Controlling the Amount of Copper Formate Shells Surrounding Cu Flakes via Wet Method and Thermo-Compression Sinter Bonding between Cu Finishes in Air Using Flakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Treatment of Cu Flakes
2.2. Paste Preparation
2.3. Sinter Bonding
2.4. Characterization
3. Results and Discussion
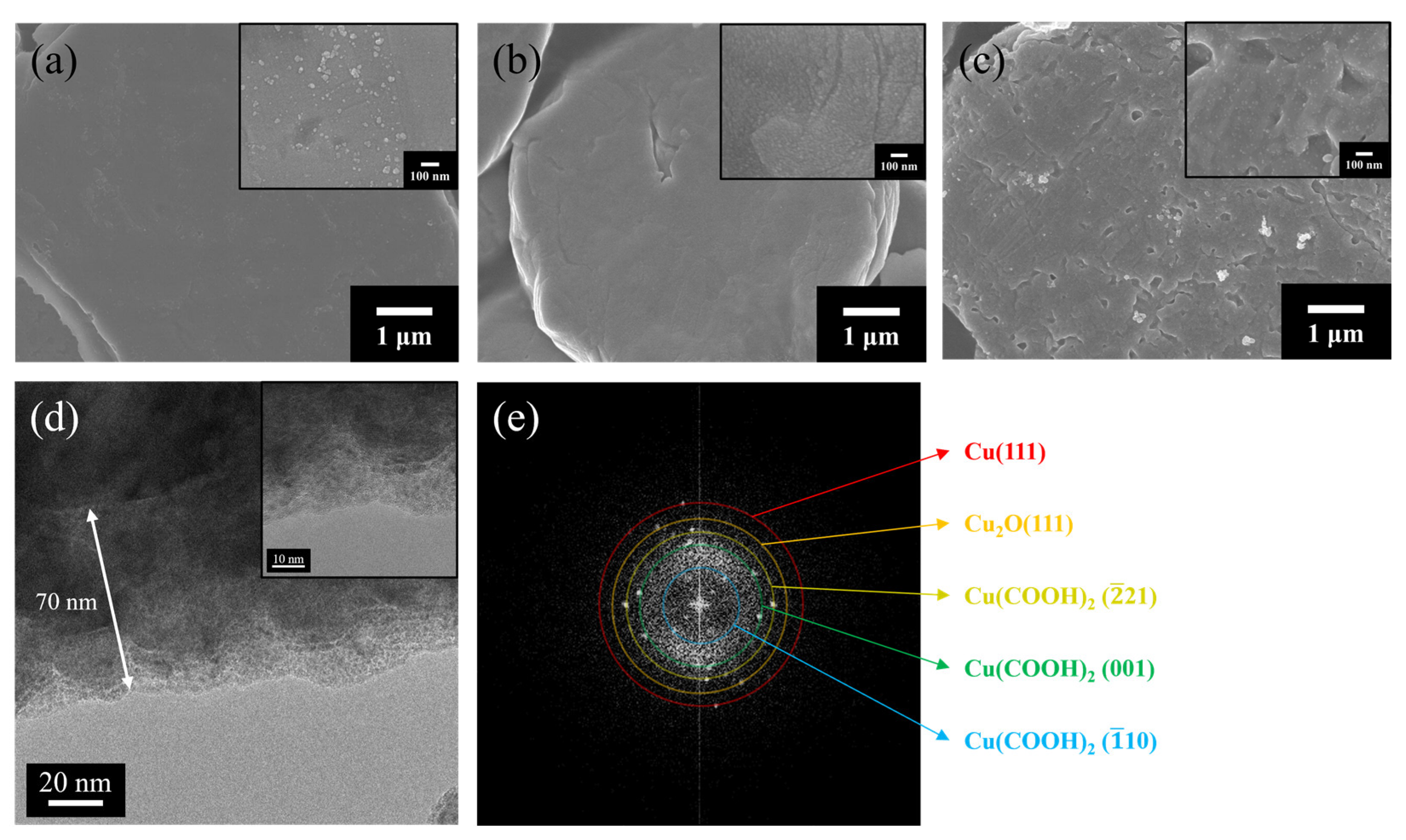
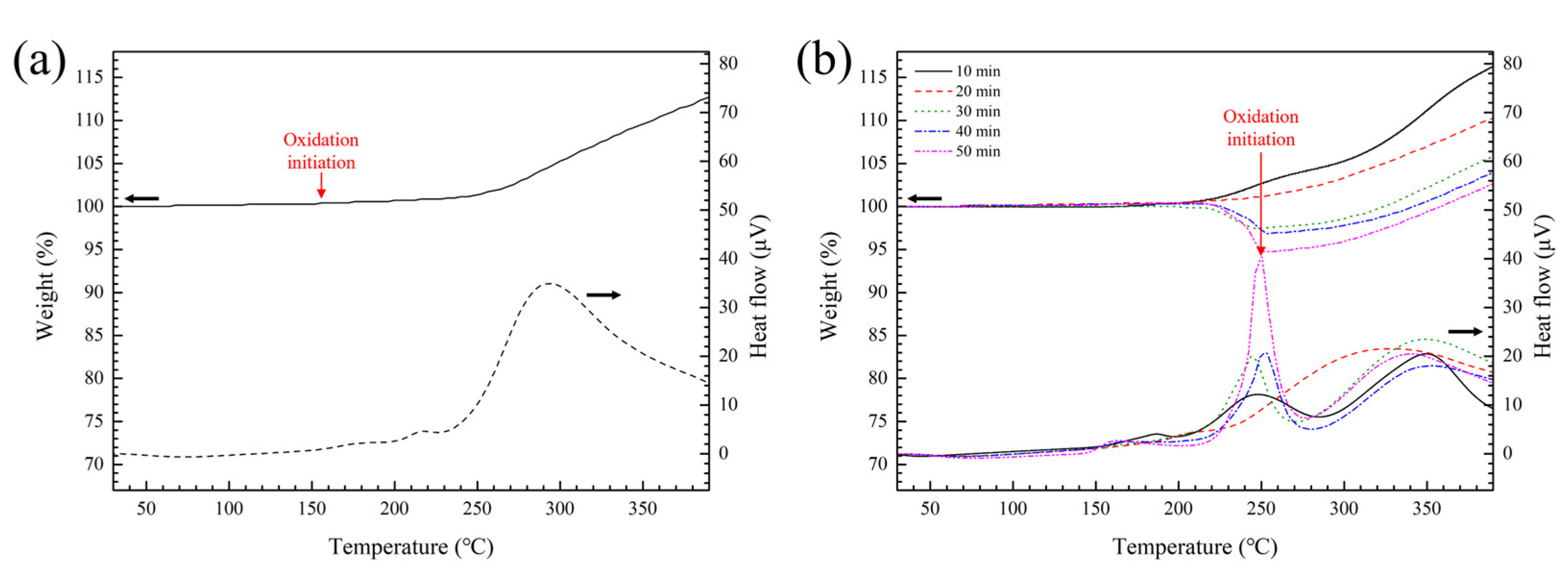
3.1. Characteristics of Cu Particles after Surface Treatment
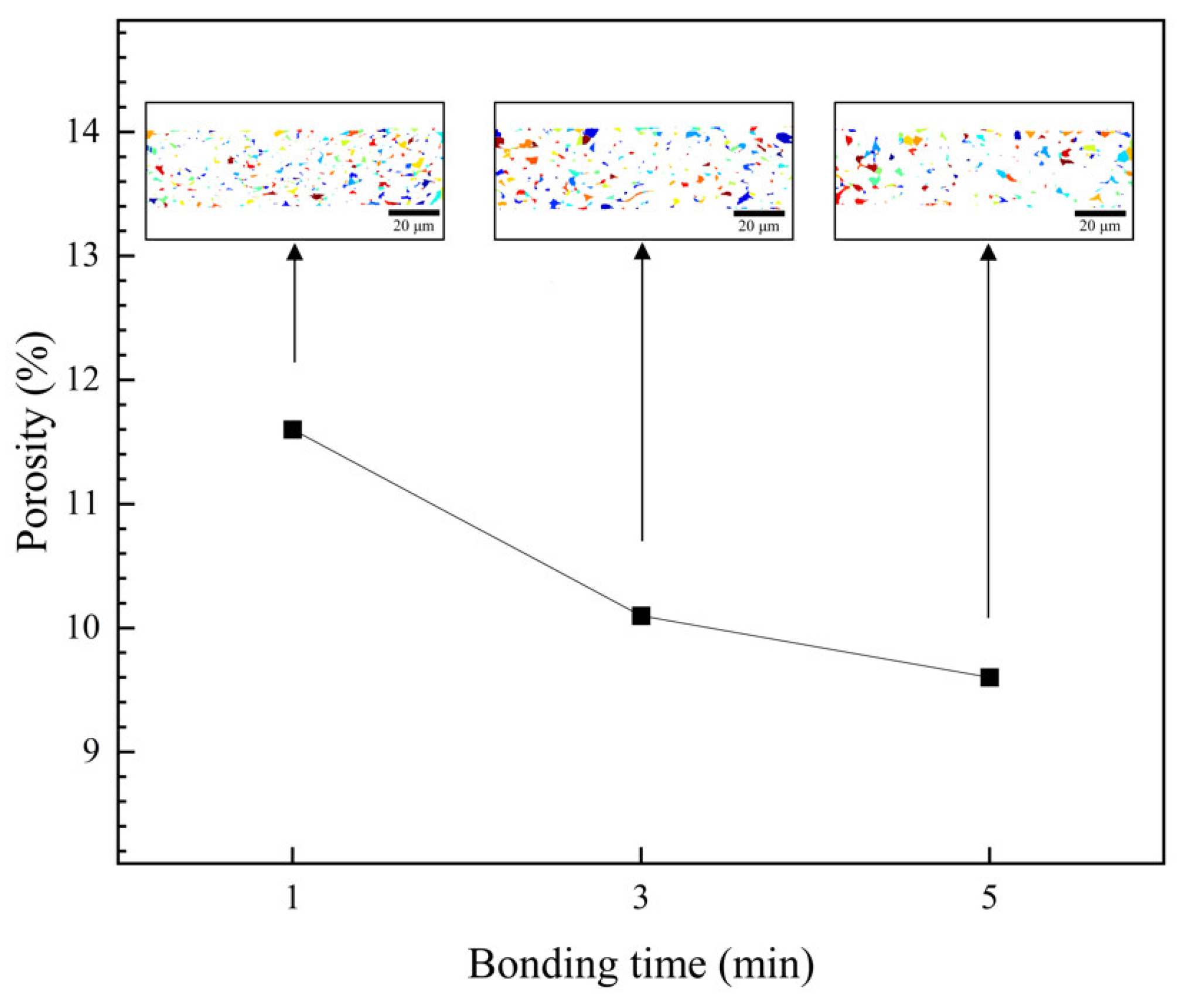
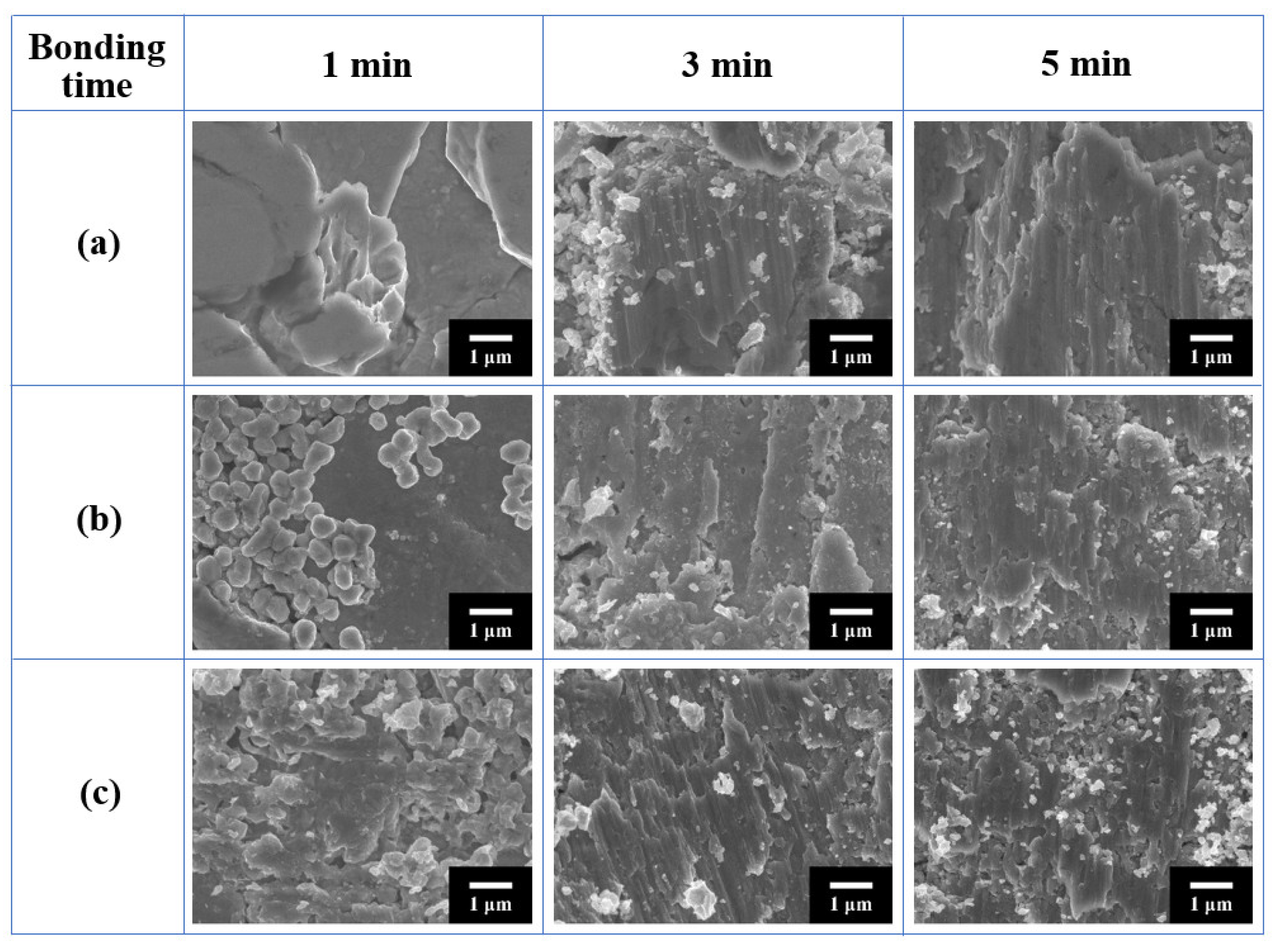
3.2. Bondline Microstructures
3.3. Shear Strength of Bondlines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roccaforte, F.; Fiorenza, P.; Greco, G.; Nigro, R.L.; Giannazzo, F.; Lucolano, F.; Saggio, M. Emerging trends in wide band gap semiconductors (SiC and GaN) technology for power devices. Microelectron. Eng. 2018, 187–188, 66–77. [Google Scholar] [CrossRef]
- Chen, T.F.; Siow, K.S. Comparing the mechanical and thermal-electrical properties of sintered copper (Cu) and sintered silver (Ag) joints. J. Alloys Compd. 2021, 866, 158783. [Google Scholar] [CrossRef]
- Mei, Y.H.; Wang, Z.; Siow, K.S. Reliability and failure mechanisms of sintered silver as die attach joint. In Die-Attach Materials for High Temperature Applications in Microelectronics Packaging; Siow, K.S., Ed.; Springer: Cham, Switzerland, 2019; pp. 125–150. [Google Scholar]
