Influence of UV Illumination on the Corrosion Behavior of New 3Ni Weathering Steel in Marine Atmospheric Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Accelerated Cycle Corrosion
2.3. Electrochemical Testing
2.4. Corrosion Product Mineral Analysis
3. Result
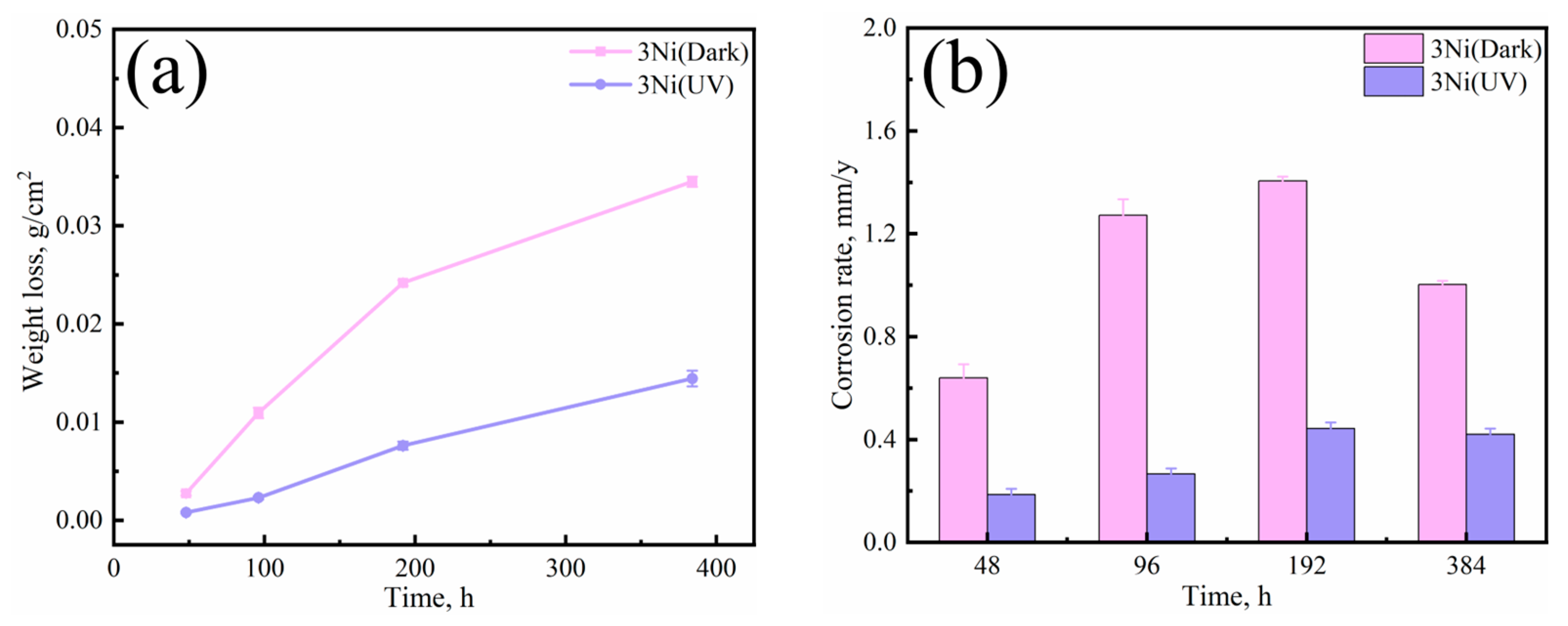
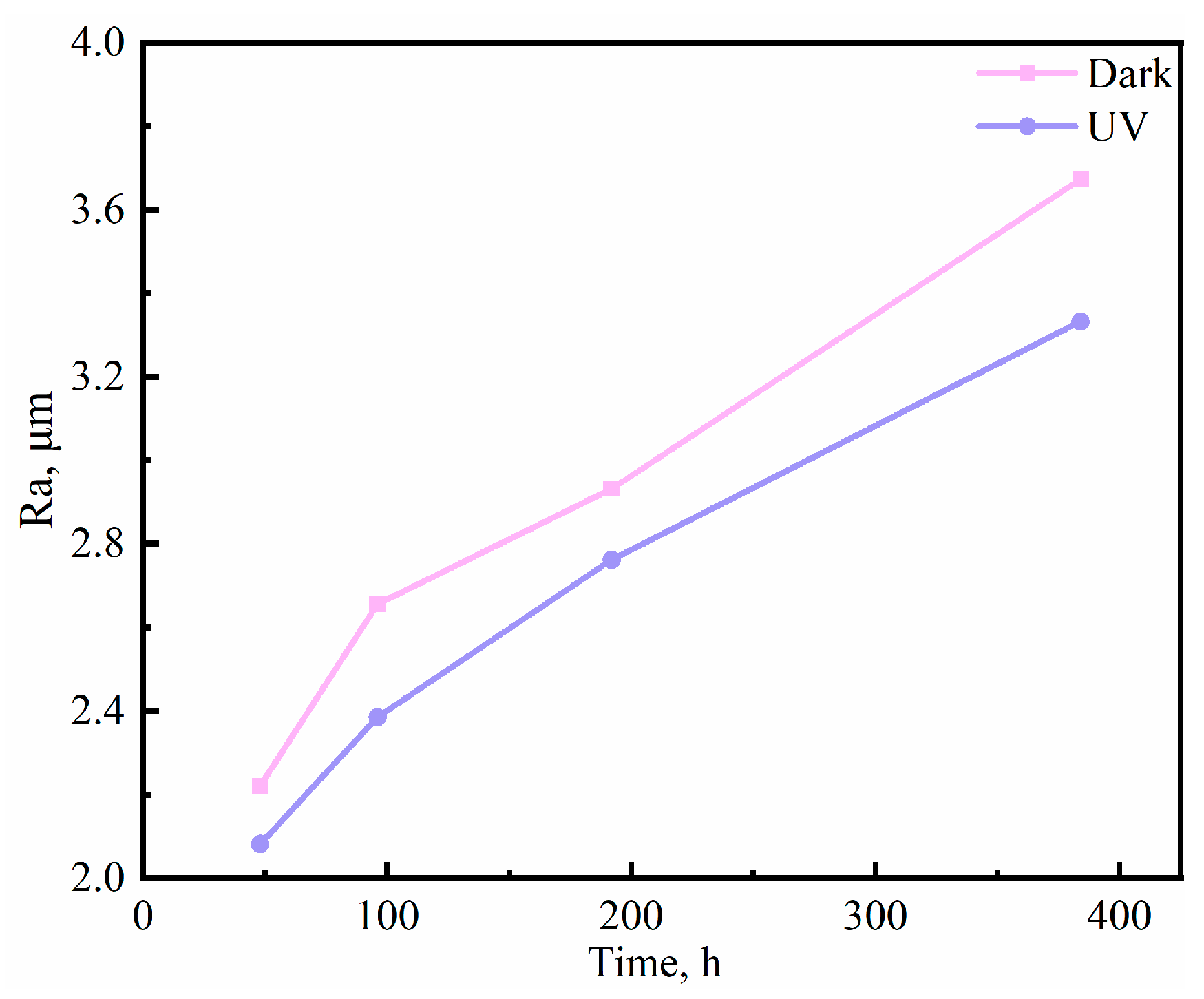
3.1. Corrosion Kinetics
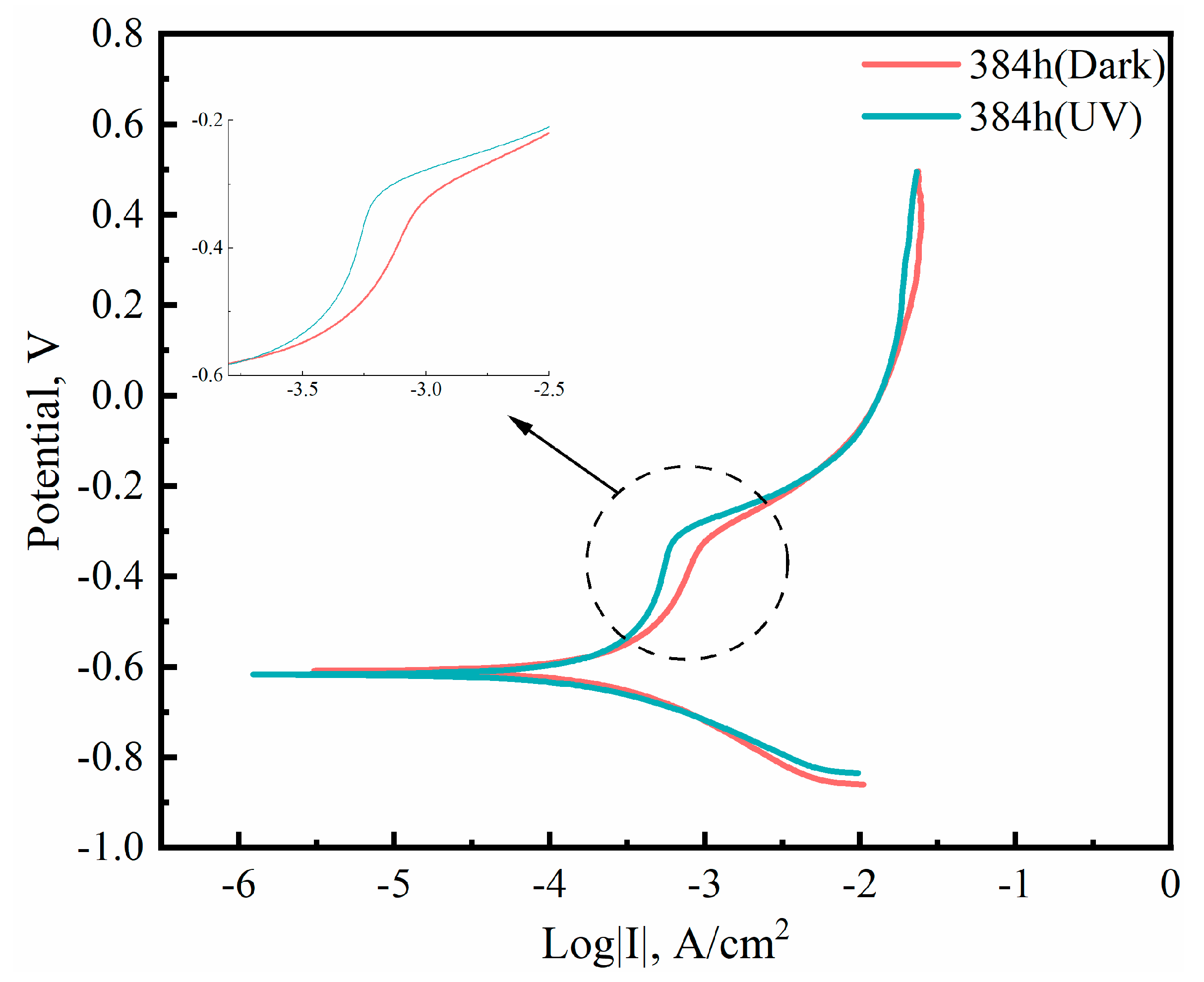
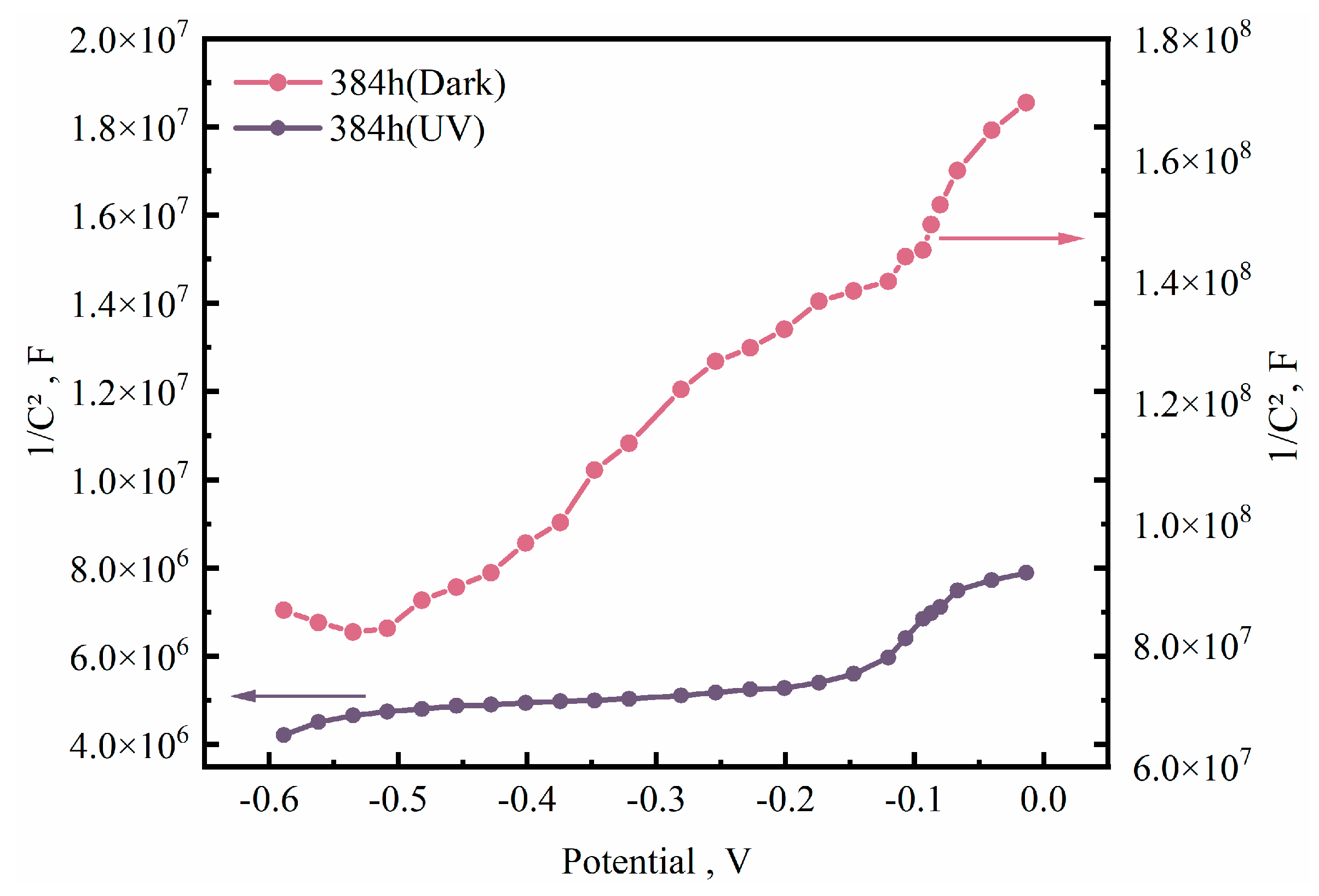
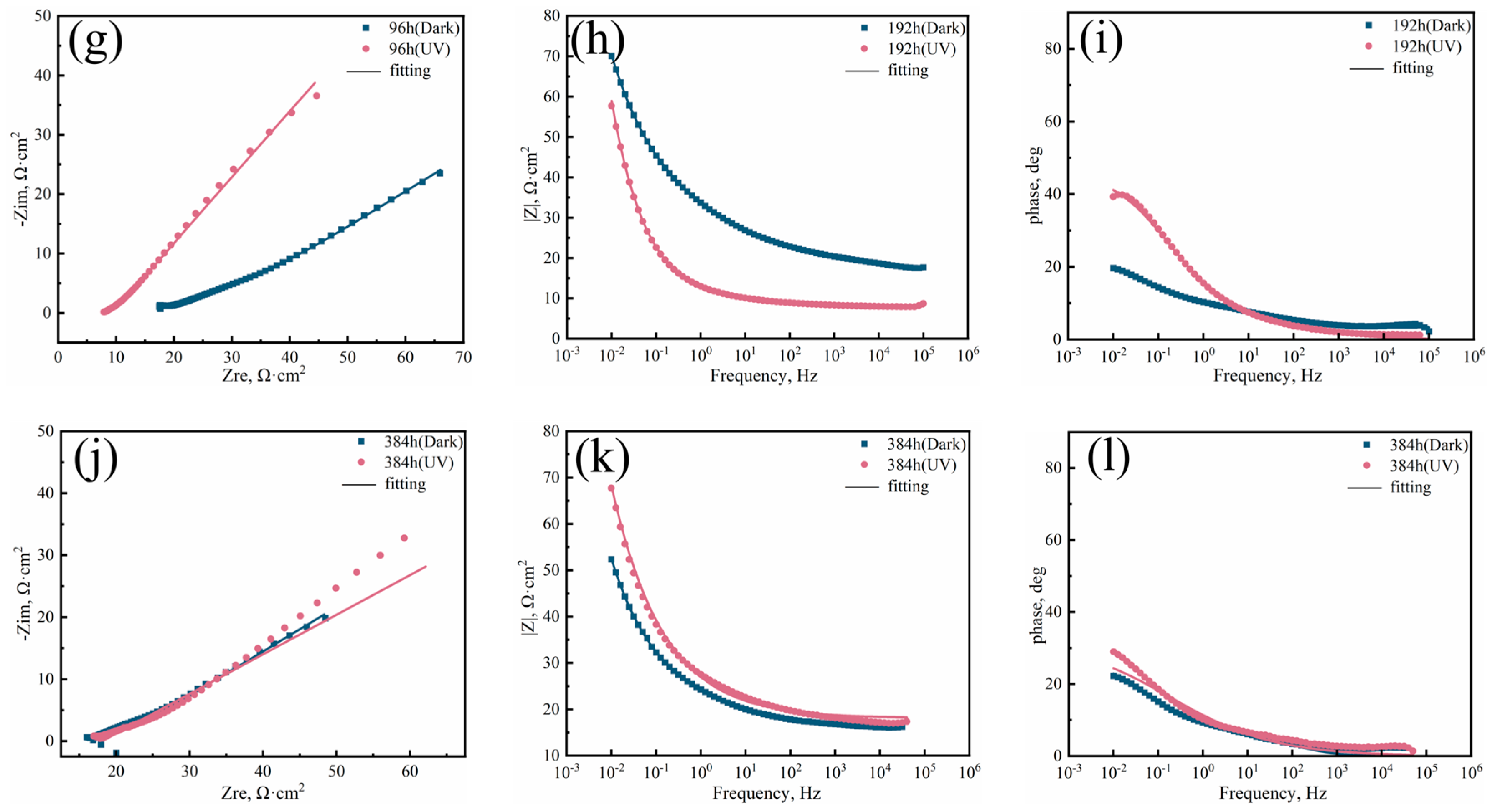
