A Novel Quantitative Analysis Method for Lead Components in Waste Lead Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Devices
2.2. Methods
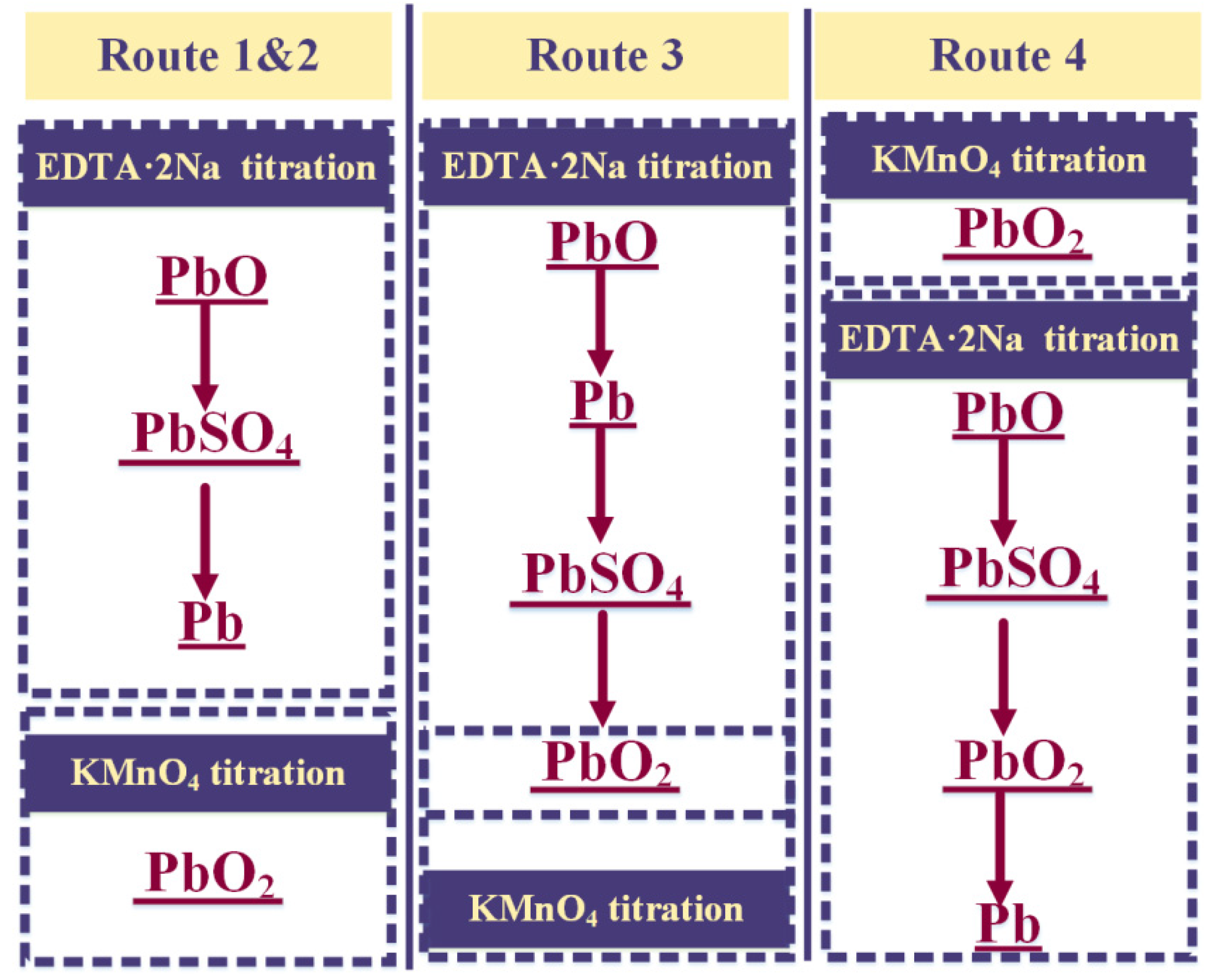
2.2.1. The Order of Determination
2.2.2. Determining the PbO2 Content in Lead Paste
2.2.3. Determining the PbO Content in Lead Paste
2.2.4. Determining the PbSO4 Content in Lead Paste
2.2.5. Determining the Pb Content in Lead Paste
3. Results and Discussion
3.1. Simulated Lead Paste Test Comparison
3.1.1. Analysis of PbO Determination Results
3.1.2. Analysis of Pb and PbO2 Determination Results
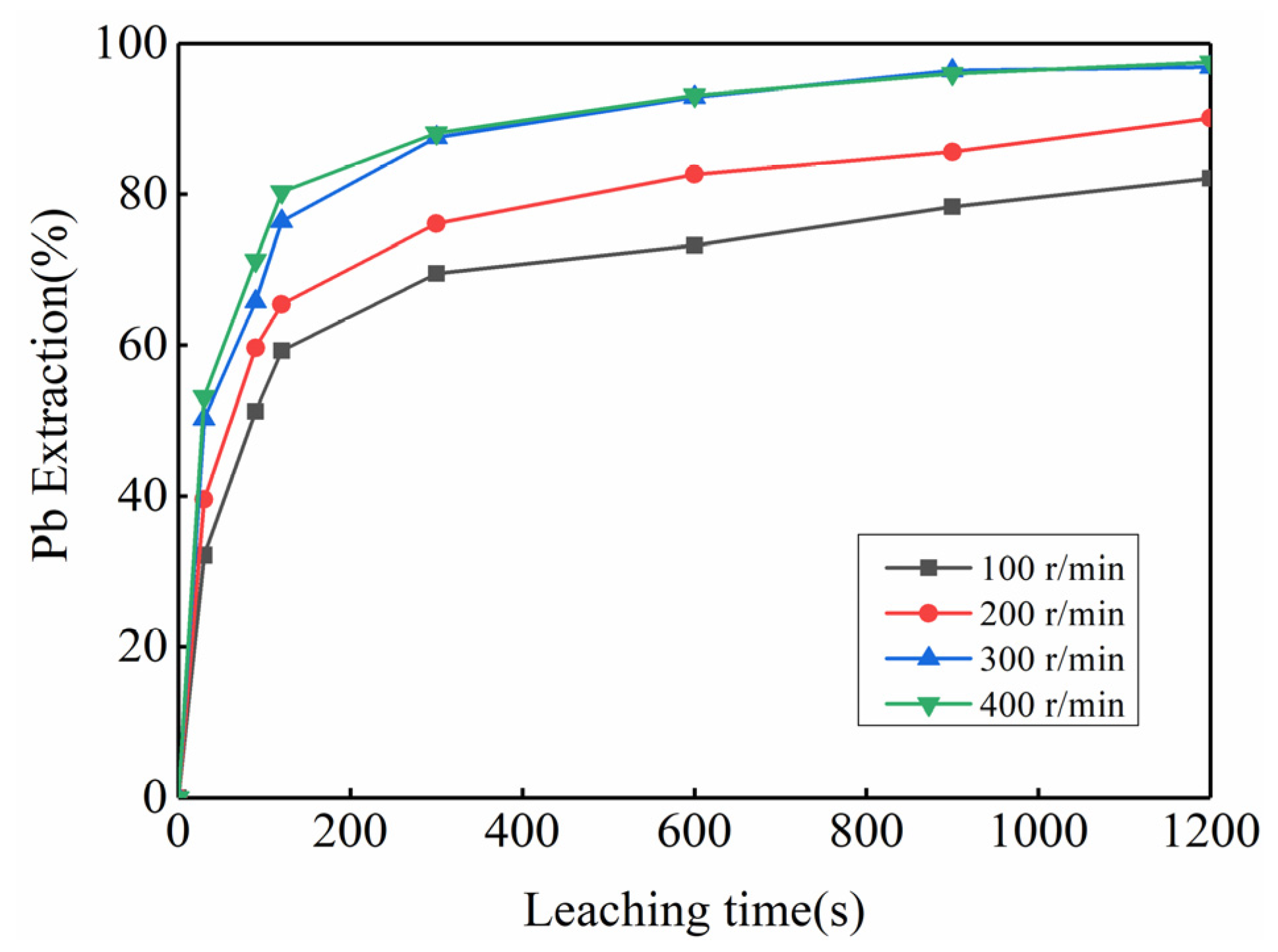
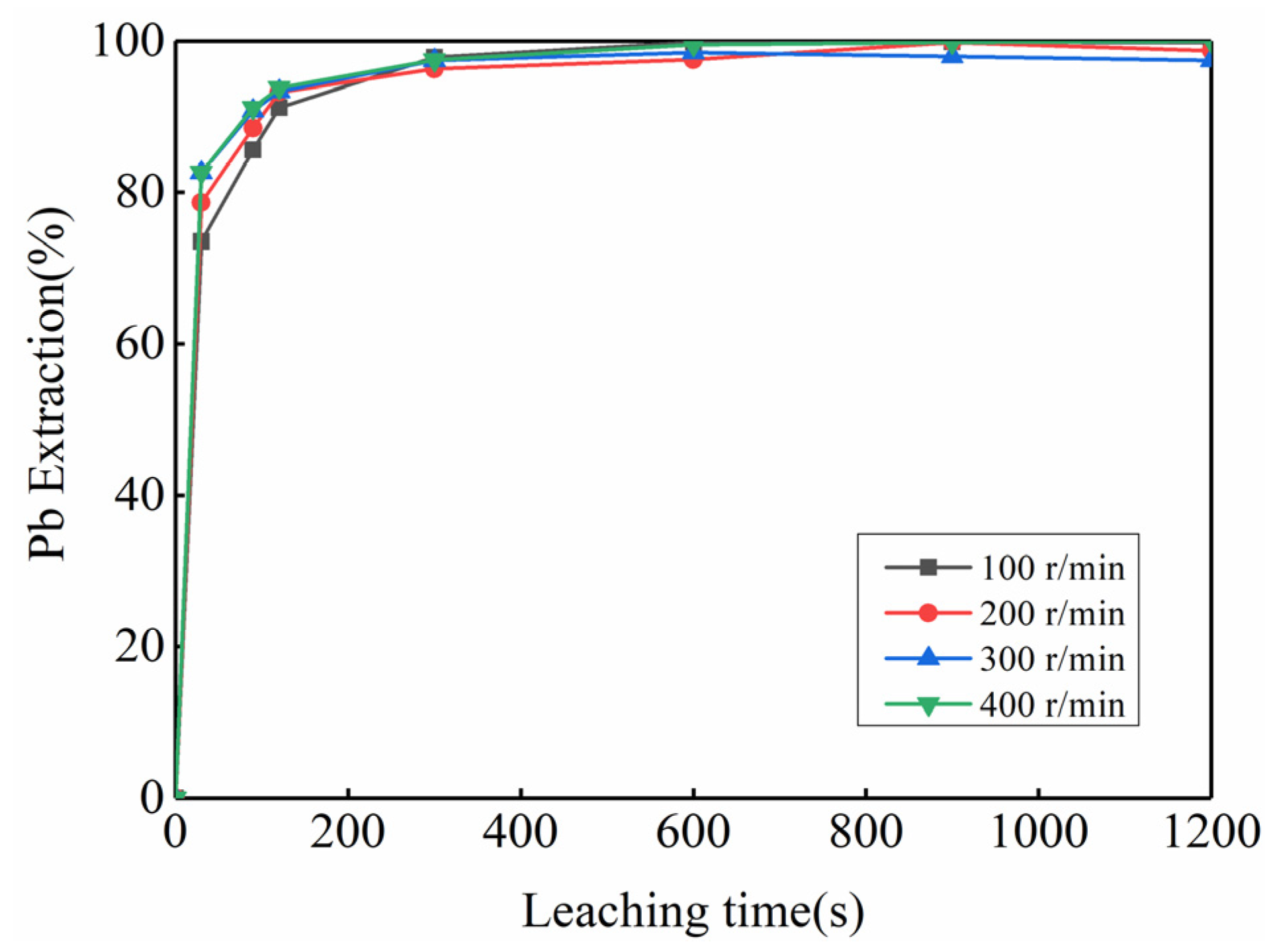
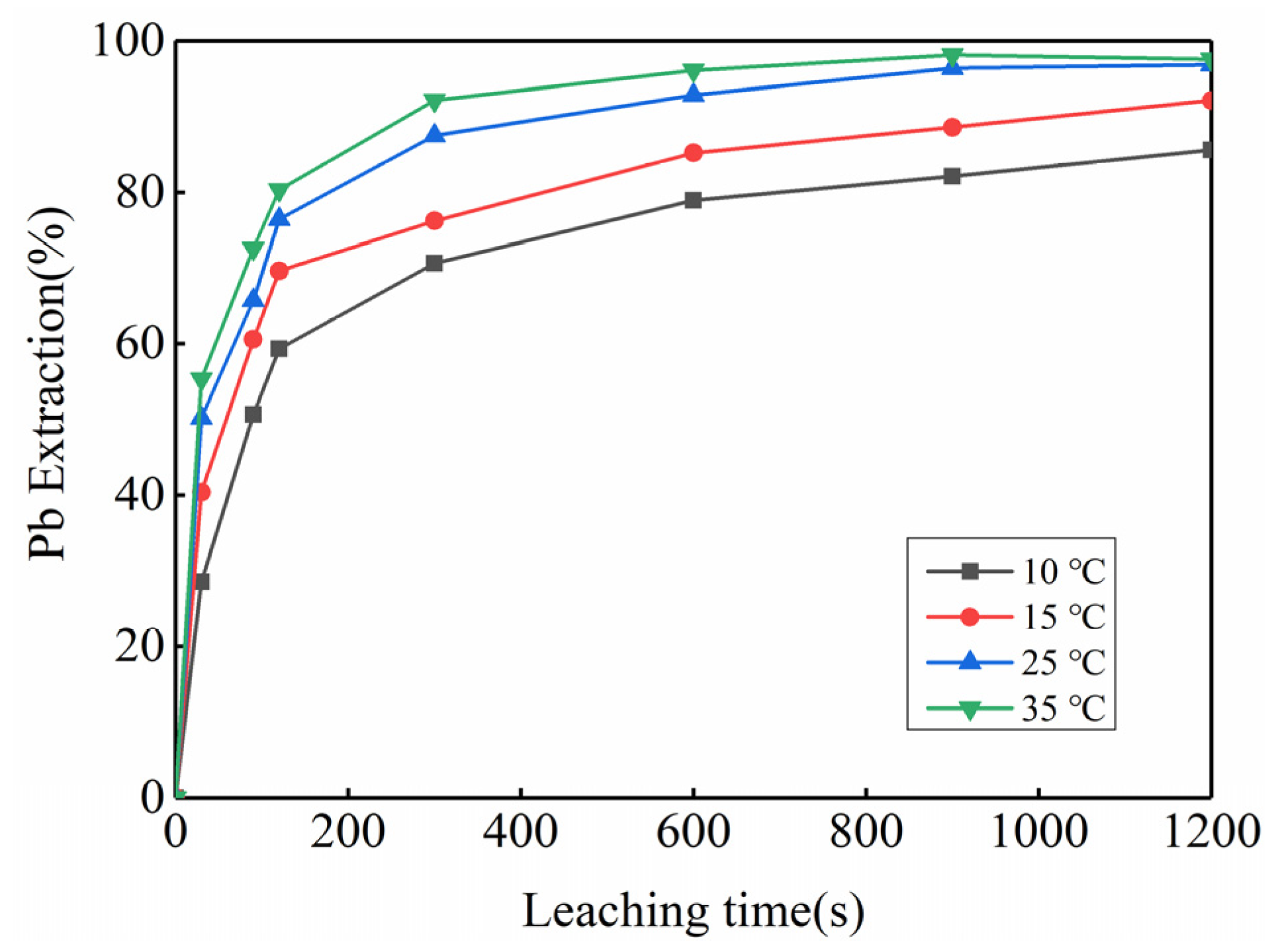
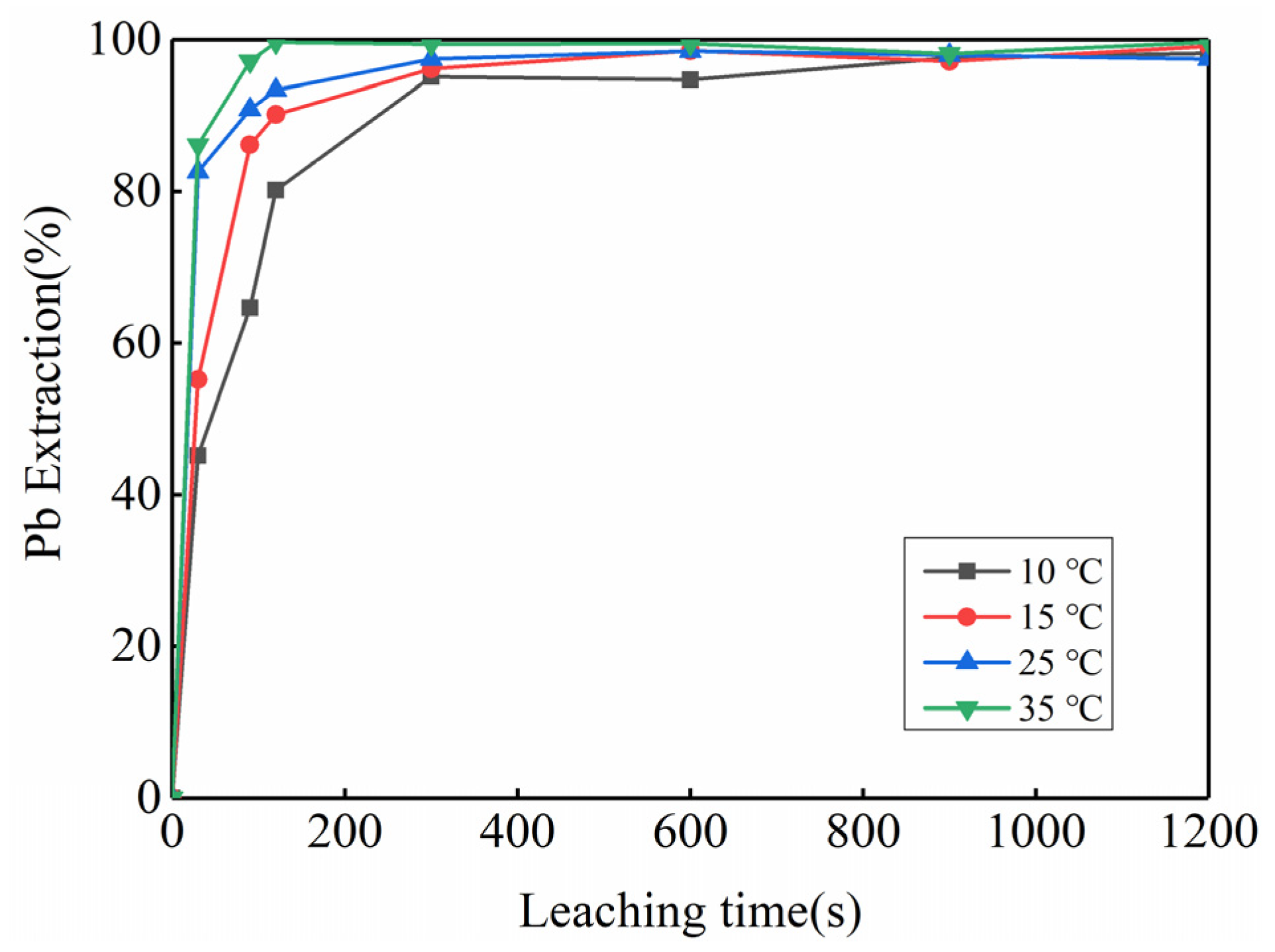
3.2. Feasibility Study of Using Kinetic Rules for PbO2 Removal
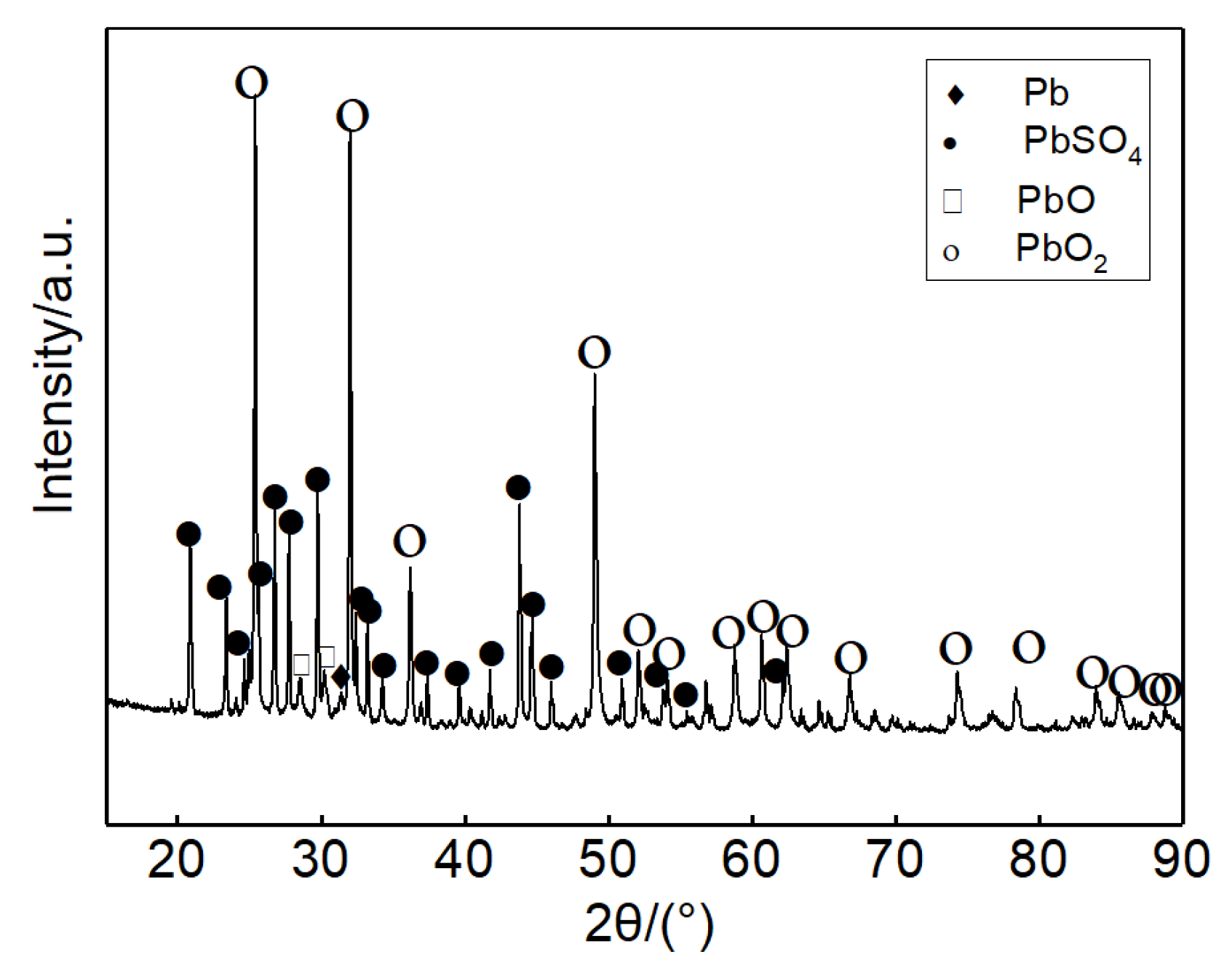
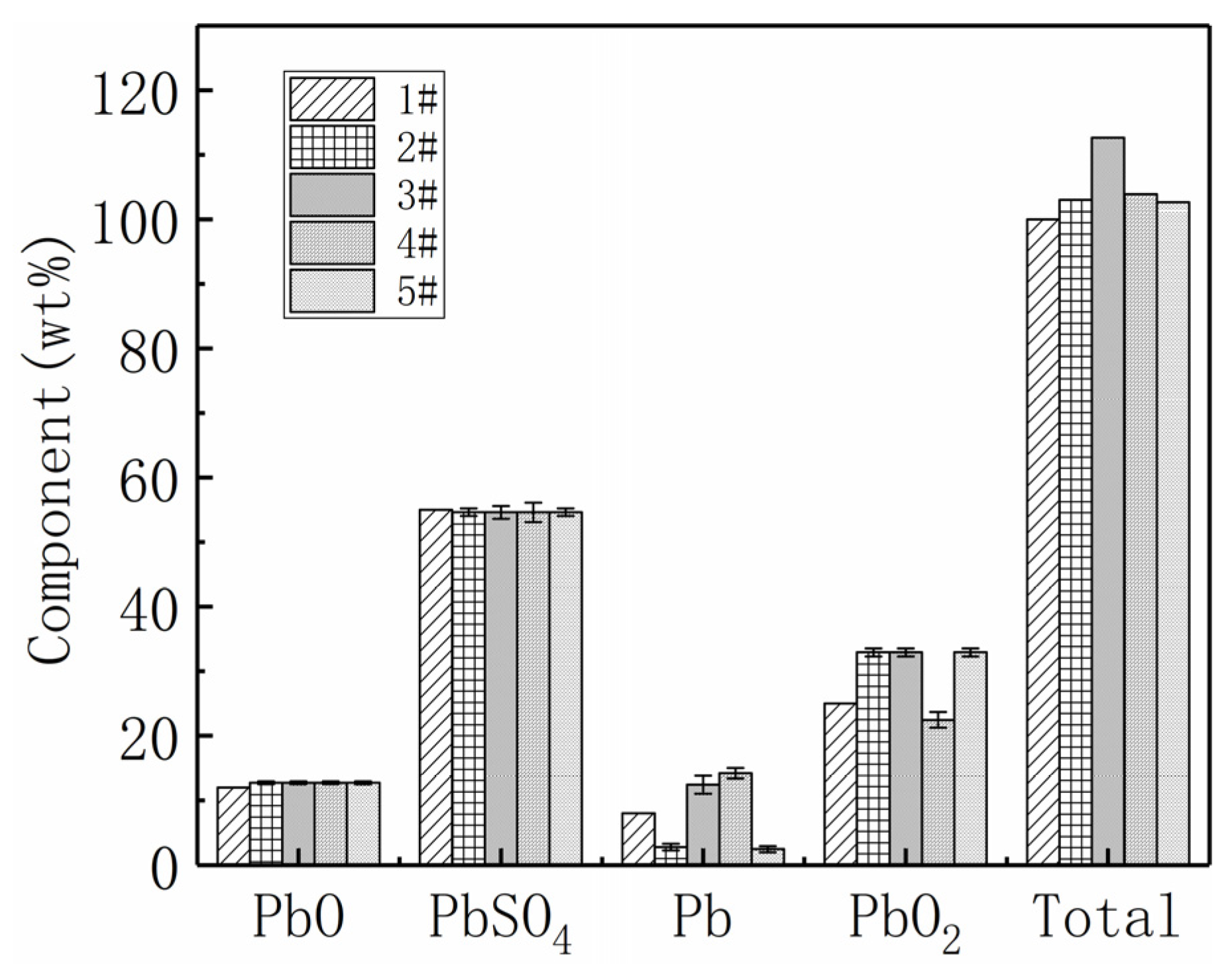
3.3. Spent Lead Paste Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ILZSG. The World Lead Factbook 2019. Available online: https://www.ilzsg.org/wp-content/uploads/SitePDFs/1_ILZSG%20Lead%20Factbook.pdf (accessed on 18 August 2023).
- Hurlbut, C.S. The 22nd Edition of the Manual of Mineral Science: (After James D. Dana); John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, France, 2001. [Google Scholar]
