Electrochemical and Surface Characterisation of Carbon Steel Exposed to Mixed Ce and Iodide Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Immersion Experiments
2.3. Electrochemical Polarization Measurements
2.4. X-ray Diffraction (XRD)
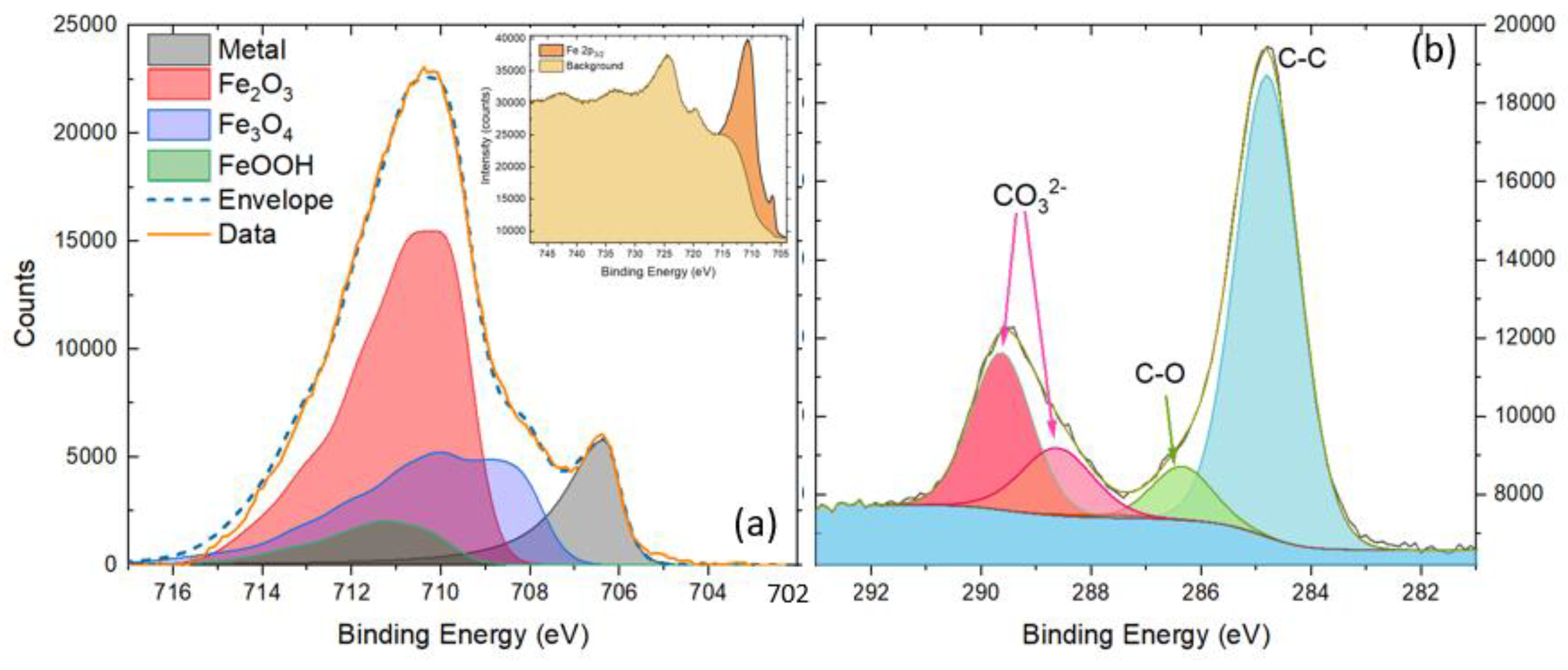
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Scanning Electron Microscopy
3. Results
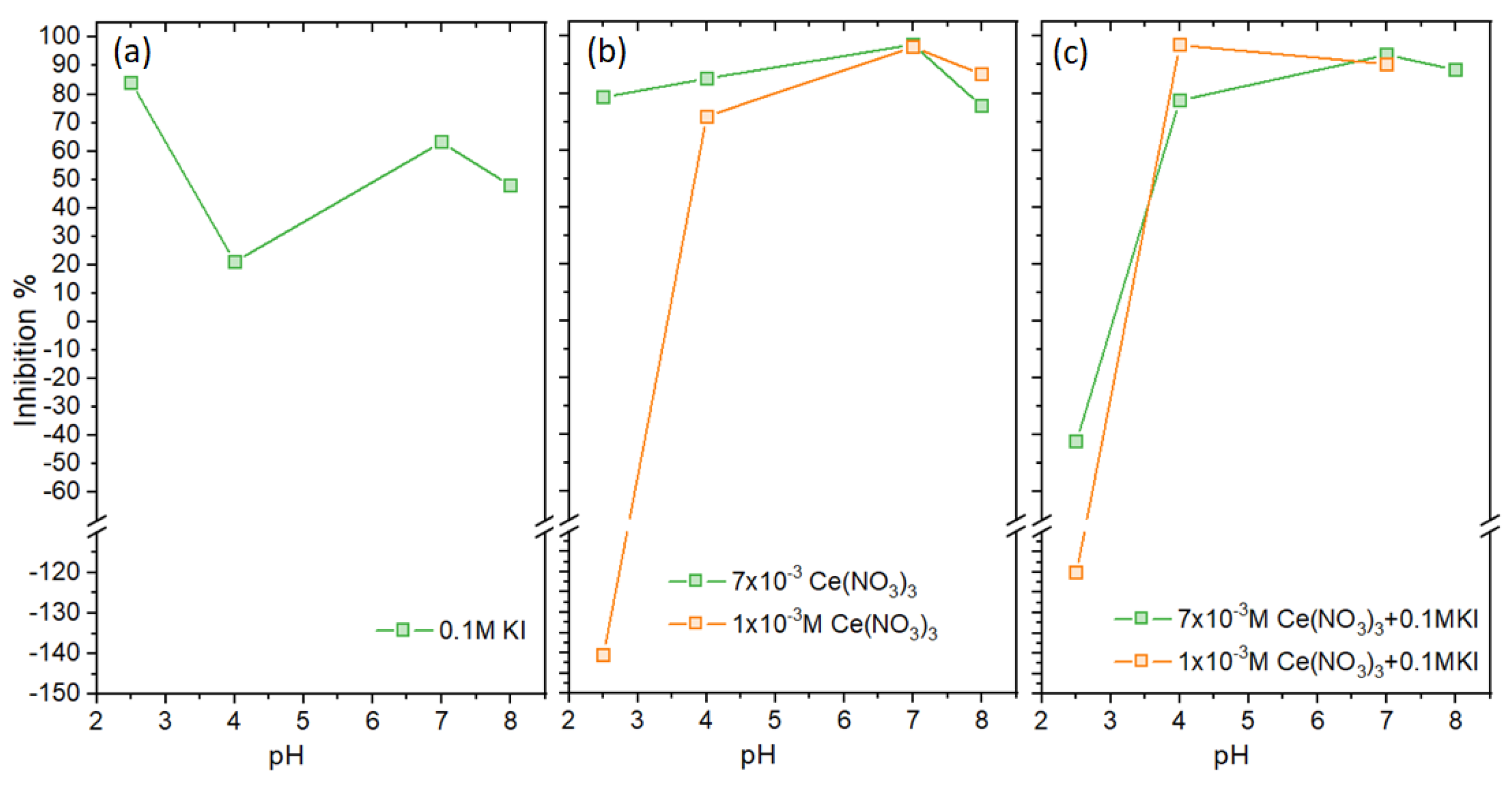