- Yoon, J.-W.; Back, J.-H. Effect of sintering conditions on the mechanical strength of Cu-sintered joints for high-power applications. Materials 2018, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.; Klingler, M.; Bigerelle, M.; Coutellier, D. Solder fatigue failures in a new designed power module under power cycling. Microelectron. Reliab. 2016, 66, 112–133. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Yang, Y.; Zhang, H.; Kim, D.; Sugahara, T.; Nagao, S.; Suganuma, K. Low-temperature and pressureless sinter joining of Cu with micron/submicron Ag particle paste in air. J. Alloys Compd. 2019, 780, 435–442. [Google Scholar] [CrossRef]
- Sun, S.; Guo, Q.; Chen, H.; Li, M.; Wang, C. Solderless bonding with nanoporous copper as interlayer for high-temperature applications. Microelectron. Reliab. 2018, 80, 198–204. [Google Scholar] [CrossRef]
- Chen, G.; Yu, L.; Mei, Y.H.; Li, X.; Chen, X.; Lu, G.Q. Reliability comparison between SAC305 joint and sintered nanosilver joint at high temperatures for power electronic packaging. J. Mater. Process Technol. 2014, 214, 1900–1908. [Google Scholar] [CrossRef]
- Tan, K.S.; Wong, Y.H.; Cheong, K.Y. Thermal characteristic of sintered Ag-Cu nanopaste for high-temperature die-attach application. Int. J. Therm. Sci. 2015, 87, 169–177. [Google Scholar] [CrossRef]
- Choi, E.B.; Lee, J.-H. Tens-of-seconds solid-state sinter-bonding technique in air using in situ reduction of surface oxide layers on easily bendable dendritic Cu particles. Appl. Surf. Sci. 2022, 580, 152347. [Google Scholar] [CrossRef]
