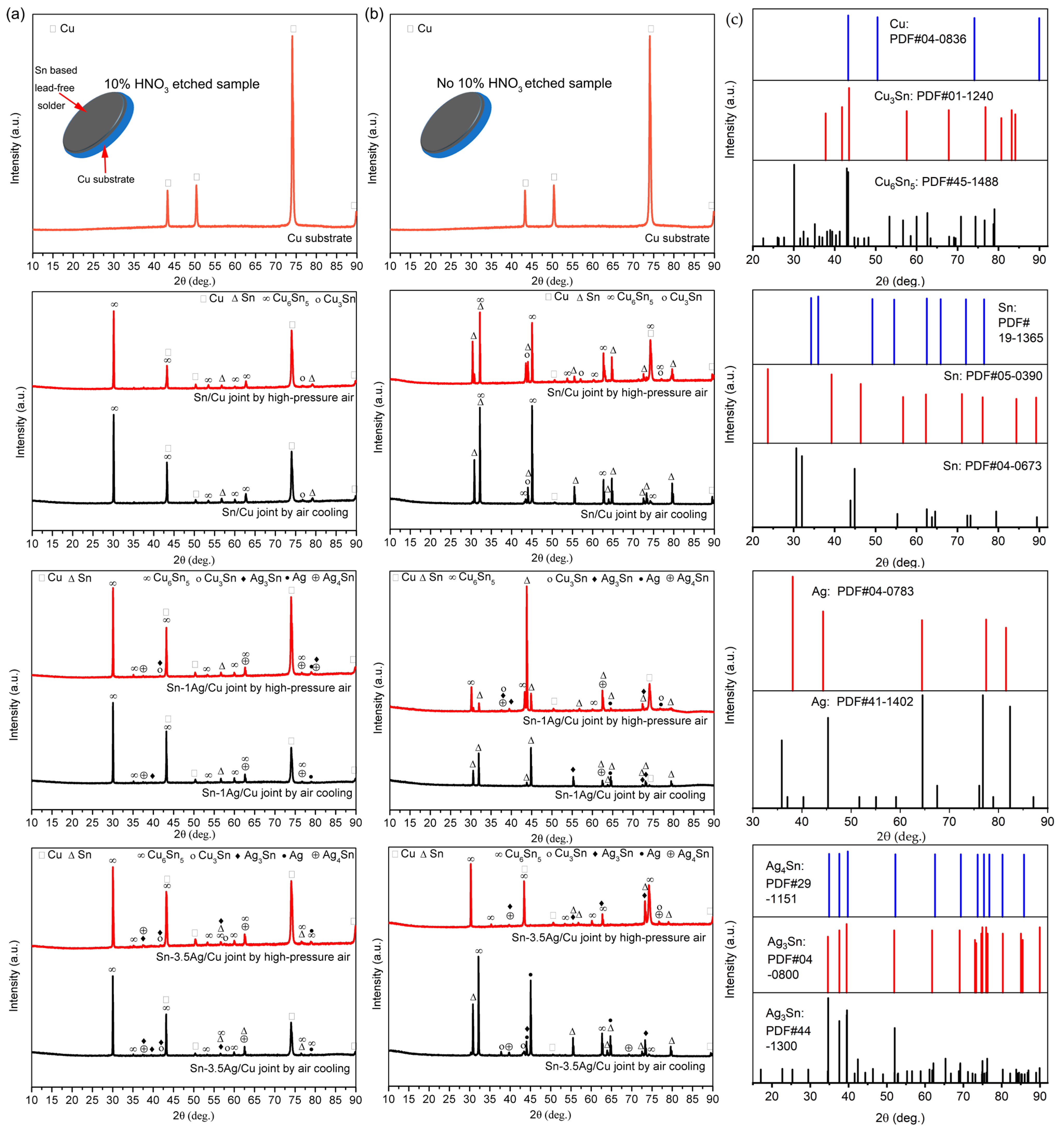
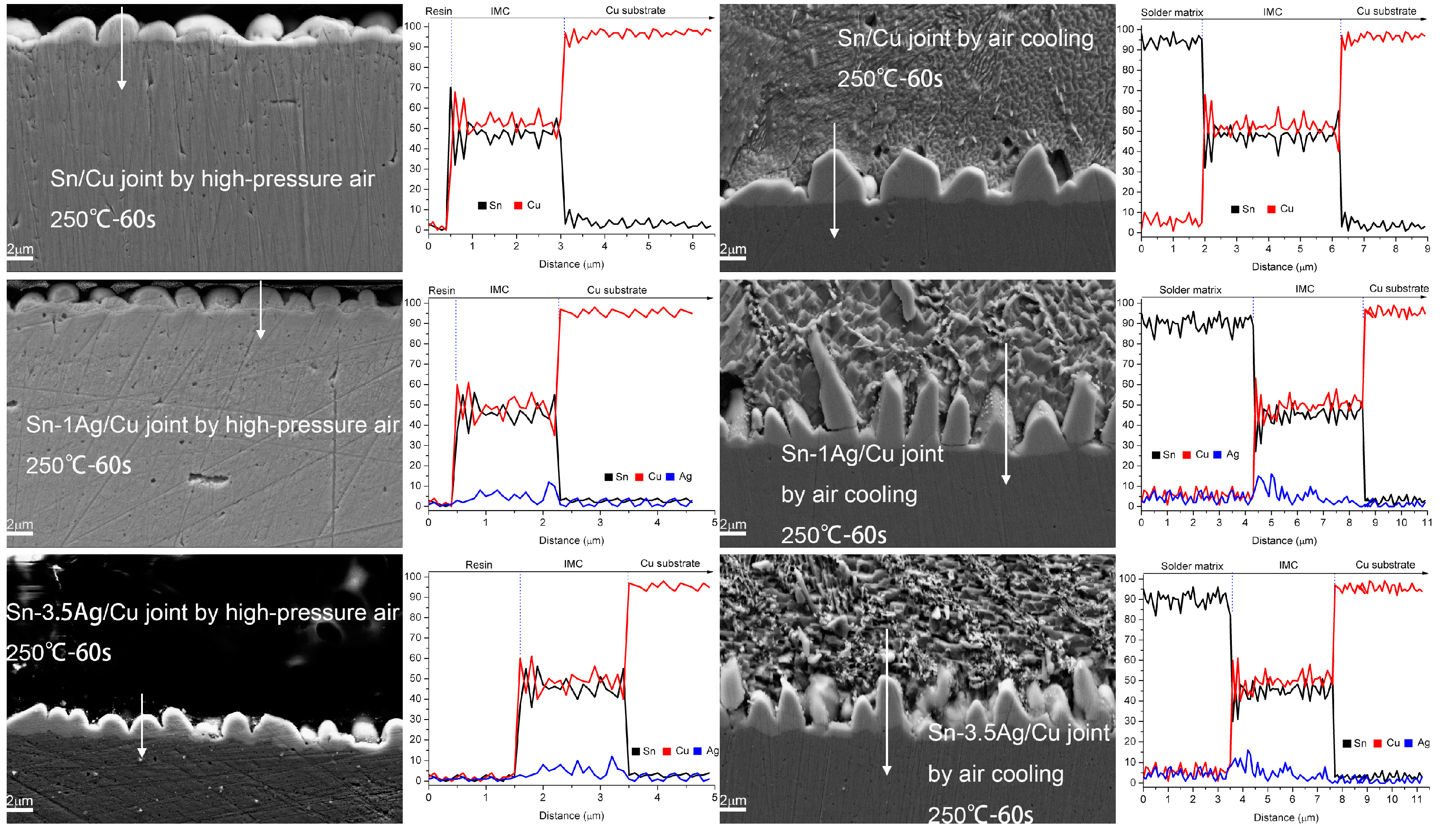
3.1. Analysis of Intermetallic Phases and Its Composition Soldering Joint Interface
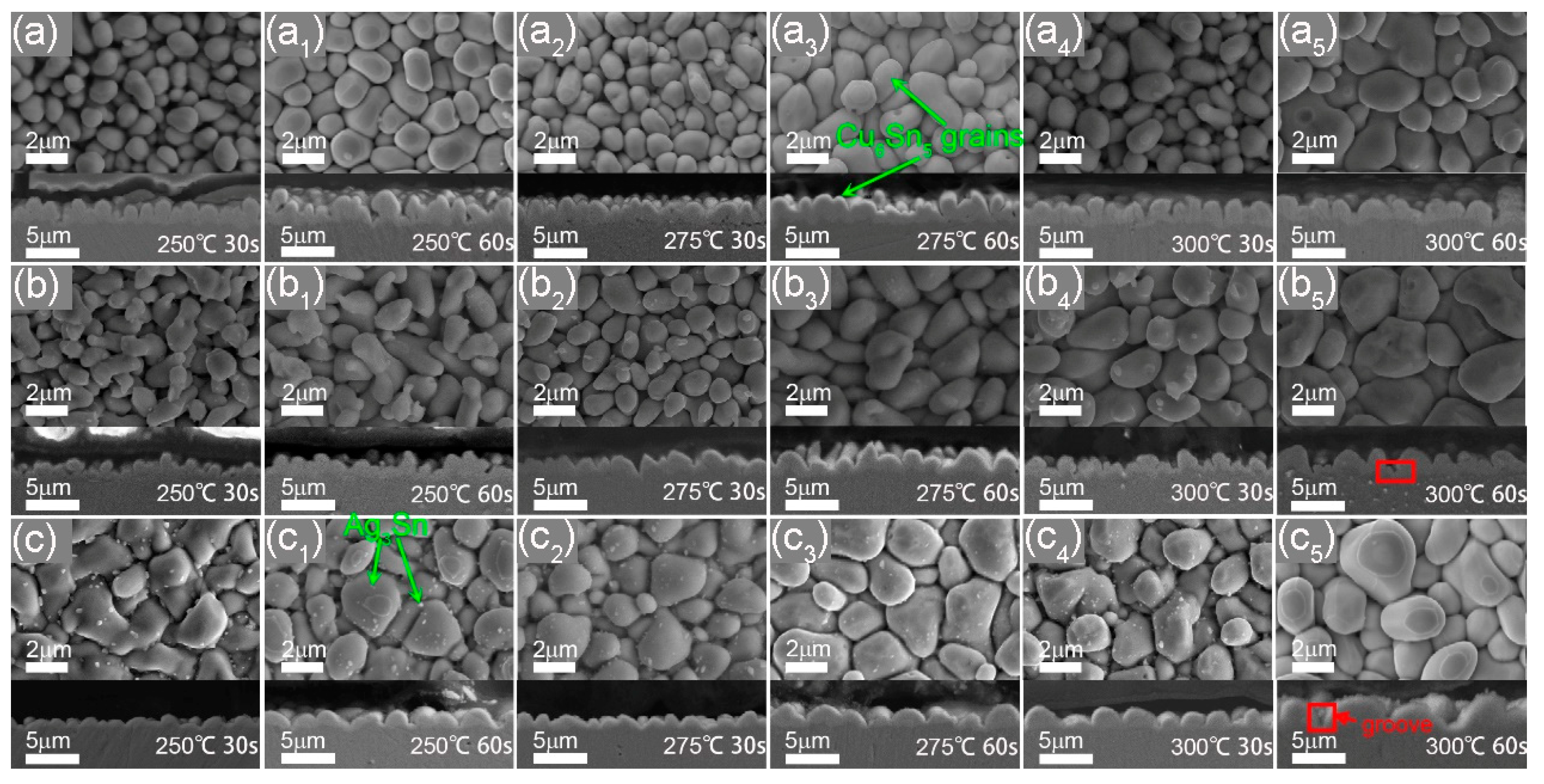
3.2. Microstructure of Interface IMC during the Heat Preservation Stage
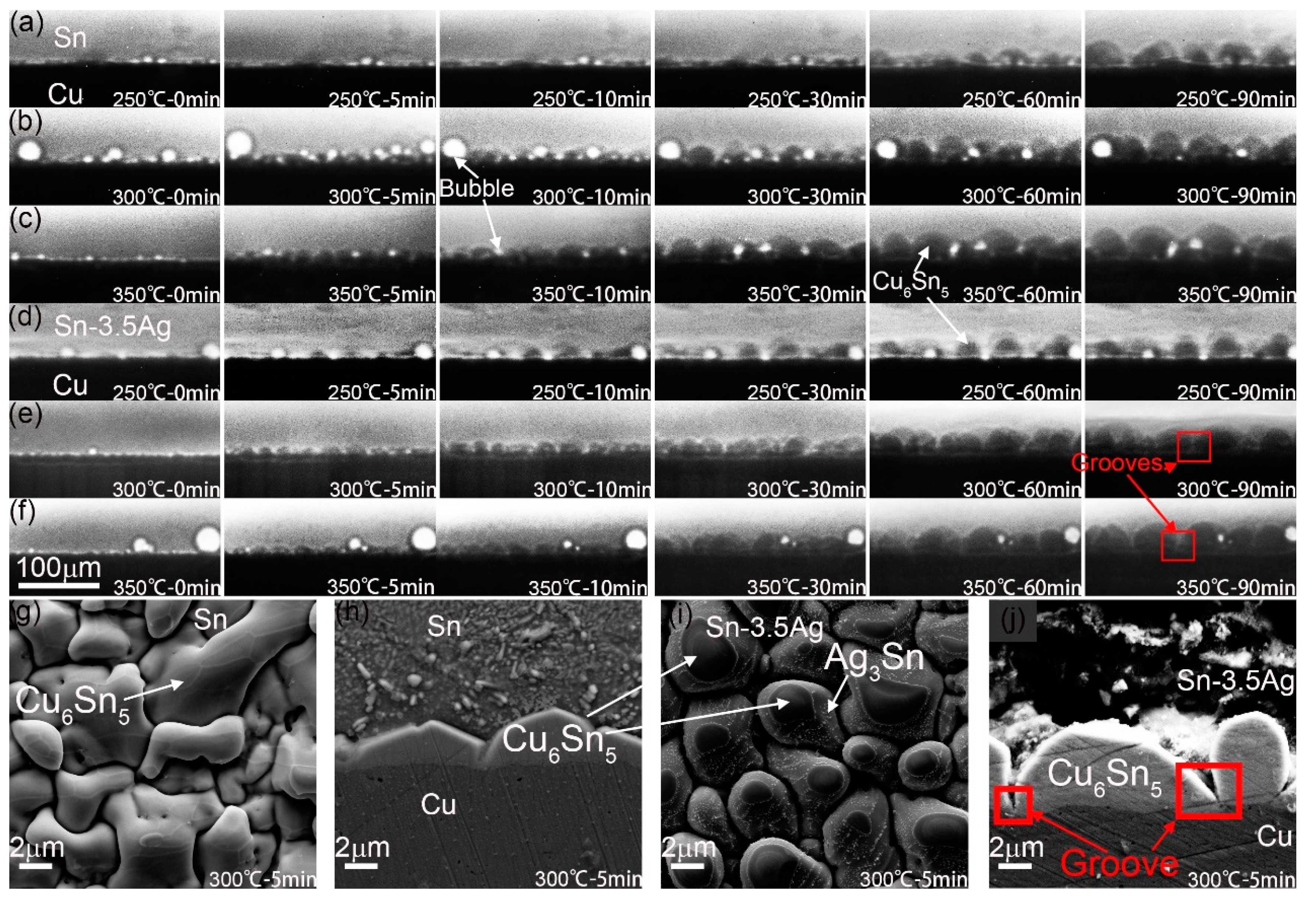
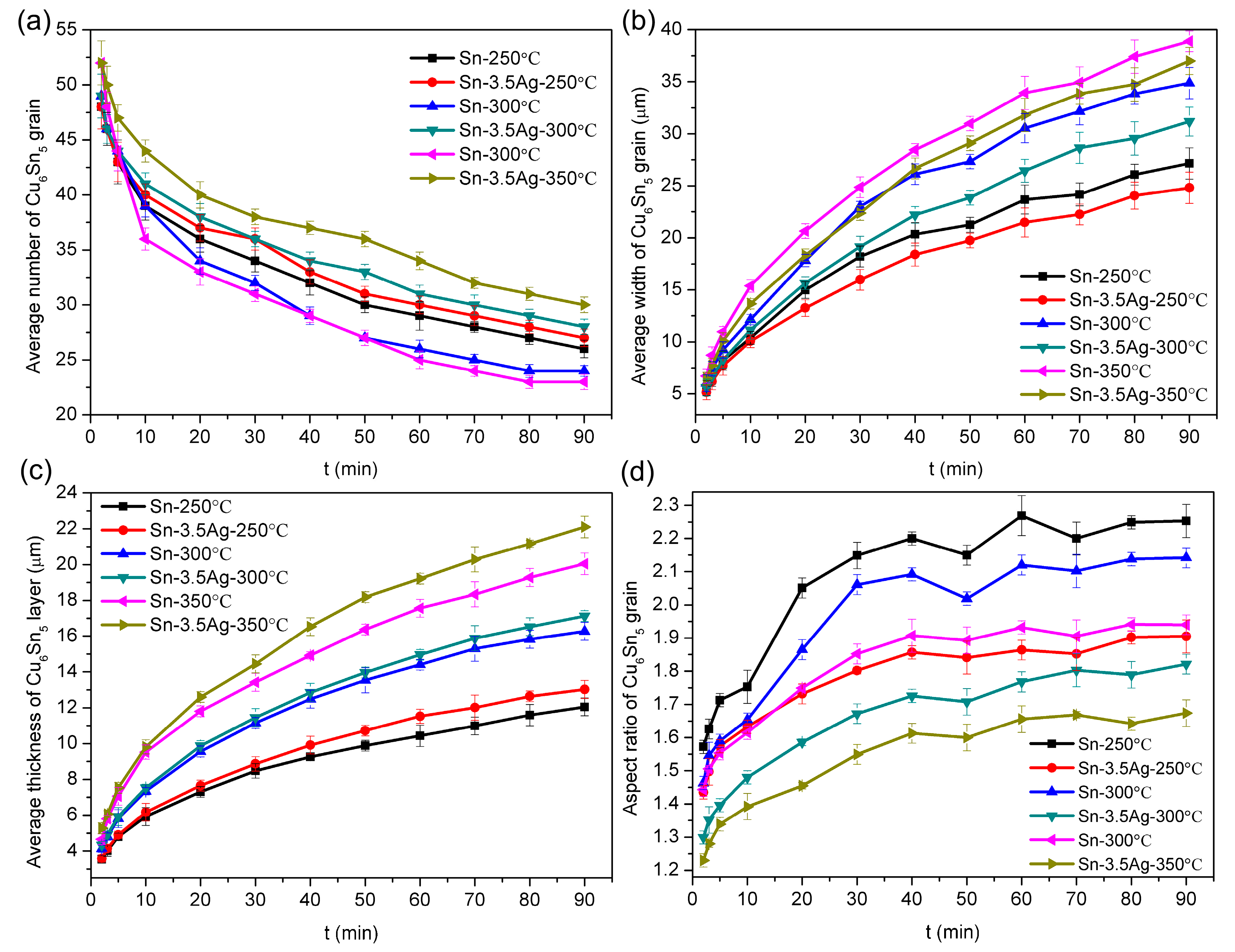
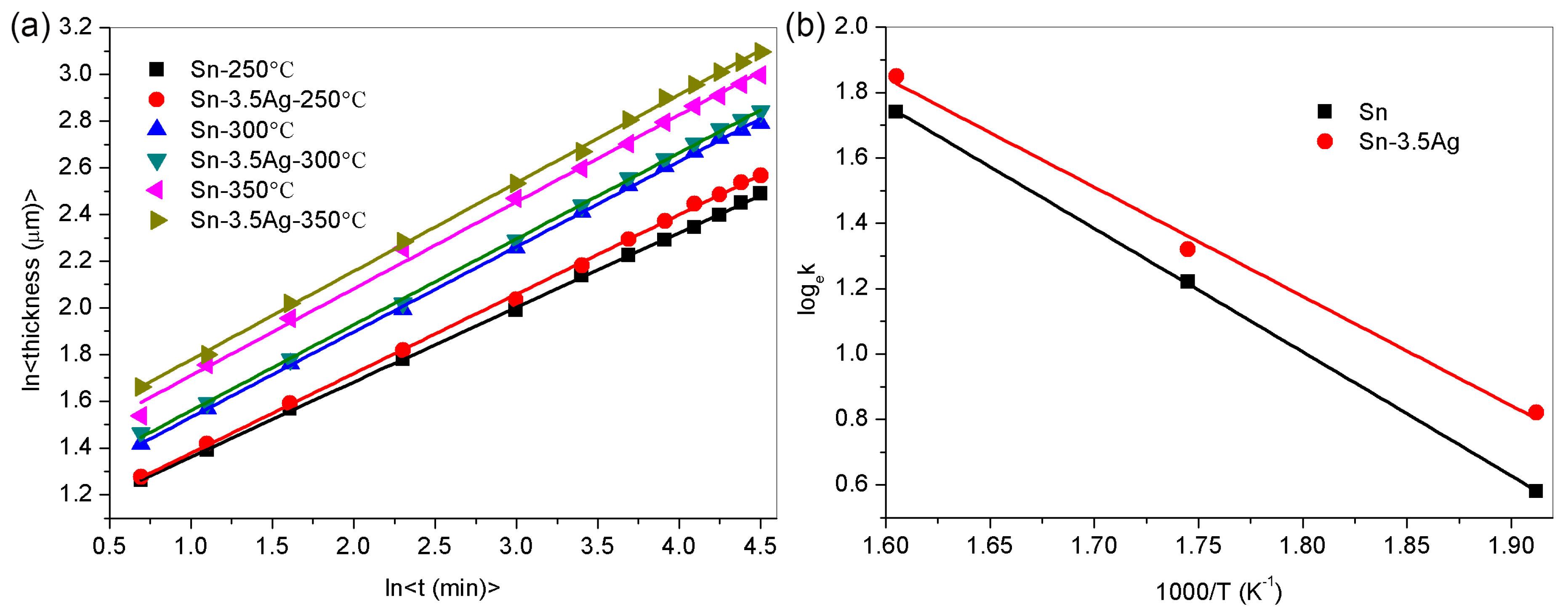
3.3. Growth Dynamics Analysis of Interface IMC during the Heat Preservation Stage
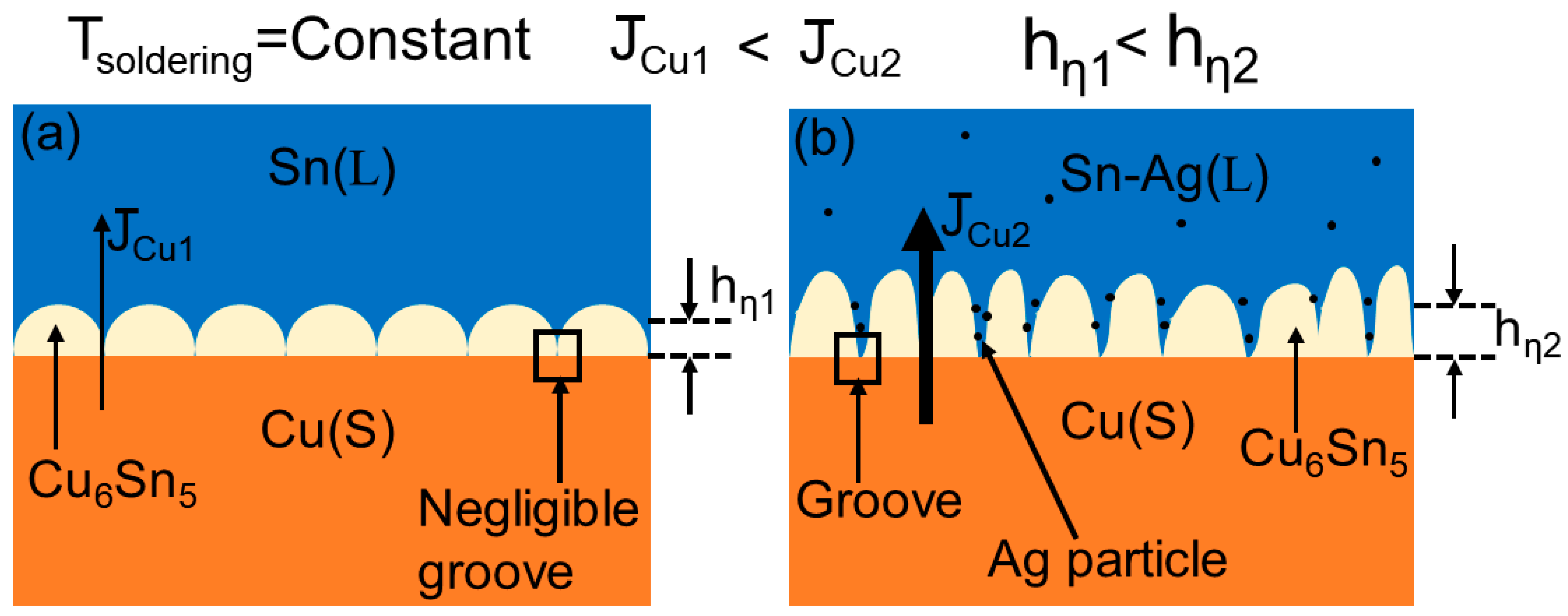
3.4. Grooving Effect of Ag Particles on the Growth Mechanism of Interface IMC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, G.; Son, K.; Lee, J.H.; Joo, Y.C.; Park, Y.B. Size effect on the electromigration characteristics of flip chip pb-free solder bumps. Electron. Mater. Lett. 2022, 18, 431–439. [Google Scholar] [CrossRef]
- Guo, B.F.; Kunwar, A.; Zhao, N.; Chen, J.; Wang, Y.P.; Ma, H.T. Effect of Ag3Sn nanoparticles and temperature on Cu6Sn5 IMC growth in Sn-xAg/Cu solder joints. Mater. Res. Bull. 2018, 99, 239–248. [Google Scholar] [CrossRef]
- Ma, H.T.; Qu, L.; Huang, M.L.; Zhao, N.; Wang, L. In-Situ study on growth behavior of Ag3Sn in Sn-3.5Ag/Cu soldering reaction by synchrotron radiation real-time imaging technology. J. Alloys Compd. 2012, 537, 286–290. [Google Scholar] [CrossRef]