- Jung, J.; Zhang, L.; Zhang, J. Lead-Acid Battery Technologies: Fundamentals, Materials, and Applications; CRC Press: Boca Raton, FL, USA, 2015; Volume 8. [Google Scholar]
- Shen, W.X. State of available capacity estimation for lead-acid batteries in electric vehicles using neural network. Energy Convers. Manag. 2007, 48, 433–442. [Google Scholar] [CrossRef]
- Ayeng’o, S.P.; Schirmer, T.; Kairies, K.-P.; Axelsen, H.; Sauer, D.U. Comparison of off-grid power supply systems using lead-acid and lithium-ion batteries. Sol. Energy 2018, 162, 140–152. [Google Scholar] [CrossRef]
- Dufo-López, R.; Lujano-Rojas, J.M.; Bernal-Agustín, J.L. Comparison of different lead–acid battery lifetime prediction models for use in simulation of stand-alone photovoltaic systems. Appl. Energy 2014, 115, 242–253. [Google Scholar] [CrossRef]
- Amrouche, S.O.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Goodenough, J.B. Rechargeable batteries: Challenges old and new. J. Solid State Electrochem. 2012, 16, 2019–2029. [Google Scholar] [CrossRef]
- Huang, K.; Liu, H.Y.; Dong, H.L.; Lin, M.; Ruan, J.J. A novel approach to recover lead oxide from spent lead acid batteries by desulfurization and crystallization in sodium hydroxide solution after sulfation. Resour. Conserv. Recycl. 2021, 167, 105385. [Google Scholar] [CrossRef]