- Mohan, K.; Shahane, N.; Raj, P.M.; Antoniou, A.; Smet, V.; Tummala, R. Low-Temperature, Organics-Free Sintering of Nanoporous Copper for Reliable, High-Temperature and High-Power Die-Attach Interconnections. In Proceedings of the 2017 IEEE Applied Power Electronics Conference and Exposition (APEC), Tampa, FL, USA, 26–30 March 2017; IEEE: Manhattan, NY, USA, 2017. [Google Scholar]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of silver nanomaterials at low temperatures: Processes, properties, and applications. ACS Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef]
- Jianfeng, Y.; Guisheng, Z.; Anming, H.; Zhou, N. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2012, 21, 15981. [Google Scholar] [CrossRef]
- Morita, T.; Ide, E.; Yasuda, Y.; Hirose, A.; Kobayashi, K. Study of bonding technology using silver nanoparticles. Jpn. J. Appl. Phys. 2008, 47, 6615–6622. [Google Scholar] [CrossRef]
- Ma, H.; Suhling, J.C. A review of mechanical properties of lead-free solders for electronic packaging. J. Mater. Sci. 2009, 44, 1141–1158. [Google Scholar] [CrossRef]
- Suganuma, K.; Kim, S.-J.; Kim, K.-S. High-temperature lead-free solders: Properties and possibilities. JOM 2009, 61, 64–71. [Google Scholar] [CrossRef]
- Soichi, S.; Suganuma, K. Low-temperature and low-pressure die bonding using thin Ag-flake and Ag-particle pastes for power devices. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 3, 923. [Google Scholar] [CrossRef]
- Kim, M.I.; Lee, J.-H. Die sinter bonding in air using Cu@Ag particulate preform and rapid formation of near-full density bondline. J. Mater. Res. Technol. 2021, 14, 1724–1738. [Google Scholar] [CrossRef]