- Ma, H.R.; Kunwar, A.; Guo, B.F.; Sun, J.H.; Jiang, C.R.; Wang, Y.P.; Song, X.G.; Zhao, N.; Ma, H.T. Effect of cooling condition and Ag on the growth of intermetallic compounds in Sn-based solder joints. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1052. [Google Scholar] [CrossRef]
- Kunwar, A.; Ma, H.R.; Ma, H.T.; Sun, J.H.; Zhao, N.; Huang, M.L. On the increase of intermetallic compound’s thickness at the cold side in liquid Sn and SnAg solders under thermal gradient. Mater. Lett. 2016, 172, 211–215. [Google Scholar] [CrossRef]
- Cabrales, H.D.N.; Arroyo, L.O.; Silva, I.C.; Caballero, I.M.; Pardavé, M.P.; González, J.A.; Borja, R.P.P. Influence of TiO2 nanoparticles content as reinforce material to enhance the mechanical and corrosion resistance properties of Sn and Sn-Ag alloy for dental applications. J. Mech. Behav. Biomed. 2023, 140, 105687. [Google Scholar] [CrossRef]
- Yakymovych, A.; Shtablavyi, I. Effect of nanosized Ni reinforcements on the structure of the Sn-3.0Ag-0.5Cu alloy in liquid and after-reflow solid states. Metals 2023, 13, 1093. