Effect of Sintering Temperature on Phase Formation and Mechanical Properties of Al–Cu–Li Alloy Prepared from Secondary Aluminum Powders
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Raw Materials
2.1.1. Primary Aluminum
2.1.2. Secondary Aluminum
2.2. Design and Manufacturing of the Alloy
2.3. Characterization of Samples
3. Results and Discussion
3.1. Thermodynamic Modeling Using Thermo-Calc
3.2. Calculation of Relative Density Using the Archimedes Method
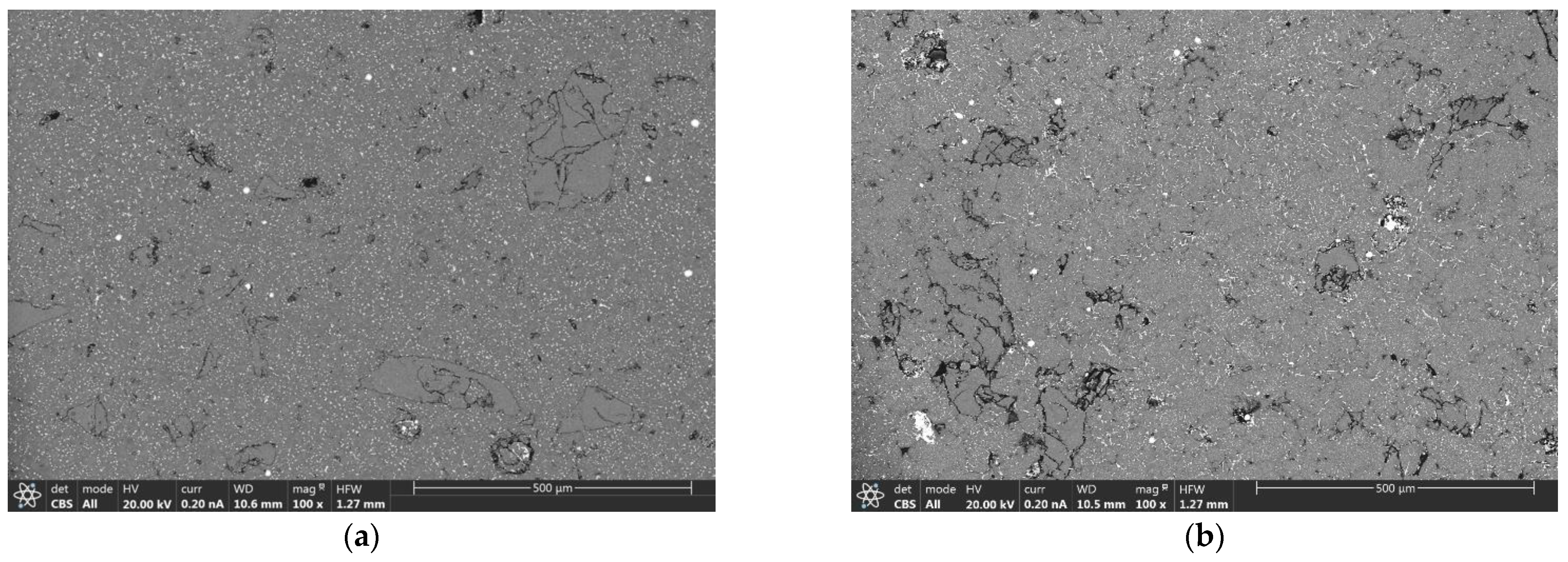
3.3. Microstructural Analysis of Sintered Components
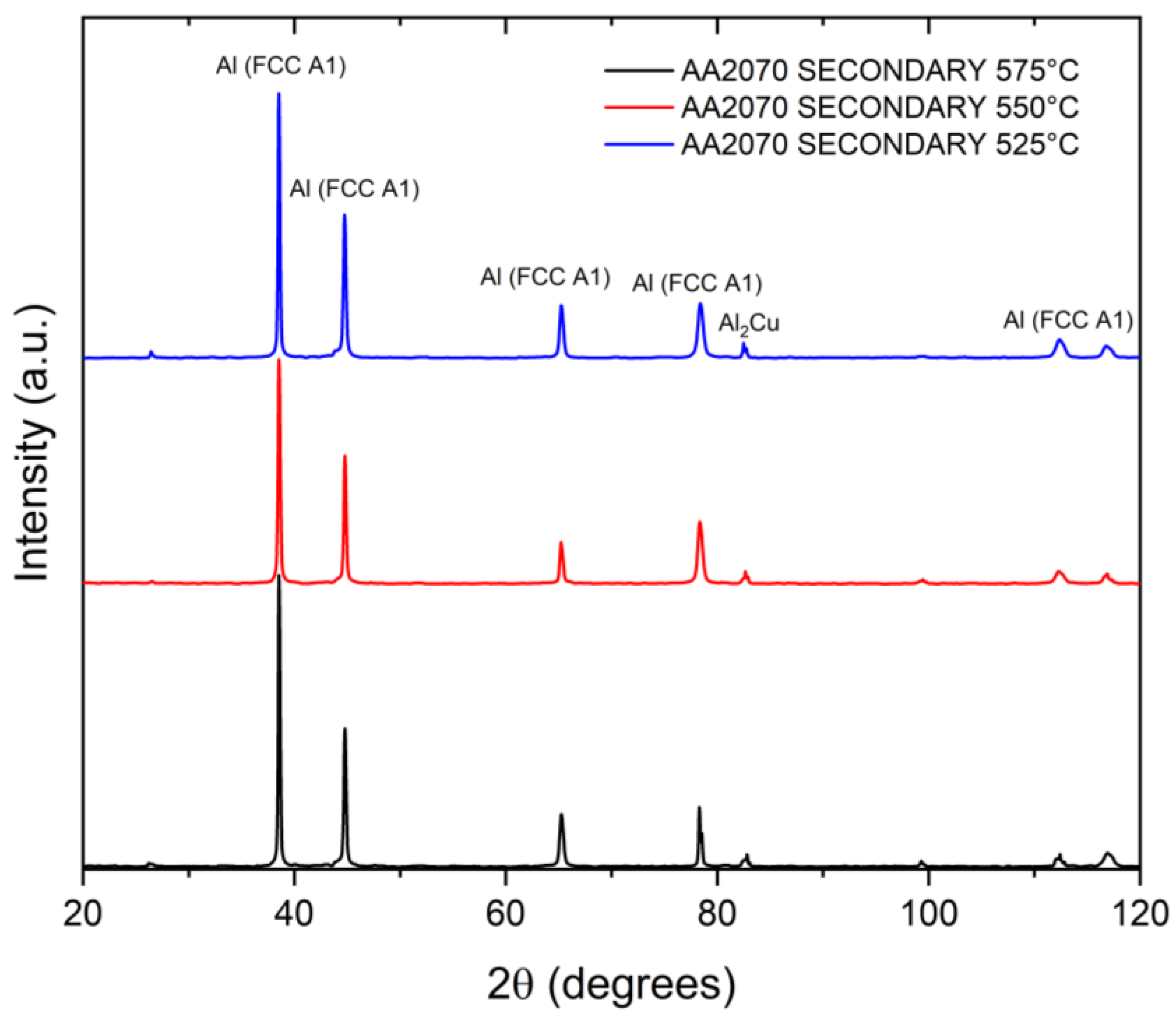
3.4. X-ray Diffraction Analysis
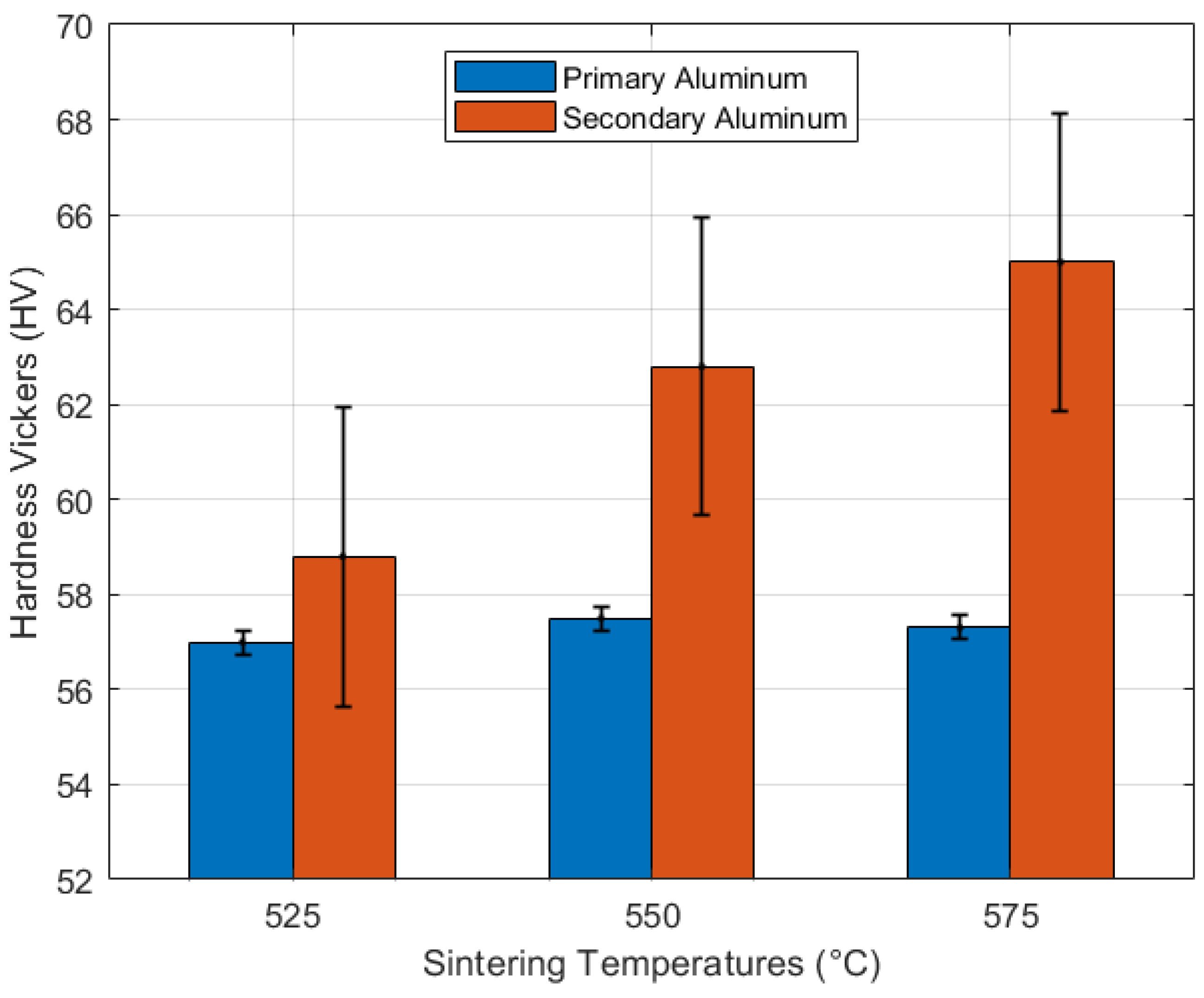
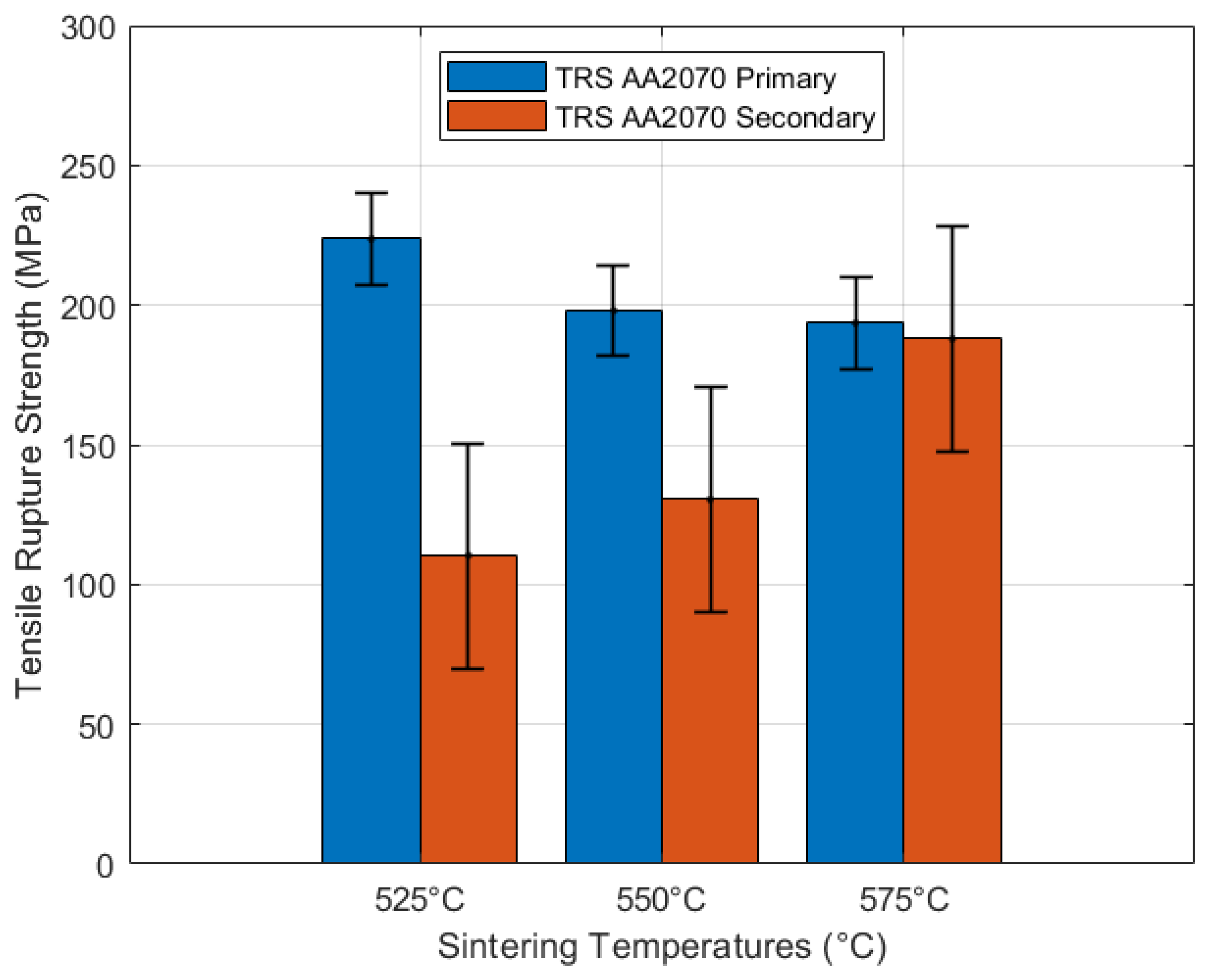
3.5. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, M.; Pandey, B.; Agrawal, M. Chapter 8—Carbon Footprint of Aluminum Production: Emissions and Mitigation; Muthu, S.S., Ed.; Butterworth-Heinemann: Boston, UK, 2018; pp. 197–228. [Google Scholar] [CrossRef]
- Pedneault, J.; Majeau-Bettez, G.; Krey, V.; Margni, M. What future for primary aluminium production in a decarbonizing economy? Glob. Environ. Chang. 2021, 69, 102316. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Haraldsson, J.; Johansson, M.T. Review of measures for improved energy efficiency in production-related processes in the aluminium industry—From electrolysis to recycling. Renew. Sustain. Energy Rev. 2018, 93, 525–548. [Google Scholar] [CrossRef]
- Wan, B.; Chen, W.; Lu, T.; Liu, F.; Jiang, Z.; Mao, M. Review of solid state recycling of aluminum chips. Resour. Conserv. Recycl. 2017, 125, 37–47. [Google Scholar] [CrossRef]
