Abstract
Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys were prepared by induction levitation melting, and the effect of substitution of Al for Ni on the microstructure and hydrogen storage properties was studied in the present work. The results illustrated that the solidification path, phase constitution, and grain size were significantly altered by Al addition. Appropriate Al addition improved abundance and grain refinement of the Mg, Mg2Ni, and Mg15NiY ternary eutectic. But as Al further increased, Mg solidified independently rather than in the formation of the ternary eutectic. More Al favored the formation of Al3Ni2Y but suppressed Mg2Ni and YMgNi4. Although the hydrogen absorption activation and the kinetic property deteriorated, the thermodynamic stability of hydrides was enhanced by adding Al. Hydrogen absorption ability under low pressure was improved, and the Mg80Ni8Al8Y4 alloy could absorb nearly 3.5 wt% hydrogen under 1 bar hydrogen at 250 °C.
1. Introduction
Conventional energy sources are gradually proving insufficient to meet contemporary energy demands due to their nonrenewable nature and the associated environmental pollution. Hydrogen energy has emerged as an ideal alternative to conventional sources due to its pollution-free characteristics, abundant availability, and high combustion efficiency. Nevertheless, the practical implementation of hydrogen energy faces persistent challenges related to the development of safe, efficient, and economically viable hydrogen storage methods.
In comparison to high-pressure and liquid hydrogen storage approaches, solid-state hydrogen storage offers considerable advantages in terms of high volumetric hydrogen storage density and enhanced safety, thereby demonstrating significant developmental potential. Among the solid-state hydrogen storage materials, magnesium-based alloys have garnered substantial attention due to their notable attributes, including a high energy density of 7.6 wt% by weight and 110 kg/m3 H2 by volume, as well as their abundant presence in the Earth’s crust [1].
However, the application of Mg-based hydrogen storage alloys remains constrained by the high thermal stability of MgH2 and the sluggish kinetics of hydrogenation/dehydrogenation processes. Various strategies, including amorphization [2,3,4], alloying [5,6], nanocrystalline structures [7,8,9,10,11], and catalysis [12,13,14,15], have demonstrated efficacy in enhancing the hydrogen storage properties of Mg-based alloys [1,16,17]. Among these strategies, the modification of phase constitution and micro-morphology through the addition of other elements is considered pivotal for optimizing hydrogen storage properties. During the hydrogenation process, rare-earth magnesium intermetallic compounds, such as REMg12, RE2Mg17, and RE5Mg41, will decompose into MgH2 and REHx nano phases [18]. The nano REHX particles formed through this disproportionation decomposition facilitate nucleation and hydrogen diffusion, thereby effectively enhancing the alloy’s hydrogen absorption/desorption kinetics. Due to the positive impact of REHX phases, the addition of RE elements to magnesium-based alloys has gained widespread acceptance.
In addition to rare-earth (RE) elements, the hydrogen storage properties of Mg-based alloys can be effectively enhanced through the alloying of B-side elements (hydrogen non-affinity elements). The impact of the third transition metals, including Cu, Fe, Co, and Ni, on the hydrogen storage properties of Mg/MgH2 has been investigated by Hanada et al. [19], with a particularly positive influence noted for Ni. Yong et al. [20] fabricated Mg90RE3Ni7 alloy by incorporating Co via mechanical milling. Three distinct hydrogen plateaus were identified, corresponding to Mg6Co2H11/Mg2CoH, Mg/MgH2, and Mg2Ni/Mg2NiH4, respectively. Improved hydrogen absorption and desorption kinetics were demonstrated, attributed to the preferential nucleation of Mg induced by the chain reaction of Mg6Co2H11/Mg2CoH5. Furthermore, Ti and V have been shown to reduce the thermal stability and activation energy of the Mg-Ce-Y-Ni alloys [21]. Overall, introducing secondary metal hydrides through the addition of transition metals can also promote hydrogen diffusion and enhance hydrogen storage properties.
In recent years, Mg-Ni-RE ternary alloys have garnered considerable attention owing to their unique microstructure and hydrogen storage properties. Xie et al. [22] reported that Mg-Ni-Ce alloys demonstrate exceptional kinetic performance, attributed to the catalytic effect of the in-situ formed CeH3. Among the Mg-Ni-RE alloys, long-period stacking ordered (LPSO) phases were usually formed in the Mg-Ni-Y alloys. Li et al. found that the 18R-type LPSO phase decomposed under 0.05 MPa hydrogen pressure at 280 °C [23]. Song et al. [24] fabricated an alloy with a composition of Mg76.87Ni12.78Y10.35 via induction melting, which included phases such as Mg2Ni, Mg, Mg15NiY, and MgNi4Y. The investigation observed that the LPSO phase (Mg15NiY) with a 14H crystal structure undergoes in-situ decomposition into nano RE hydrides during hydrogenation. These nano RE hydrides exhibit a pronounced catalytic effect, thereby enhancing hydrogenation kinetics. The addition of Ce, Ni, and Y effectively introduced multiple reaction pathways and nucleation sites, contributing to a hydrogen pump effect through the transition between Mg2Ni/Mg2NiH4 and YH2/YH3, thereby significantly improving the hydrogenation/dehydrogenation kinetics [25]. Yu et al. prepared Mg95−xNixY5 (x = 5, 10, 15) alloys by vacuum induction melting, among which Mg80Ni15Y5 alloy can absorb hydrogen at 200 °C and presented excellent hydrogen absorption and desorption properties [26].
Alloying with aluminum (Al) has found widespread applications in various hydrogen storage alloy systems, including AB5, superlattice A2B7, V-based solid solutions, and Mg-based alloys [27,28,29,30,31,32]. Notably, Mg-Al intermetallics such as Mg17Al12 and Mg2Al3 have been identified for their capacity to engage in reactions with hydrogen, thereby enhancing the decomposition kinetics of MgH2 [33,34,35,36]. In a study conducted by Lu et al. [37], the dehydrogenation enthalpy was observed to decrease from 77.9 kJ/mol to 70.8 kJ/mol for the Mg90Al10 alloy compared to pure Mg. Similar results were reported by Zhong et al. [38]. Additionally, the work of Cao et al. [39] highlighted that the introduction of Al into Mg2Ni resulted in grain refinement and improved thermodynamics (53.9 kJ/mol), attributed to the formation of AlNi.
These studies have highlighted the effectiveness of aluminum (Al) in enhancing the hydrogen storage properties of Mg-based alloys, though a comprehensive investigation is still lacking. While practical applications of Mg-based alloys are currently limited, efforts are underway to explore potential scenarios such as solid-state hydrogen storage [40]. In certain applications, such as hydrogen purification [41], the heightened dehydrogenation temperature no longer serves as the predominant limitation for magnesium-based hydrogen storage alloys. Instead, greater emphasis is placed on discerning hydrogen absorption characteristics, encompassing parameters such as hydrogen absorption temperature, plateau, and low-pressure hydrogen absorption kinetics. While considerable attention has been directed towards the dehydrogenation thermodynamics and kinetics of Mg-based alloys, hydrogen absorption properties, especially under low-pressure conditions, are frequently overlooked.
Given that dramatic alloying effects can be introduced by Al addition, substitution by Al has seldom been reported in the Mg-Ni-Y system. In the present study, we systematically investigated the effects of aluminum (Al) on the microstructure and hydrogen storage properties of Mg80Ni16−xAlxY4 alloys (x = 2, 4, 8). Effort was placed on hydrogen absorption thermodynamics and kinetics under varying temperatures and hydrogen pressures. We paid particular attention to hydrogen absorption ability under a hydrogen pressure of no more than 1 bar.
2. Experiment
2.1. Alloy Preparation
The Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys were prepared by induction levitation melting in a water-cooled copper crucible under an argon atmosphere. The purity of the raw Mg, Y, Ni, and Al elements was greater than 99.5%. Y-Ni binary alloys were first melted three times for homogeneity. Then, Mg and Al metals were added and remelted three times again. Excess Mg (10 wt%), Al (5 wt%), and Y (5 wt%) were added to compensate for the evaporative loss during melting. The three alloys were referred to as Al2, Al4, and Al8 alloys, respectively.
2.2. Characterization
Phase constitution and crystal structure of the as-cast alloys were characterized by X-ray diffraction (XRD, Rigaku-SmartLab, Rigaku, Tokyo, Japan) with Cu Kα radiation by a step of 0.02° under 40 kV and 40 mA. For data analysis, Rietveld refinement on XRD datasets was conducted with Maud (Material analysis using diffraction, Maud v.2.33 version, Luca Lutterotti Research Group, University of Trento, Trento, Italy). Firstly, the standard crystal structure (added by CIF files) was imported, and the unit cell parameters were adjusted based on the measured XRD profiles one by one. Then, the background of XRD was refined based on the instrument parameters, including the wavelength of the X-ray, diffraction type (B-B type), the range of tested angles, etc. Next, the proportion factors, peak shape parameters, lattice parameters, atomic position parameters, etc., of each phase were modified. Finally, all the phase structures were added, and the refinement was successively conducted by background, basic phase parameters, microstructure parameters, crystal structure parameters, texture factors, and strain factors. Crystallographic Information Files (CIFs) from the ICSD database were used in the procedure of the Rietveld refinement, as shown in Table S1. The Rwp value is the residual calculated directly from the XRD spectrum calculated by the model structure and the experimental data after adding weights to a specific position, which is a description of the fitted graph. The results are generally considered to be credible when the Rwp value is <15% [42].
Microstructure morphology was observed by electron probe X-ray micro-analyzer (EPMA, JEOL Ltd., Tokyo, Japan) under backscattered electron mode (BSE). The chemical information was measured by energy dispersive spectroscopy (EDS) equipped on EPMA.
2.3. Hydrogen Storage Property
Heat changes in hydrogenation and dehydrogenation were measured by high-pressure differential scanning calorimetry (HPDSC, NETZSCH-DSC 204 HP, NETZSCH, Selbu, Germany). The hydrogen storage capability and kinetic properties were measured by Setaram-PCTPRO (Hy-Energy, Newark, CA, USA) using Sievert’s method [43]. To fully activate, the samples were first pumped at 100 °C for 2 h and then cycled under 3 MPa pressure at 300 °C five times. Each hydrogenation/dehydrogenation cycle consists of absorption at 3 MPa for 1h and desorption by evacuating for 2 h at 300 °C. Pressure–composition–temperature (PCT) absorption curves were measured at 200 °C, 250 °C, and 300 °C, and the enthalpy changes were calculated based on Vant’ Hoff equation.
