The Microstructure and Magnetic Properties of a Soft Magnetic Fe-12Al Alloy Additively Manufactured via Laser Powder Bed Fusion (L-PBF)
Abstract
1. Introduction
2. Materials and Methods
2.1. Melting and Reference Materials
2.2. Powder Production
2.3. Additive Manufacturing
2.4. Heat Treatment
2.5. Characterization
3. Results
3.1. Atomized Powder
3.2. AM Laser Parameter Selection and Porosity
3.3. Crack Formation
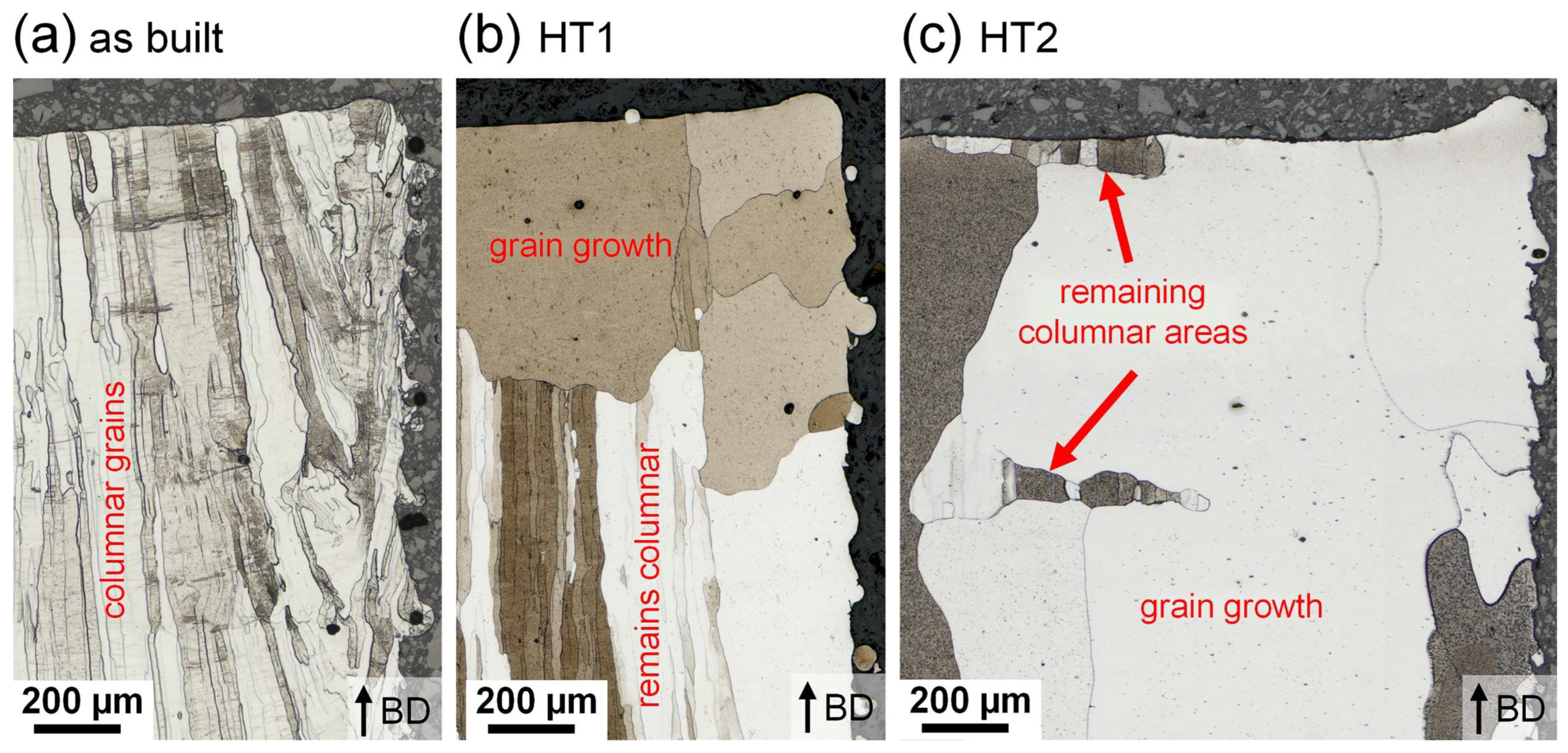
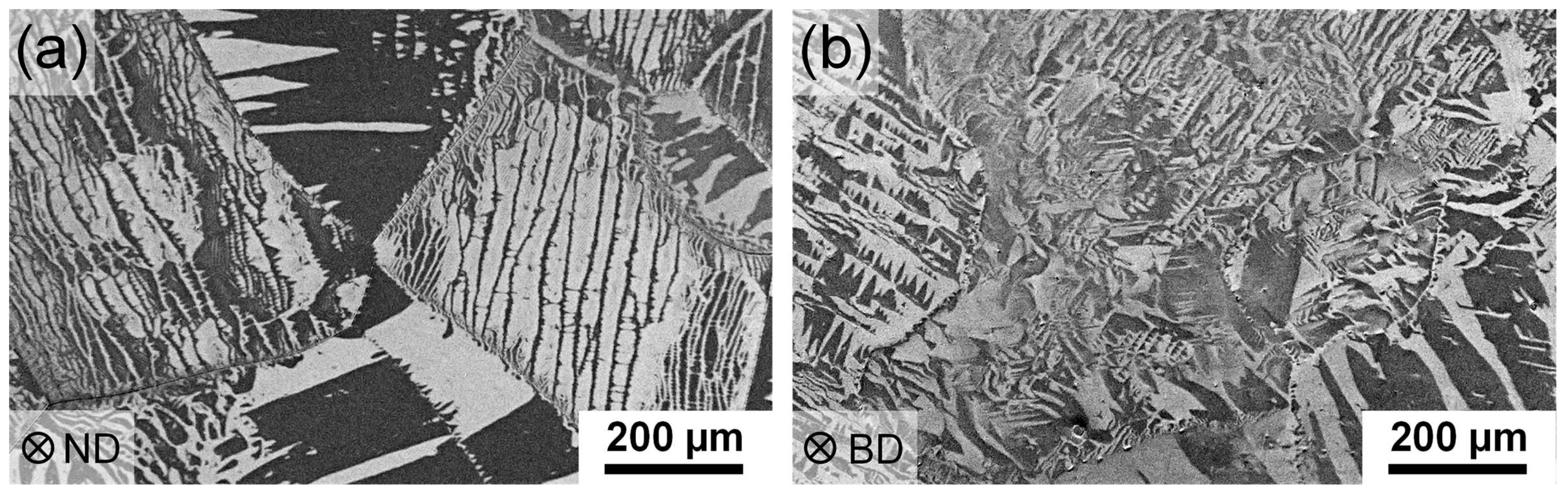
3.4. Microstructure
3.5. Specific Electrical Resistivity
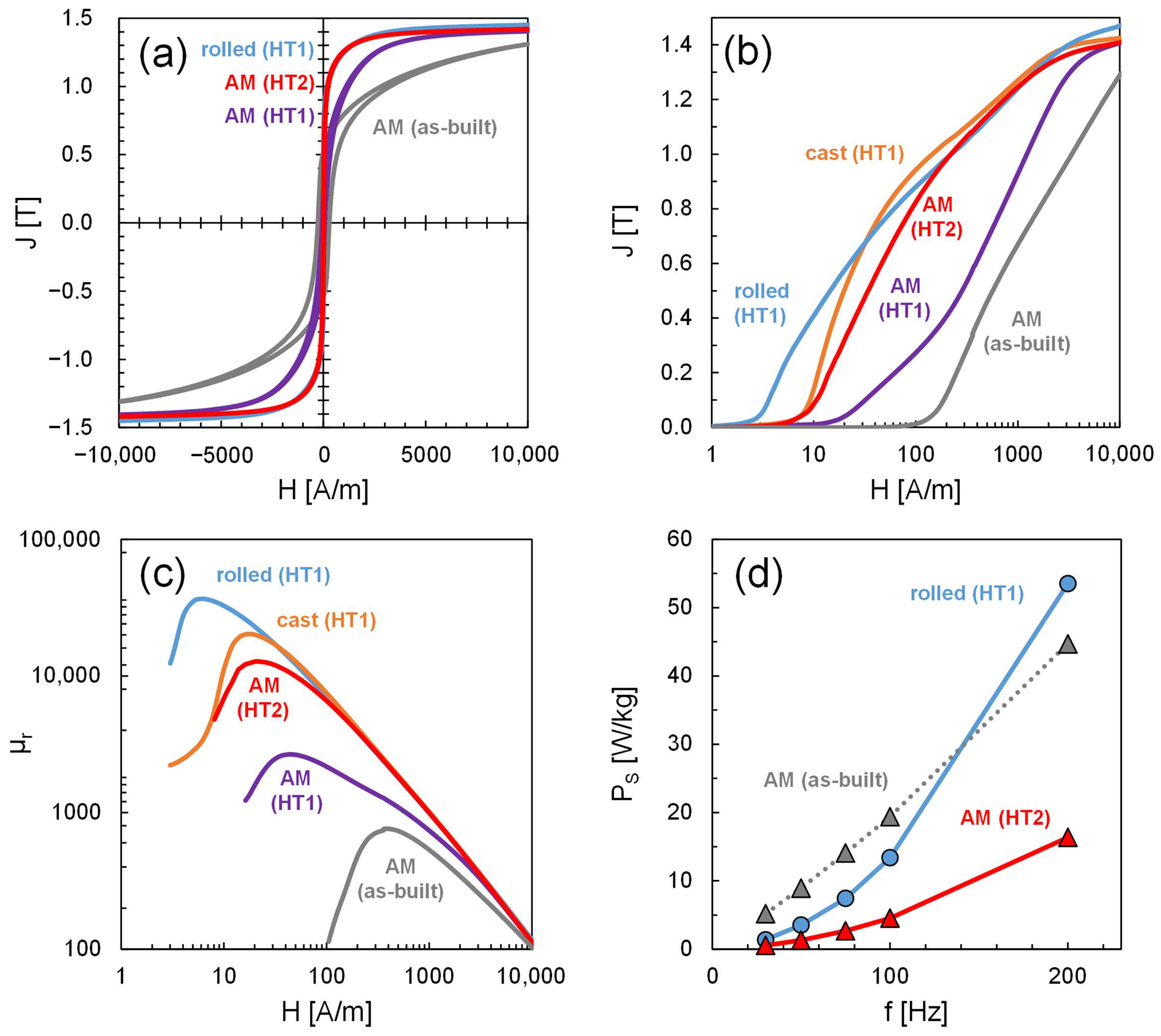
3.6. Magnetic Properties
4. Discussion
4.1. AM Laser Parameter Selection and Porosity
4.2. Crack Formation