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Wang, J.Y.; Wu, C.Y.; Chiu, C.Y.; Lee, C.H.; Huang, B.R.; Fu, K.L.; Chang, J.S.; Yen, T.H.; Lee, Y.F.; et al. Development of growth model on interfacial intermetallic compound at circular Cu/Sn3.5Ag interface. J. Mater. Sci. Mater. Electron. 2022, 33, 25580–25588. [Google Scholar] [CrossRef]
- Yang, M.; Ji, H.J.; Wang, S.; Ko, Y.H.; Lee, C.W.; Wu, J.X.; Li, M.Y. Effects of Ag content on the interfacial reactions between liquid Sn-Ag-Cu solders and Cu substrates during soldering. J. Alloys Compd. 2016, 679, 18–25. [Google Scholar] [CrossRef]
- Yang, M.; Li, M.Y.; Wang, C.Q. Interfacial reactions of eutectic Sn3.5Ag and pure tin solders with Cu substrates during liquid-state soldering. Intermetallics 2012, 25, 86–94. [Google Scholar] [CrossRef]
- Sobral, B.S.; Vieira, P.S.; Lima, T.S.; Spinelli, J.E.; Cheung, N.; Garcia, A.; Silva, B.L. Effects of Zn addition on dendritic/cellular growth, phase formation, and hardness of a Sn–3.5 wt% Ag solder alloy. Adv. Eng. Mater. 2023, 25, 2201270. [Google Scholar] [CrossRef]
- Barik, E.M.; Gillot, C.; Hodaj, F. Bubble formation and growth during Transient Liquid Phase Bonding in Cu/SnAg system for microelectronic packaging. J. Mater. Sci. Mater. Electron. 2022, 33, 2360–2374. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Li, M.L. Microstructure and properties of Sn-Ag and Sn-Sb lead-free solders in electronics packaging: A review. J. Mater. Sci. Mater. Electron. 2022, 33, 2259–2292. [Google Scholar] [CrossRef]