- Moghimian, P.; Poirié, T.; Habibnejad-Korayem, M.; Zavala, J.A.; Kroeger, J.; Marion, F.; Larouche, F. Metal powders in additive manufacturing: A review on reusability and recyclability of common titanium, nickel and aluminum alloys. Addit. Manuf. 2021, 43, 102017. [Google Scholar] [CrossRef]
- Kadir, M.I.A.; Mustapa, M.S.; Latif, N.A.; Mahdi, A.S. Microstructural Analysis and Mechanical Properties of Direct Recycling Aluminium Chips AA6061/Al Powder Fabricated by Uniaxial Cold Compaction Technique. Procedia Eng. 2017, 184, 687–694. [Google Scholar] [CrossRef]
- Fuziana, Y.F.; Warikh, A.R.M.; Lajis, M.A.; Azam, M.A.; Muhammad, N.S. Recycling aluminium (Al 6061) chip through powder metallurgy route. Mater. Res. Innov. 2014, 18, S6-354–S6-358. [Google Scholar] [CrossRef]
- Pulido-Suárez, P.A.; Uñate-González, K.S.; Tirado-González, J.G.; Esguerra-Arce, A.; Esguerra-Arce, J. The evolution of the microstructure and properties of ageable Al-Si-Zn-Mg alloy during the recycling of milling chips through powder metallurgy. J. Mater. Res. Technol. 2020, 9, 11769–11777. [Google Scholar] [CrossRef]
- Sandeep, B.; Kannan, T.T.M.; Chandradass, J.; Ganesan, M.; John Rajan, A. Scope of 3D printing in manufacturing industries—A review. Mater. Today Proc. 2021, 45, 6941–6945. [Google Scholar] [CrossRef]
- Sauerwein, M.; Doubrovski, E.; Balkenende, R.; Bakker, C. Exploring the potential of additive manufacturing for product design in a circular economy. J. Clean. Prod. 2019, 226, 1138–1149. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of ‘dirty’ alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Hettiarachchi, B.D.; Brandenburg, M.; Seuring, S. Connecting additive manufacturing to circular economy implementation strategies: Links, contingencies and causal loops. Int. J. Prod. Econ. 2022, 246, 108414. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Tosone, R.; Uggutwitzer, P.J.; Pogatscher, S. On the potential of aluminum crossover alloys. Prog. Mater. Sci. 2022, 124, 100873. [Google Scholar] [CrossRef]