- Ide, E.; Angata, S.; Hirose, A.; Kobayashi, K.F. Metal-metal bonding process using Ag metallo-organic nanoparticles. Acta Mater. 2005, 53, 2385–2393. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, D.; Zou, G.; Liu, L.; Bai, H.; Wu, A.; Zhou, Y.N. Sintering bonding process with Ag nanoparticle paste and joint properties in high temperature environment. J. Nanomater. 2016, 2016, 5284048. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Lin, T.; Zhang, P.; He, P.; Paik, K.W. Highly mechanical and high-temperature properties of Cu–Cu joints using citrate-coated manosized Ag paste in Air. Prog. Nat. Sci. 2021, 31, 129–140. [Google Scholar] [CrossRef]
- Iijima, J.; Lim, J.W.; Hong, S.H.; Suzuki, S.; Mimura, K.; Isshiki, M. Native oxidation of ultra high purity Cu bulk and thin films. Appl. Surf. Sci. 2006, 253, 2825–2829. [Google Scholar] [CrossRef]
- Fehlner, F.P.; Mott, N.F. Low-temperature oxidation. Oxid. Met. 1970, 2, 59–99. [Google Scholar] [CrossRef]
- Aromaa, J.; Kekkonen, M.; Mousapour, M.; Jokilaakso, A.; Lundström, M. The Oxidation of copper in air at temperatures up to 100 °C. Corros. Mater. Degrad. 2021, 2, 625–640. [Google Scholar] [CrossRef]
- Zuo, Y.; Carter-Searjeant, S.; Green, M.; Mills, L.; Mannan, S.H. High bond strength Cu joints fabricated by rapid and pressureless in situ reduction-sintering of Cu nanoparticles. Mater. Lett. 2020, 276, 128260. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Gao, Y.; Hu, D.; Li, C.-F.; Zhang, H.; Jiu, J.; Nagao, S.; Suganuma, K. Highly conductive copper films based on submicron copper particles/copper complex inks for printed electronics: Microstructure, resistivity, oxidation resistance, and long-term stability. J. Alloys Compd. 2018, 732, 240–247. [Google Scholar] [CrossRef]