3. Experimental Results and Discussion
3.1. Phase Composition of the Original as-Cast Alloys
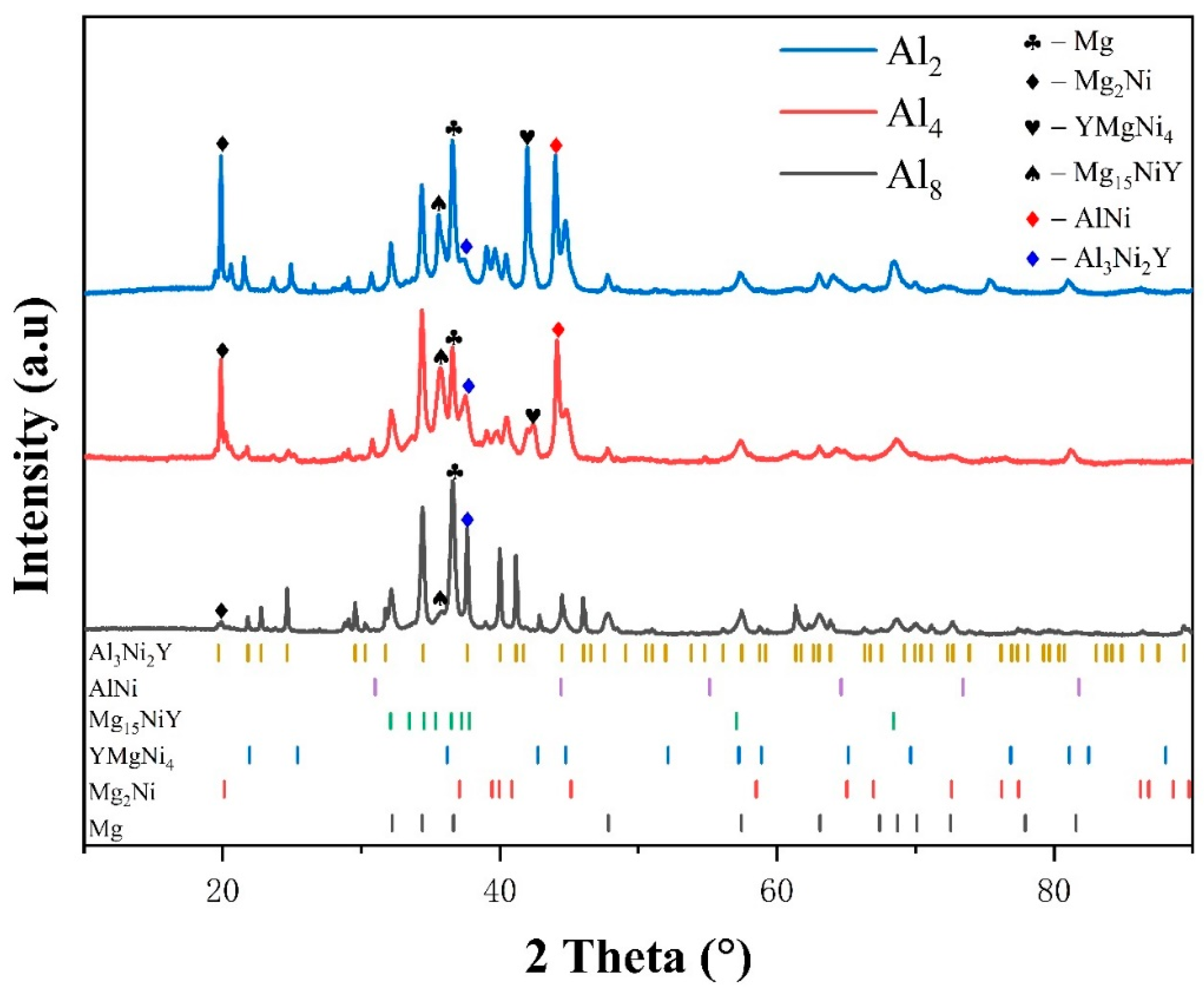
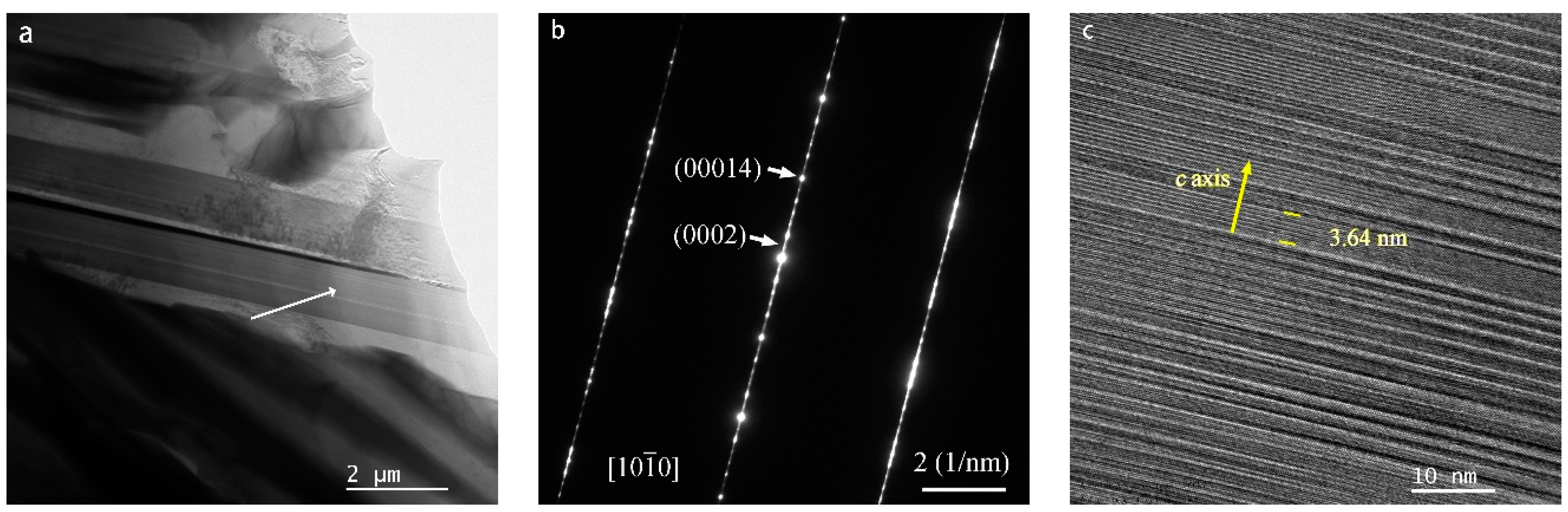
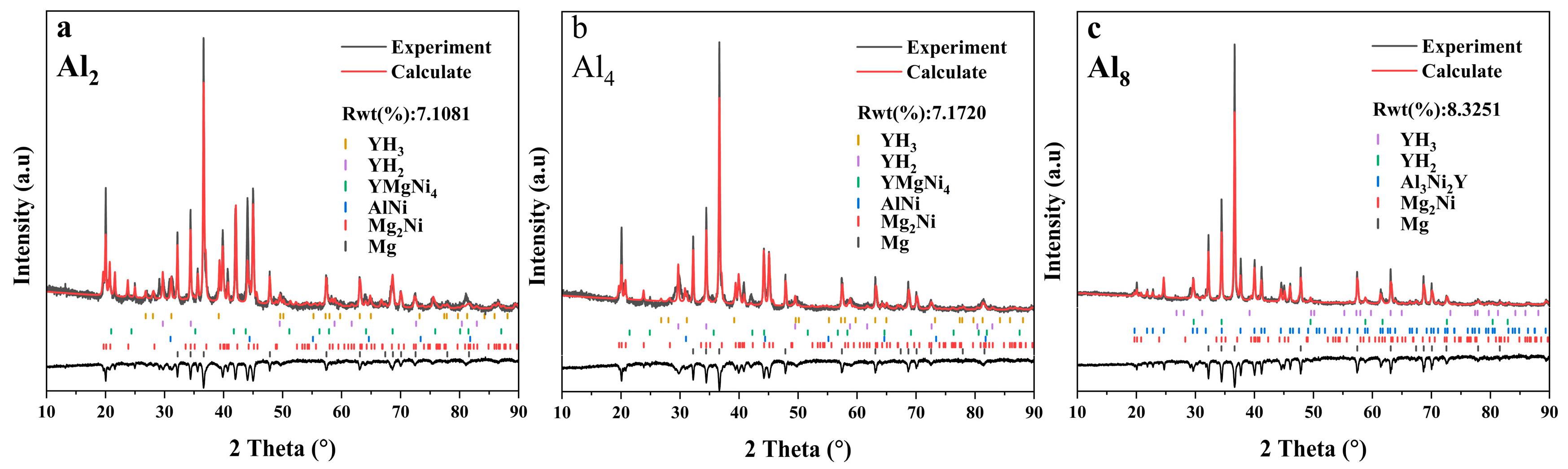
Presented in Figure 1 is the X-ray diffraction (XRD) pattern of the as-cast Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys. Analysis of the XRD pattern reveals Mg, Mg2Ni, YMgNi4, Mg15NiY, and AlNi phases in the Mg80Ni14Al2Y4 alloy. Additionally, minor Al3Ni2Y can also be identified through comparison with the XRD curve of the Mg80Ni8Al8Y4 alloy. The standard crystal structures from ICSD (The Inorganic Crystal Structure Database) are listed in Table S1 in the Supplementary Materials. The cell parameters of the phase structures are refined and displayed in Table S2 in the Supplementary Materials. Since the standard structure of LPSO is still unclear, the determination of the unit cell parameters of LPSO was ignored. Unit cells of Mg and Mg2Ni are larger than the standard crystal structure, which may be caused by a trace of solid solution of Y and Al. The Mg15NiY phase was characterized by TEM, as shown in Figure 2. From electron diffraction and HRTEM analyses, Mg15NiY was identified as a typical 14H-type LPSO [44]. Different from the ternary Mg-Ni-Y alloys, the Al-Ni or Al-Ni-Y phases were introduced by Al substitution. In addition, compared with the similar Mg80Ni15Y5 alloy [26], where the main phases were also Mg and Mg2Ni, the Y-Ni phase was reported in the ternary alloy.
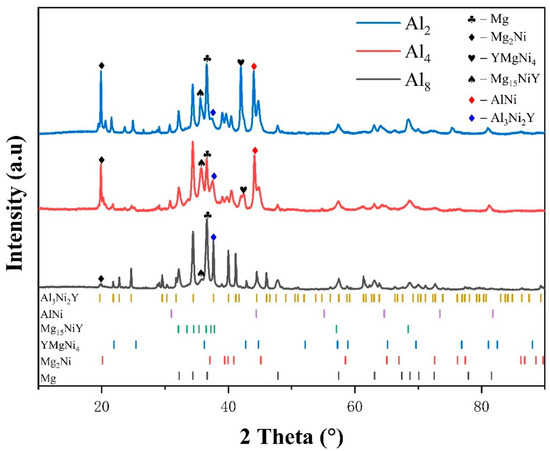
Figure 1.
XRD patterns of as-cast Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys.
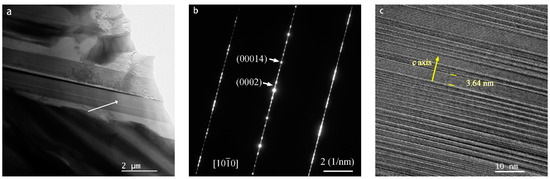
Figure 2.
The TEM results of as-cast Mg80Ni8Al8Y4 alloy: (a) bright field image; (b) diffraction pattern; and (c) HRTEM of the LPSO Mg15NiY.
As Al substitution increases (i.e., the Ni content decreases), there is a notable alteration in the content and type of constituent phases. The phase composition of the Mg80Ni12Al4Y4 alloy remains identical to that of the Mg80Ni14Al2Y4 alloy. However, with the preferential binding of elemental nickel by aluminum, there is a reduction in the Mg2Ni phase. In the case of the Mg80Ni8Al8Y4 alloy, there is a substantial emergence of the Al3Ni2Y phase. Additionally, this is accompanied by a notable reduction in the Mg2Ni phase, along with the disappearance of the YMgNi4 and AlNi phases. All three alloys contain Mg, Mg2Ni, and Mg15NiY phases, and the contents of the Mg2Ni and Mg15NiY phases are inversely proportional to the amount of Al substitution. In addition, the cell parameters of Mg and Mg2Ni gradually decrease (as indicated in Table S2) as Al increases. This may result from a decrease in Y or Al dissolving due to the consumption of Y-Al compounds.