3.1. Inhibition as a Function of Cerium Concentration
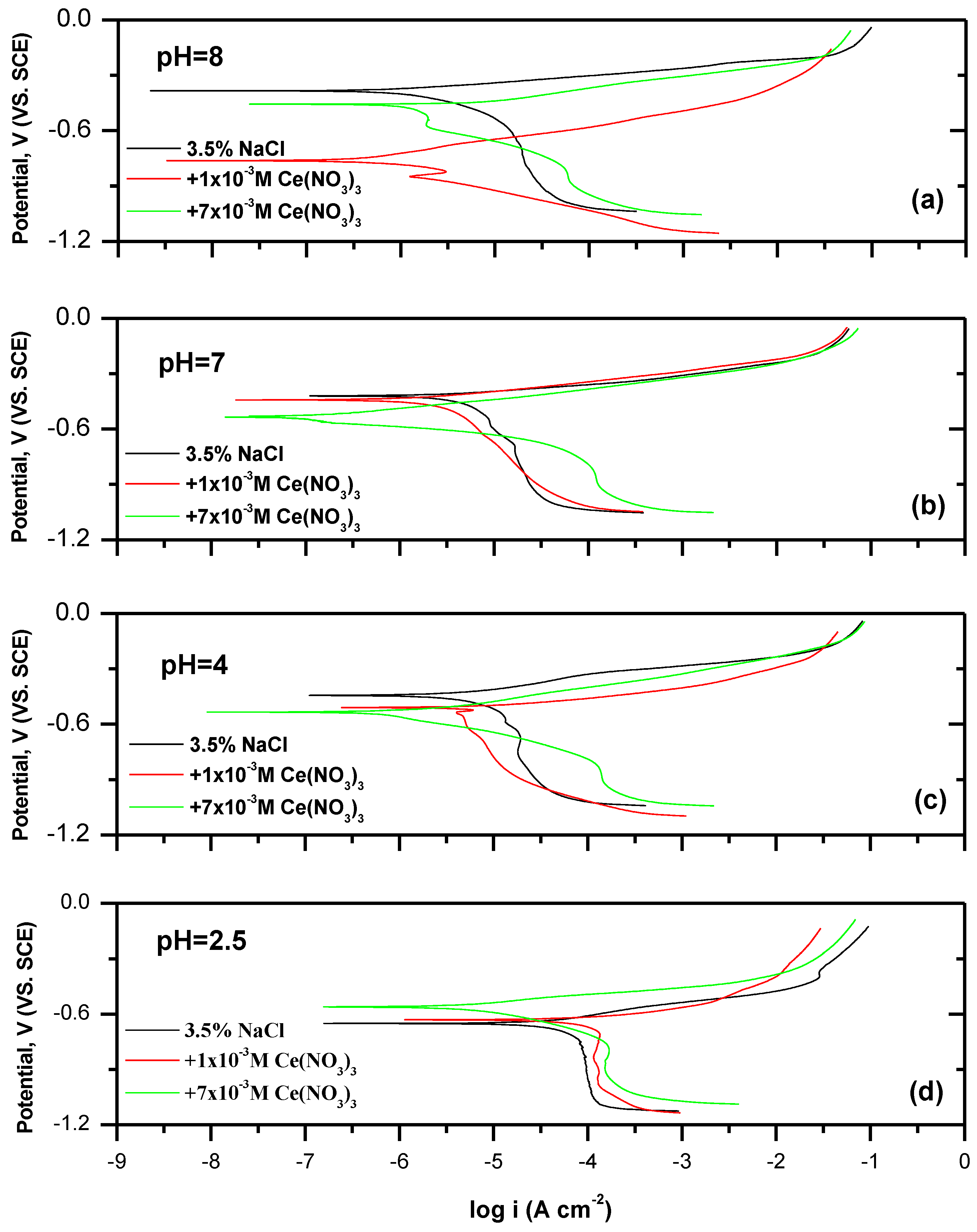
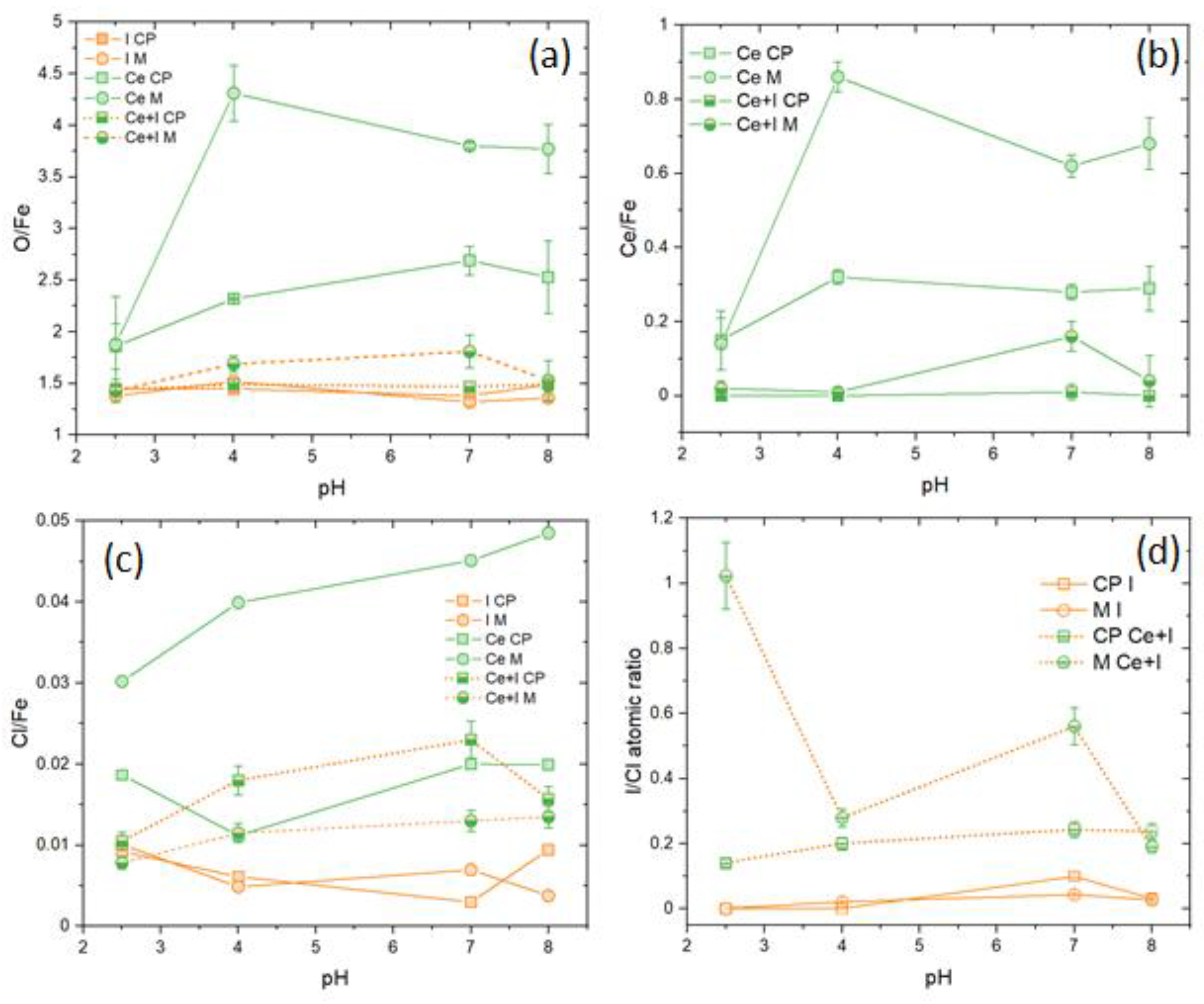
3.2. Inhibition of Ce, and Ce+I-Electrolytes as a Function of pH
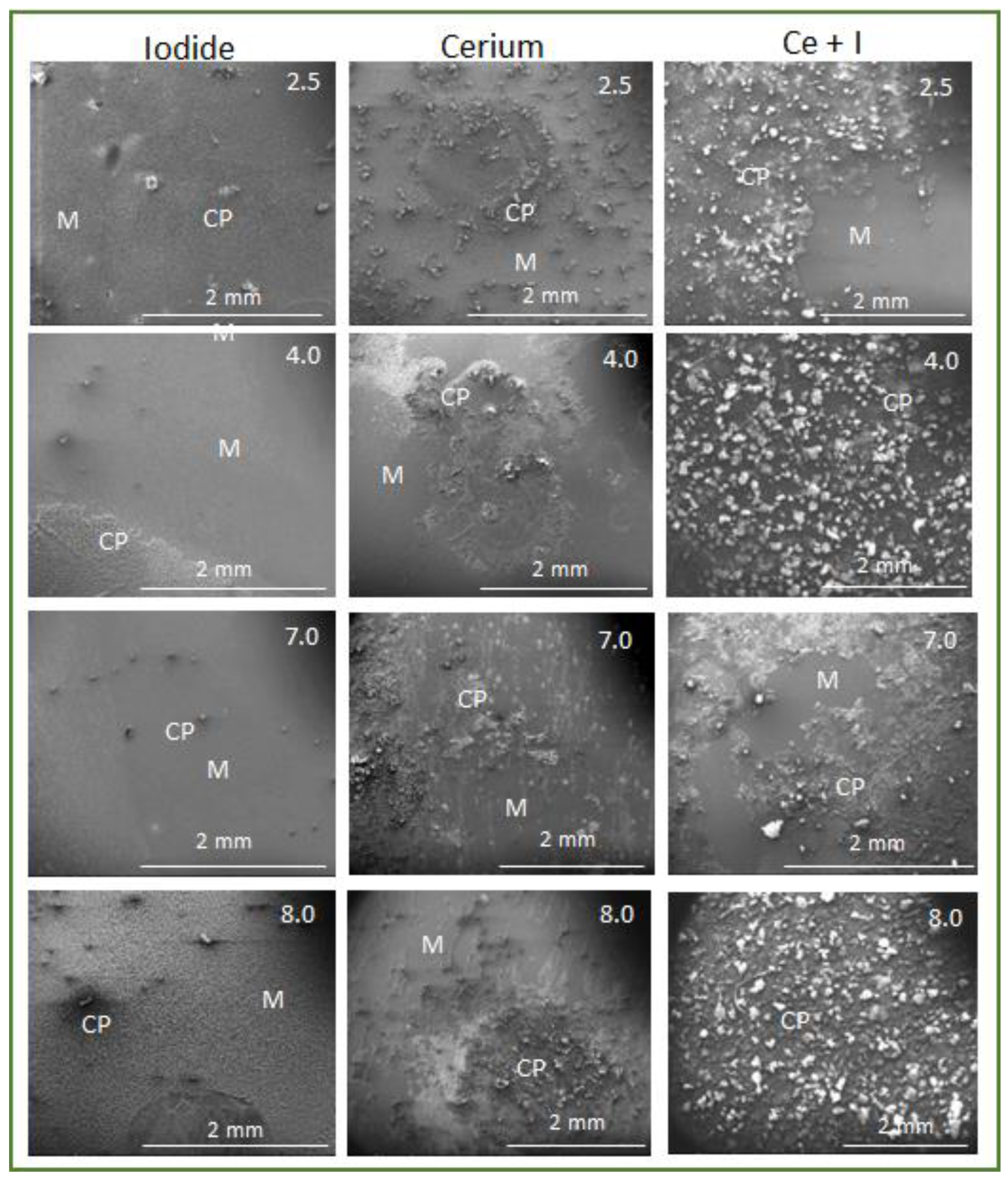
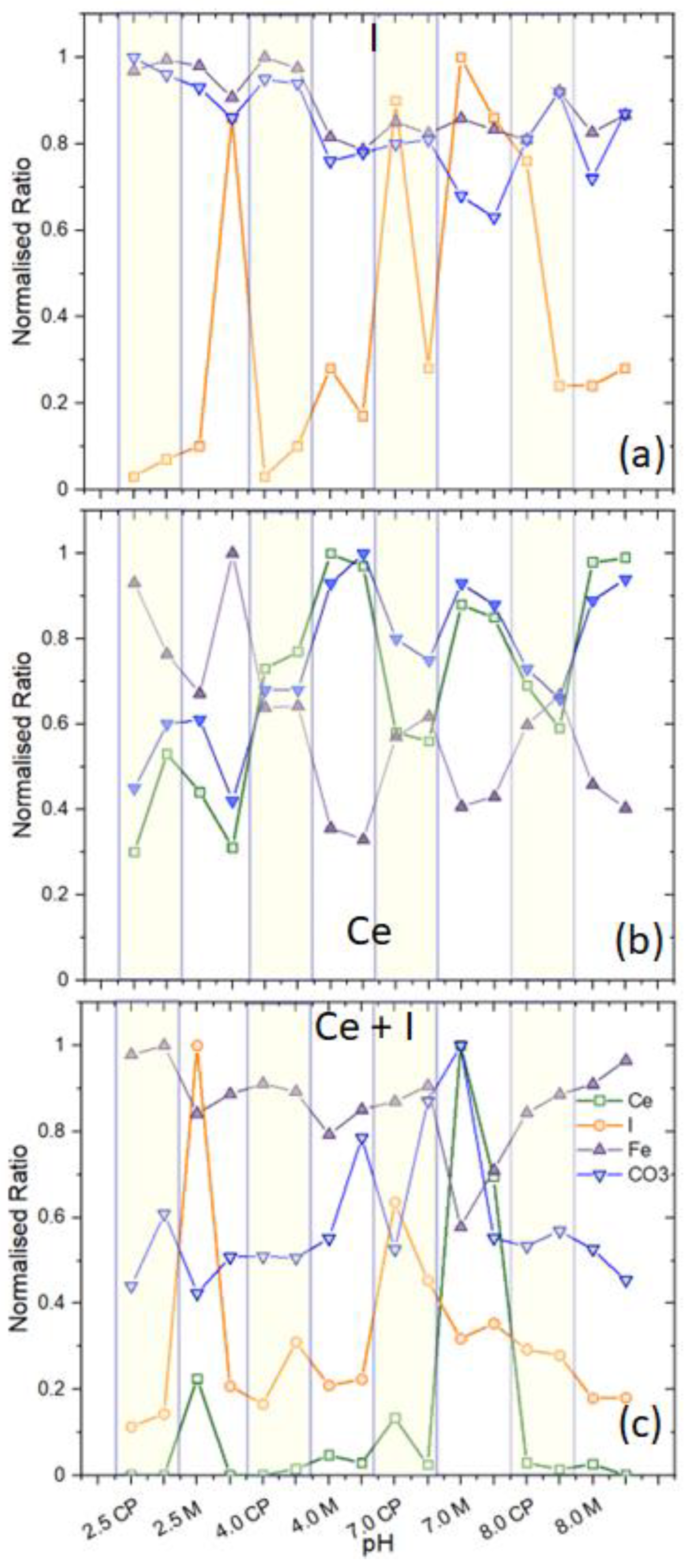
3.3. SEM Characterisation of Surfaces Exposed to Electrolytes as a Function of pH at 24 h
3.4. X-ray Diffraction