4.3. Microstructure in the as-Built and Heat-Treated Condition
4.4. Specific Electrical Resistivity
4.5. DC Magnetic Properties in the as-Built and Heat-Treated Condition
4.6. Iron Losses and Magnetic Domain Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawlek, F. Magnetische Werkstoffe; Springer: Berlin/Heidelberg, Germany, 1952; ISBN 9783642532993. [Google Scholar]
- Bozorth, R.M. Ferromagnetism; IEEE Press: Piscataway, NJ, USA, 1978; ISBN 0-7803-1032-2. [Google Scholar]
- Heck, C. Magnetic Materials and Their Applications; Butterworths: London, UK, 1974; ISBN 978-0-408-70399-4. [Google Scholar]
- Palm, M.; Stein, F.; Dehm, G. Iron Aluminides. Annu. Rev. Mater. Res. 2019, 49, 297–326. [Google Scholar] [CrossRef]
- Tebble, R.S.; Craik, D.J. Magnetic Materials; Wiley-Interscience: Hoboken, NJ, USA, 1969; ISBN 9780608100289. [Google Scholar]
- Cahn, R.W.; Haasen, P. (Eds.) Physical Metallurgy, 4th ed.; North-Holland: Amsterdam, NY, USA, 1996; ISBN 978-0-444-89875-3. [Google Scholar]
- Masumoto, H.; Saito, H. On the Effect of Heat Treatment on the Magnetic Properties of Iron-Aluminium Alloys. III: The Changes of Electric Resistance and Magnetostriction Due to Water Quenching; Science Reports of the Research Institutes, Tohoku University. Ser. A, Physics, Chemistry and Metallurgy No. 42. 1952. Available online: http://hdl.handle.net/10097/26514 (accessed on 7 July 2023).