3.2. Microstructure of the Original as-Cast Alloys
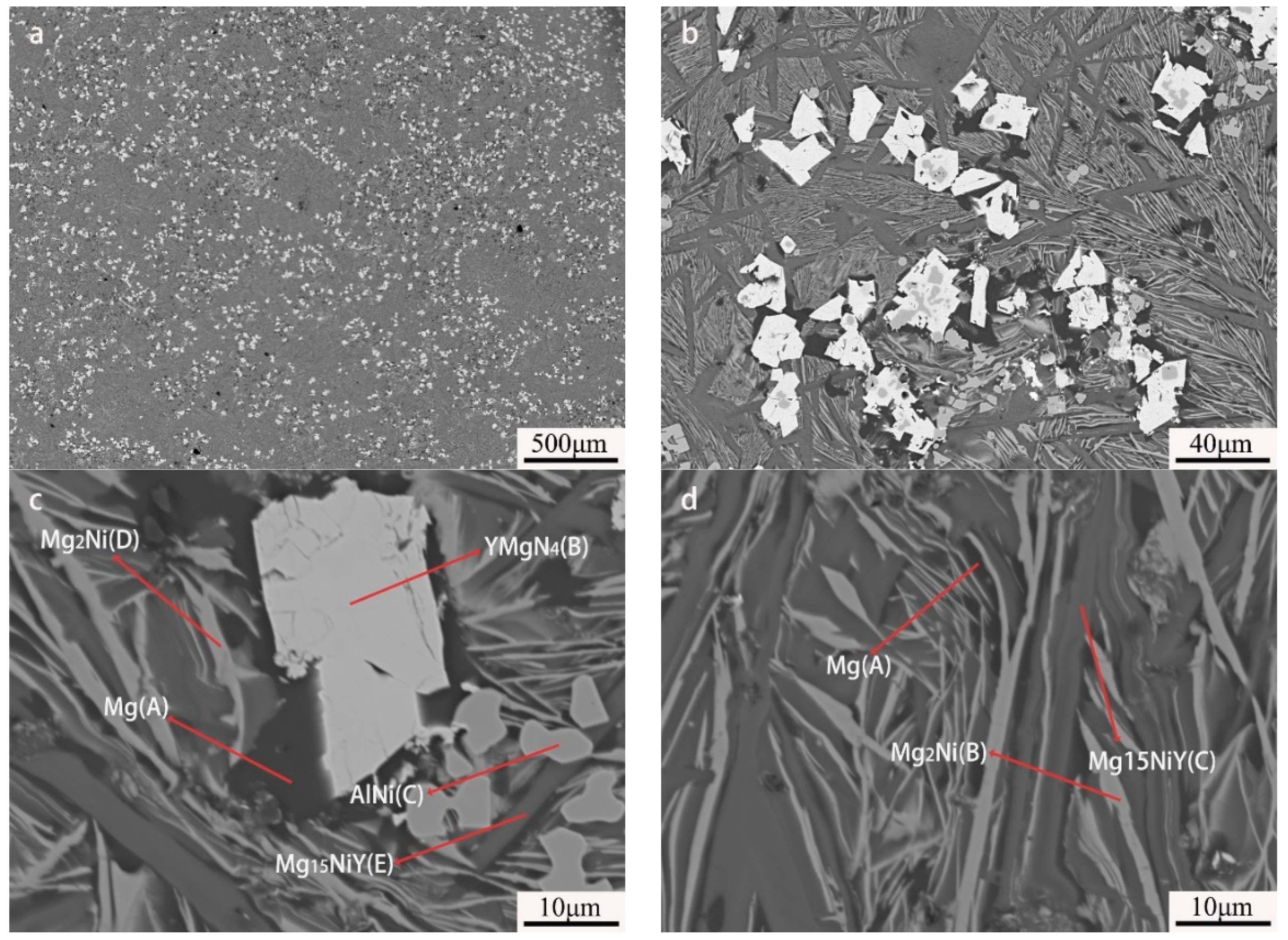
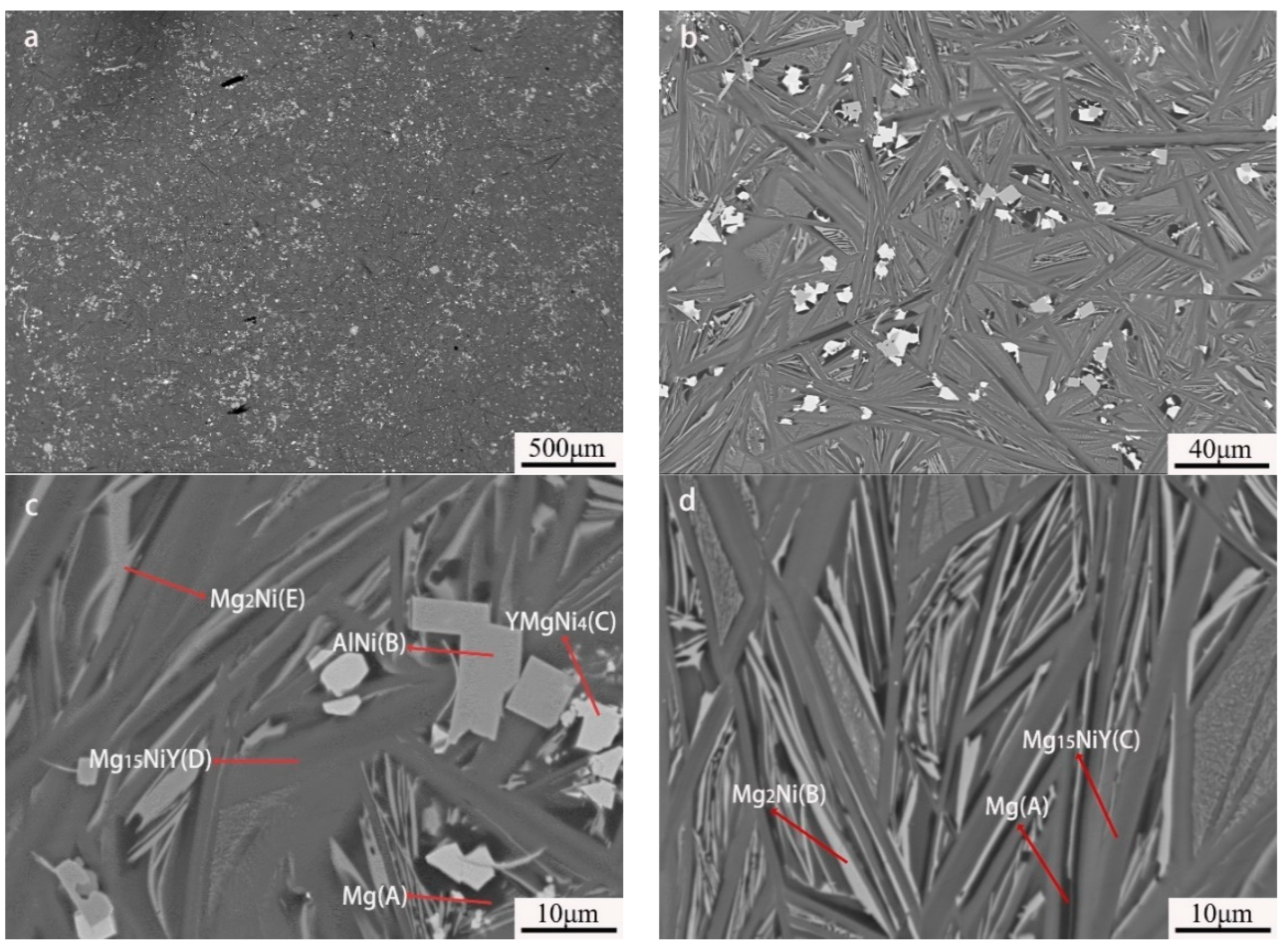
EPMA-BSE images showed five different contrasts in the as-cast Mg80Ni14Al2Y4 alloy, as displayed in Figure 3. Combined with the EDS results (listed in Table 1), the large block phase presents a bright contrast and is identified as YMgNi4 (marked with “B”), deduced by the quantitative chemical measurement. Moreover, the AlNi phase with relatively bright contrast exhibited a little square shape marked with “C”. Furthermore, the ternary eutectic composed of Mg, Mg2Ni, and Mg15NiY is illustrated in Figure 3d. Mg presents a black contrast marked with “A”, which is attributed to the low average atomic number. This morphology was also reported in other works on Mg-RE-Ni-based alloys [24,45,46,47,48] It should be noted that large strip shape Mg15NiY can also be observed in the as-cast Mg80Ni14Al2Y4 alloy, indicating Mg15NiY solidified by both proeutectic and eutectic processes. In addition, Al3Ni2Y could not be observed in the EPMA test, possibly due to its low content.
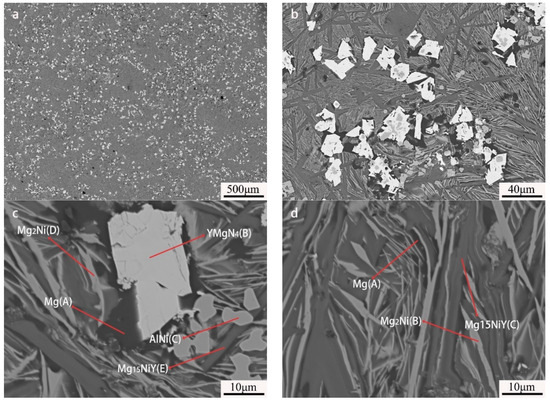
Figure 3.
BSE images of Mg80Ni14Al2Y4 as-cast alloy: (a) 40×; (b) 500×; (c,d) 2000×.

Table 1.
EDS data of Mg80Ni16−xAlxY4 (x = 2, 4, 8) as-cast alloys.
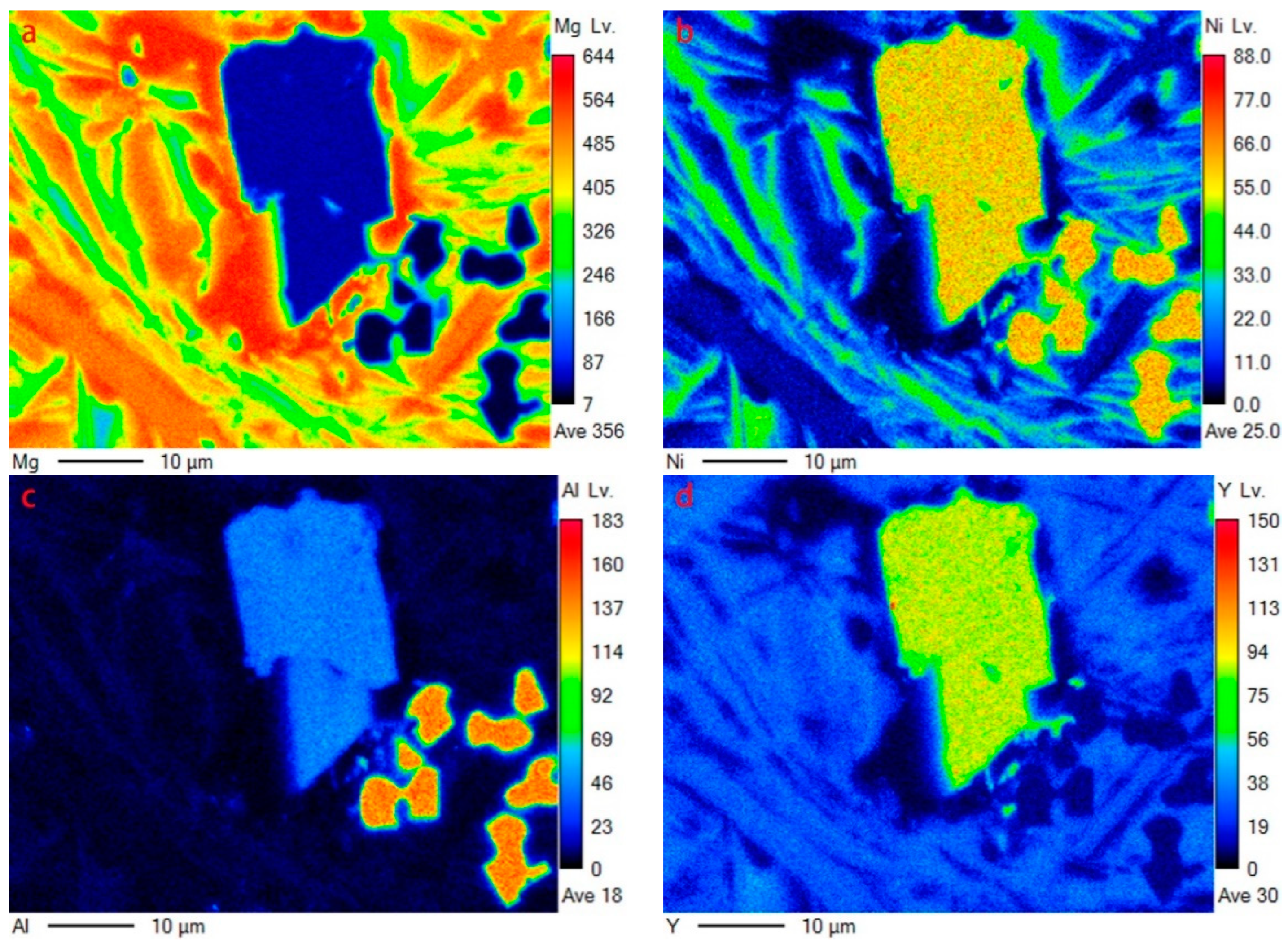
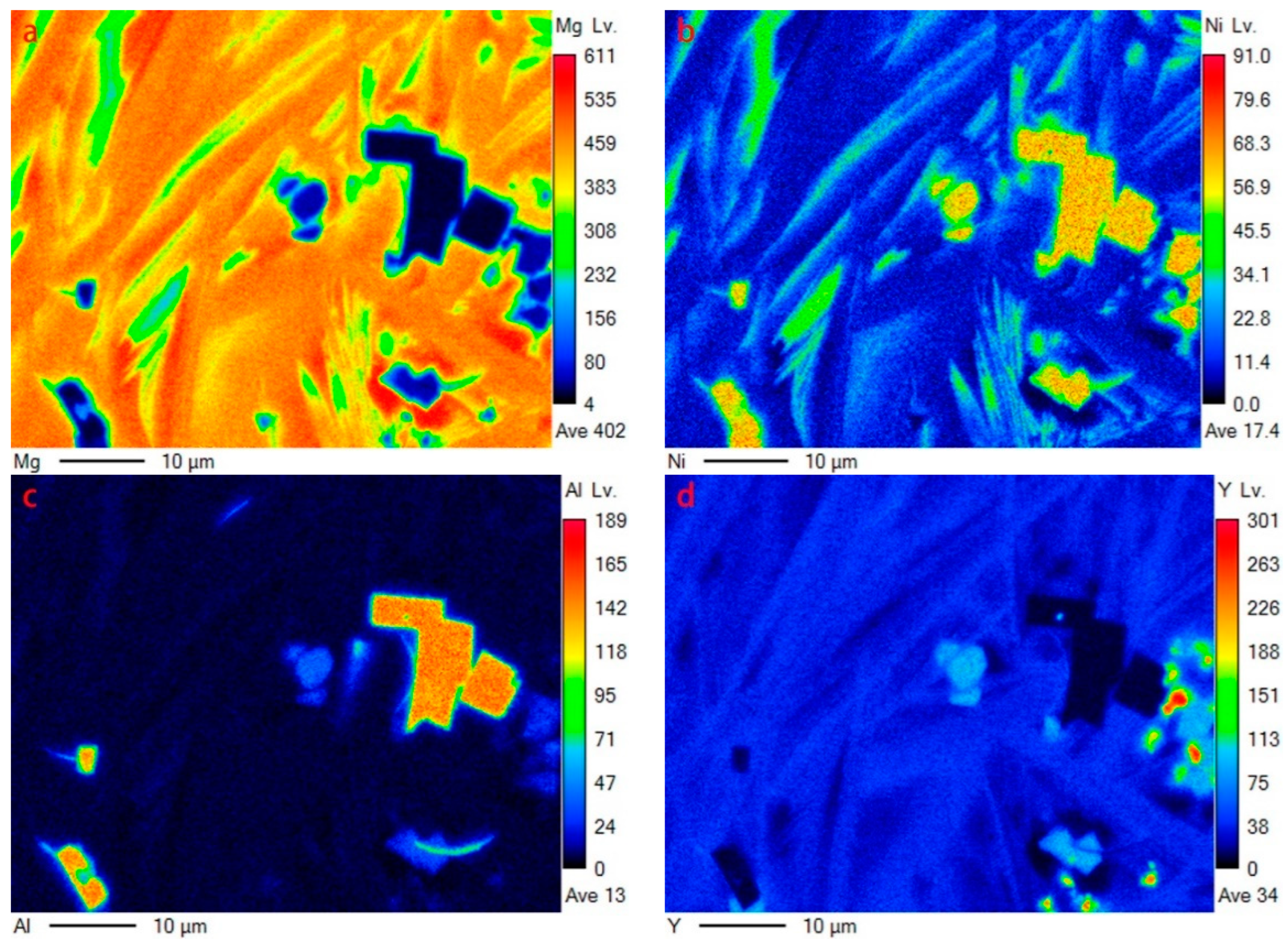
The same characteristics can be illustrated by EDS mapping (corresponding to the region in Figure 3c), as shown in Figure 4. Y element is mainly dissolved in YMgNi4 and Mg15Ni (see Figure 4d). Furthermore, Y can be detected in Mg2Ni, as illustrated by both the EDS quantitative test and mapping characterization, indicating a solid solution of Y in Mg2Ni, while Y can hardly be detected dissolving in Mg, which is attributed to the low solid solution ability of Y in Mg. In addition, the Al element is mainly distributed in AlNi and YMgNi4, and little Al can also be observed in Mg2Ni, as displayed in Figure 4c.