- Liu, X.; Nishikawa, H. Low-pressure Cu-Cu bonding using in-situ surface-modified microscale Cu particles for power device packaging. Scr. Mater. 2016, 120, 80–84. [Google Scholar] [CrossRef]
- Peng, Y.; Mou, Y.; Liu, J.; Chen, M. Fabrication of high-strength Cu–Cu joint by low-temperature sintering micron–nano Cu composite paste. J. Mater. Sci. 2020, 31, 8456–8463. [Google Scholar] [CrossRef]
- Li, J.J.; Cheng, C.L.; Shi, T.L.; Fan, J.H.; Yu, X.; Cheng, S.Y.; Liao, G.L.; Tang, Z.R. Surface effect induced Cu-Cu bonding by Cu nanosolder paste. Mater. Lett. 2016, 184, 193–196. [Google Scholar] [CrossRef]
- Li, J.; Liang, Q.; Shi, T.; Fan, J.; Gong, B.; Feng, C.; Fan, J.; Liao, G.; Tang, Z. Design of Cu nanoaggregates composed of ultra-small Cu nanoparticles for Cu-Cu thermocompression bonding. J. Alloys Compd. 2019, 772, 793–800. [Google Scholar] [CrossRef]
- Kim, I.; Kim, Y.; Woo, K.; Ryu, E.-H.; Yon, K.-Y.; Cao, G.; Moon, J. Synthesis of oxidation-resistant core-shell copper nanoparticles. RSC Adv. 2013, 3, 15169–15177. [Google Scholar] [CrossRef]
- Schwarzer, C.; Chew, L.M.; Schnepf, M.; Stoll, T. Investigation of Copper Sinter Material for Die Attach. In Proceedings of the SMTA International, Rosemont, IL, USA, 14–18 October 2018; SMTA: San Jose, CA, USA, 2018. [Google Scholar]
- Diaz-Droguett, D.E.; Espinoza, R.; Fuenzalida, V.M. Copper nanoparticles grown under hydrogen: Study of the surface oxide. Appl. Surf. Sci. 2011, 257, 4597–4602. [Google Scholar] [CrossRef]