- Hilzinger, R.; Rodewald, W. Magnetic Materials: Fundamentals, Products, Properties, Applications; Publicis Publishing: Erlangen, Germany, 2013; ISBN 3895783528. [Google Scholar]
- Song, B.; Dong, S.; Coddet, P.; Liao, H.; Coddet, C. Fabrication and microstructure characterization of selective laser-melted FeAl intermetallic parts. Surf. Coat. Technol. 2012, 206, 4704–4709. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Liao, H.; Coddet, C. Characterisations of TiC particle reinforced FeAl composite part fabricated by selective laser melting. Mater. Res. Innov. 2013, 18, 50–56. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Liao, H.; Coddet, C. Manufacture of Fe–Al cube part with a sandwich structure by selective laser melting directly from mechanically mixed Fe and Al powders. Int. J. Adv. Manuf. Technol. 2013, 69, 1323–1330. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.J.; Liao, H.L.; Coddet, C. Morphology evolution mechanism of single tracks of FeAl intermetallics in selective laser melting. Mater. Res. Innov. 2013, 16, 321–325. [Google Scholar] [CrossRef]
- Rolink, G.; Vogt, S.; Senčekova, L.; Weisheit, A.; Poprawe, R.; Palm, M. Laser metal deposition and selective laser melting of Fe–28 at.% Al. J. Mater. Res. 2014, 29, 2036–2043. [Google Scholar] [CrossRef]
- Michalcová, A.; Senčekova, L.; Rolink, G.; Weisheit, A.; Pešička, J.; Stobik, M.; Palm, M. Laser additive manufacturing of iron aluminides strengthened by ordering, borides or coherent Heusler phase. Mater. Des. 2017, 116, 481–494. [Google Scholar] [CrossRef]
- Karczewski, K.; Durejko, T.; Czujko, T. The Microstructure Evolution of a Fe3Al Alloy during the LENS Process. Materials 2018, 11, 390. [Google Scholar] [CrossRef]