- Jie, X.; Yao, Z.; Wang, C.; Qiu, D.; Chen, Y.; Zhang, Y.; Ma, B.; Gao, W. Progress in Waste Lead Paste Recycling Technology from Spent Lead–Acid Battery in China. J. Sustain. Metall. 2022, 8, 978–993. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Liang, S.; Hou, H.; Hu, J.; Liu, B.; Kumar, R.V. Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J. Power Sources 2019, 436, 226853. [Google Scholar] [CrossRef]
- Khan, M.A.; Ramzani, P.M.A.; Zubair, M.; Rasool, B.; Khan, M.K.; Ahmed, A.; Khan, S.A.; Turan, V.; Iqbal, M. Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Sci. Total Environ. 2020, 710, 136294. [Google Scholar] [CrossRef]
- Yadav, S.K.; Patil, G.P.; Virmagami, A.; Bijalwan, V.; Devi, K.; Chauhan, A.; Gupta, S.K.; Fathima, S.; Naorem, C.D.; Yadav, S.; et al. Occupational lead exposure is an independent modulator of hypertension and poor pulmonary functions: A cross-sectional comparative study in lead-acid battery recycling workers. Toxicol. Ind. Health 2022, 38, 139–150. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, Y.; Chen, M.J.; Huang, T.Y.; Yang, F.H.; Wang, Z.J. Current status and technological progress in lead recovery from electronic waste. Int. J. Environ. Sci. Technol. 2023, 20, 1037–1052. [Google Scholar] [CrossRef]