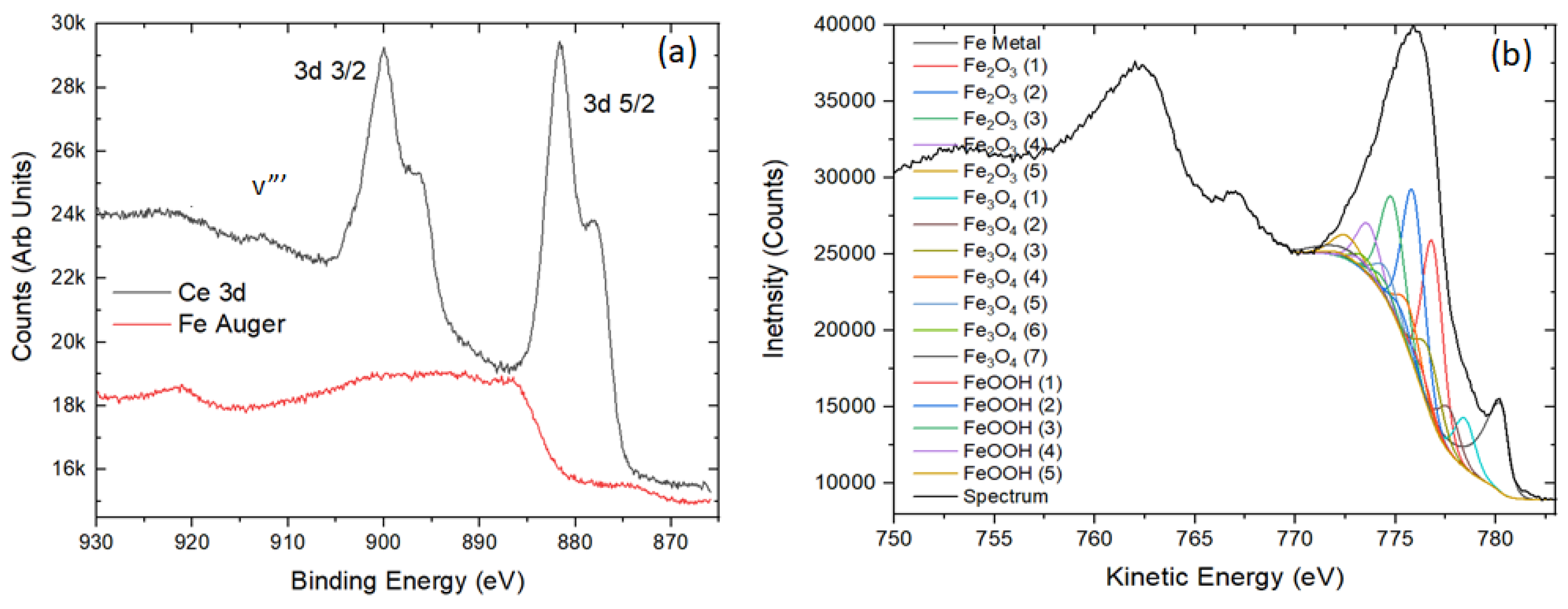
3.5. XPS Analyses
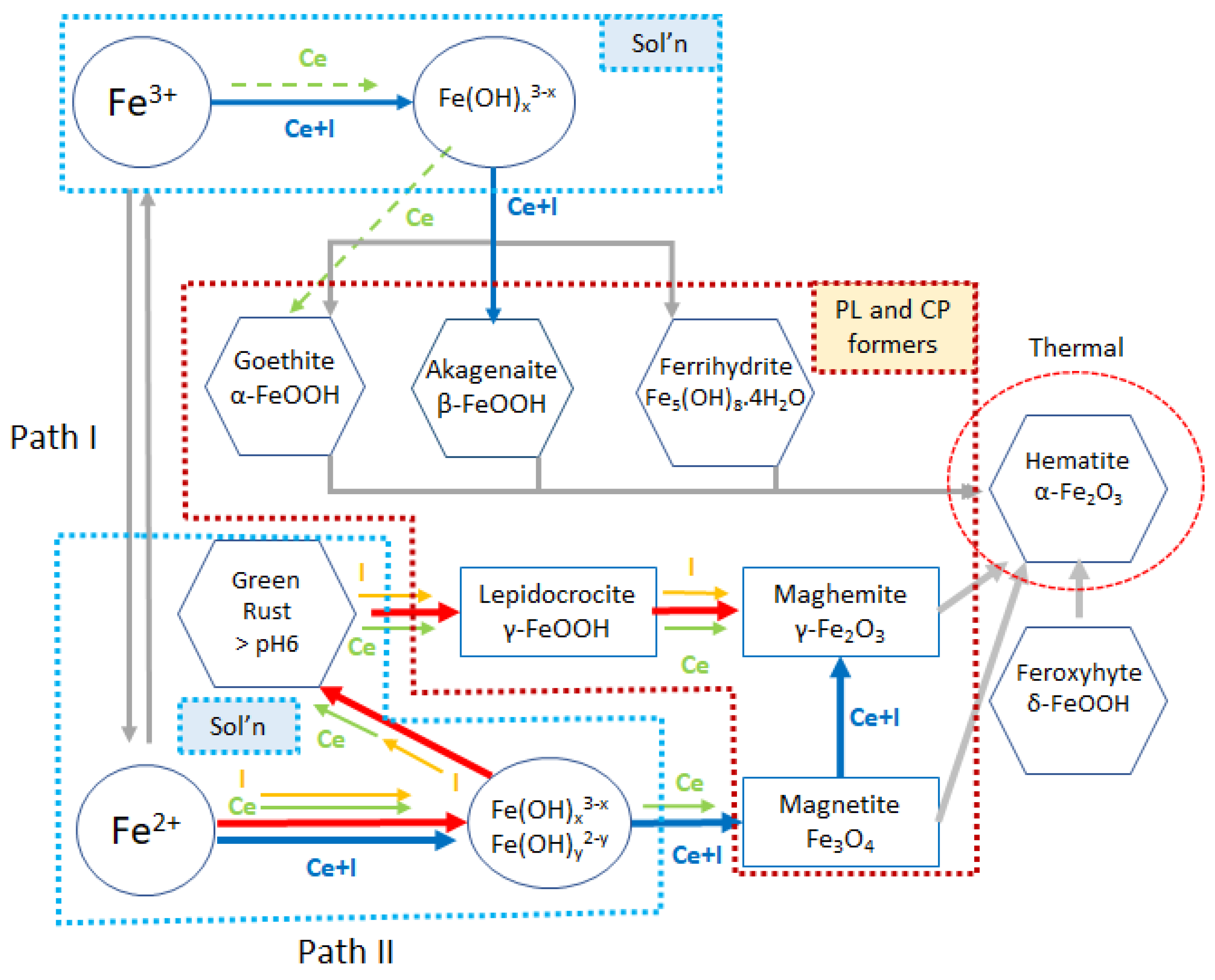
4. Discussion
5. Conclusions
- First, the optimum Ce concentration of 7 × 10−3 M at pH 7 was obtained by determining the inhibitor efficiency (IE%) from potentiodynamic polarization by examining a range of concentrations from 1 × 10−4 M to 10 × 10−3 M. An optimum of 97% was obtained for 7 × 10−3 M at pH 7.