- Cano, E.; Torres, C.L.; Bastidas, J.M. An XPS study of copper corrosion originated by formic acid vapor at 40% and 80% relative humidity. Mater. Corros. 2001, 52, 667–676. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Lee, B.; Kim, J.; Moon, J. Effect of carboxylic acid on sintering of inkjet-printed copper nanoparticulate films. ACS Appl. Mater. Interfaces 2011, 3, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.L.; Kim, Y.S.; Lee, K.S.; Lee, J.-H. Characterization of the die-attach process via low-temperature reduction of Cu formate in Air. J. Mater. Sci. 2019, 30, 9806–9813. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, J.-H. Die Sinter bonding in air using copper formate preform for formation of full-density bondline. Trans. Nonferrous Met. Soc. China. 2021, 31, 1717–1728. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Li, W.; Jiu, J.; Nagao, S.; Sugahara, T.; Suganuma, K. Die bonding performance using bimodal Cu particle paste under different sintering atmospheres. J. Electron. Mater. 2017, 46, 4575–4581. [Google Scholar] [CrossRef]
- Wu, L.; Qian, J.; Zhang, F.; Yu, J.; Wang, Z.; Guo, H.; Chen, X. Low-temperature sintering of Cu/functionalized multiwalled carbon nanotubes composite paste for power electronic packaging. IEEE Trans. Power Electron. 2022, 37, 1234–1243. [Google Scholar]
- Wang, X.; Zhang, Z.; Feng, Y.; Xiao, F. Anti-oxidative copper nanoparticle paste for Cu–Cu bonding at low temperature in air. J. Mater. Sci. Mater. Electron. 2022, 33, 817–823. [Google Scholar] [CrossRef]