- Li, S.; Yue, X.; Li, Q.; Peng, H.; Dong, B.; Liu, T.; Yang, H.; Fan, J.; Shu, S.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Abd El-Aty, A.; Xu, Y.; Guo, X.; Zhang, S.-H.; Ma, Y.; Chen, D. Strengthening mechanisms, deformation behavior, and anisotropic mechanical properties of Al-Li alloys: A review. J. Adv. Res. 2018, 10, 49–67. [Google Scholar] [CrossRef]
- Azarniya, A.; Taheri, A.K.; Taheri, K.K. Recent advances in ageing of 7xxx series aluminum alloys: A physical metallurgy perspective. J. Alloys Compd. 2019, 781, 945–983. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Umer, R.; Khan, K.A. Review of recent trends and developments in aluminium 7075 alloy and its metal matrix composites (MMCs) for aircraft applications. Results Eng. 2023, 20, 101372. [Google Scholar] [CrossRef]
- Starke, E.A. Historical Development and Present Status of Aluminum–Lithium Alloys. In Aluminum-Lithium Alloys: Processing, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–26. [Google Scholar] [CrossRef]
- Dorin, T.; Vahid, A.; Lamb, J. Aluminium Lithium Alloys. In Fundamentals of Aluminium Metallurgy: Recent Advances; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–438. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Hajjioui, E.A.; Bouchaâla, K.; Faqir, M.; Essadiqi, E. A review of manufacturing processes, mechanical properties and precipitations for aluminum lithium alloys used in aeronautic applications. Heliyon 2023, 9, e12565. [Google Scholar] [CrossRef]