- Helser, J.; Vassilieva, E.; Cappuyns, V. Environmental and human health risk assessment of sulfidic mine waste: Bioaccessibility, leaching and mineralogy. J. Hazard. Mater. 2022, 424, 127313. [Google Scholar] [CrossRef]
- Chowdhury, K.I.A.; Nurunnahar, S.; Kabir, M.L.; Islam, M.T.; Baker, M.; Islam, M.S.; Rahman, M.; Hasan, M.A.; Sikder, A.; Kwong, L.H.; et al. Child lead exposure near abandoned lead acid battery recycling sites in a residential community in Bangladesh: Risk factors and the impact of soil remediation on blood lead levels. Environ. Res. 2021, 194, 110689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rahman, M.A.; Islam, M.R.; Hashem, M.A.; Rahman, M.M. Lead and other elements-based pollution in soil, crops and water near a lead-acid battery recycling factory in Bangladesh. Chemosphere 2022, 290, 133288. [Google Scholar] [CrossRef]
- Ferracin, L.C.; Chácon-Sanhueza, A.E.; Davoglio, R.A.; Rocha, L.O.; Caffeu, D.J.; Fontanetti, A.R.; Rocha-Filho, R.C.; Biaggio, S.R.; Bocchi, N. Lead recovery from a typical Brazilian sludge of exhausted lead-acid batteries using an electrohydrometallurgical process. Hydrometallurgy 2002, 65, 137–144. [Google Scholar] [CrossRef]
- Andrews, D.; Raychaudhuri, A.; Frias, C. Environmentally sound technologies for recycling secondary lead. J. Power Sources 2000, 88, 124–129. [Google Scholar] [CrossRef]