- Next, the IE% at different pHs was determined for two Ce concentrations of 1 × 10−3 M to 7 × 10−3 M, confirming that 7 × 10−3 M Ce had the highest overall inhibitor efficiency across the pH range. The IE% of 0.1 M KI in 3.5% NaCl electrolyte was examined. It varied between 21% and 84%, with the latter observed at pH 2.
- The IE% of mixed was generally above 90%. The exception was at pH 2.5, where IE% was negative, i.e., there was promotion of corrosion. This was also reflected in weight loss experiments (see Appendix A). At higher pH values, IE% was similar to or better than the I- or Ce-electrolytes alone. Indeed, synergy was found between these two ions at pH 4, 7, and 8. Importantly, the mixture improved IE% at pH 4.
- At pH 4 and above, the formation of akageneite (β-FeOOH) was the dominant species as opposed to lepidocrocite observed in I-electrolytes and Ce electrolytes. γ-Fe2O3 was observed in both cases. This appears to form a very compact protective layer. Additionally, the widespread heterogeneous precipitation of cerium (III) compounds is probably mainly hydroxides since only Ce(III) was observed. As at pH 4, this was largely Ce(OH)3. At pH 7 and 8, greater amounts of carbonate were observed, also indicating the presence of hydroxycarbonates. Iron carbonates were not observed in XRD or XPS. It should be noted that carbonate is not part of the electrolyte, and its presence was proposed to be due to the absorption of CO2 from the atmosphere. Because it is a significant component of the surface oxides, it is reported here.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Weight Loss Measurements at pH 2.5
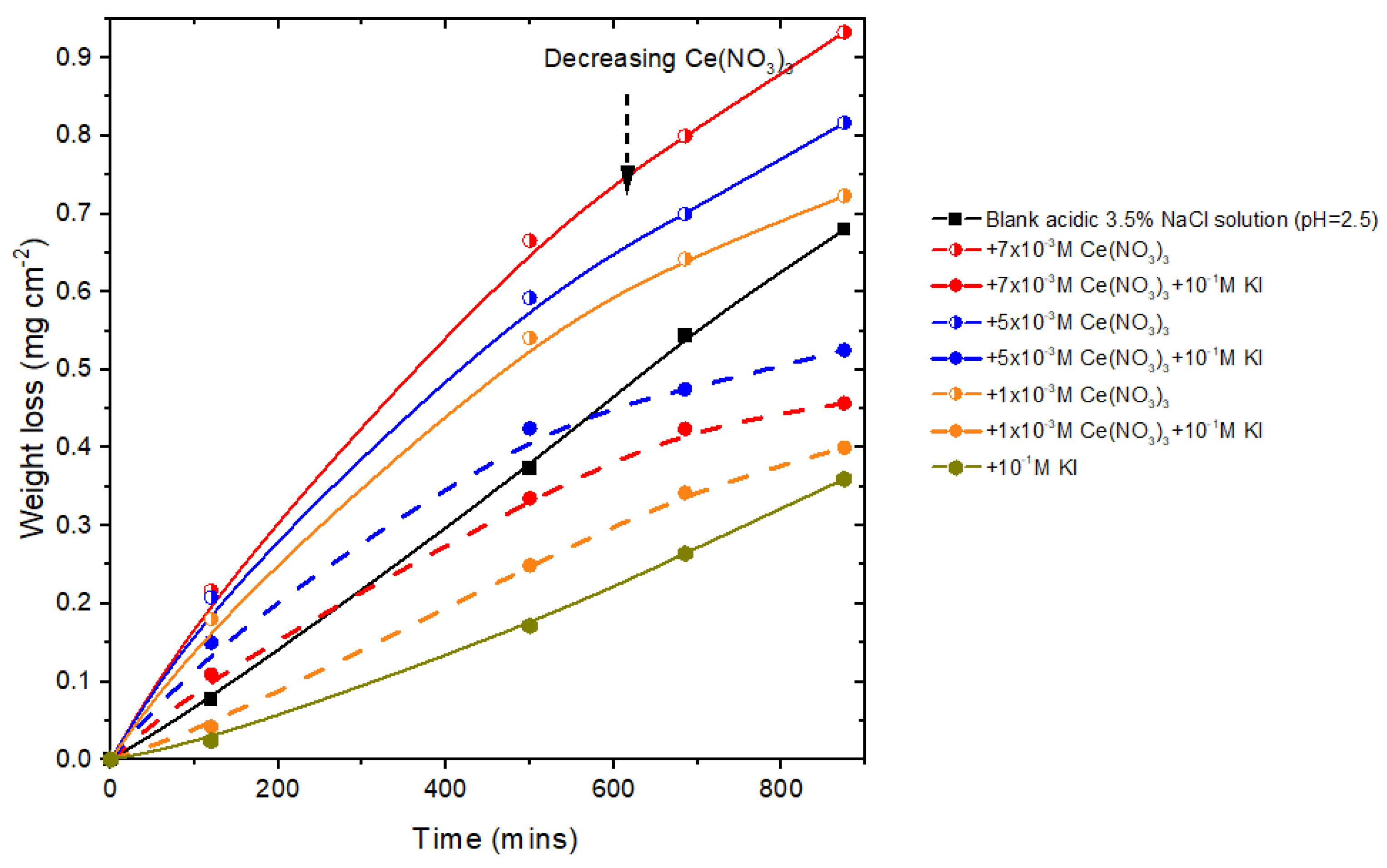
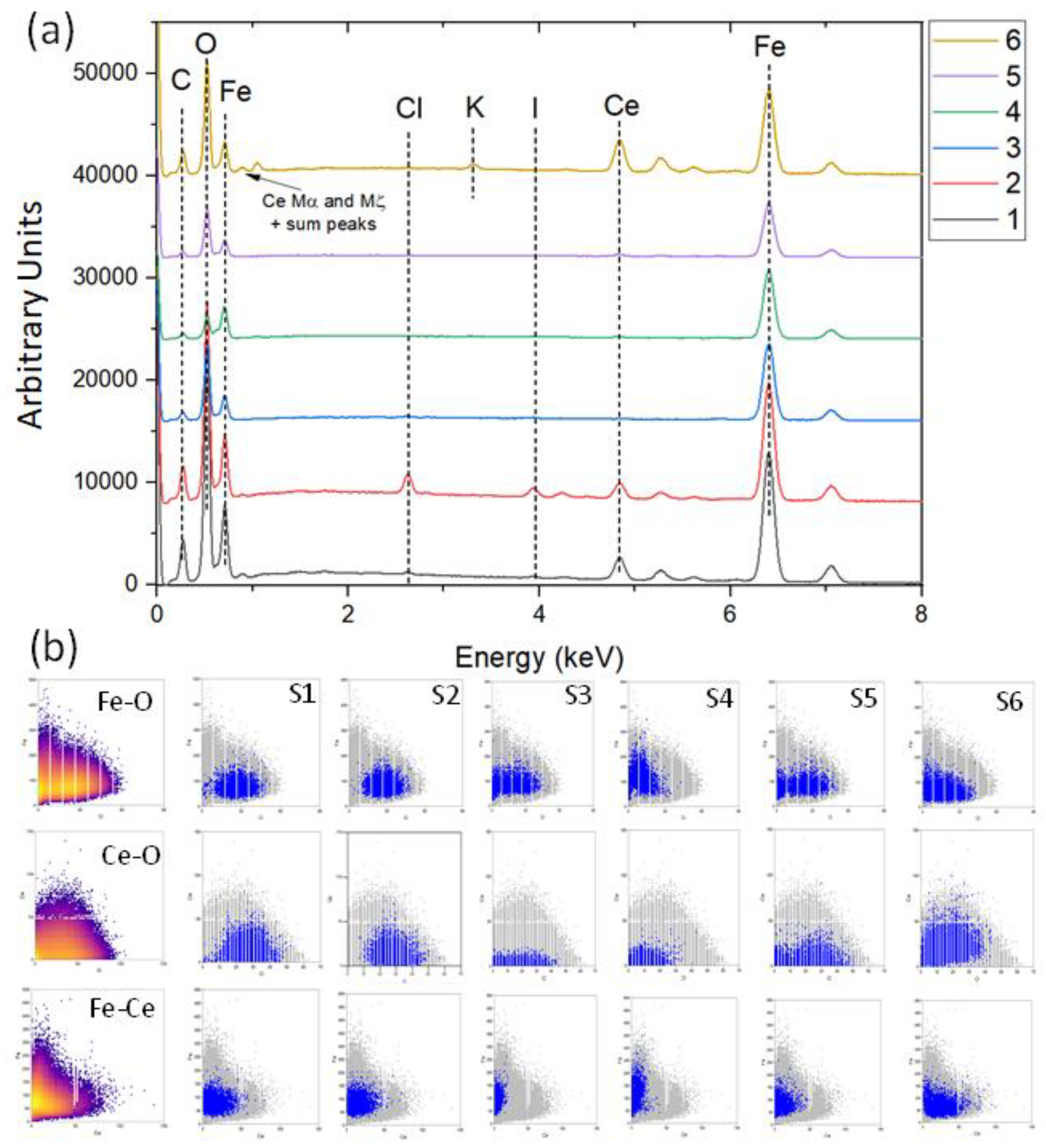
Appendix A.2. EDS Area Measurements of Individual Sites by Classification
| Feature Type | Fe | O | C | I | Ce | Cl | S |
|---|---|---|---|---|---|---|---|
| Matrix | 92.60 (S2) | 1.51 | 2.19 | - | - | 0.02 | - |
| 83.5 (S3) | 3.0 | 12.7 | - | - | - | - | |
| 83.3 (S4) | 3.2 | 12.7 | - | - | - | - | |
| 61.3 (S6) | 17.1 | 19.2 | - | 1.9 | 0.09 | 0.05 | |
| 55.4 (S7) | 18.8 | 22.7 | - | 2.6 | - | - | |
| 69.7 (S8) | 9.7 | 19.1 | - | 0.92 | - | - | |
| 76.0 (S9) | 4.5 | 18.0 | 0.01 | 0.16 | - | 0.05 | |
| 73.8 (S10) | 12.4 | 13.2 | 0.02 | 0.03 | 0.08 | - | |
| 76.9 (S11) | 13.4 | 8.4 | 0.05 | 0.22 | 0.2 | - | |
| 54.7 (S12) | 3.4 | 12.5 | - | - | - | - | |
| Surface Particulates | 78.6 (S2) | 20.24 | 2.70 | 0.06 | - | ||
| 67.1 (S4) | 11.45 | 20.3 | - | - | 0.06 | - | |
| 32.8 (S7) | 40.0 | 18.7 | - | 8.0 | 0.21 | 0.15 | |
| 25.6 (S7) | 43.0 | 19.7 | - | 10.8 | 0.54 | 0.20 | |
| 37.9 (S9) | 39.7 | 21.4 | 0.01 | 0.87 | 0.08 | - | |
| 42.8 (S11) | 35.4 | 6.5 | 0.54 | 9.9 | 4.08 | 0.12 | |
| Flaky | 31.2 (S6) | 54.5 | 12.4 | - | 1.5 | 0.11 | 0.04 |
| 35.9 (S7) | 49.9 | 12.6 | - | 1.2 | 0.21 | - | |
| 32.7 (S10) | 47.0 | 13.4 | - | 5.4 | 0.14 | - | |
| 41.9 (S11) | 49.5 | 5.0 | 0.23 | 0.19 | 3.0 | - | |
| 39.9 (S12) | 50.6 | 9.1 | - | - | 0.40 | - | |
| 38.0 (S12) | 50.5 | 9.5 | - | - | 0 | - | |
| 34.7 (S12) | 49.2 | 11.6 | - | 4.3 | 0.2 | - | |
| Mud Crack | 32.6 (S6) | 50.53 | 144.9 | - | 1.7 | - | 0.03 |
| 24.8 (S7) | 50.46 | 20.4 | 0.0 | 3.8 | 0.21 | 0.02 | |
| 39.9 (S8) | 50.53 | 11.4 | - | - | - | - | |
| 76.0 (S9) | 4.48 | 18.1 | 0.01 | 0.16 | - | - | |
| 34.3 (S10) | 52.96 | 5.1 | 0.05 | 0.59 | - | - | |
| 53.7 (S11) | 39.33 | 12.2 | 0.1 | 0.12 | 0.63 | - | |
| Surface Growths | 34.6 (6) | 46.21 | 16.2 | - | 2.2 | 0.41 | 0.03 |
| 36.3 (6fi) * | 45.5 | 15.6 | - | 0.95 | 1.3 | 0.02 | |
| 37.6 (S7) | 50.98 | 10.8 | - | 0.28 | - | - | |
| 34.3 (S8) | 50.0 | 14.3 | - | 0.66 | 0.47 | - | |
| 37.8 (S9) | 39.5 | 21.3 | 0.01 | 0.87 | 0.08 | - | |
| 53.2 (S10) | 34.65 | 11.5 | 0.12 | 0.37 | 1.9 | - | |
| 53.2 (S11) | 40.25 | 5.1 | 0.02 | 0.46 | 0.19 | - | |
| 38.0 (S12) | 50.53 | 9.5 | - | - | 0.385 | - | |
| Particles Embedded | 18.4 (S6) | 53.59 | 18.1 | 0.01 | 9.6 | 0.21 | 0.1 |
| 29.4 (S8) | 44.9 | 21.5 | - | 3.7 | 0.21 | 0.0 | |
| 26.5 (S10) | 51.84 | 18.7 | 0.54 | 1.4 | 0.9 | - | |
| 34.7 (S12) | 49.22 | 11.6 | - | 4.3 | 0.20 | - | |
| S7-dtmap |
References
- Montemor, M.F.; Ferreira, M.G.S. A Review on the Use of Nanostructures and Functional Organosilane Coatings Modified with Corrosion Inhibitors as Environmentally Friendly Pre-Treatments for Metallic Substrates. In Self-Healing Properties of New Surface Treatments (EFC 58); European Federation of Corrosion Series; The Institute of Materials, Minerals and Mining: London, UK, 2011; Volume 58. [Google Scholar]
- Montemor, M.F. Fostering green inhibitors for corrosion prevention. In Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2016; Volume 233, pp. 107–137. [Google Scholar] [CrossRef]