- Wanhill, R.J.H.; Bray, G.H. Chapter 12—Fatigue Crack Growth Behavior of Aluminum–Lithium Alloys; Eswara Prasad, N., Gokhale, A.A., Wanhill, R.J.H., Eds.; Butterworth-Heinemann: Boston, UK, 2014; pp. 381–413. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Lavernia, E.J.; Eswara Prasad, N.; Kutumbarao, V.V. Chapter 10—Quasi-Static Strength, Deformation, and Fracture Behavior of Aluminum–Lithium Alloys; Eswara Prasad, A.N., Gokhale, A., Wanhill, R.J.H., Eds.; Butterworth-Heinemann: Boston, UK, 2014; pp. 305–339. [Google Scholar] [CrossRef]
- Semenov, A.M.; Sinyavskiy, V.; Kalinin, V.D. Corrosion resistance of aluminum-lithium alloys under various climatic conditions. Prot. Met. Phys. Chem. Surfaces 2014, 50, 236–243. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Li, J. Microstructures evolution and mechanical properties disparity in 2070 Al-Li alloy with minor Sc addition. Trans. Nonferrous Met. Soc. China 2018, 28, 2151–2161. [Google Scholar] [CrossRef]
- Staroselsky, A.; Borkowski, L. Microstructural Characterization and Simulation of High Temperature Inelastic Deformation and Fracture of Al-Li 2070. J. Mater. Eng. Perform. 2019, 28, 6942–6957. [Google Scholar] [CrossRef]
- Lumley, R.N.; Schaffer, G.B. The effect of solubility and particle size on liquid phase sintering. Scr. Mater. 1996, 35, 589–595. [Google Scholar] [CrossRef]
- Schaffer, G.B.; Sercombe, T.B.; Lumley, R.N. Liquid phase sintering of aluminum alloys. Mater. Chem. Phys. 2001, 67, 85–91. [Google Scholar] [CrossRef]
- ASTM B311-17; Standard Test Method for Density of Powder Metallurgy (PM) Materials Containing Less Than Two Percent Porosity. ASTM International: West Conshohocken, PA, USA, 2017.
- UNE EN ISO 3325; Sintered Metal Materials, Excluding Hardmetals—Determination of Transverse Rupture Strength. ISO: Geneva, Switzerland, 2002.