- Yu, J.J.; Cao, J.; Zhou, S.Q.; Wu, L.; Chen, Z.Y.; Fu, F.B.; Rao, Y.Z.; Zhang, R. Directly Recovering Lead and Recycling Electrolyte via Electrolyzing Desulfurized Lead Paste with PVDF as Binder. J. Sustain. Metall. 2023, 9, 172–182. [Google Scholar] [CrossRef]
- Dai, F.S.; Huang, H.; Chen, B.M.; Zhang, P.P.; He, Y.P.; Guo, Z.C. Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Sep. Purif. Technol. 2019, 224, 237–246. [Google Scholar] [CrossRef]
- Sun, X.; Yang, J.; Zhang, W.; Zhu, X.; Hu, Y.; Yang, D.; Yuan, X.; Yu, W.; Dong, J.; Wang, H.; et al. Lead acetate trihydrate precursor route to synthesize novel ultrafine lead oxide from spent lead acid battery pastes. J. Power Sources 2014, 269, 565–576. [Google Scholar] [CrossRef]
- Ye, L.; Duan, L.; Liu, W.; Hu, Y.; Ouyang, Z.; Yang, S.; Xia, Z. Facile method for preparing a nano lead powder by vacuum decomposition from spent lead-acid battery paste: Leaching and desulfuration in tartaric acid and sodium tartrate mixed lixivium. Hydrometallurgy 2020, 197, 105450. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, P.Y.; Yang, J.K.; Li, Z.Y.; Liang, S.; Yu, W.H.; Li, M.Y.; Tong, Y.X.; Hu, J.P.; Hou, H.J.; et al. A closed-loop acetic acid system for recovery of PbO@C composite derived from spent lead-acid battery. Resour. Conserv. Recycl. 2022, 184, 106391. [Google Scholar] [CrossRef]