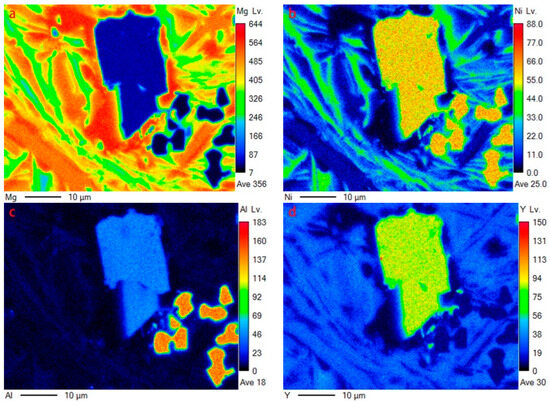
Figure 4.
EDS mapping of Figure 3c: (a) Mg; (b)Ni; (c)Al; (d)Y.
As to the Mg80Ni12Al4Y4 alloy, five contrasts can be observed, as shown in Figure 5a,b, which are similar to that of the Mg80Ni14Al2Y4, based on the chemical composition quantitative analysis. It is obvious that block-shaped YMgNi4 decreased, and the size became small, as shown in Figure 5b,c, which is consistent with the XRD characterization. Combined with the morphology and EDS mapping observation (see Figure 6), an abundance of the Mg15NiY phase increased. In addition, the ternary eutectic microstructure tends to be more fine, indicating the grain refinement effect of adding Al. Furthermore, AlNi became coarser and squarer, as illustrated in Figure 5c. The result indicated that increasing Al promoted the development of AlNi.
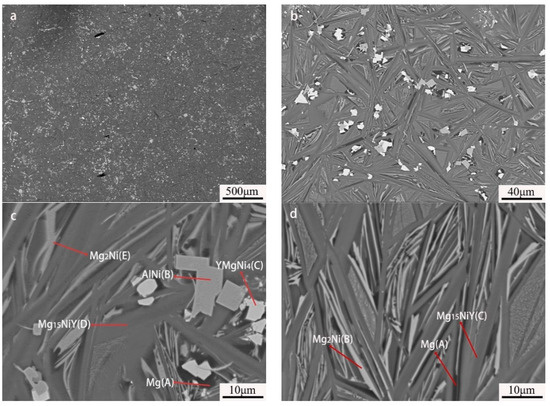
Figure 5.
BSE images of Mg80Ni12Al4Y4 as-cast alloy: (a) 40×; (b) 500×; (c,d) 2000×.
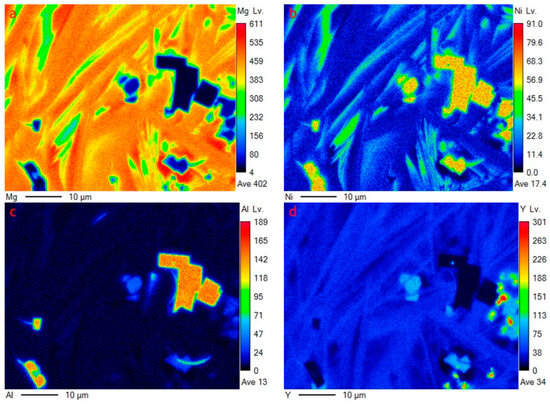
Figure 6.
EDS mapping of Figure 5c: (a) Mg; (b)Ni; (c)Al; (d)Y.
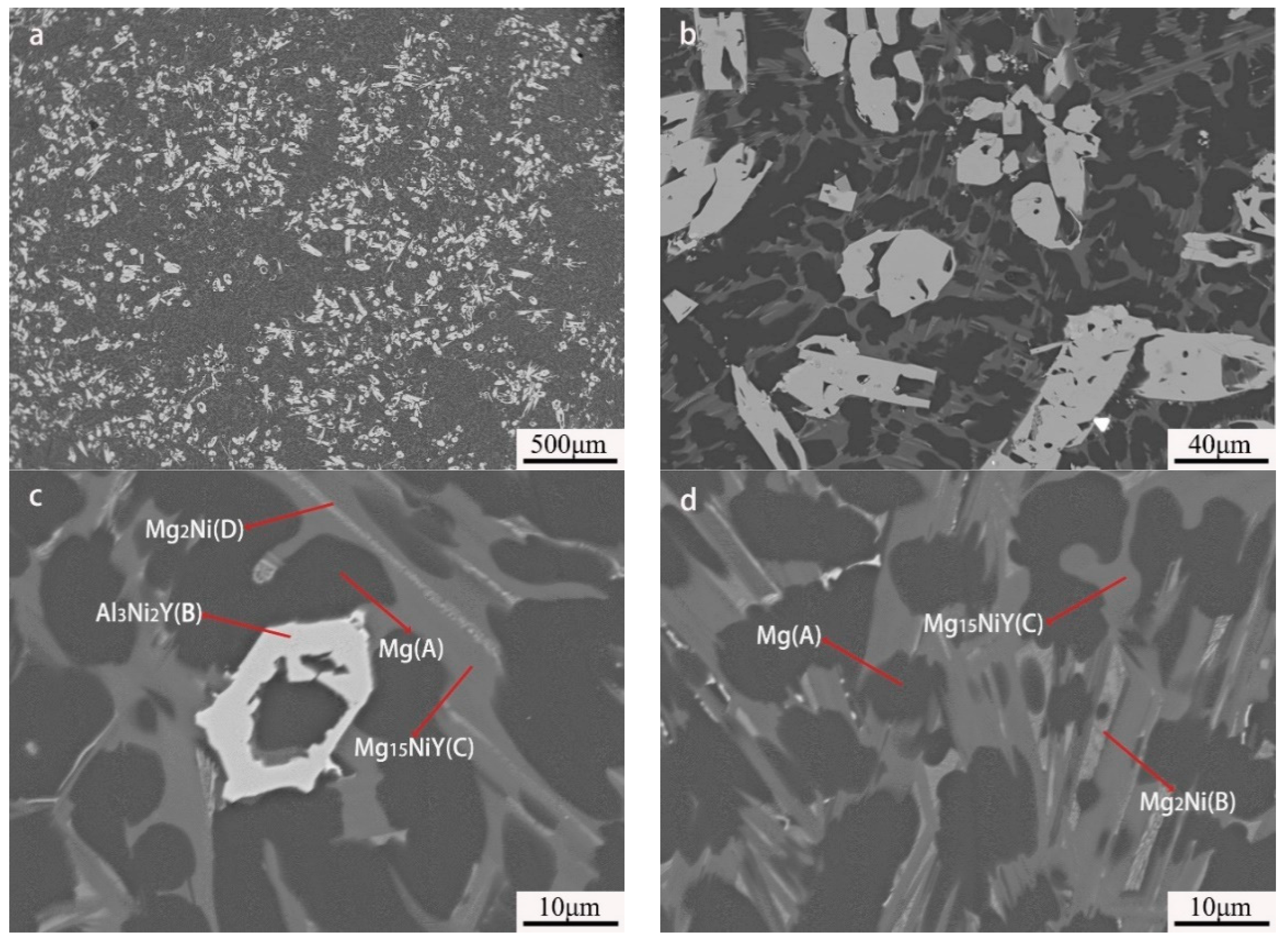
By further increasing Al, the microstructure morphology significantly changed, as displayed in Figure 7. A large number of squared phases with bright contrast can be observed in the Mg80Ni8Al8Y4 alloy. Chemical composition analysis of the bright phases confirmed the formation of Al3Ni2Y (marked with “B”). Different from the Al2 and Al4 alloys, Mg presented block grains instead of strip eutectic morphology. Mg2Ni and Mg15NiY dramatically decreased according to EDS mapping (Figure 8b,d), which agreed well with the XRD result. This resulted from the great expropriation of Y and Ni by the abundant formation of Al3Ni2Y. In addition, ternary eutectic microstructure was absent, but Mg2Ni and Mg15NiY binary eutectic appeared. Obviously, adding more Al led to a change in the solidification routes, where the formation of Mg separated from the ternary eutectic. On the contrary, grain coarsening was caused by adding more Al.
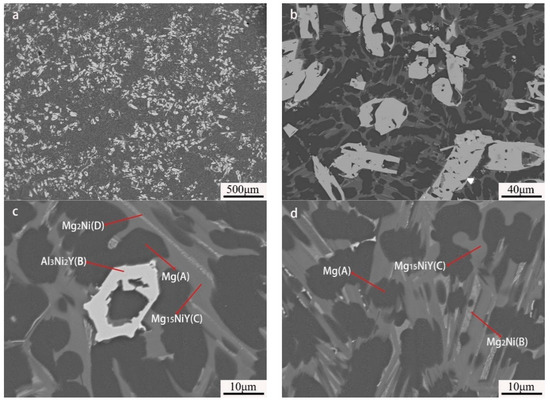
Figure 7.
BSE images of Mg80Ni8Al8Y4 as-cast alloy: (a) 40×; (b) 500×; (c,d) 2000×.