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Chapter 5—Alloys of the Al–Cu–Mn–(Mg, Fe, Si) System; Belov, N.A., Eskin, D.G., Aksenov, A.A., Eds.; Elsevier: Oxford, UK, 2005; pp. 159–192. [Google Scholar] [CrossRef]
- Jiao, S.; Cheng, X.; Shen, S.; Wang, X.; He, B.; Liu, D.; Wang, H. Microstructure evolution and mechanical behavior of Al–Li alloy fabricated by laser melting deposition technique. J. Alloys Compd. 2020, 821, 153125. [Google Scholar] [CrossRef]
- McPhee, W.A.G.; Schaffer, G.B.; Drennan, J. The effect of iron on liquid film migration and sintering of an Al–Cu–Mg alloy. Acta Mater. 2003, 51, 3701–3712. [Google Scholar] [CrossRef]
- Reddy, M.P.; Manakari, V.; Parande, G.; Shakoor, R.A.; Mohamed AM, A.; Gupta, M. Structural, mechanical and thermal characteristics of Al-Cu-Li particle reinforced Al-matrix composites synthesized by microwave sintering and hot extrusion. Compos. Part. B Eng. 2019, 164, 485–492. [Google Scholar] [CrossRef]
- Partridge, P.G. Oxidation of aluminium-lithium alloys in the solid and liquid states. Int. Mater. Rev. 1990, 35, 37–58. [Google Scholar] [CrossRef]
- Qi, M.; Chen, C.; Wei, J.; Mei, X.; Sun, C.; Su, G.; Zhang, C.; Yan, M.; Yang, F.; Guo, Z. Superior mechanical properties and microstructural evolution of powder metallurgy 2195 Al-Li alloy subjected to hot extrusion. J. Alloys Compd. 2023, 962, 171184. [Google Scholar] [CrossRef]
- Rodríguez-González, P.; Ruiz-Navas, E.M.; Gordo, E. Effect of Heat Treatment Prior to Direct Hot-Extrusion Processing of Al-Cu-Li Alloy. Metals 2022, 12, 1046. [Google Scholar] [CrossRef]
- Zhang, D.; Zou, H.; Cai, S. Effect of Iron Coating on Thermal Properties of Aluminum-Lithium Alloy Powder. Propellants Explos. Pyrotech. 2017, 42, 953–959. [Google Scholar] [CrossRef]