- Forsyth, M.; Seter, M.; Hinton, B.; Deacon, G.; Junk, P. New ‘Green’ Corrosion Inhibitors Based on Rare Earth Compounds. Aust. J. Chem. 2011, 64, 812–819. [Google Scholar] [CrossRef]
- Forsyth, M.; Wilson, K.; Behrsing, T.; Forsyth, C.; Deacon, G.B.; Phanasgoankar, A. Effectiveness of rare-earth metal compounds as corrosion inhibitors for steel. Corrosion 2002, 58, 953–960. [Google Scholar] [CrossRef]
- Seumo Tchekwagep, P.M.; Aksaray, G.; Farsak, M.; Kardaş, G. Surface modification of mild steel using 4-carboxyphenyl diazonium in sulfuric and hydrochloric acids. A corrosion study. RSC Adv. 2023, 13, 16789–16796. [Google Scholar] [CrossRef] [PubMed]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Fouda, A.S.; Mostafa, H.A.; Elewady, G.Y.; El-Hashemy, M.A. Low molecular weight straight-chain diamines as corrosion inhibitors for SS type 304 in HCl solution. Chem. Eng. Commun. 2008, 195, 934–947. [Google Scholar] [CrossRef]
- Sax, N.I. Dangerous Properties of Industrial Materials, 5th ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1979. [Google Scholar]
- Buchheit, R.G.; Hughes, A.E. Chromate and Chromate-Free Coatings. In Corrosion: Fundamentals, Testing and Protection; Moosbrugger, C., Cramer, D., Covino, B.S.J., Eds.; ASM Handbook; ASM International: Materials Park, OH, USA, 2003; Volume 13A, pp. 720–735. [Google Scholar]
- Gad, S.M.; Emad, S.; Zhou, X.; Lyon, S.B.; Jin, Z.; Dagwa, I.M. Effectiveness of strontium zinc phosphosilicate on the corrosion protection of AA2198-T851 aluminium alloy in sodium chloride solution. Corros. Sci. 2022, 209, 110725. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Hughes, A.E.; Gengenbach, T.; Glenn, A.M.; Cole, I.S. Combined influence of Ce(III) and iodide ions for corrosion protection of AA 2024-T3 in acidic to neutral chloride-rich environments: Electrochemical and surface characterization studies. J. Rare Earths 2023, 41, 309–320. [Google Scholar] [CrossRef]
- Mishra, A.K.; Balasubramaniam, R. Corrosion inhibition of aluminium by rare earth chlorides. Mater. Chem. Phys. 2007, 103, 385–393. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M. CeCl3 and LaCl3 binary solutions as environment-friendly corrosion inhibitors of AA5083 Al-Mg alloy in NaCl solutions. J. Alloys Compd. 2001, 323, 855–858. [Google Scholar] [CrossRef]
- Arab, S.T.; Al-Turkustani, A.M.; Al-Dhahiri, R.H. Synergistic effect of Azadirachta Indica extract and iodide ions on the corrosion inhibition of aluminium in acid media. J. Korean Chem. Soc. 2008, 52, 281–294. [Google Scholar] [CrossRef][Green Version]
- Bouklah, M.; Hammouti, B.; Aouniti, A.; Benkaddour, M.; Bouyanzer, A. Synergistic effect of iodide ions on the corrosion inhibition of steel in 0.5 M H2SO4 by new chalcone derivatives. Appl. Surf. Sci. 2006, 252, 6236–6242. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Venkatachari, G. Influence of halide ions on the adsorption of diphenylamine on iron in 0.5 M H2SO4 solutions. Electrochim. Acta 2006, 51, 4080–4088. [Google Scholar] [CrossRef]
- Larabi, L.; Harek, Y.; Traisnel, M.; Mansri, A. Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1M HCl. J. Appl. Electrochem. 2004, 34, 833–839. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloric acid corrosion of aluminium. Corros. Sci. 2009, 51, 275–282. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Rehim, S.S.A.; Hazzazi, O.A.; Amin, M.A.; Khaled, K.F. On the corrosion inhibition of low carbon steel in concentrated sulphuric acid solutions. Part I: Chemical and electrochemical (AC and DC) studies. Corros. Sci. 2008, 50, 2258–2271. [Google Scholar] [CrossRef]
- Momoh-Yahaya, H.; Eddy, N.O.; Oguzie, E.E. Inhibitive, adsorptive and thermodynamic study of hypoxanthine against the corrosion of aluminium and mild steel in sulphuric acid. J. Mater. Environ. Sci. 2014, 5, 237–244. [Google Scholar]
- Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate-Chloride Solution. Materials 2016, 9, 868. [Google Scholar] [CrossRef]
- Bernal, S.; Botana, F.J.; Calvino, J.J.; Marcos, M.; Pérez-Omil, J.A.; Vidal, H. Lanthanide salts as alternative corrosion inhibitors. J. Alloys Compd. 1995, 225, 638–641. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Lanthanide salts as corrosion inhibitors for AA5083. Mechanism and efficiency of corrosion inhibition. J. Electrochem. Soc. 2008, 155, C169–C177. [Google Scholar] [CrossRef]