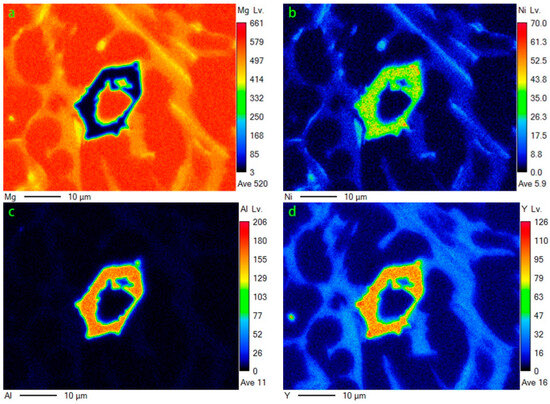
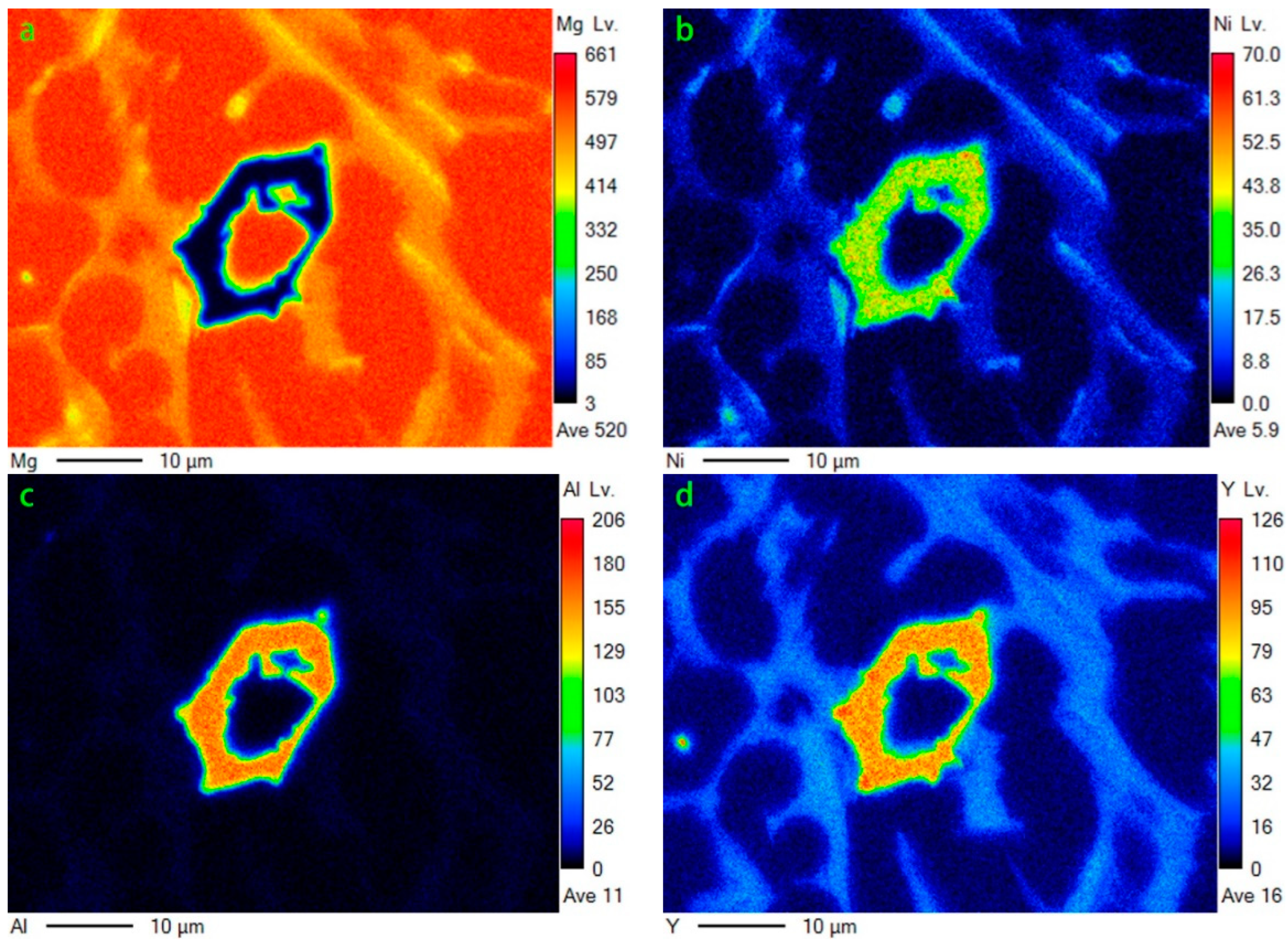
Figure 8.
EDS mapping of Figure 7c: (a) Mg; (b)Ni; (c)Al; (d)Y.
3.3. Hydrogen Absorption/Desorption Activation and Kinetic Properties
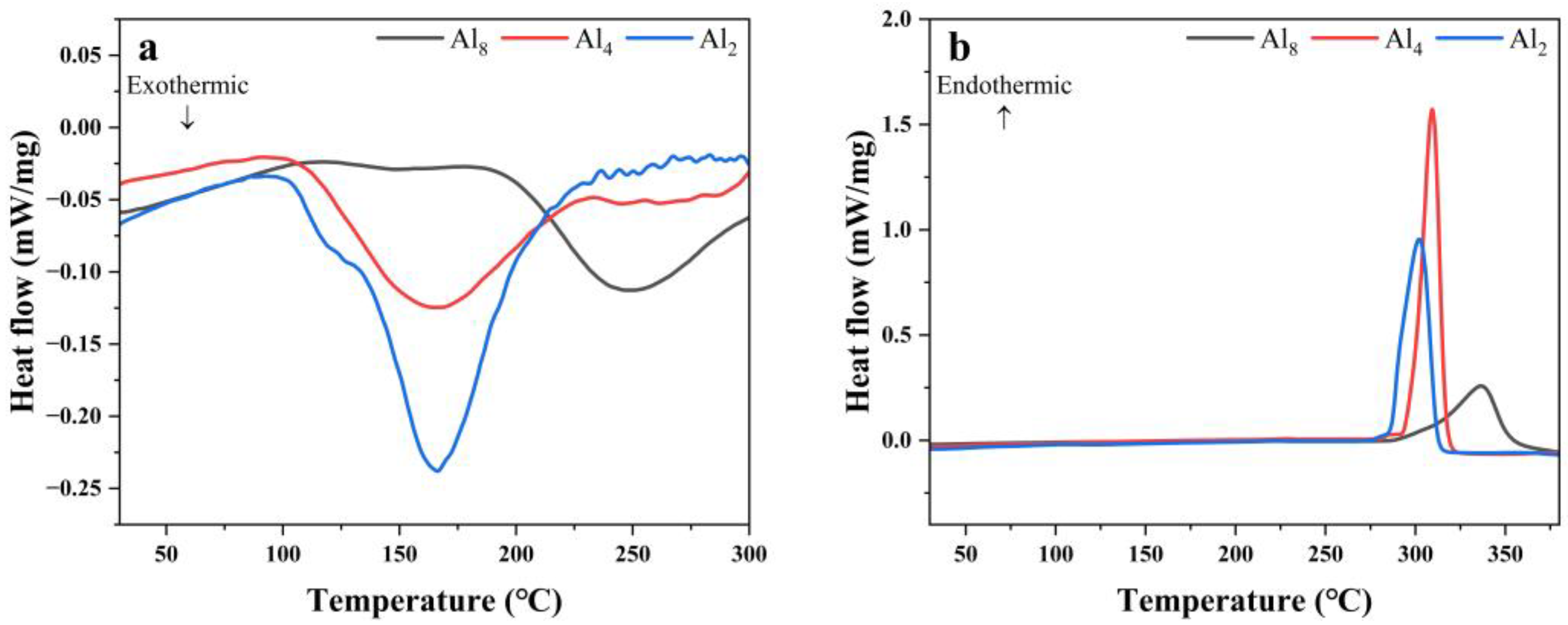
Figure 9 shows the PDSC curves of the first hydrogenation and dehydrogenation of the three alloys. Al2 alloy presents an exothermic peak of around 160 °C when heating under hydrogen pressure, which corresponds with the hydrogenation process. A similar exothermic peak can be observed for the Al4 alloy. However, a delayed exothermic peak around 250 °C occurred for the Al8 alloy. This may be attributed to the coarse microstructure or dense oxidation coating due to the addition of a lot of Al. The dehydrogenation behaviors of the three alloys exhibit a decreasing trend with decreasing Al content, indicating a negative effect of Al on the activation property.
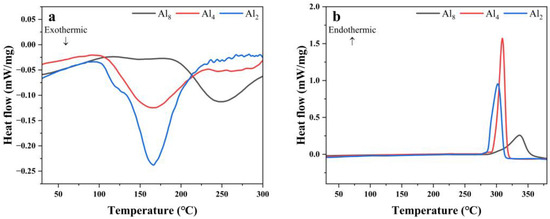
Figure 9.
PDSC curves of Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloy at 2 °C/min: (a) hydrogen absorption; (b) hydrogen desorption.
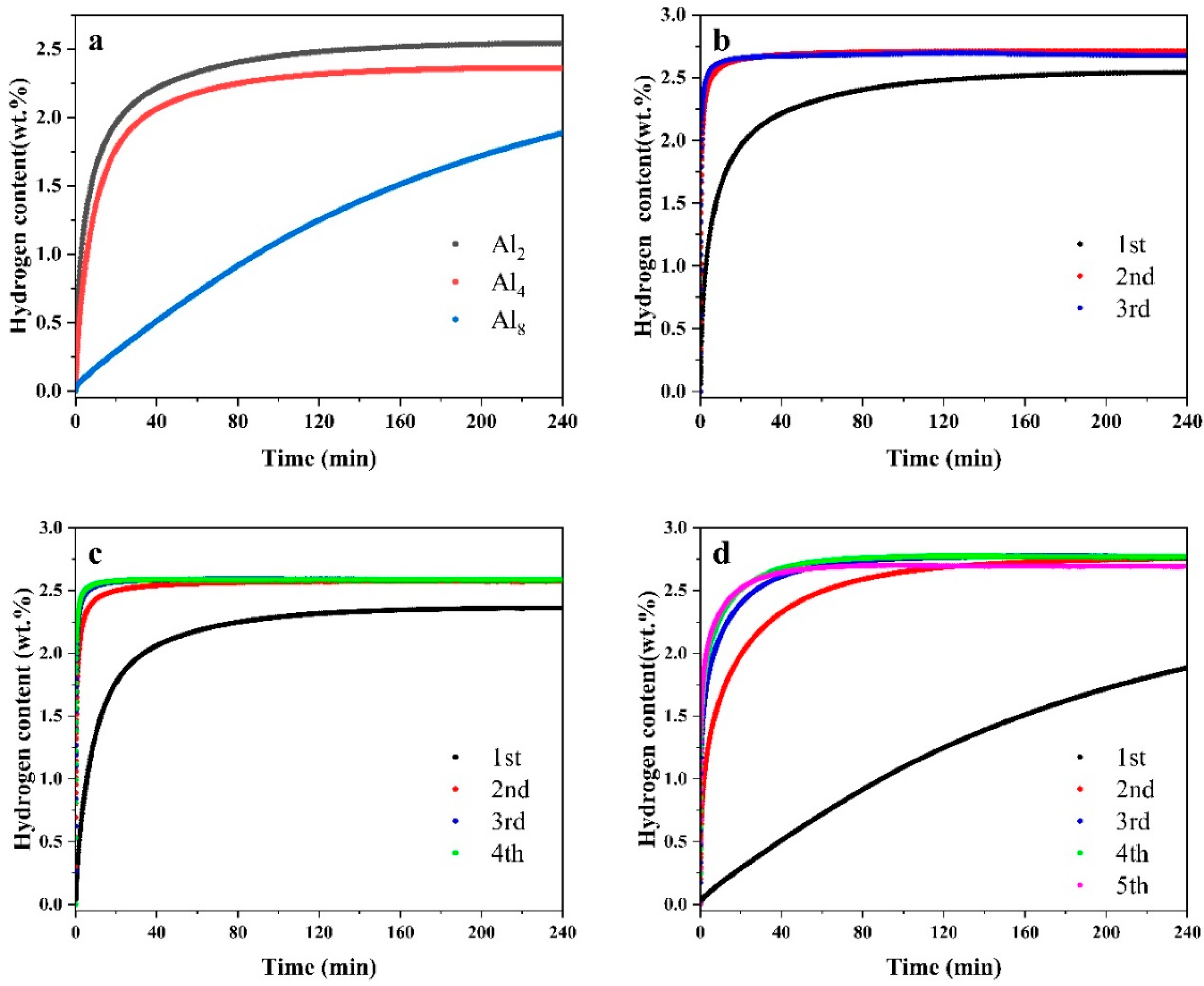
Figure 10 illustrates the first hydrogen absorption and isothermal cycling hydrogen absorption curves during the activation process of the as-cast alloy Mg80Ni16−xAlxY4 (x = 2, 4, 8). It is evident that the initial hydrogen absorption rate exhibits marked variations. Figure 10a presents the initial hydrogen absorption curves of the three alloys under conditions of 300 °C and 3 MPa hydrogen pressure. Substantial disparities are evident among the initial activation hydrogen absorption curves of the three alloys. Specifically, the Al2 and Al4 alloys exhibit no activation period, enabling rapid hydrogen absorption, whereas Mg80Ni12Al8Y4 demonstrates a more prolonged activation period. The Al2, Al4, and Al8 alloys exhibit initial hydrogen absorption capacities of 1.97 wt.%, 1.77 wt.%, and 0.29 wt.% within the first 20 min, respectively. It is evident that the initial hydrogen absorption property of the alloys gradually declines with an increase in Al substitution.
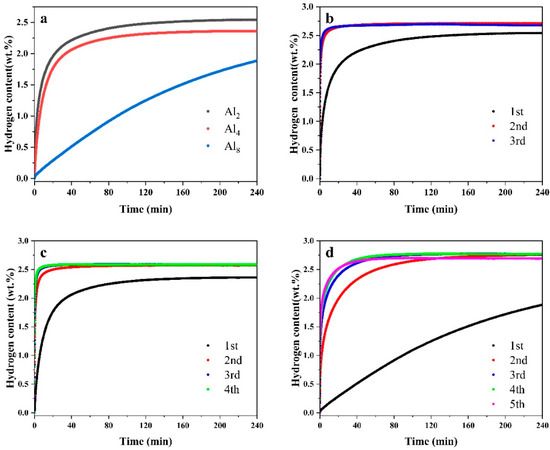
Figure 10.