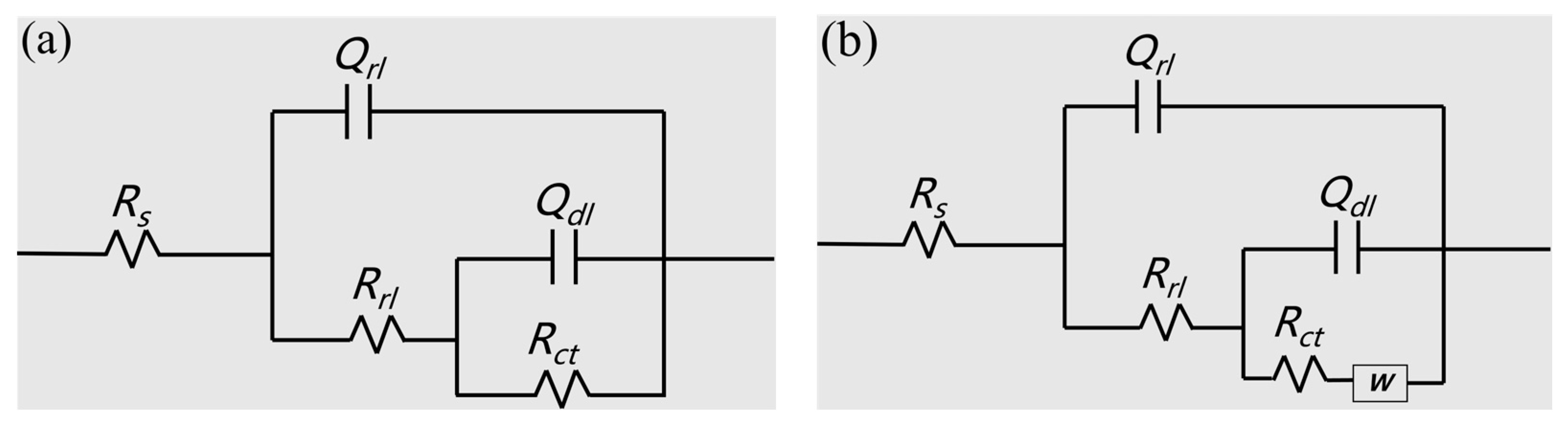
3.2. Electrochemical Testing
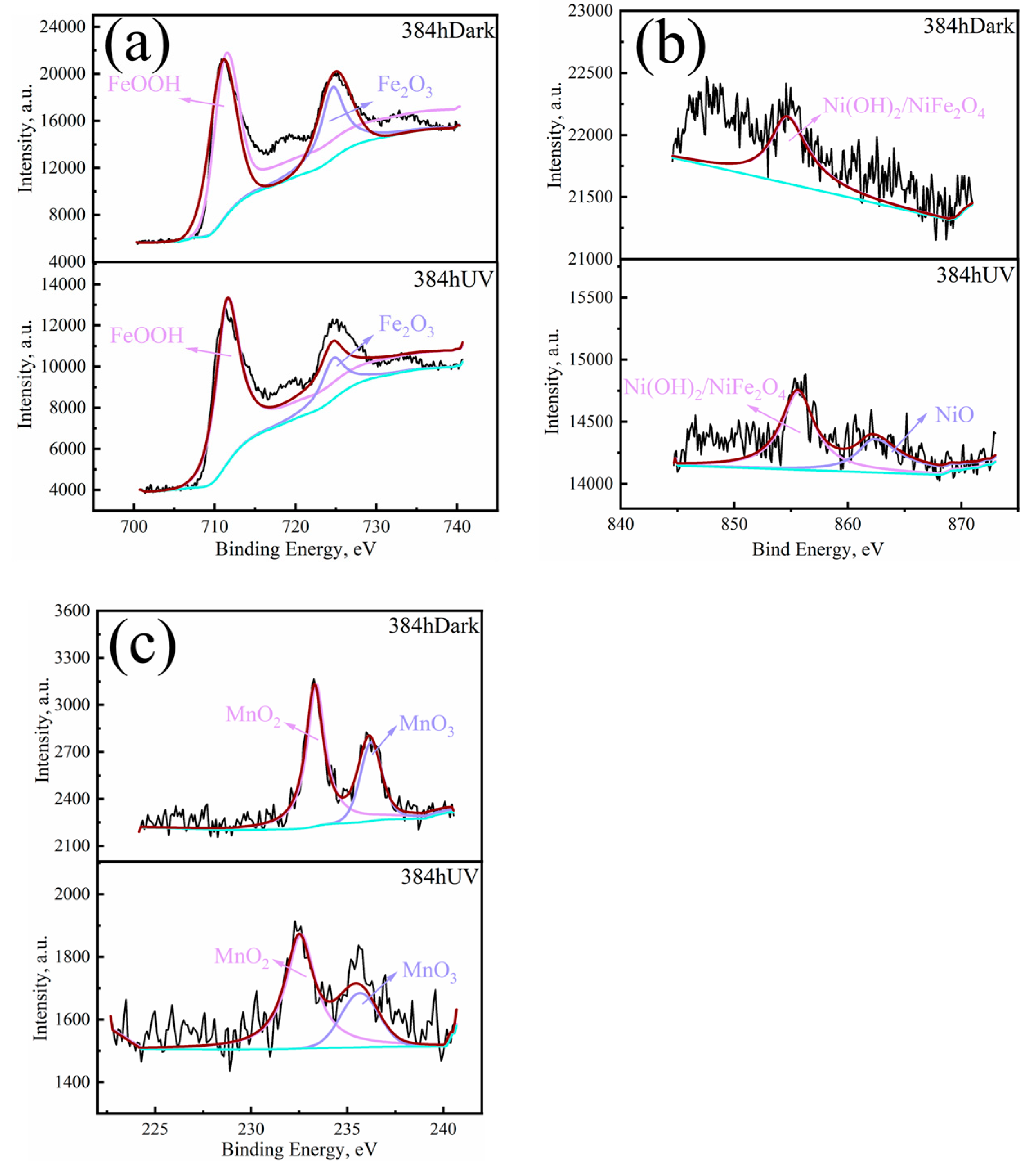
3.3. Composition of the Material Phase
3.4. CPF Analysis
4. Discussion
5. Conlusions
- (1)
- Comparing the corrosion process of 3Ni steel in two marine atmospheres with darkness and UV illumination, it can be seen that 3Ni steel in the two environments show different corrosion rates, corrosion morphology, physical composition of corrosion products and properties.
- (2)
- The influence of the marine atmospheric environment on the corrosion process of 3Ni steel with the involvement of UV illumination is related to the photovoltaic effect of corrosion products with semiconductor properties.