- Somers, A.E.; Hinton, B.R.W.; de Bruin-Dickason, C.; Deacon, G.B.; Junk, P.C.; Forsyth, M. New, environmentally friendly, rare earth carboxylate corrosion inhibitors for mild steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Dastgheib, A.; Mohammadzadeh Attar, M.; Zarebidaki, A. Evaluation of Corrosion Inhibition of Mild Steel in 3.5 wt% NaCl Solution by Cerium Nitrate. Met. Mater. Int. 2020, 26, 1634–1642. [Google Scholar] [CrossRef]
- Boudellioua, H.; Hamlaoui, Y.; Tifouti, L.; Pedraza, F. Effect of the temperature of cerium nitrate–NaCl solution on corrosion inhibition of mild steel. Mater. Corros. 2020, 71, 1300–1309. [Google Scholar] [CrossRef]
- Tian, F.; Han, B.; Liu, Y.; Yin, X.S.; Tang, Y.M.; Yang, W.Z. Corrosion inhibition of carbon steel by cerium citrate compound in NaCl medium. Corros. Prot. 2009, 30, 279–282. [Google Scholar]
- Al-Turkustani, A.M.; Arab, S.T.; Al-Dahiri, R.H. Effect of temperature on the performance of aloe extract and azadirachta indica extract in absence and presence of iodide ions on aluminum corrosion in hydrochloric acid. J. Mater. Environ. Sci. 2012, 3, 1163–1176. [Google Scholar]
- Ebenso, E.E.; Alemu, H.; Umoren, S.A.; Obot, I.B. Inhibition of mild steel corrosion in sulphuric acid using alizarin yellow GG dye and synergistic iodide additive. Int. J. Electrochem. Sci. 2008, 3, 1325–1339. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Chapter 14—Nitrate as corrosion inhibitor. In Inorganic Anticorrosive Materials; Verma, C., Aslam, J., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–296. [Google Scholar]
- Feng, X.; Tang, Y.; Zhao, X.; Zuo, Y. The influence of Ce(NO3)3·6H2O on the inhibitive effect of Ca(NO2)2 in simulated concrete pore solution. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2012, 27, 994–998. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum group metals: A review of resources, production, and usage with a focus on catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Margalef-Marti, R.; Carrey, R.; Benito, J.A.; Marti, V.; Soler, A.; Otero, N. Nitrate and nitrite reduction by ferrous iron minerals in polluted groundwater: Isotopic characterization of batch experiments. Chem. Geol. 2020, 548, 119691. [Google Scholar] [CrossRef]
- Wen, C.; Tian, Y.W.; Yang, D.Y.; Wang, G.; Deng, P.C.; Dong, C.F. Controlled release mechanism and inhibition performance of smart inhibitor LDH-NO2 in the reinforced concrete structures. Gongcheng Kexue Xuebao/Chin. J. Eng. 2022, 44, 1368–1378. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.K.; Lee, H.-S.; Ismail, M.A.; Park, W.-J. Effect of LiNO2 inhibitor on corrosion characteristics of steel rebar in saturated Ca(OH)2 solution containing NaCl: An electrochemical study. Constr. Build. Mater. 2017, 133, 387–396. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K.; Watkins, W.L.H.; Graham, G.W.; Belitz, R.K.; Gandhi, H.S. Characterization of Pd/γ-alumina catalysts containing ceria. J. Catal. 1988, 114, 23–33. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Gouda, V.K.; Sayed, S.M. Corrosion behaviour of steel in stagnant salt solutions. Br. Corros. J. 1973, 8, 71–75. [Google Scholar] [CrossRef]
- Moghaddam, P.N.; Amini, R.; Kardar, P.; Ramezanzadeh, B. Synergistic corrosion inhibition effects of the non-hazardous cerium nitrate and tannic acid polyphenolic molecules on the surface of mild-steel in chloride-containing solution: Detailed surface and electrochemical explorations. J. Mol. Liq. 2021, 322, 114510. [Google Scholar] [CrossRef]
- Hinton, B.R.W. Corrosion Inhibition with Rare-Earth-Metal Salts. J. Alloys Compd. 1992, 180, 15–25. [Google Scholar] [CrossRef]
- Ivušić, F.; Lahodny-Šarc, O.; Ćurković, H.O.; Alar, V. Synergistic inhibition of carbon steel corrosion in seawater by cerium chloride and sodium gluconate. Corros. Sci. 2015, 98, 88–97. [Google Scholar] [CrossRef]
- Tran, M.; Dubot, P.; Sutter, E.M.M. Activation of water reduction in the presence of REM salts in aqueous solution. Int. J. Hydrogen Energy 2008, 33, 937–944. [Google Scholar] [CrossRef]
- Lin, P.; Yang, X.; Werner, J.M.; Honaker, R.Q. Application of Eh-pH Diagrams on Acid Leaching Systems for the Recovery of REEs from Bastnaesite, Monazite and Xenotime. Metals 2021, 11, 734. [Google Scholar] [CrossRef]
- Khaled, K.F.; Ebenso, E. Cerium salt as green corrosion inhibitor for steel in acid medium. Res. Chem. Intermed. 2015, 41, 49–62. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Tan, W.; Feng, X.; Koopal, L.K. Transformation of hydroxycarbonate green rust into crystalline iron (hydr)oxides: Influences of reaction conditions and underlying mechanisms. Chem. Geol. 2013, 351, 57–65. [Google Scholar] [CrossRef]
- Inoue, K.; Kwon, S.K.; Kimijima, K.; Kanie, K.; Muramatsu, A.; Shinoda, K.; Suzuki, S.; Waseda, Y. Analysis of iron oxyhydroxides and oxides converted from green rust in aqueous solution. ISIJ Int. 2007, 47, 453–457. [Google Scholar] [CrossRef]