- Mishra, R.S.; Sidhar, H. Chapter 2—Physical Metallurgy of 2XXX Aluminum Alloys; Mishra, R.S., Sidhar, H., Eds.; Butterworth-Heinemann: Boston, UK, 2017; pp. 15–36. [Google Scholar] [CrossRef]
- Madhusudhan Reddy, G.; Gokhale, A.A. Chapter 9—Welding Aspects of Aluminum–Lithium Alloys; Prasad, N.E., Gokhale, A.A., Wanhill, R.J.H., Eds.; Butterworth-Heinemann: Boston, UK, 2014; pp. 259–302. [Google Scholar] [CrossRef]
- Xun, C.; Li, X.; Wen, K.; Zhu, K.; Gao, G.; Li, Z.; Zhang, Y.; Xiong, B. Influence of enhanced Li content on the as-cast eutectic phase features and the evolution during homogenization of Al-Cu-Li alloys. J. Mater. Res. Technol. 2023, 26, 8555–8568. [Google Scholar] [CrossRef]
- Tsubakino, H.; Nozato, R.; Sakurai, T.; Hasegawa, Y.; Hayashi, Y. Precipitation of metastable δ′ in Al–1.9Li–2.5Mg alloy. Mater. Sci. Technol. 1994, 10, 222–226. [Google Scholar] [CrossRef]
- Bo, L.; Xiangxiang, H.; Rui, X.; Yuliang, Z.; Yemao, L.; Huaqiang, X. Evolution of iron-rich intermetallics and its effect on the mechanical properties of Al–Cu–Mn–Fe–Si alloys after thermal exposure and high-temperature tensile testing. J. Mater. Res. Technol. 2023, 23, 2527–2541. [Google Scholar] [CrossRef]
- Madhankumar, S.; Sivakumar, K.; Chandradass, J.; Addanki, R.S.; Rodriguez, V.A.; Saroshkumar, U. Enhancement of mechanical properties and tribological properties of aluminum using Iron(Fe) and Nickel(Ni) additives. Mater. Today Proc. 2021, 45, 6852–6856. [Google Scholar] [CrossRef]
- Novák, P.; Benediktová, D.; Mestek, S.; Tsepeleva, A.; Kopeček, J. Aluminum alloys with natural ratio of alloying elements manufactured by powder metallurgy. J. Alloys Compd. 2023, 931, 167440. [Google Scholar] [CrossRef]