- Guo, B.F.; Ma, H.T.; Kunwar, A.; Chu, X.H. Effect of the degree of supercooling on growth mechanism of Cu6Sn5 in pure Sn/Cu solder joint. J. Mater. Sci. Mater. Electron. 2021, 32, 7528–7540. [Google Scholar] [CrossRef]
- Ma, H.T.; Kunwar, A.; Sun, J.H.; Guo, B.F.; Ma, H.R. In Situ study on the increase of intermetallic compound thickness at anode of molten tin due to electromigration of copper. Scr. Mater. 2015, 107, 88–91. [Google Scholar] [CrossRef]
- Guo, B.F.; Kunwar, A.; Jiang, C.R.; Zhao, N.; Sun, J.H.; Chen, J.; Wang, Y.P.; Huang, M.L.; Ma, H.T. Synchrotron radiation imaging study on the rapid IMC growth of Sn-xAg solders with Cu and Ni substrates during the heat preservation stage. J. Mater. Sci. Mater. Electron. 2017, 33, 36–72. [Google Scholar] [CrossRef]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.M. Kinetics of Ag3Sn growth in Ag-Sn-Ag system during transient liquid phase soldering process. Acta Mater. 2010, 58, 3429–3443. [Google Scholar] [CrossRef]
- Dai, W.; Liu, Y.Y.; Huang, N.G.; Lan, R.L.; Zheng, K.K.; Lu, Y.X.; Li, J.; Jiang, Y.M.; Sun, Y.T. Selective corrosion of β-Sn and intermetallic compounds in an Ag–Sn alloy at different potentials in NaCl and Na2SO4 solutions. Corros. Sci. 2023, 212, 110958. [Google Scholar] [CrossRef]
- Schmuelling, G.; Oehl, N.; Fromma, O.; Knipper, M.; Olesiak, J.K.; Plaggenborg, T.; Parisi, J.; Winter, M.; Placke, T. Synthesis and electrochemical characterization of nano-sized Ag4Sn particles as anode material for lithium-ion batteries. Electrochim. Acta 2016, 196, 597–602. [Google Scholar] [CrossRef]
- Manasijevi, D.; Balanovi, L.; Markovi, I.; Gorgievski, M.; Stamenkovíc, U.; Đorđevíc, A.; Miníc, D.; Cosovi, V. Structural and thermal properties of Sn-Ag alloys. Solid State Sci. 2021, 119, 106685. [Google Scholar] [CrossRef]
- Shalaby, R.M. Development of holmium doped eutectic Sn-Ag lead-free solder for electronic packaging. Solder. Surf. Mt. Technol. 2022, 34, 277–286. [Google Scholar] [CrossRef]
- Hou, N.; Xian, J.W.; Sugiyama, A.; Yasuda, H.; Gourlay, C.M. Ag3Sn morphology transitions during eutectic growth in Sn–Ag alloys. Metall. Mater. Trans. A 2023, 54 Pt A, 909–927. [Google Scholar] [CrossRef]
- Cui, Y.; Xian, J.W.; Zois, A.; Marquardt, K.; Yasuda, H.; Gourlay, C.M. Nucleation and growth of Ag3Sn in Sn-Ag and Sn-Ag-Cu solder alloys. Acta Mater. 2023, 249, 118831. [Google Scholar] [CrossRef]