- (3)
- The marine atmospheric environment, in which UV exposure occurs, increases the conductivity of corrosion products through the photovoltaic effect of the corrosion products, which in turn alters the physical phase composition of the corrosion products and improves the densification of the corrosion products.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Zhang, T.; Chen, L.; Fan, Y.; Zhao, Y.; Li, H.; Yang, W.; Sun, Y. Clarifying the effect of a small amount of Cr content on the corrosion of Ni-Mo steel in tropical marine atmospheric environment. Corros. Sci. 2023, 210, 110813. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Wu, W.; Jiang, S.; Yang, J.; Liu, Z. Optimizing the resistance of Ni-advanced weathering steel to marine atmospheric corrosion with the addition of Al or Mo. Constr. Build. Mater. 2021, 279, 122341. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; He, Z.; Lu, L.; Huang, Y. Influence of chloride deposition rate on rust layer protectiveness and corrosion severity of mild steel in tropical coastal atmosphere. Mater. Chem. Phys. 2021, 259, 123971. [Google Scholar] [CrossRef]
- Li, Z.; Song, J.; Chen, J.; Yu, Q.; Xiao, K. Corrosion behavior of a high-strength steel E690 in aqueous electrolytes with different chloride concentrations. J. Mater. Res. Technol. 2023, 22, 596–604. [Google Scholar] [CrossRef]
- Li, G.; Wu, W.; Chai, P.; Yang, X.; Song, L. Influence of Cr and Ni elements on the electrochemical and early corrosion behavior of FeMnAlC low-density steel. J. Mater. Res. Technol. 2023, 23, 5892–5906. [Google Scholar] [CrossRef]
- Di Sarno, L.; Majidian, A.; Karagiannakis, G. The Effect of Atmospheric Corrosion on Steel Structures: A State-of-the-Art and Case-Study. Buildings 2021, 11, 571. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.; Xu, H.; Cai, Y.; Yan, X. Corrosion Behavior and Mechanical Properties of 30CrMnSiA High-Strength Steel under an Indoor Accelerated Harsh Marine Atmospheric Environment. Materials 2022, 15, 629. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, G.-I.; Son, G.-H.; Yoo, Y.-H.; Hong, S.; Kim, J.-G. New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials 2023, 16, 3132. [Google Scholar] [CrossRef]
- Krivy, V.; Kubzova, M.; Kreislova, K.; Urban, V. Characterization of Corrosion Products on Weathering Steel Bridges Influenced by Chloride Deposition. Metals 2017, 7, 336. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Song, L.; Ma, X.; Chen, Z.; Hou, B. The role of UV illumination on the initial atmospheric corrosion of 09CuPCrNi weathering steel in the presence of NaCl particles. Corros. Sci. 2014, 87, 427–437. [Google Scholar] [CrossRef]
- Jing, J.; Chen, Z.; Feng, C. Using the photoinduced volt-ampere curves to study the p/n types of the corrosion products with semiconducting properties. J. Electroanal. Chem. 2021, 881, 114961. [Google Scholar] [CrossRef]
- Guo, M.; Lu, X.; Tang, J.; Pan, C.; Wang, Z. Corrosion Behavior of 316 Stainless Steel Exposed to a Simulated Salt lake Atmospheric Environment under UV Illumination. Int. J. Electrochem. Sci. 2021, 16, 210457. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Z.; Cheng, X.; Li, X.; Liu, B. Atmospheric Corrosion Behavior and Mechanism of a Ni-Advanced Weathering Steel in Simulated Tropical Marine Environment. J. Mater. Eng. Perform. 2017, 26, 6075–6086. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Liu, X.; Hou, J.; Sun, M.; Zeng, R. Study on the Mechanism of the Photoelectrochemical Effect on the Initial NaCl-Induced Atmospheric Corrosion Process of Pure Copper Exposed in Humidified Pure Air. J. Electrochem. Soc. 2018, 165, C608–C617. [Google Scholar] [CrossRef]