- Inoue, K.; Shinoda, K.; Saito, M.; Suzuki, S.; Waseda, Y. Atomic-scale structure and morphology of γ-FeOOH particles formed during corrosion of Fe-based alloys in aqueous solution. E-J. Surf. Sci. Nanotechnol. 2008, 49–53. [Google Scholar]
- Suzuki, S. Characterization of Formation of Ferrous and Ferric Oxides in Aqueous Solution from a Multidisciplinary Viewpoint. ISIJ Int. 2022, 62, 800–810. [Google Scholar] [CrossRef]
- Glotch, T.D.; Kraft, M.D. Thermal transformations of akaganéite and lepidocrocite to hematite: Assessment of possible precursors to Martian crystalline hematite. Phys. Chem. Miner. 2008, 35, 569–581. [Google Scholar] [CrossRef]
- Easton, C.D.; Kinnear, C.; McArthur, S.L.; Gengenbach, T.R. Practical guides for X-ray photoelectron spectroscopy: Analysis of polymers. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 023207. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Korolkov, D.V. XPS study of group IA carbonates. Cent. Eur. J. Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef]
- Bruce, L.A.; Hoang, M.; Hughes, A.E.; Turner, T.W. Surface area control during the synthesis and reduction of high area ceria catalyst supports. Appl. Catal. A-Gen. 1996, 134, 351–362. [Google Scholar] [CrossRef]
- Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. PH-distribution of cerium species in aqueous systems. J. Rare Earths 2012, 30, 559–562. [Google Scholar] [CrossRef]
- Forsyth, M.; Forsyth, C.M.; Wilson, K.; Behrsing, T.; Deacon, G.B. ATR characterisation of synergistic corrosion inhibition of mild steel surfaces by cerium salicylate. Corros. Sci. 2002, 44, 2651–2656. [Google Scholar] [CrossRef]
- Karimian, N.; Burton, E.D.; Johnston, S.G.; Hockmann, K.; Choppala, G. Humic acid impacts antimony partitioning and speciation during iron(II)-induced ferrihydrite transformation. Sci. Total Environ. 2019, 683, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wren, J.C.; Glowa, G.A.; Merritt, J. Corrosion of stainless steel by gaseous I2. J. Nucl. Mater. 1999, 265, 161–177. [Google Scholar] [CrossRef]
- Wang, W.; Howe, J.Y.; Gu, B. Structure and Morphology Evolution of Hematite (α-Fe2O3) Nanoparticles in Forced Hydrolysis of Ferric Chloride. J. Phys. Chem. C 2008, 112, 9203–9208. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE and Cebelcor: Houston, TX, USA, 1974. [Google Scholar]
- Xu, H.; Lee, S.; Xu, H. Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field. Am. Mineral. 2017, 102, 711–719. [Google Scholar] [CrossRef]
- McClean, R.G.; Schofield, M.A.; Kean, W.F.; Sommer, C.V.; Robertson, D.P.; Toth, D.; Gajdardziska-Josifovska, M. Botanical iron minerals: Correlation between nanocrystal structure and modes of biological self-assembly. Eur. J. Mineral. 2001, 13, 1235–1242. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 71st ed.; CRC Press: Boston, MA, USA, 1990. [Google Scholar]
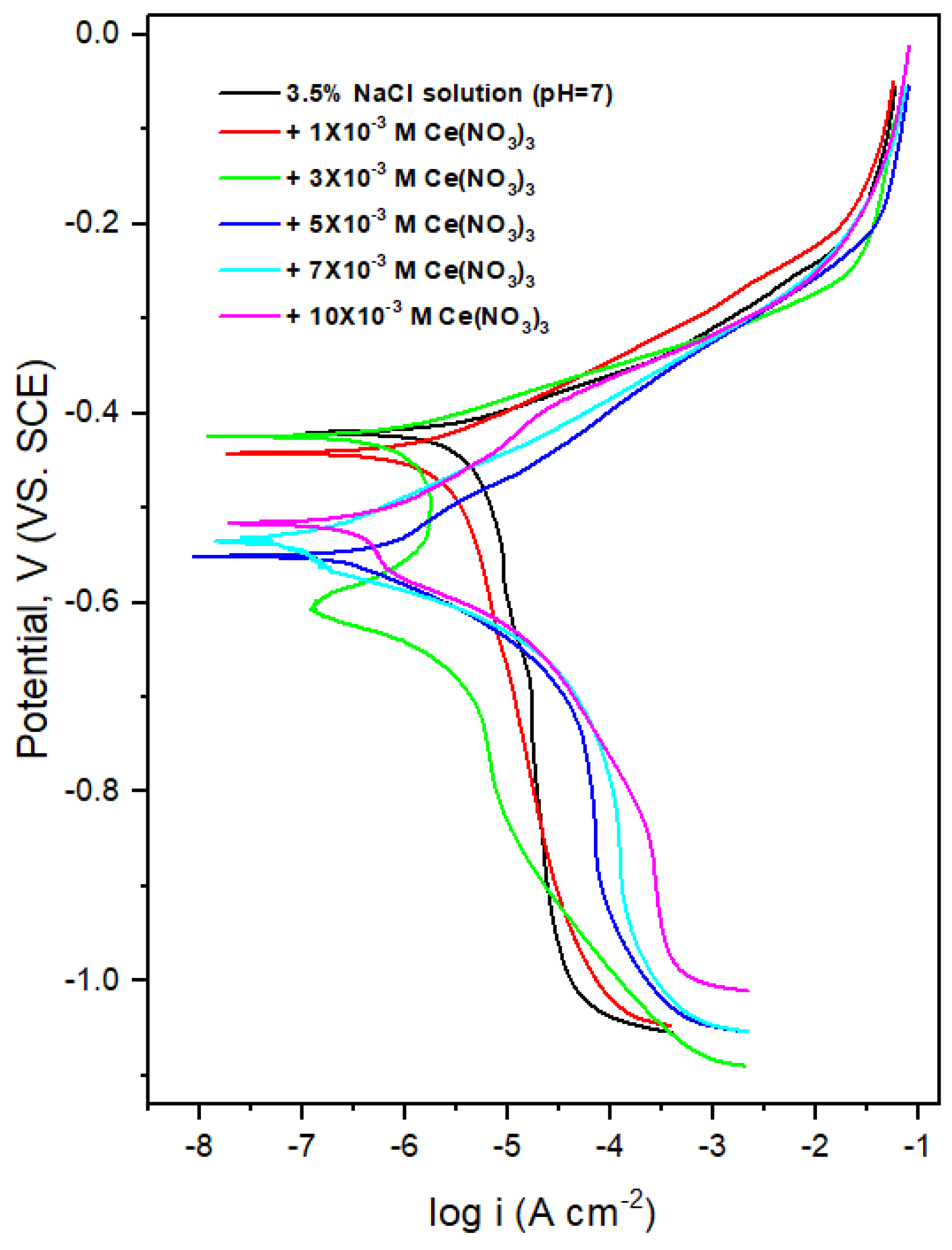
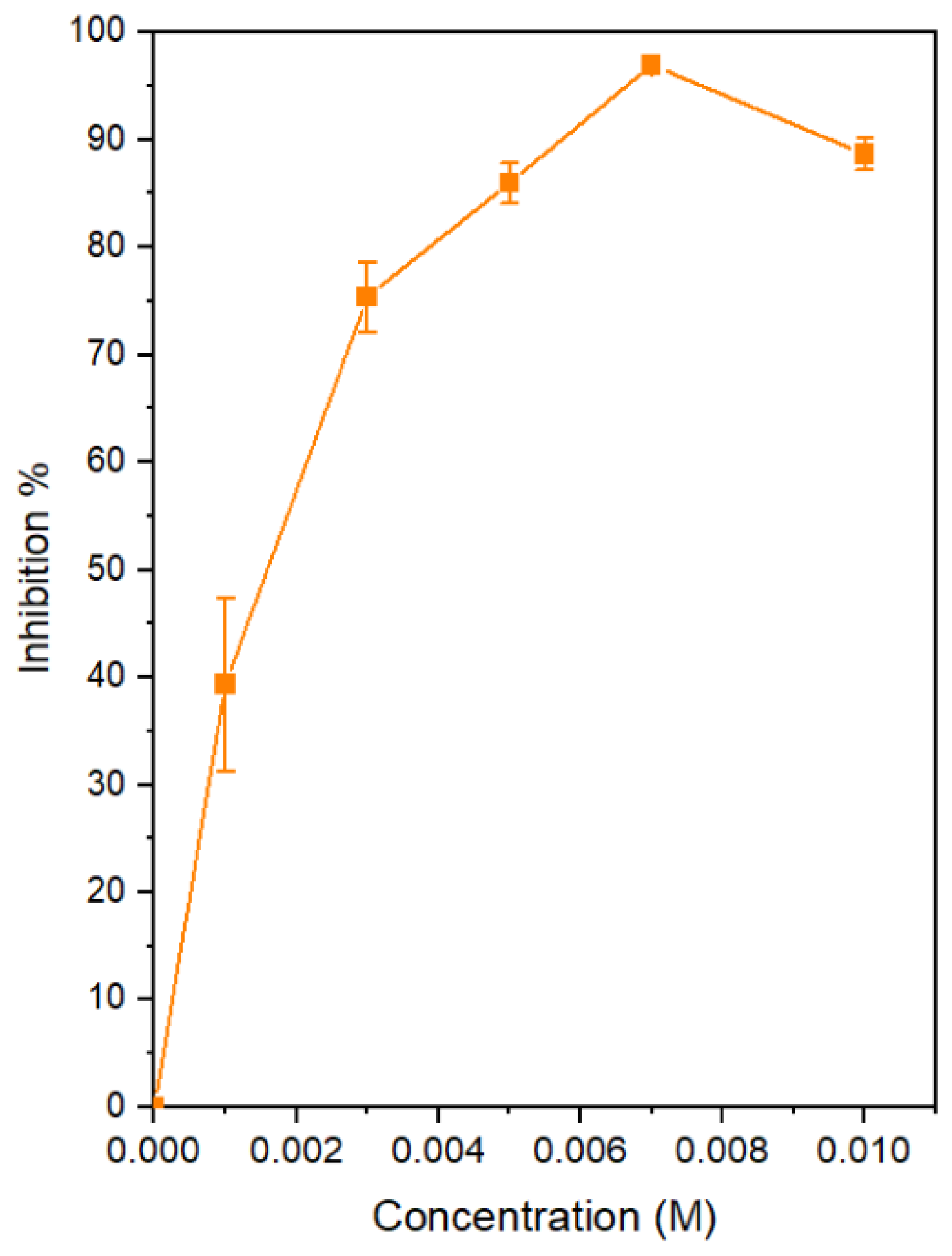




| Ce(NO3)3 Moles | icorr μA | Ecorr mV | βa | βc | Θ | IE% |
|---|---|---|---|---|---|---|
| 0 | 2.30 | −420.30 | 38.78 | −142.71 | 0.00 | 0.00 |
| 1 × 10−3 | 1.40 | −441.73 | 52.18 | −142.24 | 0.39 | 39.35 |
| 3 × 10−3 | 0.57 | −427.06 | 34.11 | −80.81 | 0.75 | 75.42 |
| 5 × 10−3 | 0.32 | −548.52 | 39.87 | −59.61 | 0.86 | 86.00 |
| 7 × 10−3 | 0.07 | −527.46 | 24.12 | −87.12 | 0.97 | 96.97 |
| 10 × 10−3 | 0.26 | −509.21 | 24.77 | −126.08 | 0.89 | 88.69 |
| pH | Concentrations Ce(NO3)3 M | icorr μA/cm2 | Ecorr mV | βa mV/dec−1 | βc mV/dec−1 | IE% |
|---|---|---|---|---|---|---|
| 2.5 | 0 | 10.44 | −669.36 | 47.84 | −92.55 | 0.00 |
| 1 × 10−3 | 41.03 | −629.96 | 29.08 | −93.98 | −292.79 | |
| 4 | 0 | 3.62 | −446.90 | 75.11 | −147.34 | 0.00 |
| 1 × 10−3 | 3.82 | −512.00 | 30.93 | −37.57 | −5.31 | |
| 7 | 0 | 2.30 | −420.30 | 38.78 | −142.71 | 0.00 |
| 1 × 10−3 | 1.40 | −441.73 | 52.18 | −142.24 | 39.35 | |
| 8 | 0 | 1.49 | −522.51 | 37.58 | −99.86 | 0.00 |
| 1 × 10−3 | 0.53 | −750.94 | 89.08 | −88.10 | 64.29 | |
| 2.5 | 0 | 10.44 | −669.36 | 47.84 | −92.55 | 0.00 |
| 7 × 10−3 | 6.94 | −552.59 | 69.92 | −126.51 | 33.56 | |
| 4 | 0 | 3.62 | −446.90 | 75.11 | −147.34 | 0.00 |
| 7 × 10−3 | 0.00 | −537.81 | 39.73 | −85.82 | 85.24 | |
| 7 | 0 | 2.30 | −420.30 | 38.78 | −142.71 | 0.00 |
| 7 × 10−3 | 0.07 | −527.46 | 24.12 | −87.12 | 96.97 | |
| 8 | 0 | 1.49 | −522.51 | 37.58 | −99.86 | 0.00 |
| 7 × 10−3 | 0.78 | −458.36 | 13.25 | −104.00 | 47.57 |
| Solution Compositions | IE% |
|---|---|
| 1 × 10−3 M Ce(NO3)3 | −292.79 |
| 1 × 10−3 M Ce(NO3)3 + 1 × 10−1 M KI | −37.67 |