- Choi, W.K.; Jang, S.Y.; Kim, J.H.; Paik, K.W.; Lee, H.M. Grain morphology of intermetallic compounds at solder joints. J. Mater. Res. 2002, 17, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Zhao, N.; Zhao, H.J.; Huang, M.L.; Ma, H.T. In situ study of the real-time growth behavior of Cu6Sn5 at the Sn/Cu interface during the soldering reaction. Scr. Mater. 2014, 72–73, 43–46. [Google Scholar] [CrossRef]
- Schaefer, M.; Fournelle, R.A.; Liang, J. Theory for intermetallic phase growth between cu and liquid Sn-Pb solder based on grain boundary diffusion control. J. Electron. Mater. 1998, 27, 1167–1176. [Google Scholar] [CrossRef]
- Kim, H.K.; Tu, K.N. Kinetic analysis of the soldering reaction between eutectic SnPb alloy and Cu accompanied by ripening. Phys. Rev. B 1996, 53, 16027–16034. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Yang, F. Size effect model on kinetics of interfacial reaction between Sn-xAg-yCu solders and Cu substrate. Sci. Rep. 2014, 4, 7117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, R.E.; Goho, S.M.; DeHoff, R.T. The role of thermal grooving, thermotransport and electrotransport on the failure of thin film metallizations. In Proceedings of the 22nd International Reliability Physics Symposium, Las Vegas, NV, USA, 3–5 April 1984; pp. 234–241. [Google Scholar]