- Navas EM, R.; Palenzuela, B.R. Sintering of Aluminum Alloys. Processing and Properties. Encycl. Mater. Met. Alloys 2022, 3, 343–352. [Google Scholar]
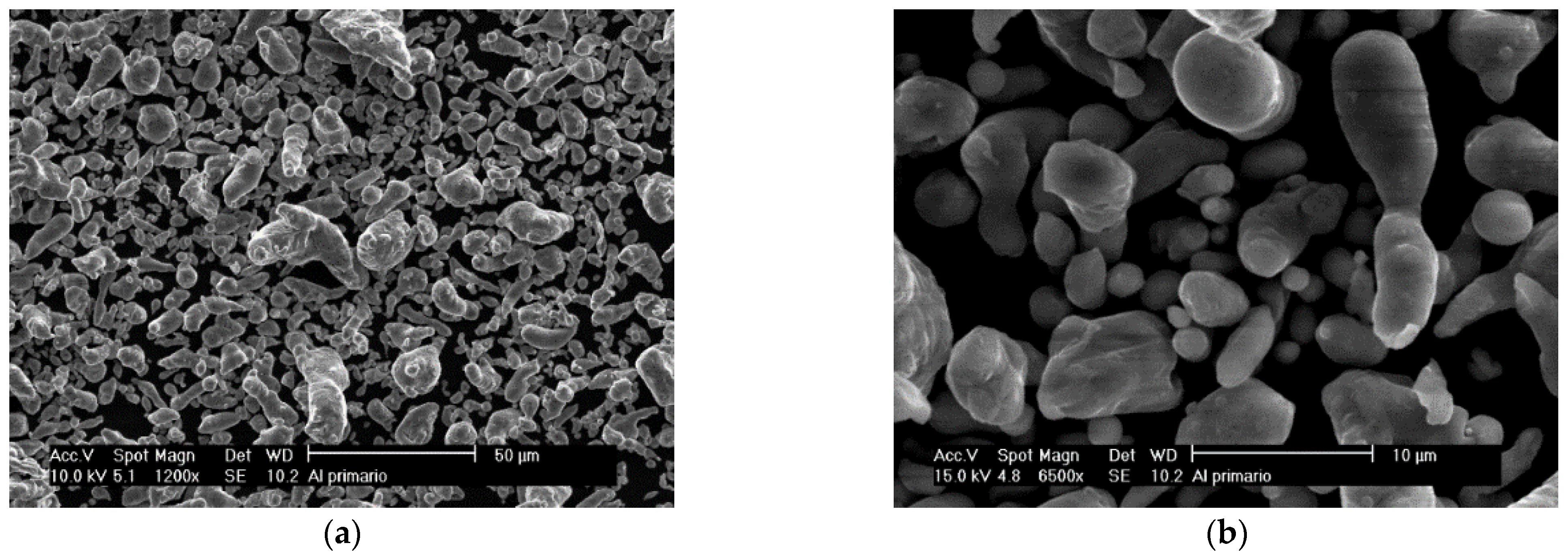

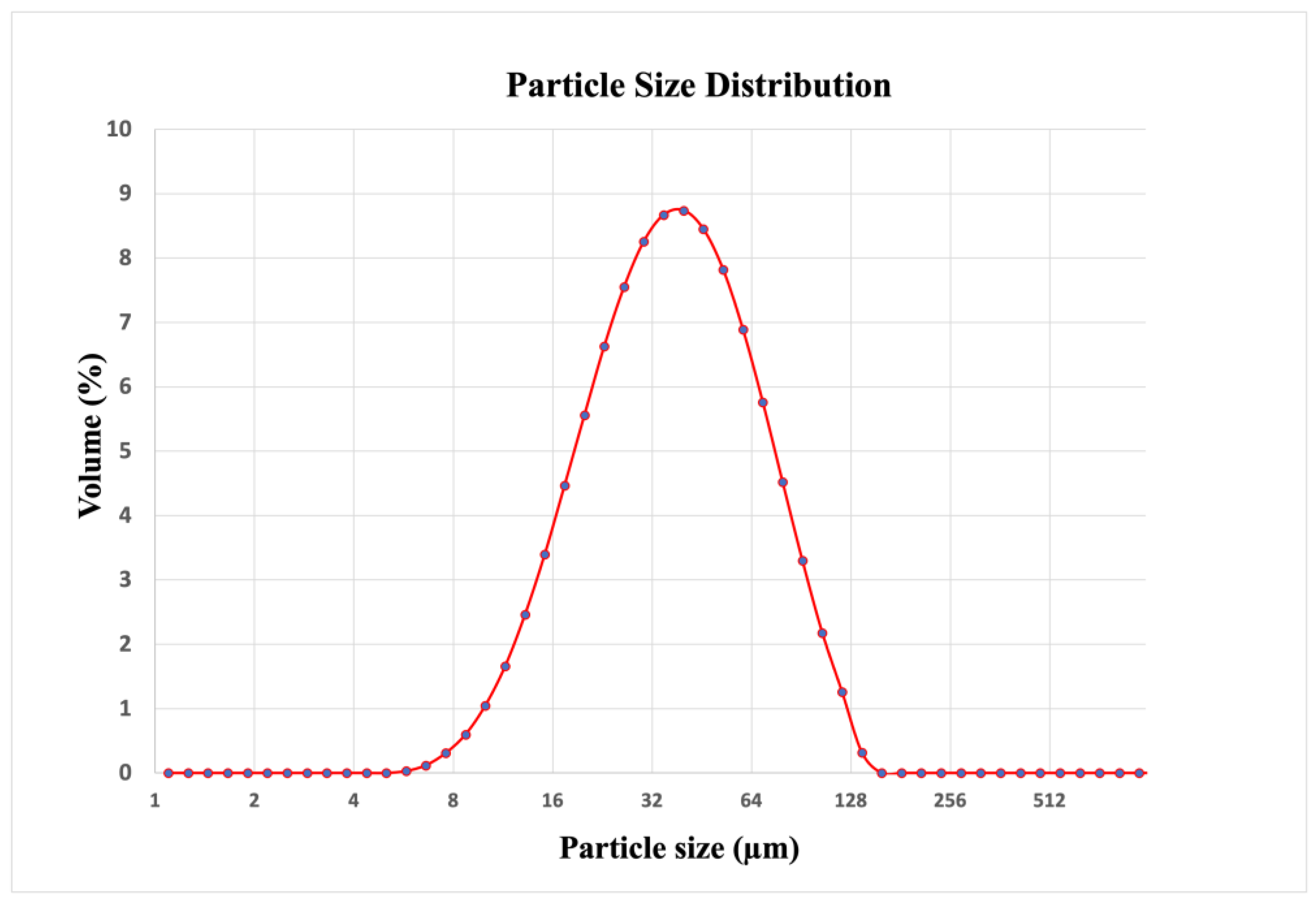
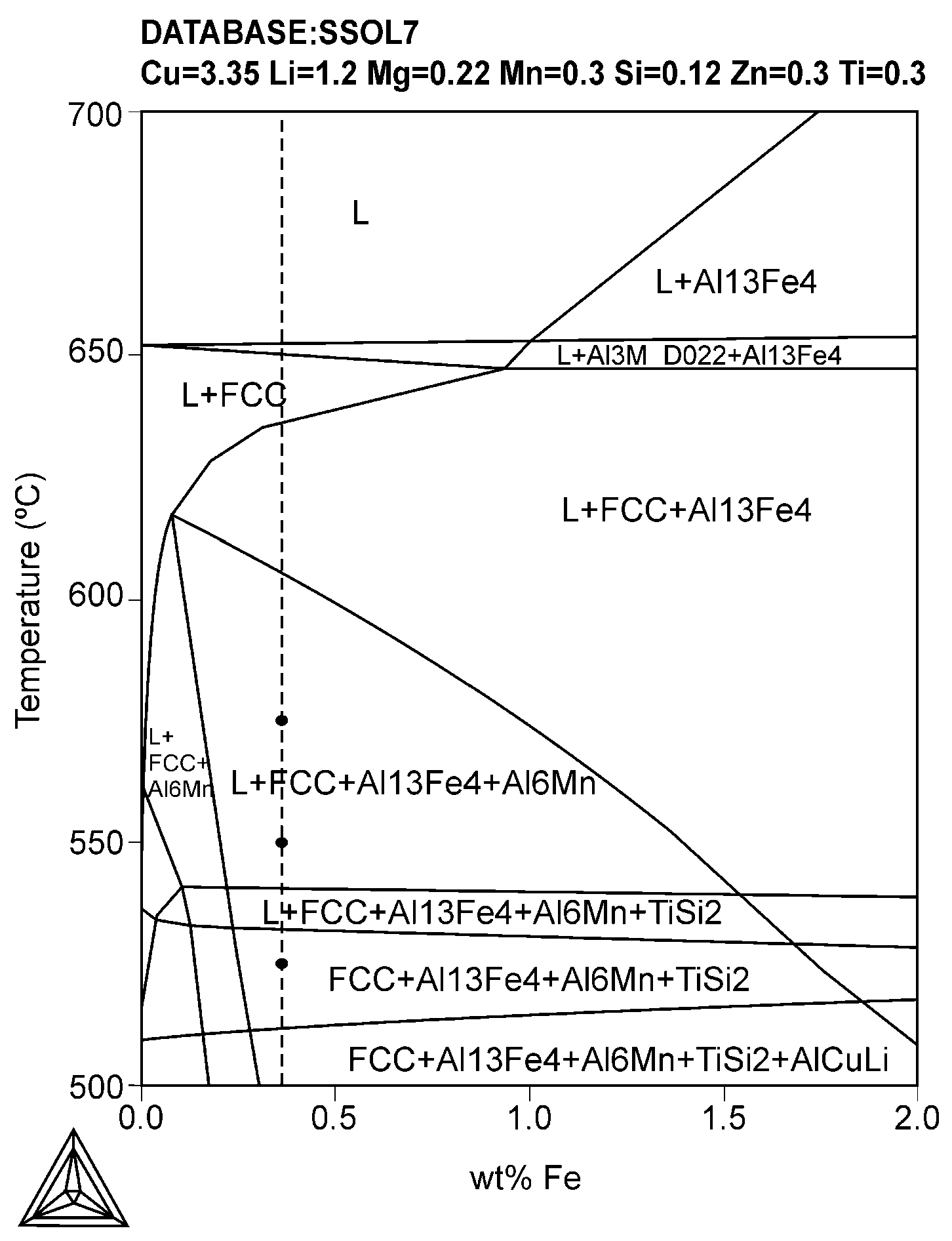
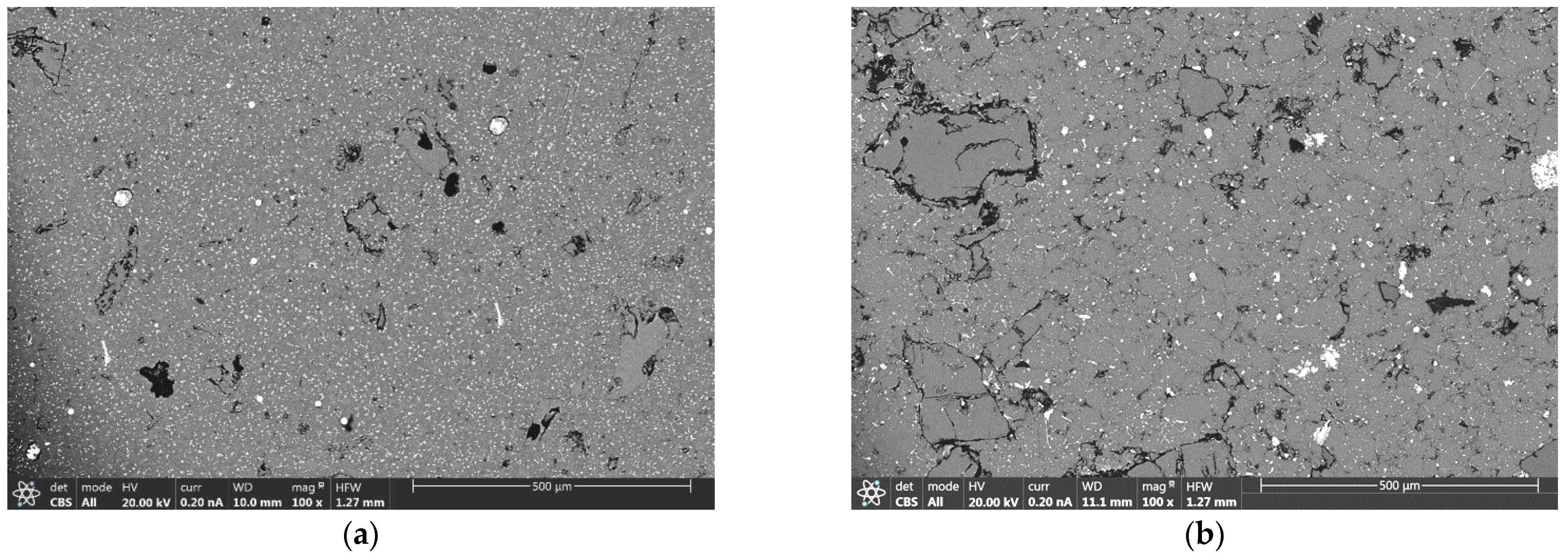

| Secondary Aluminum Composition (wt.%) | |||||
|---|---|---|---|---|---|
| Al | Si | Fe | Mg | Cu | Others |
| 98.61 | 0.32 | 0.38 | 0.35 | 0.08 | 0.34 |
| Weight % | Cu | Li | Mg | Si | Fe | Zn | Mn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Chemical Composition Range for AA2070 | 2.9–3.8 | 1.0–1.4 | 0.05–0.4 | 0.12 | 0.15 | 0.1–0.5 | 0.1–0.5 | 0.1 | Bal. |
| Average Chemical Composition Primary AA2070 | 3.28 | 1.17 | 0.25 | 0.04 | 0.07 | 0.28 | 0.34 | 0.12 | Bal. |
| Average Chemical Composition Secondary AA2070 | 3.45 | 1.20 | 0.42 | 0.31 | 0.36 | 0.35 | 0.37 | 0.14 | Bal. |
| PHASES | Al | Li | Cu | Mg | Mn | Fe | Si | Zn | Ti | Mass Fraction |
|---|---|---|---|---|---|---|---|---|---|---|
| at.% | ||||||||||
| Al13Fe4 | 60.97 | - | - | - | 4.30 | 34.66 | - | - | - | 0.008 |
| Al6Mn | 74.50 | - | - | - | 11.98 | 13.52 | - | - | - | 0.015 |
| FCC_A2 | 94.54 | 1.23 | 3.43 | 0.23 | 0.08 | 0.008 | 0.09 | 0.3 | 0.08 | 0.976 |
| TiSi2 | - | - | - | - | - | - | 53.98 | - | 46.02 | 0.0006 |
| PHASES | Al | Li | Cu | Mg | Mn | Fe | Si | Zn | Ti | Mass Fraction |
|---|---|---|---|---|---|---|---|---|---|---|
| at.% | ||||||||||
| Liquid | 77.38 | 2.04 | 24.22 | 0.78 | 0.03 | 0.03 | 1.52 | 0.61 | 0.003 | 0.032 |
| Al13Fe4 | 60.96 | - | - | - | 4.51 | 34.53 | - | - | - | 0.011 |
| Al6Mn | 74.50 | - | - | - | 12.18 | 13.32 | - | - | - | 0.010 |
| FCC_A2 | 95.25 | 1.20 | 0.027 | 2.72 | 0.13 | 0.01 | 0.08 | 0.296 | 1.06 | 0.946 |
| AA2070 Alloy | Temperature (°Celsius) | Average Density (g/cm3) | Average ρrelative (%) | Average Porosity (%) |