- Son, J.; Yu, D.-Y.; Kim, Y.-C.; Byun, D.; Bang, J. Effect of bimodal Cu paste on interfacial properties and mechanical strength of sintered joints. J. Electron. Mater. 2022, 51, 7326–7336. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Fan, J.; Jiang, J.; Liu, Y.; Ye, H.; Zhang, G. Microstructural evolution, fracture behavior and bonding mechanisms study of copper sintering on bare DBC substrate for SiC power electronics packaging. J. Mater. Res. Technol. 2022, 18, 1407–1421. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.L.; Lee, J.-H. Controlling the Amount of Copper Formate Shells Surrounding Cu Flakes via Wet Method and Thermo-Compression Sinter Bonding between Cu Finishes in Air Using Flakes. Metals 2023, 13, 1516. https://doi.org/10.3390/met13091516
Choi WL, Lee J-H. Controlling the Amount of Copper Formate Shells Surrounding Cu Flakes via Wet Method and Thermo-Compression Sinter Bonding between Cu Finishes in Air Using Flakes. Metals. 2023; 13(9):1516. https://doi.org/10.3390/met13091516
Chicago/Turabian StyleChoi, Woo Lim, and Jong-Hyun Lee. 2023. "Controlling the Amount of Copper Formate Shells Surrounding Cu Flakes via Wet Method and Thermo-Compression Sinter Bonding between Cu Finishes in Air Using Flakes" Metals 13, no. 9: 1516. https://doi.org/10.3390/met13091516
APA StyleChoi, W. L., & Lee, J.-H. (2023). Controlling the Amount of Copper Formate Shells Surrounding Cu Flakes via Wet Method and Thermo-Compression Sinter Bonding between Cu Finishes in Air Using Flakes. Metals, 13(9), 1516. https://doi.org/10.3390/met13091516