- Karczewski, K.; Pęska, M.; Ziętala, M.; Polański, M. Fe-Al thin walls manufactured by Laser Engineered Net Shaping. J. Alloys Compd. 2017, 696, 1105–1112. [Google Scholar] [CrossRef]
- Shishkovsky, I.; Missemer, F.; Kakovkina, N.; Smurov, I. Intermetallics Synthesis in the Fe–Al System via Layer by Layer 3D Laser Cladding. Crystals 2013, 3, 517–529. [Google Scholar] [CrossRef]
- Goll, D.; Schuller, D.; Martinek, G.; Kunert, T.; Schurr, J.; Sinz, C.; Schubert, T.; Bernthaler, T.; Riegel, H.; Schneider, G. Additive manufacturing of soft magnetic materials and components. Addit. Manuf. 2019, 27, 428–439. [Google Scholar] [CrossRef]
- Goll, D.; Schurr, J.; Trauter, F.; Schanz, J.; Bernthaler, T.; Riegel, H.; Schneider, G. Additive manufacturing of soft and hard magnetic materials. Procedia CIRP 2020, 94, 248–253. [Google Scholar] [CrossRef]
- Hohs, D.; Klett, S.; Löffler, R.; Trier, F.; Goll, D.; Schneider, G. Visualization of magnetization reversal processes by dynamic Kerr microscopy. Pract. Metallogr. 2023, 60, 37–52. [Google Scholar] [CrossRef]
- Zanni, M.; Ceschini, L.; Fortunato, A.; Valli, G.; Del Bianco, L.; Spizzo, F. Relationship between microstructure, mechanical and magnetic properties of pure iron produced by laser powder bed fusion (L-PBF) in the as-built and stress relieved conditions. Prog. Addit. Manuf. 2022, 7, 1195–1212. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Yadroitsev, I.; Matope, S. Effect of Process Parameters on Residual Stresses, Distortions, and Porosity in Selective Laser Melting of Maraging Steel 300. Metals 2019, 9, 1042. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Dimitrov, D.; Matope, S.; Yadroitsev, I. Evaluation of the impact of scanning strategies on residual stresses in selective laser melting. Int. J. Adv. Manuf. Technol. 2019, 102, 2441–2450. [Google Scholar] [CrossRef]