- Song, S.; Chen, Z. Effect of UV Illumination on the NaCl-Induced Atmospheric Corrosion of Pure Zinc. J. Electrochem. Soc. 2014, 161, C288–C293. [Google Scholar] [CrossRef]
- Lin, H.; Frankel, G.S. Accelerated Atmospheric Corrosion Testing of Ag. Corrosion 2013, 69, 1060–1072. [Google Scholar] [CrossRef]
- Toshiyasu Nishimura, H.K.K.N.; Kodama, T. Effect of Co and Ni on the corrosion behavior of low alloy steel in wet/dry environments. Corros. Sci. 2000, 9, 1611–1621. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. A study of the evolution of rust on Mo–Cu-bearing fire-resistant steel submitted to simulated atmospheric corrosion. Corros. Sci. 2012, 54, 244–250. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of atmospheric corrosion of MnCuP weathering steel in a simulated coastal-industrial atmosphere. Corros. Sci. 2012, 59, 270–276. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Electronic structure and pitting susceptibility of passive film on carbon steel. Electrochim. Acta 1999, 17, 2947–2957. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng, X.; Dong, C.; Xu, L.; Li, X. Passivity of 316L stainless steel in borate buffer solution studied by Mott–Schottky analysis, atomic absorption spectrometry and X-ray photoelectron spectroscopy. Corros. Sci. 2010, 52, 3646–3653. [Google Scholar] [CrossRef]
- Stratmann, M.; Bohnenkamp, K.; Engell, H.-J. An electrochemical study of phase-transitions in rust layers. Corros. Sci. 1983, 23, 969–985. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Zhao, J.; Li, X. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, L.; Zhang, L.; Wang, Q.; Wang, T. Structure of the rust layer of weathering steel in A high chloride environment: A detailed characterization via HRTEM, STEM-EDS, and FIB-SEM. Corros. Sci. 2020, 177, 108997. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, C.; Niu, D.; Xu, J.; Li, M. Influence of alloyed chromium on the atmospheric corrosion resistance of weathering steel. Corros. Sci. 2013, 74, 424–429. [Google Scholar] [CrossRef]
- Rubim, J.C.; Sousa, M.H.; Silva, J.C.O.; Tourinho, F.A. Raman spectroscopy as a powerful technique in the characterization of ferrofluids. Braz. J. Phys. 2001, 31, 402–408. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Cano, H.; Lopesino, P.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steel in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Chen, L.; Dong, B.; Yang, W.; Fan, Y.; Zhao, Y. On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl concentrations. Corros. Sci. 2021, 192, 109851. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, H.; Gu, Y. Effect of alloyed Cr on corrosion behavior of low-alloy steel in wet atmosphere. Mater. Corros. 2022, 73, 918–931. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, X.; Zhang, Y.; Wu, H.; Guo, H. Research on corrosion mechanism of microstructure on low alloy steel in humid atmosphere. Mater. Today Commun. 2022, 31, 103715. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; Yang, J.; Xiao, D.; Luo, D.; Zhou, W.; Tuo, C.; Wu, H.; Niu, G. Effect of tempering on corrosion behavior and mechanism of low alloy steel in wet atmosphere. J. Mater. Res. Technol. 2022, 20, 4077–4096. [Google Scholar] [CrossRef]