Activation hydrogen absorption curve of Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloy: (a) the first activation of hydrogen absorption and cycles of (b) Al2, (c) Al4, and (d) Al8 alloys.
Figure 10b,c depicts the cyclic activation hydrogen absorption curves. Friedrichs et al. [49] reported that an unavoidable oxide layer, approximately 3–4 nm thick, forms on the surface of Mg during experimental processes, even when protected by inert gas. During the initial hydrogen absorption stage, hydrogen must traverse the oxide layer before penetrating the alloy’s interior, severely impeding hydrogen absorption performance. Following the completion of the initial hydrogen absorption, the oxide layer ruptured, leading to a significant increase in the rate and capacity of subsequent hydrogen absorption. The third hydrogen absorption curve of the Mg80Ni14Al2Y4 alloy essentially overlaps with the second curve, indicating completion of activation. In contrast, the Al4 and Al8 alloys require four and five hydrogen absorption/desorption cycles, respectively, for full activation. In summary, Mg80Ni14Al2Y4 alloy has the best activation properties; Mg80Ni12Al4Y4 is the next best, and Mg80Ni8Al8Y4 is the worst.
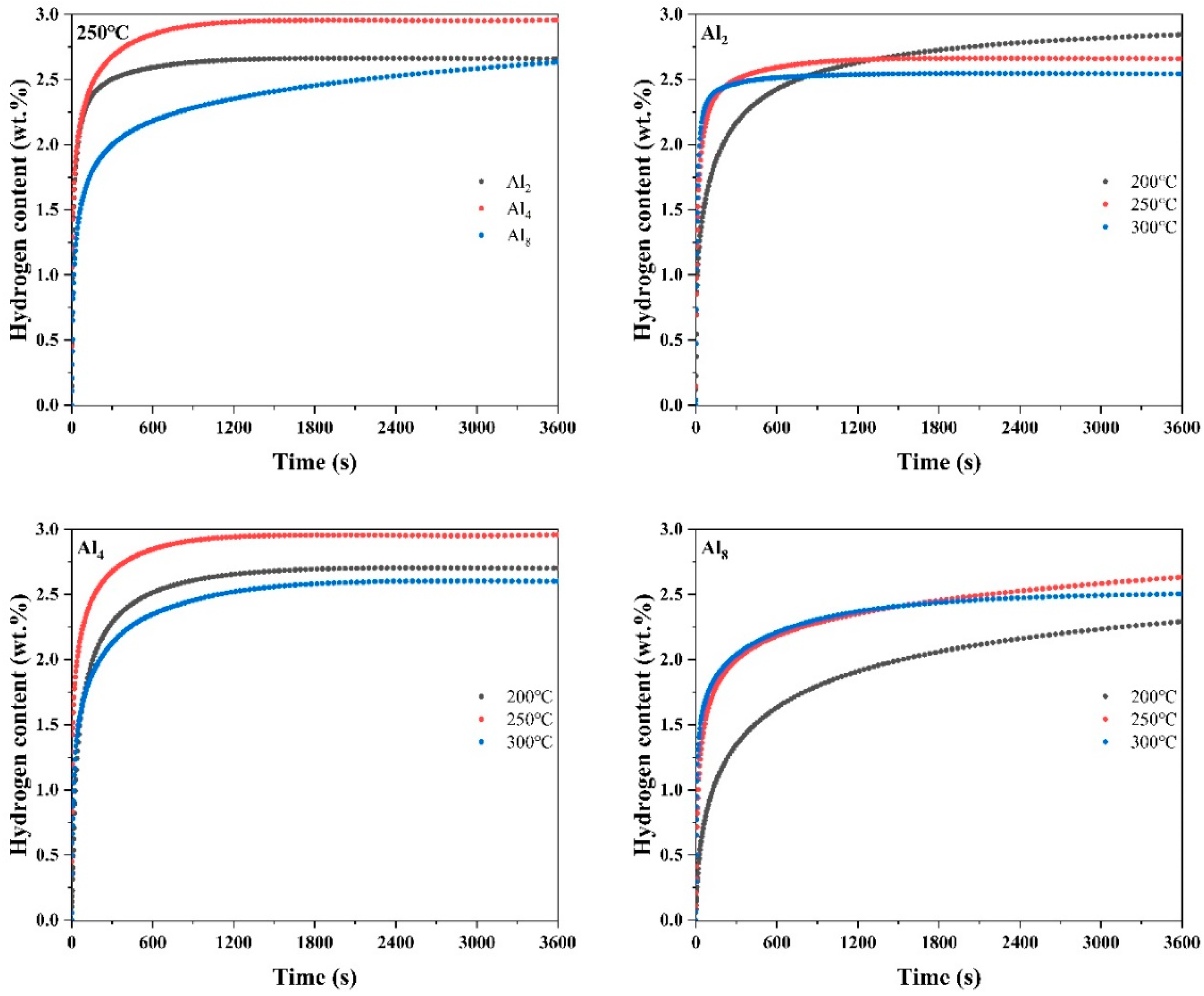
Figure 11 illustrates the hydrogen absorption curves of fully activated Mg80Ni16−xAlxY4 (x = 2, 4, 8) as-cast alloys under a hydrogen pressure of 3 MPa and temperatures of 200, 250, and 300 °C. The results indicate that the hydrogen absorption kinetics curves of the alloys exhibit nearly identical processes, particularly at temperatures of 250 and 300 °C.
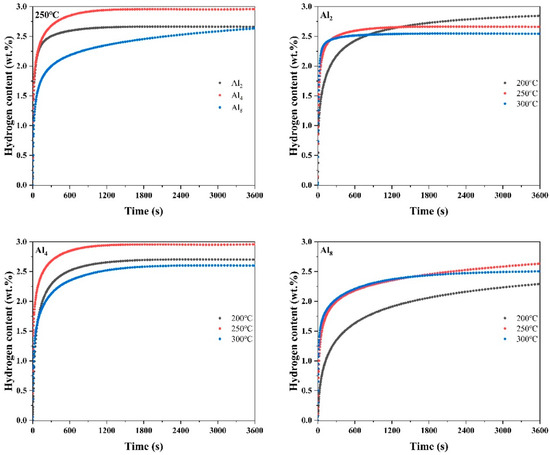
Figure 11.
Hydrogen absorption curves of Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloy at different temperatures.
The temperature plays a crucial role in the hydrogen absorption process. Firstly, the absorption is an exothermic process, and a decrease in temperature favors the progression of the hydrogen absorption reaction. However, the reduction in temperature is accompanied by the suppression of hydrogen diffusion rates, thereby weakening hydrogen absorption kinetics. Therefore, there exists an optimal hydrogen absorption temperature for the absorption process. As depicted in Figure 11, the optimal hydrogen absorption temperature for all three alloys is 250 °C. In Figure 11, at 250 °C, the as-cast alloys of Al2, Al4, and Al8 can absorb 2.66 wt.%, 2.96 wt.%, and 2.63 wt.% H2 in 3600 s, respectively. The Al4 alloy exhibits the highest hydrogen-absorbing capacity. However, it is noteworthy that the elevated Al substitution decelerates the hydrogen absorption rate, especially at low temperatures.
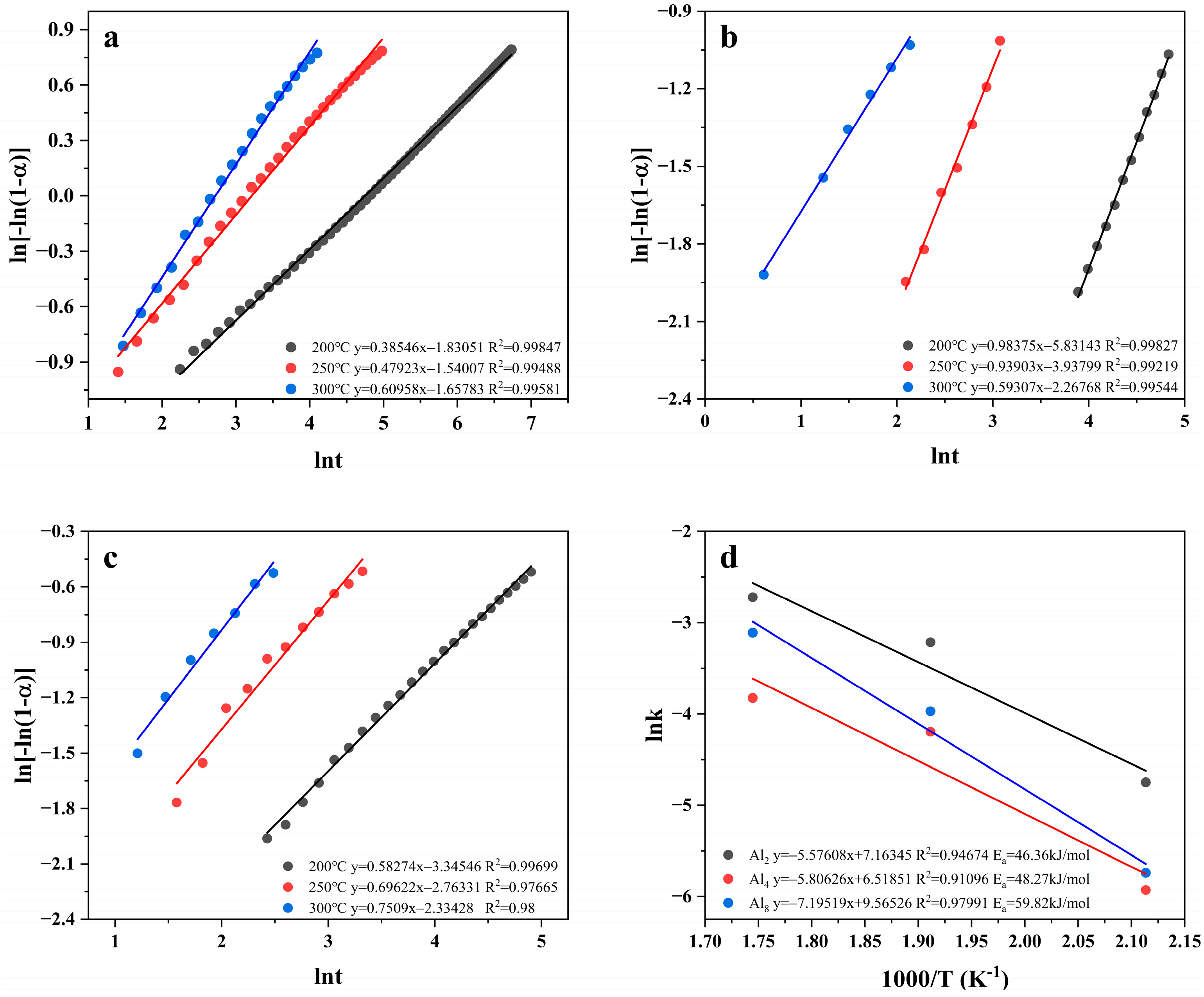
To assess the kinetic characters further, the apparent activation energy was calculated based on the Johnson–Mehl–Avrami (JMAK) [50] kinetic model using the Arrhenius equation [51], where T is the hydrogen absorption temperature; R is the gas constant, and A is the frequency factor.
where α (0–1) denotes the fraction of Mg converted to MgH2 at moment t (i.e., hydrogen uptake/maximum hydrogen uptake); k is a kinetic parameter, and η denotes the Avrami index.
Figure 12d shows the Arrhenius plots of Mg80Ni16−xAlxY4 (x = 2, 4, 8) as-cast alloys; the corresponding data are listed in Table S3 in the Supplementary Materials. The apparent activation energies of hydrogen absorption for Mg80Ni14Al2Y4, Mg80Ni12Al4Y4, and Mg80Ni8Al8Y4 alloys are 46.36, 48.27, and 59.82 kJ/mol, respectively. The three alloys have significantly lower hydrogen absorption activation energies compared to pure MgH2 (70.76 kJ/mol) [52]. The apparent activation energies of hydrogen absorption of the alloys are linearly related to the Al content, and Al2 has the lowest apparent activation energy of hydrogen absorption.
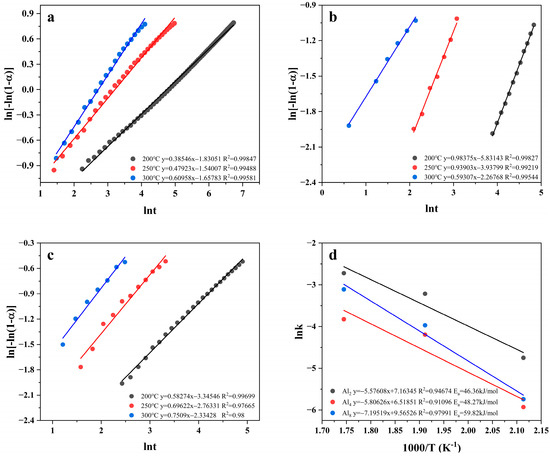
Figure 12.