- Zhu, X.F. Study on Leaching Process of Spent Lead Battery Paste with Organic Acid and Preparation of Ultrafine Lead Oxide by Calcination at Low Temperature. Ph.D. Dissertation, Huazhong University of Science and Technology, Wuhan, China, 2012. (In Chinese). [Google Scholar]
- Wang, Y. Study on the New Hydrometallurgical Process Ofpreparing Lead Chemical Products from Diachylum in Waste Lead Storage Battery. Master’s Thesis, Hefei University of Technology, Hefei, China, 2010. (In Chinese). [Google Scholar]
- Zhang, X. Study on New Recovery Technology and Electrochemical performance of High Purity Lead and α-PbO from the Spent Lead Acid Batteries. Ph.D. Dissertation, Beijing University of Chemical Technology, Beijing, China, 2017. (In Chinese). [Google Scholar]
- Liu, G.L. Introduction to Lead-Acid Battery Processing; Machine Press: Beijing, China, 2009. [Google Scholar]
- Yang, T.Z.; Xie, B.Y.; Liu, W.F.; Zhang, D.C.; Chen, L. An environment-friendly process of lead recovery from spent lead paste. Sep. Purif. Technol. 2020, 233, 116035. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, Z.; Yu, Q.; Zhu, W.; Li, S.T.; Han, L.; Li, S.T.; Lu, X.; Yuan, J.L.; Lv, Z.; et al. Preparation of high-purity lead carbonate and lead oxide from spent lead paste. J. Clean. Prod. 2022, 372, 133786. [Google Scholar] [CrossRef]