- Moelans, N.; Blanpain, B.; Wollants, P. Pinning effect of second-phase particles on grain growth in polycrystalline films studied by 3-D phase field simulations. Acta Mater. 2007, 55, 2173–2182. [Google Scholar] [CrossRef]
- Tonks, M.R.; Zhang, Y.; Butterfield, A.; Bai, X.M. Development of a grain boundary pinning model that considers particle size distribution using the phase field method. Model. Simul. Mat. Sci. Eng. 2015, 23, 045009. [Google Scholar] [CrossRef]
- Sharps, P.R.; Tomsia, A.P.; Pask, J.A. Wetting and spreading in the Cu-Ag system. Acta Metall. 1981, 29, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhao, L.Y.; Liang, H.X.; Zhang, Y.; Li, X.X.; Teng, C.Y.; Wang, H.; Bai, H.L. A review on phase field modeling for formation of η-Cu6Sn5 intermetallic. Metals 2022, 12, 2043. [Google Scholar] [CrossRef]
- Moon, K.W.; Boettinger, W.J.; Kattner, U.R.; Biancaniello, F.S.; Handwerker, C.A. Experimental and thermodynamic assessment of Sn-Ag-Cu solder alloys. J. Electron. Mater. 2000, 29, 1122–1136. [Google Scholar] [CrossRef]