|---|---|---|---|---|
| Primary | 525 | 2.52 | 95.90 ± 0.25 | 4.10 |
| 550 | 2.52 | 95.81 ± 0.24 | 4.19 | |
| 575 | 2.47 | 93.85 ± 0.33 | 6.15 | |
| Secondary | 525 | 2.49 | 94.84 ± 0.27 | 5.16 |
| 550 | 2.49 | 94.61 ± 0.44 | 5.39 | |
| 575 | 2.49 | 94.68 ± 0.18 | 5.32 |
| Sintering Temperature | 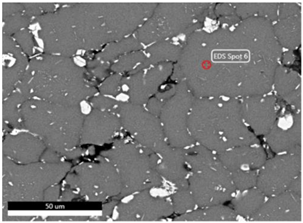 | |||
| Element (at.%) | 525 °C | 550 °C | 575 °C | |
| Al | 96.6 | 98.3 | 98.7 | |
| Cu | 3.4 | 1.7 | 1.3 | |
| Sintering Temperature | 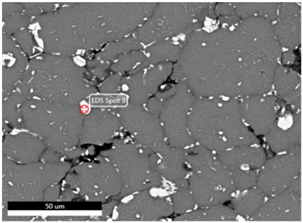 | |||
| Element (at.%) | 525 °C | 550 °C | 575 °C | |
| Al | 78.5 | 77.4 | 76.0 | |
| Cu | 16.6 | 3.0 | 3.5 | |
| Mn | - | 1.7 | 1.8 | |
| Fe | 4.9 | 12.3 | 13.2 | |
| Si | - | 5.6 | 5.5 | |
| Sintering Temperature | 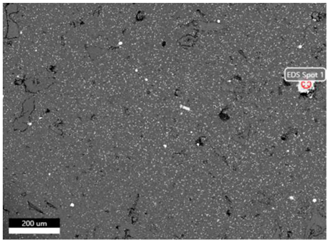 | |
| Element (at.%) | 525 °C | |
| Al | 62.8 | |
| Mn | 23.0 | |
| Cu | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadilla, A.; Sanhueza, J.P.; Montalba, C.; Ruiz-Navas, E.M. Effect of Sintering Temperature on Phase Formation and Mechanical Properties of Al–Cu–Li Alloy Prepared from Secondary Aluminum Powders. Metals 2024, 14, 12. https://doi.org/10.3390/met14010012
Cañadilla A, Sanhueza JP, Montalba C, Ruiz-Navas EM. Effect of Sintering Temperature on Phase Formation and Mechanical Properties of Al–Cu–Li Alloy Prepared from Secondary Aluminum Powders. Metals. 2024; 14(1):12. https://doi.org/10.3390/met14010012
Chicago/Turabian StyleCañadilla, Antonio, Juan Pablo Sanhueza, Cristóbal Montalba, and Elisa María Ruiz-Navas. 2024. "Effect of Sintering Temperature on Phase Formation and Mechanical Properties of Al–Cu–Li Alloy Prepared from Secondary Aluminum Powders" Metals 14, no. 1: 12. https://doi.org/10.3390/met14010012