- Gong, K.; Wu, M.; Liu, G. Comparative study on corrosion behavior of rusted X100 steel in dry/wet cycle and immersion environments. Constr. Build. Mater. 2020, 235, 117440. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Nishimura, T.; Tanaka, K.-I.; Shimizu, Y. Effect of NaCl on rusting of steel in wet and dry corrosion cycle. Tetsu-Hagané 1995, 11, 1079–1084. [Google Scholar] [CrossRef]
- Watkinson, D.; Lewis, M.R.T. The Role of βFeOOH in the Corrosion of Archaeological Iron. MRS Proc. 2004, 852, OO1-6. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeuchi, K.; Kandori, K. Nakayama Transformation of γ-FeOOH to α-FeOOH in acidic solutions containing metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 266, 155–159. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment. Mater. Chem. Phys. 2008, 112, 844–852. [Google Scholar] [CrossRef]
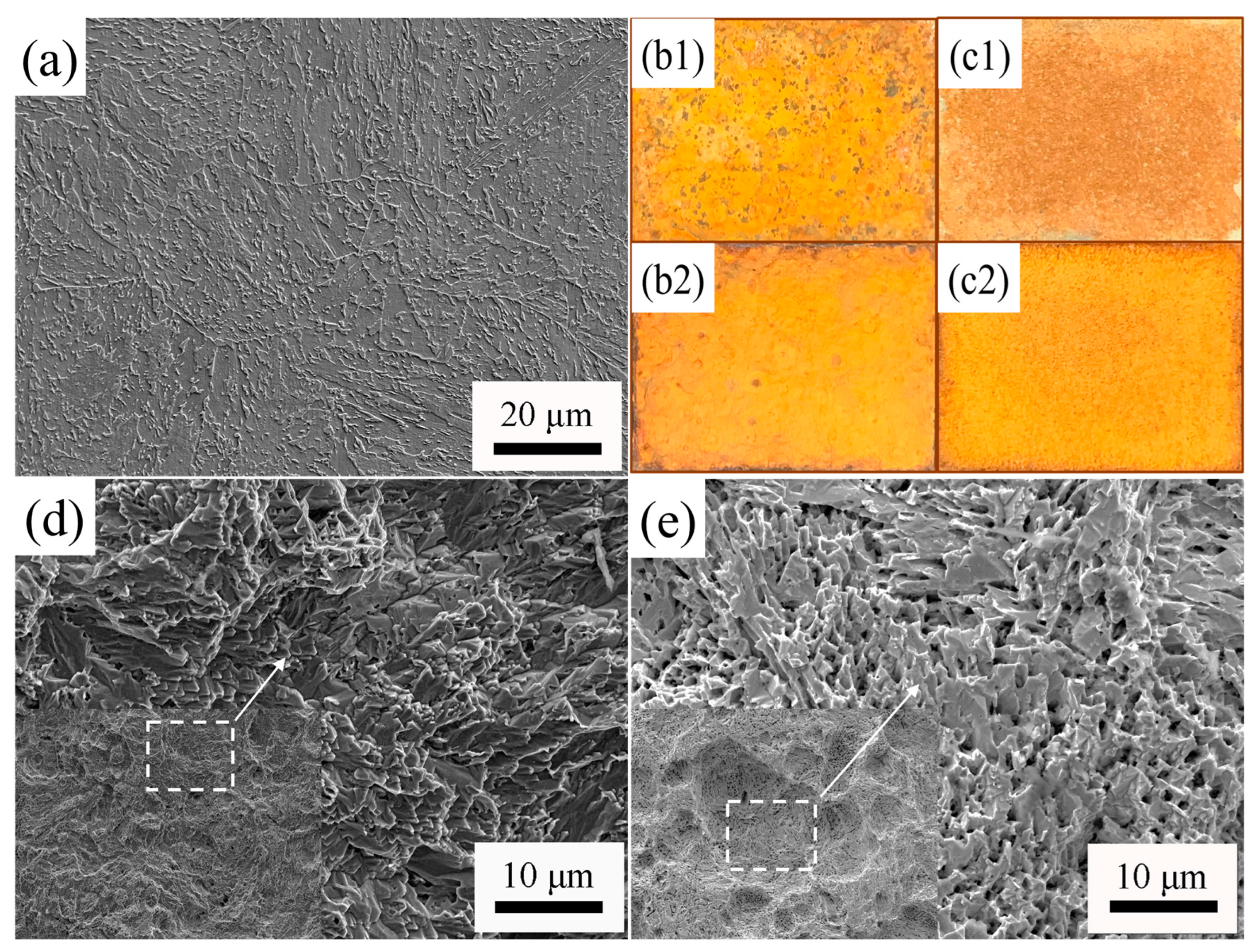






| Steel | C | Si | Mn | P | S | Ni | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 3Ni | 0.08 | 0.25 | 1.02 | 0.09 | 0.04 | 3.17 | 1.98 | 0.23 | Bal |
| Steel | Rs (Ω·cm2) | CPF (Ω−1 cm−2.sn) | Rrl (Ω·cm2) | CPF (Ω−1 cm−2.sn) | Rct (Ω·cm2) | W × 10−3 | |||
|---|---|---|---|---|---|---|---|---|---|
| Qrl | n1 | Qdl | n2 | ||||||
| 3Ni Dark | 48 | 7.961 | 0.137 | 0.6184 | 2.877 | 2.608 | 0.834 | 1847 | \ |
| 96 | 10.14 | 0.1141 | 0.4414 | 2.487 | 9.948 | 0.8204 | 1051 | \ | |
| 192 | 6.845 | 0.00382 | 0.1614 | 15.05 | 0.0266 | 0.3112 | 67.3 | 0.0263 | |
| 384 | 17.19 | 0.0007 | 0.3398 | 0.2655 | 0.05693 | 0.3631 | 36.49 | 0.0295 | |
| 3Ni UV | 48 | 6.518 | 0.06327 | 0.7141 | 228.2 | 2.008 | 0.6349 | 359.5 | \ |
| 96 | 5.43 | 0.09089 | 0.5965 | 4.538 | 6.229 | 0.6751 | 2739 | \ | |
| 192 | 8.001 | 0.03467 | 0.4869 | 4.576 | 0.04949 | 0.5734 | 2911 | \ | |
| 384 | 18.08 | 0.052 | 0.3619 | 4.534 | 1.521 | 0.8481 | 1002 | 1.009 | |
| Position | Cl | Ni | Mo | Cu | HV | |
|---|---|---|---|---|---|---|
| Dark | 1 | 0.62 | 1.66 | 1.18 | 0.69 | 71 |
| 2 | 1.44 | 1.95 | 0.84 | \ | 96 | |
| 3 | 1.94 | 0.55 | 0.73 | \ | 51 | |
| UV | 4 | 1.25 | 2.1 | 3.97 | 0.97 | 69 |
| 5 | 3.46 | 2.34 | 2.55 | \ | 116 | |
| 6 | 2.18 | 1.66 | 1.55 | \ | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Yuan, R.; Zhang, Z.; Yang, Y.; Wang, Y.; Zhang, P.; Mao, X.; Wu, H. Influence of UV Illumination on the Corrosion Behavior of New 3Ni Weathering Steel in Marine Atmospheric Environments. Metals 2023, 13, 1543. https://doi.org/10.3390/met13091543
Xu H, Yuan R, Zhang Z, Yang Y, Wang Y, Zhang P, Mao X, Wu H. Influence of UV Illumination on the Corrosion Behavior of New 3Ni Weathering Steel in Marine Atmospheric Environments. Metals. 2023; 13(9):1543. https://doi.org/10.3390/met13091543
Chicago/Turabian StyleXu, Hongliang, Rui Yuan, Zhihui Zhang, Ying Yang, Yubo Wang, Pengcheng Zhang, Xinping Mao, and Huibin Wu. 2023. "Influence of UV Illumination on the Corrosion Behavior of New 3Ni Weathering Steel in Marine Atmospheric Environments" Metals 13, no. 9: 1543. https://doi.org/10.3390/met13091543