- Hamasha, M.M.; Hamasha, K.; Hamasha, S. Impact of isothermal aging on mechanical properties of 92.8%Sn-3%Ag-0.5%Cu-3.3%Bi (cyclomax) solder joints. Metals 2023, 13, 591. [Google Scholar] [CrossRef]


| Temperature (°C) | Sn/Cu | Sn-3.5Ag/Cu | ||
|---|---|---|---|---|
| k (μm/min−n) | n | k (μm/min−n) | n | |
| 250 | 2.84 | 0.33 | 2.84 | 0.34 |
| 300 | 3.20 | 0.36 | 3.30 | 0.36 |
| 350 | 3.64 | 0.36 | 4.03 | 0.37 |
| Q/(KJ·mol−1) | 35.32 | 28.88 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Ma, H.; Kunwar, A.; Wang, R.; Zheng, H. In Situ Study the Grooving Effect Induced by Ag Particles on Rapid Growth of Cu6Sn5 Grain at Sn-xAg/Cu Soldering Interface during the Heat Preservation Stage. Metals 2023, 13, 1445. https://doi.org/10.3390/met13081445
Guo B, Ma H, Kunwar A, Wang R, Zheng H. In Situ Study the Grooving Effect Induced by Ag Particles on Rapid Growth of Cu6Sn5 Grain at Sn-xAg/Cu Soldering Interface during the Heat Preservation Stage. Metals. 2023; 13(8):1445. https://doi.org/10.3390/met13081445
Chicago/Turabian StyleGuo, Bingfeng, Haitao Ma, Anil Kunwar, Rongzhi Wang, and Han Zheng. 2023. "In Situ Study the Grooving Effect Induced by Ag Particles on Rapid Growth of Cu6Sn5 Grain at Sn-xAg/Cu Soldering Interface during the Heat Preservation Stage" Metals 13, no. 8: 1445. https://doi.org/10.3390/met13081445