| PbO2 | PbO | PbSO4 | Pb | Reference |
|---|---|---|---|---|
| 15 mL 50% (v/v) HNO3; 5 mL 2.5% (v/v) H2O2; Gently shake for 30 min | 60 mL 5% CH3COOH; Dissolve with stirring for 30 min | 150 mL 25% NaCl solution; Dissolve with continuous stirring for 1 h or leave overnight after stirring | 50 mL 2% (v/v) HNO3; Dissolve with continuous stirring for 30 min | [26] |
| 10 mL 20% (v/v) HNO3; 5 mL 1% H2O2; Gently shake | 40 mL 5% CH3COOH; Dissolve with stirring for 3~5 min; leave for 15 min | 25% NaCl solution; Dissolve with continuous stirring and leave for 1 h | Slightly hot 30 mL 20% (v/v) HNO3 solution; Rinsed in several times | [27] |
| 3 wt.% H2O2 | 20 vol.% CH3COOH; | 20 wt.% ammonium acetate; Dissolved by heating, slightly boiled for 5 min | 40 vol.% HNO3; Dissolve by heating, boil slightly for 5 min | [28] |
| Number | Simulated Lead Paste g | Determination of PbO g | |
|---|---|---|---|
| Pb | PbO2 | ||
| 1# | 0.250 | / | 0.02 |
| 2# | / | 0.750 | 0.00 |
| 3# | 0.250 | 0.750 | 0.15 |
| Number | Simulated Lead Paste g | Determination of PbO2 g | |
|---|---|---|---|
| Pb | PbO2 | ||
| 1# | 0.033 | 0.100 | 0.13 g |