- Andreiev, A.; Hoyer, K.-P.; Hengsbach, F.; Haase, M.; Tasche, L.; Duschik, K.; Schaper, M. Powder bed fusion of soft-magnetic iron-based alloys with high silicon content. J. Mater. Process. Technol. 2023, 317, 117991. [Google Scholar] [CrossRef]
- Brandt, M. (Ed.) Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Elsevier Science: Kent, UK, 2016; ISBN 9780081004333. [Google Scholar]
- Lejček, P.; Roudnická, M.; Čapek, J.; Dvorský, D.; Drahokoupil, J.; Šimek, D.; Čížek, J.; Svora, P.; Molnárová, O.; Vojtěch, D. Selective laser melting of pure iron: Multiscale characterization of hierarchical microstructure. Mater. Charact. 2019, 154, 222–232. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Hayakawa, Y. Mechanism of secondary recrystallization of Goss grains in grain-oriented electrical steel. Sci. Technol. Adv. Mater. 2017, 18, 480–497. [Google Scholar] [CrossRef]
- Taguchi, S.; Sakakura, A.; Matsumoto, F.; Takashima, K.; Kuroki, K. The development of grain-oriented silicon steel with high permeability. J. Magn. Magn. Mater. 1975, 2, 121–131. [Google Scholar] [CrossRef]
- Wilson, F.G.; Gladman, T. Aluminium nitride in steel. Int. Mater. Rev. 1988, 33, 221–286. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, J.; Li, J.; Ning, J. Effect of Nb on solution and precipitation of inhibitors in grain-oriented silicon steel. J. Magn. Magn. Mater. 2017, 426, 89–94. [Google Scholar] [CrossRef]
- Riipinen, T.; Pippuri-Mäkeläinen, J.; Que, Z.; Metsä-Kortelainen, S.; Antikainen, A.; Lindroos, T. The effect of heat treatment on structure and magnetic properties of additively manufactured Fe-Co-V alloys. Mater. Today Commun. 2023, 36, 106437. [Google Scholar] [CrossRef]