| 5 × 10−3 M Ce(NO3)3 | 25.22 |
| 5 × 10−3 M Ce(NO3)3 + 1 × 10−1 M KI | 59.24 |
| 7 × 10−3 M Ce(NO3)3 | 33.56 |
| 7 × 10−3 M Ce(NO3)3 + 1 × 10−1 M KI | 84.60 |
| 1 × 10−1 M KI | 73.68 |
| pH | Electrolytes | icorr μA cm−2 | Ecorr mV | IE% | S |
|---|---|---|---|---|---|
| 2.5 | Blank (3.5% NaCl) | 10.44 | − | 0.00 | |
| 7 × 10−3 M Ce(NO3)3 + 0.1 M KI | 1.61 | −487.78 | 84.60 | 1.14 | |
| 0.1 M KI | 2.75 | −559.61 | 73.68 | ||
| 4 | Blank (3.5% NaCl) | 3.62 | −446.90 | 0.00 | |
| 7 × 10−3 M Ce(NO3)3 + 0.1 M KI | 0.51 | −579.19 | 85.84 | 0.74 | |
| 0.1 M KI | 2.58 | −422.51 | 28.67 | ||
| 7 | Blank (3.5% NaCl) | 2.30 | −420.30 | 0.00 | |
| 7 × 10−3 M Ce(NO3)3 + 0.1 M KI | 0.19 | −524.87 | 91.61 | 0.09 | |
| 0.1 M KI | 0.58 | −389.99 | 74.91 | ||
| 8 | Blank (3.5% NaCl) | 1.49 | −522.51 | 0.00 | |
| 7 × 10−3 M Ce(NO3)3 + 0.1 M KI | 0.20 | −582.94 | 86.60 | 5.10 | |
| 0.1 M KI | 1.95 | −396.42 | −30.35 |
| Sample | Lepidocrocite γ-FeOOH Red-Reddish Brown | Akaganeite β-FeOOH Metallic-Brown | Goethite α-FeOOH Brownish Yellow to Orange-Yellow | Luogufengite Fe2O3Rust-Red |
|---|---|---|---|---|
| Morphology | Scales aggregated into rossettes | Rod like | ||
| KI | ||||
| 2.5 | Major | Minor | ||
| 4.0 | Major | Minor | ||
| 7.0 | Trace | - | ||
| 8.0 | Major | Minor | ||
| Ce | ||||
| 2.5 | ||||
| 4.0 | Major | Trace | - | Amorphous |
| 7.0 | ||||
| 8.0 | Major | |||
| Ce+I | ||||
| 2.5 | Major | Minor | - | Poorly Crystalline |
| 4.0 | Major | Major | - | Poorly Crystalline |
| 7.0 | Minor | Major | - | Poorly Crystalline |
| 8.0 | Minor | Major | - | Poorly Crystalline |
| Element | pH 2.5 | pH 4 | pH 7 | pH 8 | ||||
|---|---|---|---|---|---|---|---|---|
| CP | M | CP | M | CP | M | CP | M | |
| I-Electrolyte | ||||||||
| Fe | 35.8 ± 0.5 | 34.4 ± 1.3 | 36.0 ± 0.4 | 29.2 ± 0.5 | 30.5 ± 0.5 | 30.8 ± 0.5 | 31.5 ± 1.0 | 30.8 ± 0.7 |
| I (×102) | 1.51 ± 0.4 | 14.0 ± 0.7 | 2.0 ± 1.0 | 6.5 ± 1.5 | 17.0 ± 0.9 | 27.0 ± 9.0 | 14.5 ± 7.5 | 7.5 ± 0.5 |
| O | 51.8 ± 0.1 | 47.3 ± 1.9 | 52.3 ± 0.3 | 44.3 ± 0.3 | 42.0 ± 0.2 | 40.5 ± 0.1 | 46.9 ± 3.0 | 41.9 ± 0.1 |
| C | 11.7 ± 0.6 | 17.5 ± 2.1 | 11.0 ± 0.1 | 23.4 ± 1.0 | 24.6 ± 0.3 | 26.6 ± 0.5 | 20.7 ± 4.5 | 26.2 ± 0.9 |
| Cl | 0.33 ± 0.01 | 0.35 ± 0.10 | 0.22 ± 0.01 | 0.15 ± 0.15 | 0.09 ± 0.09 | 0.22 ± 0.04 | 0.26 ± 0.08 | 0.12 ± 0.05 |
| Ce-electrolyte | ||||||||
| Fe | 21.6 ± 2.5 | 21.8 ± 4.7 | 16.6 ± 0.2 | 8.5 ± 0.4 | 14.8 ± 0.6 | 10.1 ± 0.1 | 16.1 ± 1.0 | 10.7 ± 0.6 |
| Ce | 3.0 ± 0.8 | 2.9 ± 0.4 | 5.4 ± 0.3 | 7.3 ± 0.1 | 4.1 ± 0.1 | 6.3 ± 0.1 | 4.6 ± 0.4 | 7.2 ± 0.2 |
| O | 39.9 ± 1.1 | 39.7 ± 1.8 | 38.4 ± 0.2 | 36.7 ± 0.3 | 39.7 ± 0.1 | 38.4 ± 0.3 | 40.4 ± 1.3 | 40.4 ± 0.5 |
| C | 34.8 ± 2.6 | 34.6 ± 6.0 | 39.1 ± 0.5 | 46.7 ± 0.1 | 40.6 ± 0.5 | 44.5 ± 0.3 | 38.5 ± 0.7 | 40.6 ± 0.7 |
| Cl | 0.39 ± 0.04 | 0.62 ± 0.06 | 0.19 ± 0.02 | 0.34 ± 0.01 | 0.30 ± 0.01 | 0.46 ± 0.01 | 0.33 ± 0.10 | 0.61 ± 0.06 |
| Ce+I electrolyte | ||||||||
| Fe | 34.9 ± 1.2 | 31.0 ± 2.1 | 32.2 ± 0.9 | 28.8 ± 0.8 | 32.1 ± 1.4 | 22.3 ± 2.3 | 31.6 ± 1.8 | 31.3 ± 3.3 |
| Ce | - | 0.5 ± 0.6 | - | 0.4 ± 0.3 | 0.5 ± 0.5 | - | 0.04 ± 0.03 | 1.0 ± 1.9 |
| I | 0.05 ± 0.01 | 0.25 ± 0.21 | 0.12 ± 0.03 | 0.16 ± 0.05 | 0.18 ± 0.10 | 0.16 ± 0.01 | 0.12 ± 0.02 | 0.08 ± 0.01 |
| O | 50.4 ± 0.86 | 44.4 ± 6.0 | 48.1 ± 1.3 | 48.6 ± 1.9 | 47.2 ± 2.0 | 40.2 ± 1.3 | 47.0 ± 0.8 | 47.3 ± 0.9 |
| C | 13.9 ± 1.7 | 19.4 ± 4.4 | 18.6 ± 2.0 | 19.8 ± 2.6 | 18.8 ± 2.5 | 32.5 ± 2.7 | 20.0 ± 2.9 | 19.0 ± 1.5 |
| Cl | 0.37 ± 0.17 | 0.27 ± 0.10 | 0.58 ± 0.14 | 0.33 ± 0.12 | 0.74 ± 0.34 | 0.29 ± 0.19 | 0.50 ± 0.08 | 0.42 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hashemy, M.A.; Easton, C.D.; Seeber, A.; Cole, I.S.; Hughes, A.E. Electrochemical and Surface Characterisation of Carbon Steel Exposed to Mixed Ce and Iodide Electrolytes. Metals 2023, 13, 1553. https://doi.org/10.3390/met13091553
El-Hashemy MA, Easton CD, Seeber A, Cole IS, Hughes AE. Electrochemical and Surface Characterisation of Carbon Steel Exposed to Mixed Ce and Iodide Electrolytes. Metals. 2023; 13(9):1553. https://doi.org/10.3390/met13091553
Chicago/Turabian StyleEl-Hashemy, Mohammed A., Christopher D. Easton, Aaron Seeber, Ivan S. Cole, and Anthony E. Hughes. 2023. "Electrochemical and Surface Characterisation of Carbon Steel Exposed to Mixed Ce and Iodide Electrolytes" Metals 13, no. 9: 1553. https://doi.org/10.3390/met13091553
APA StyleEl-Hashemy, M. A., Easton, C. D., Seeber, A., Cole, I. S., & Hughes, A. E. (2023). Electrochemical and Surface Characterisation of Carbon Steel Exposed to Mixed Ce and Iodide Electrolytes. Metals, 13(9), 1553. https://doi.org/10.3390/met13091553