Avrami plots and Arrhenius plots and apparent activation energy of hydrogen absorption of Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloy: (a) Al2; (b) Al4; (c) Al8; (d) Arrhenius Plots.
3.4. Hydrogen Absorption Thermodynamics and Low-Pressure Hydrogenation Properties
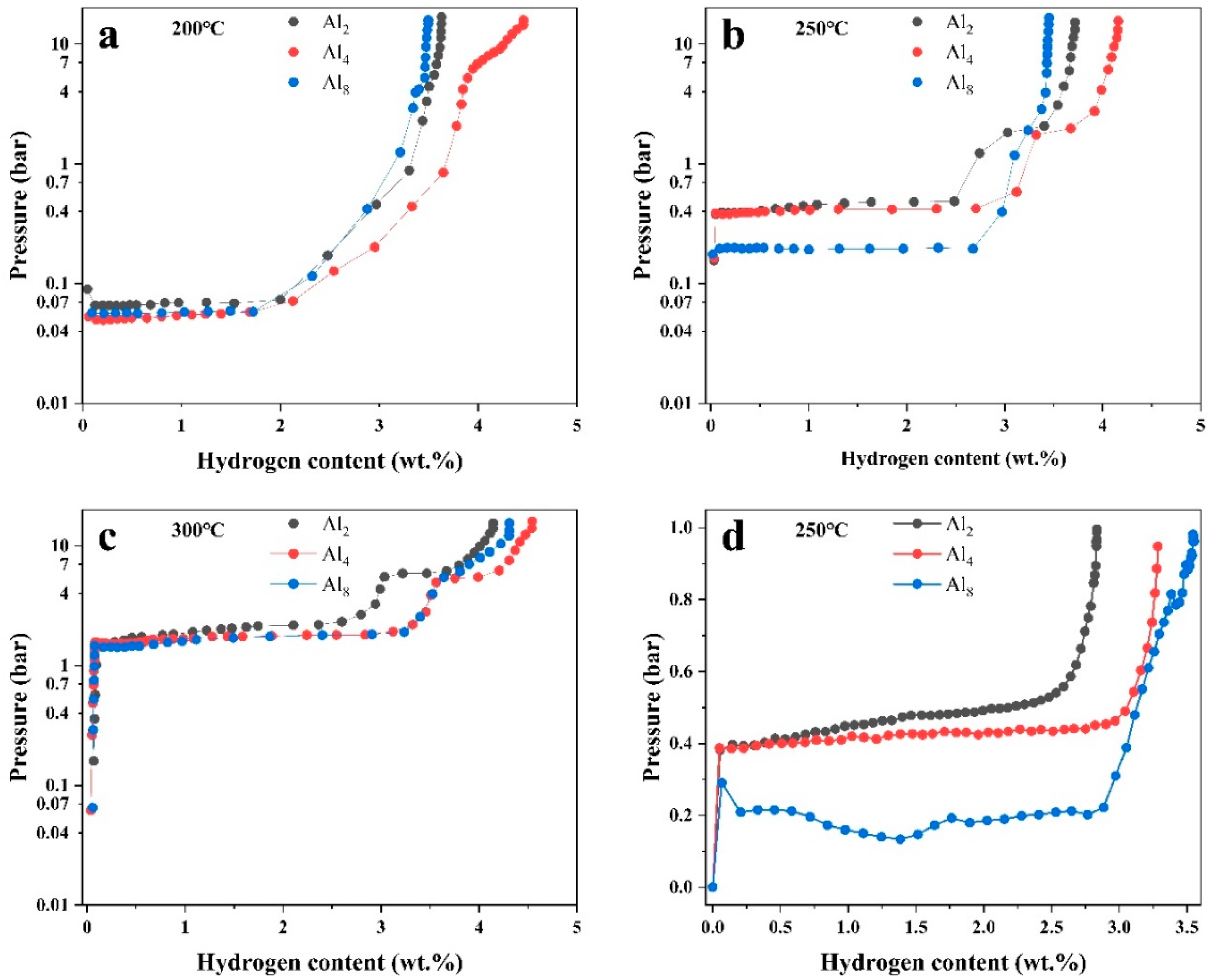
P-C-T curves were measured at 200 °C, 250 °C, and 300 °C to explore the thermodynamics of the three alloys, as shown in Figure 13. The characteristic P-C-T data are summarized in Table 2. Two plateaus existed in the Al2 and Al4 alloys, especially at 250 °C and 300 °C. The higher plateau corresponds to the Mg2Ni phase and the lower one belongs to Mg. The higher plateau of Mg2Ni of the Al8 alloy is faint, which is consistent with the microstructure characterization that Mg2Ni significantly decreased with added Al. Plateaus below 250 °C of all three alloys are lower than 1 bar, indicating close affinity with hydrogen. The Al4 alloy presents the highest hydrogen absorption ability at all three temperatures, which is consistent with the result of kinetic measurement. In addition, the plateau pressure gradually decreased with increasing Al, indicating an increase in the thermodynamic stability. The hydrogen absorption enthalpies were calculated based on Vant’ Hoff equation [17]:
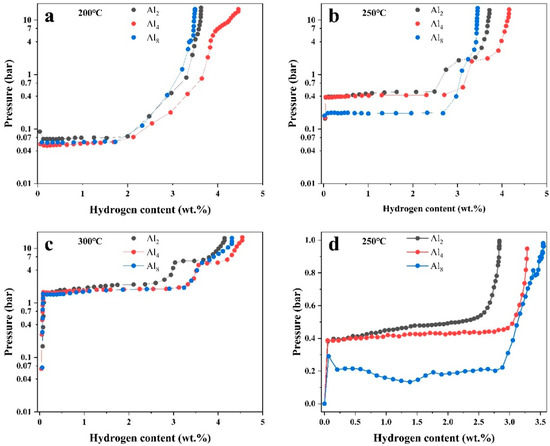
Figure 13.
P-C-T curves of (a) Al2, (b) Al4, and (c) Al8 alloys at different temperatures and (d) pressure hydrogen absorption contents under less than 1 bar.

Table 2.
Characteristic P-C-T data obtained from the corresponding P-C-T curves of the Mg80Ni16−xAlxY4 (x = 2, 4, 8) as-cast alloys.
is the equilibrium pressure of hydrogen absorption; is the standard atmospheric pressure; R is the gas constant; T is the absolute temperature, and and are changes in the enthalpy and entropy, respectively.
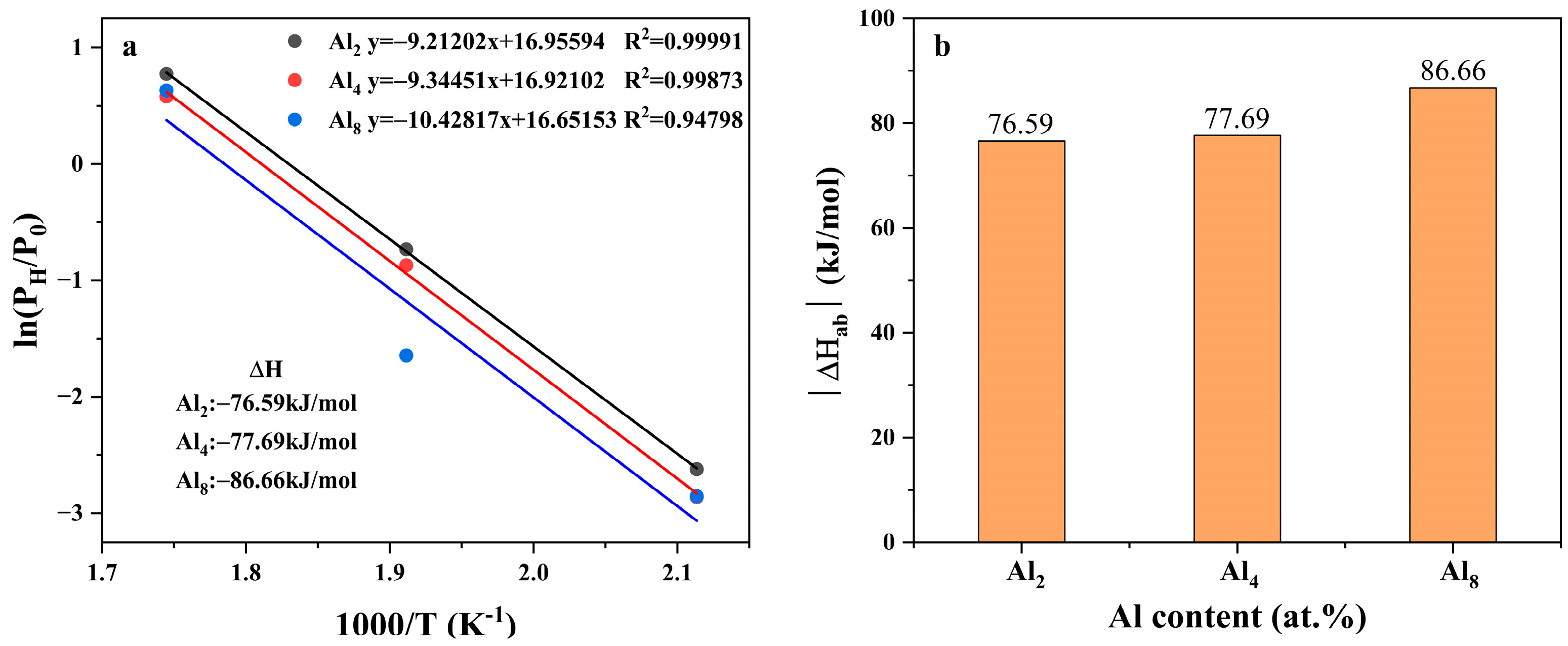
can be calculated by the slope according to Vant’ Hoff equation, as displayed in Figure 14; the corresponding data are listed in Table S4 in the Supplementary Materials. Hydrogen absorption enthalpies of the three alloys were lower than the pure MgH2 (72.29 kJ/mol as reported by ref. [53]). This trend agrees well with the change in equilibrium pressure of hydrogen absorption of the three alloys, demonstrating the enhancement of thermodynamic stability of the hydrides. Yu et al. reported that the hydrogen absorption enthalpy of the unalloyed Mg80Ni15Y5 alloy [26] was −62.6 kJ/mol H2, indicating the decreasing trend by adding Al. This trend agrees well with the change in equilibrium pressure of hydrogen absorption of the three alloys, demonstrating the enhancement of thermodynamic stability of the hydrides.
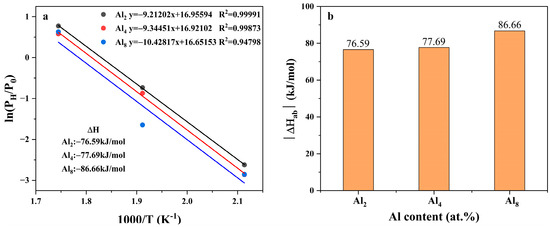
Figure 14.
Vant’ Hoff plots and relationship between the absolute enthalpy of hydrogen absorption of Mg80Ni16−xAlxY4 (x = 2, 4, 8) and Al content: (a) Vant’ Hoff plots; (b) The relationship between hydrogen absorption enthalpy and Al content.
Theoretically, Al can hardly dissolve into the crystal lattice of Mg; therefore, the increase in thermodynamic stability is considered to not be directly related to the increase in Al. Instead, Y is reported to solid solute into Mg to a certain extent, which is, thus, suggested to be responsible for the change in the plateau pressure. Although the exact mechanism is still confused, speculation is that the solid solution of Y in Mg may be related to the solidification route and Y content. As mentioned above, block Mg rather than strip eutectic was observed in the Al8 alloy, which illustrated the preferential formation of Mg. Therefore, more Y was likely to dissolve into Mg in the early stage of the solidification process.
For some applications, such as industrial residual of hydrogen, the export hydrogen pressure is quite low, which is the approximate atmosphere. Hydrogen absorption ability at low hydrogen pressure is, thus, considered to be critical to the usability of the Mg-based alloys. To examine the hydrogen absorption ability at low pressure, hydrogen absorption tests were measured by staged p-hydrogenation experiments at 250 °C, where the hydrogen pressure was set at 1 bar for every hydrogen absorption test. The pressure after hydrogenation and the total hydrogen absorption content were recorded, as shown in Figure 13d. The result shows that the Al8 alloy presents minimum residual pressure at a low pressure of no more than 1 bar. Furthermore, nearly 3.5 wt.% hydrogen can be absorbed in the Al8 alloy. This is consistent with the thermodynamics assessment, as discussed above, illustrating improved low-pressure hydrogen absorption ability by increasing Al.