- Goodall, A.D.; Chechik, L.; Mitchell, R.L.; Jewell, G.W.; Todd, I. Cracking of soft magnetic FeSi to reduce eddy current losses in stator cores. Addit. Manuf. 2023, 70, 103555. [Google Scholar] [CrossRef]
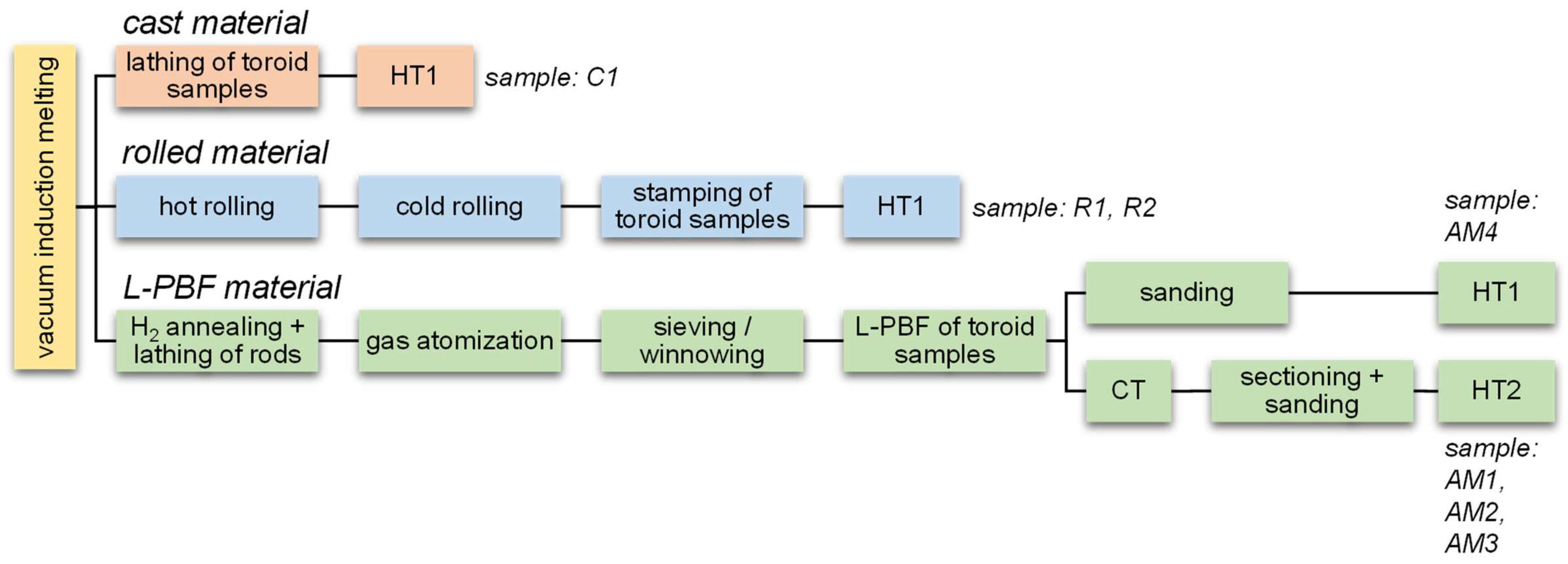
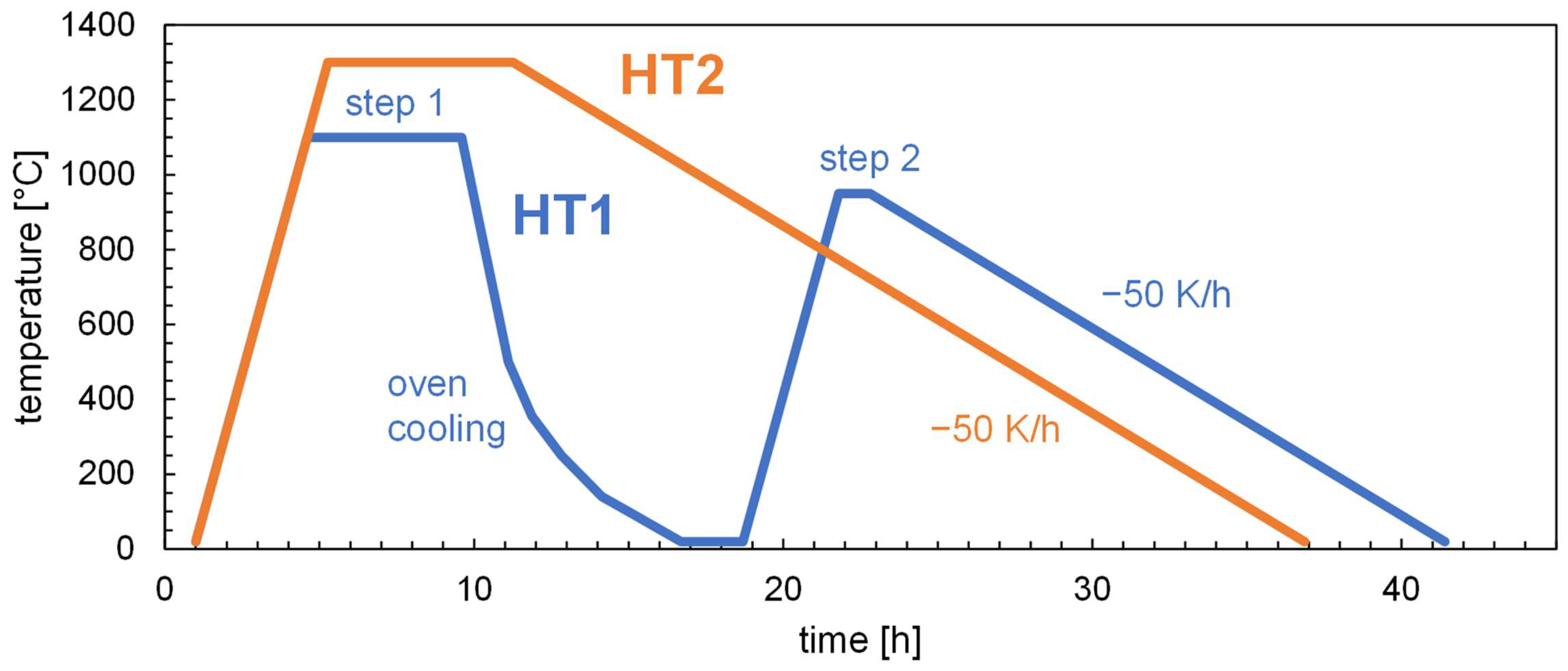
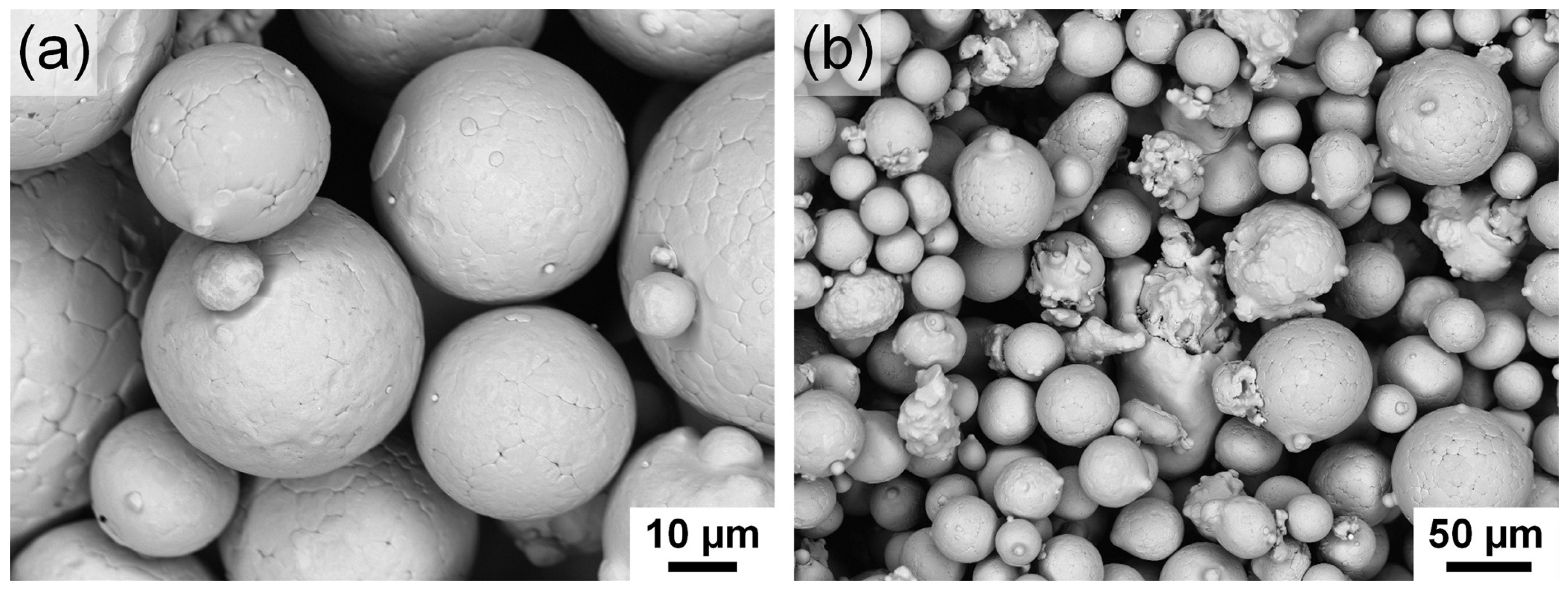





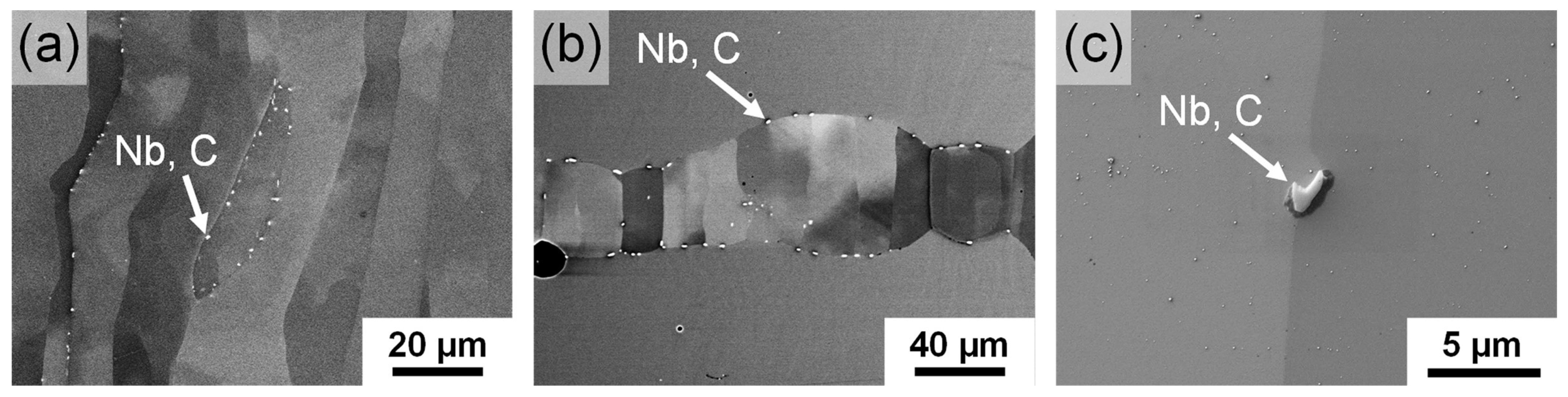

| Process | Sample | Targeted thickness t (mm) | Heat treatment | Atmosphere |
|---|---|---|---|---|
| Cast | C1 | massive | HT1 | dry H2 |
| Rolled | R1 | 1.0 | HT1 | dry H2 |
| R2 | 2.0 | HT1 | dry H2 | |
| L-PBF | AM1 | 1.0 | HT2 | Ar + 5% H2 |
| AM2 | 2.0 | HT2 | Ar + 5% H2 | |
| AM3 | 3.0 | HT2 | Ar + 5% H2 | |
| AM4 | 4.0 | HT1 | Ar + 5% H2 |
| Element | As-Built | HT1 (Core Region) | HT1 (Surface Region) | HT2 |
|---|---|---|---|---|
| Al (wt%) | 11.5 | 11.4 | 11.4 | 11.3 |
| Mn (wt%) | 0.7 | 0.6 | 0.6 | 0.6 |
| Fe (wt%) | 87.8 | 88.0 | 88.0 | 88.1 |
| Process | Sample | Heat Treatment | Thickness t (mm) | Density (g/cm3) | Relative Density (%) | HC (A/m) | J100 (T) | µmax (×103) | PS (W/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Cast | C1 | HT1, step 1 | massive | 6.77 | 100.0 | 15.9 | 1.43 | 24.4 | - |
| HT1, step 2 | massive | 6.77 | 100.0 | 12.4 | 1.43 | 20.3 | - | ||
| Rolled | R1 | HT1, step 1 | 1.0 | 6.77 | 100.0 | 4.5 | 1.47 | 26.6 | - |
| HT1, step 2 | 1.0 | 6.77 | 100.0 | 3.2 | 1.47 | 36.6 | 3.5 | ||
| R2 | HT1, step 1 | 2.1 | 6.77 | 100.0 | 5.8 | 1.45 | 26.7 | - | |
| HT1, step 2 | 2.1 | 6.77 | 100.0 | 4.1 | 1.45 | 40.4 | - | ||
| L-PBF | AM1 | As-built | 1.1 | 6.71 | 99.2 | 278 | 1.29 | 0.76 | 8.9 |