| Number | Simulated Lead Paste g | Determination of Pb g | |
|---|---|---|---|
| Pb | PbO2 | ||
| 1# | 0.250 | / | 0.07 |
| 2# | / | 0.750 | 0.01 |
| 3# | 0.250 | 0.750 | 0.35 |
| Temperature | Pb | PbO2 | ||
|---|---|---|---|---|
| Fitted Equation | R2 | Fitted Equation | R2 | |
| 10 °C | 1 − (1 − α)1/3 = 0.0155 + 0.0022 × t | 0.9619 | 1 − (1 − α)1/3 = 0.0338 + 0.0032 × t | 0.9512 |
| 15 °C | 1 − (1 − α)1/3 = 0.0298 + 0.0028 × t | 0.9137 | 1 − (1 − α)1/3 = 0.0316 + 0.0052 × t | 0.9701 |
| 25 °C | 1 − (1 − α)1/3 = 0.0460 + 0.0031 × t | 0.8436 | 1 − (1 − α)1/3 = 0.1109 + 0.0055 × t | 0.7455 |
| 35 °C | 1 − (1 − α)1/3 = 0.0508 + 0.0036 × t | 0.8584 | 1 − (1 − α)1/3 = 0.1076 + 0.0071 × t | 0.8401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xie, F.; Wang, W.; Bai, Y.-L. A Novel Quantitative Analysis Method for Lead Components in Waste Lead Paste. Metals 2023, 13, 1517. https://doi.org/10.3390/met13091517
Zhang Z, Xie F, Wang W, Bai Y-L. A Novel Quantitative Analysis Method for Lead Components in Waste Lead Paste. Metals. 2023; 13(9):1517. https://doi.org/10.3390/met13091517
Chicago/Turabian StyleZhang, Zhuang, Feng Xie, Wei Wang, and Yun-Long Bai. 2023. "A Novel Quantitative Analysis Method for Lead Components in Waste Lead Paste" Metals 13, no. 9: 1517. https://doi.org/10.3390/met13091517