| HT2 | 1.1 | 6.76 | 99.8 | 11.3 | 1.41 | 12.7 | 1.3 | ||
| AM2 | As-built | 2.1 | 6.73 | 99.4 | 313 | 1.25 | 0.53 | 10.6 | |
| HT2 | 2.1 | 6.75 | 99.7 | 18.8 | 1.42 | 13.1 | 3.2 | ||
| AM3 | As-built | 3.0 | 6.72 | 99.3 | 333 | 1.23 | 0.45 | 11.8 | |
| HT2 | 3.0 | 6.76 | 99.8 | 14.1 | 1.42 | 10.2 | 5.9 | ||
| AM4 | As-built | 4.1 | 6.71 | 99.2 | 301 | 1.20 | 0.50 | 12.7 | |
| HT1, step 1 | 4.1 | 6.75 | 99.6 | 36.7 | 1.39 | 2.0 | 4.5 | ||
| HT1, step 2 | 4.0 | 6.74 | 99.6 | 61.4 | 1.41 | 2.7 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunert, T.; Kresse, T.; Fohr, F.; Volbers, N.; Schneider, G.; Goll, D. The Microstructure and Magnetic Properties of a Soft Magnetic Fe-12Al Alloy Additively Manufactured via Laser Powder Bed Fusion (L-PBF). Metals 2024, 14, 117. https://doi.org/10.3390/met14010117
Kunert T, Kresse T, Fohr F, Volbers N, Schneider G, Goll D. The Microstructure and Magnetic Properties of a Soft Magnetic Fe-12Al Alloy Additively Manufactured via Laser Powder Bed Fusion (L-PBF). Metals. 2024; 14(1):117. https://doi.org/10.3390/met14010117
Chicago/Turabian StyleKunert, Torsten, Thomas Kresse, Frederik Fohr, Niklas Volbers, Gerhard Schneider, and Dagmar Goll. 2024. "The Microstructure and Magnetic Properties of a Soft Magnetic Fe-12Al Alloy Additively Manufactured via Laser Powder Bed Fusion (L-PBF)" Metals 14, no. 1: 117. https://doi.org/10.3390/met14010117
APA StyleKunert, T., Kresse, T., Fohr, F., Volbers, N., Schneider, G., & Goll, D. (2024). The Microstructure and Magnetic Properties of a Soft Magnetic Fe-12Al Alloy Additively Manufactured via Laser Powder Bed Fusion (L-PBF). Metals, 14(1), 117. https://doi.org/10.3390/met14010117