3.5. Phase Transformation during Hydrogen Ab/Desorption
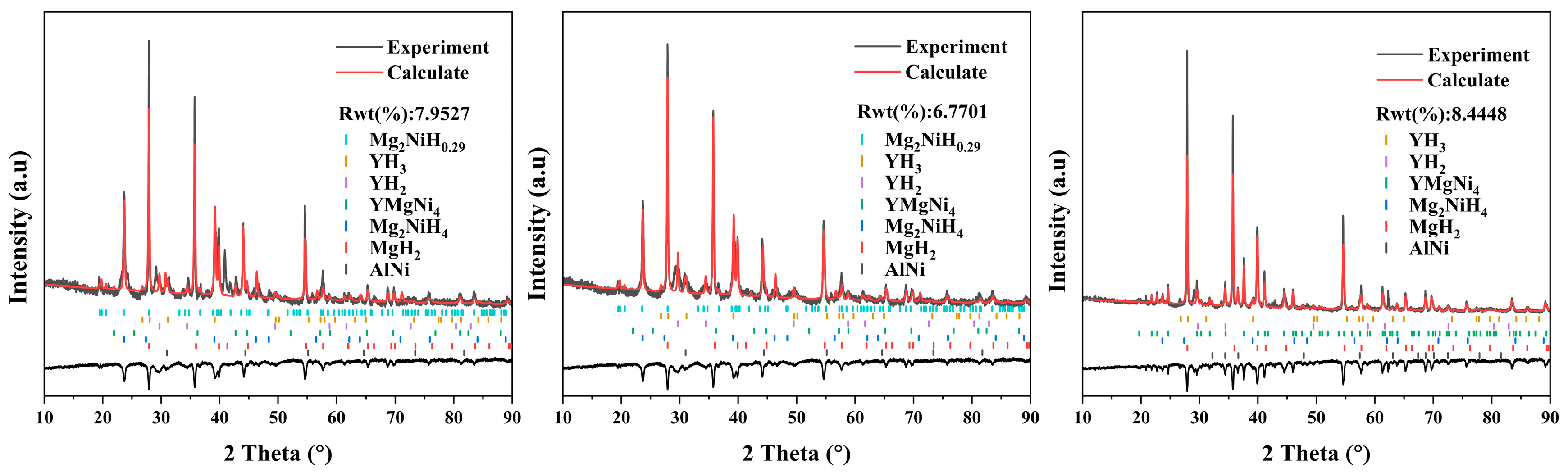
XRD patterns of the three alloys after hydrogenation are displayed in Figure 15; the measured profiles were refined based on the Rietveld method. The phase compositions of hydrogenated alloys are listed in Table 3. The hydrogenated Mg80Ni14Al2Y4 alloy consists of MgH2, Mg2NiH4, Mg2NiH0.29, YH2, YH3, YMgNi4, and AlNi. The Mg2NiH0.29 is the intermediate product of Mg2Ni hydrogenating to Mg2NiH4 in the hydrogenation process. The existence of YHx is attributed to the disproportionation of Mg15NiY upon hydrogenation, which has been reported by Song et al. [24]. The result indicated that YMgNi4 and AlNi did not participate in hydrogenation. As reported, YMgNi4 reacted with hydrogen only under a pressure higher than 4 MPa when the temperature was 80 °C [54], thus retained in the present work due to the low hydrogen pressure.
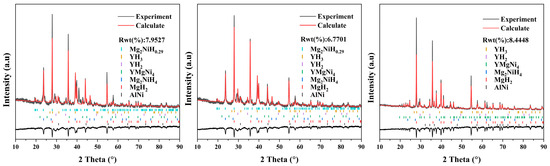
Figure 15.
XRD patterns of hydrogenated as-cast Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys.

Table 3.
Contents of phases of Mg80Ni16−xAlxY (x = 2, 4, 8) as-cast alloys after hydrogenation/dehydrogenation.
For Al4 alloy, the phase composition is identical to that of the Al2 alloy except for a change in content. Due to the rise in Al content and the combination of Al and Ni elements, the Al4 alloy may have a higher Mg content, which leads to an increase in the hydrogen uptake of the alloy compared to the Al2 alloy. As the Al content continues to increase, the phase composition of the alloy changes. The hydrogenated Al8 alloy consists of Mg, MgH2, Mg2NiH4, YH2, YH3, and Al3Ni2Y phases. Abundant Al3Ni2Y can be detected, indicating nonreaction of Al3Ni2Y with hydrogen. In addition, the diffraction peaks of Mg can be observed due to the unfavorable effect of the increased Al content on the hydrogen absorption kinetics, making incomplete hydrogenation of Mg. In general, MgH2 increases, but Mg2NiH4 decreases after hydrogenation. This is consistent with the trend of the phase constitution of the as-cast alloys that shows that adding Al decreases the abundance of Mg2Ni.
Figure 16 shows the XRD profiles after dehydrogenation. The content of each phase in the alloys after dehydrogenation is also summarized in Table 3.
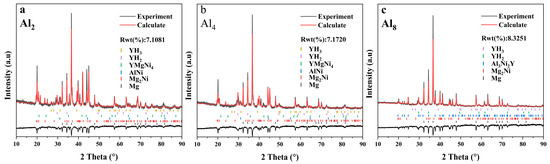
Figure 16.
XRD patterns of dehydrogenated as-cast Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys: (a) Mg80Ni14Al2Y4; (b) Mg80Ni12Al4Y4; (c) Mg80Ni8Al8Y4.
The dehydrogenated Al2 alloy is composed of Mg, Mg2Ni, YMgNi4, AlNi, YH2, and YH3. The presence of YHx indicates that the hydride remains stable at the experimental temperature and has a high thermodynamic stability. The YMgNi4 phase is also stabilized during the hydrogen absorption/del absorption process, illustrating the negative effect on the hydrogen storage property of YMgNi4. The dehydrogenated Al4 alloy has the same phase structure as the Al2 alloy, with an increased Mg content and a decreased Mg2Ni phase, which leads to an increase in the hydrogen uptake of the alloy compared to the Al2 alloy. The YMgNi4 phase content decreases substantially due to the increase in Al and the combination of Al and Ni. As to the dehydrogenated Al8 alloy, more Mg and less Mg2Ni were illustrated, which corresponded with the phase constitution of the as-cast alloy. Although Al8 alloy presents the highest Mg content, a large number of residual Al3Ni2Y results in low hydrogen absorption content. A decrease in YHx with an increase in Al resulted from the formation of Al3Ni2Y, which did not participate in the hydrogenation reaction.
4. Conclusions
- Mg80Ni16−xAlxY4 (x = 2, 4, 8) alloys present multi-phase microstructure, including Mg, Mg2Ni, YMgNi4, AlNi, Mg15NiY, and Al3Ni2Y. An increase in Al resulted in a decrease in Mg2Ni and YMgNi4 due to the reduction in Ni while promoting Al3Ni2Y;
- The Al4 alloy showed fine Mg, Mg2Ni, and Mg15NiY ternary eutectic. However, grain coarsening was induced by a further increase in Al. Instead of ternary eutectic, the Al8 alloy revealed binary eutectic and independently solidified Mg caused by alternation of the solidification path;
- The Al2 and Al4 alloys can be hydrogenated at no more than 200 °C. But increase in Al elevated the apparent activation energy and worsened the activation and hydrogen absorption kinetic property;
- More than 4 wt.% hydrogen can be absorbed by all the alloys at 300 °C. Al reduced the hydrogen absorption enthalpy, leading to an increase in the thermodynamic stability of MgH2. Al improved low-pressure hydrogen absorption ability, and 3.5 wt.% hydrogen could be absorbed by Al4 alloy under only 1 bar hydrogen pressure at 250 °C.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met14010126/s1, Table S1: Crystal structures of intermetallic compounds in the present study. Table S2: Unit cell parameters of the original alloy. Table S3: Kinetic data points. Table S4: Thermodynamic data points.
Author Contributions
Conceptualization, Y.L., Z.L., G.Z. and F.Y.; formal analysis, X.D., Y.L. and Y.Z.; investigation, X.D., Y.L. and Y.Z.; resources, Z.L., G.Z. and F.Y.; data curation, X.D. and Y.Z.; methodology, Y.L., Z.L., G.Z. and F.Y.; supervision, Y.L., Z.L., G.Z. and F.Y.; writing—original draft, X.D. and Y.L.; writing—review and editing, X.D., Y.L., Y.Z. and Z.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (51961032 and 51962028), Innovation Foundation of Inner Mongolia University of Science and Technology (2019YQL03), Major Science and Technology Project of Inner Mongolia (2021ZD0029), Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23005, NJYT23007), Inner Mongolia Natural Science Foundation (2022LHMS05021), Research Science Project of Colleges and Universities in Inner Mongolia (NJZY21399).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing research on this topic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shang, Y.Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Han, B.; Yu, S.B.; Wang, H.; Lu, Y.S.; Lin, H.J. Nanosize effect on the hydrogen storage properties of Mg-based amorphous alloy. J. Scr. Mater. 2022, 216, 114736. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Botta, W.J.; Estrin, Y.; Floriano, R.; Fruchart, D.; Grosdidier, T.; Horita, Z.; Huot, J.; Li, H.W.; et al. Impact of severe plastic deformation on kinetics and thermodynamics of hydrogen storage in magnesium and its alloys. J. Mater. Sci. 2023, 146, 221–239. [Google Scholar] [CrossRef]
- Guo, M.C.; Wang, H.; Liu, J.W.; Ouyang, L.Z. Improving hydrogen-induced crystallization and electrochemical hydrogen storage properties of MgNi amorphous alloy with CoB addition. J. Non-Cryst. Solids 2022, 588, 121646. [Google Scholar] [CrossRef]
- Xu, N.; Yuan, Z.R.; Ma, Z.H.; Guo, X.L.; Zhu, Y.F.; Zou, Y.J.; Zhang, Y. Effects of highly dispersed Ni nanoparticles on the hydrogen storage performance of MgH2. Int. J. Miner. Met. Mater. 2023, 30, 54–62. [Google Scholar] [CrossRef]
- Cao, W.C.; Ding, X.; Zhang, Y.; Zhang, J.X.; Chen, R.R.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Enhanced de-/hydrogenation kinetics of a hyper-eutectic Mg85Ni15- xAgx alloy facilitated by Ag dissolving in Mg2Ni. J. Alloys Compd. 2022, 917, 165457. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhou, C.S.; Fang, Z.G.Z.; Robert, C.B.J.; Lu, J.; Chai, R. Isothermal hydrogenation kinetics of ball-milled nano-catalyzed magnesium hydride. Materialia 2019, 5, 100227. [Google Scholar] [CrossRef]
- Imamura, H.; Masanari, K.; Kushara, M.; Katsuoto, H.; Sumi, T.; Sakata, Y. High hydrogen storage capacity of nanosized magnesium synthesized by high energy ball-milling. J. Alloys Compd. 2005, 386, 211–216. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Schüth, F. Hydrogen storage properties of nanostructured MgH2/TiH2 composite prepared by ball milling under high hydrogen pressure. Int. J. Hydrogen Energy 2011, 36, 10828–10833. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, C.S.; Ma, H.; Chen, J. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef]
- Sui, Y.Q.; Yuan, Z.M.; Zhou, D.S.; Zhai, T.T.; Li, X.M.; Feng, D.C.; Li, Y.M.; Zhang, Y.H. Recent progress of nanotechnology in enhancing hydrogen storage performance of magnesium-based materials: A review. Int. J. Hydrogen Energy 2022, 47, 30546–30566. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tian, Z.H.; Yao, P.F.; Zhao, H.M.; Xia, C.Q.; Yang, T. Improved hydrogen storage kinetic properties of magnesium-based materials by adding Ni2P. Renew. Energy 2022, 189, 559–569. [Google Scholar]
- Lu, Z.Y.; Yu, H.J.; Lu, X.; Song, M.C.; Wu, F.Y.; Zheng, J.G.; Yuan, Z.F.; Zhang, L.T. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Kuamr, S.; Jain, A.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Mukherjee, A.; Dey, G.K.; Kojima, Y. Surface modification of MgH2 by ZrCl4 to tailor the reversible hydrogen storage performance. Int. J. Hydrogen Energy 2017, 42, 6152–6159. [Google Scholar] [CrossRef]
- Tan, X.F.; Kim, M.; Yasuda, K.; Nogita, K. Strategies to enhance hydrogen storage performances in bulk Mg-based hydrides. J. Mater. Sci. Technol. 2023, 153, 139–158. [Google Scholar] [CrossRef]
- Yang, X.L.; Lu, X.H.; Zhang, J.Q.; Hou, Q.H.; Zou, J.H. Progress in improving hydrogen storage properties of Mg-based materials. Mater. Today Adv. 2023, 19, 100387. [Google Scholar]
- Darriet, B.; Pezat, M.; Hbika, A.; Hagenmuller, P. Application of magnesium rich rare-earth alloys to hydrogen storage. Int. J. Hydrogen Energy 1980, 5, 173–178. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Yong, H.; Wang, S.; Ma, J.W.; Zhang, K.W.; Zhao, D.L.; Hu, J.F.; Zhang, Y.H. Dual-tuning of de/hydrogenation kinetic properties of Mg-based hydrogen storage alloy by building a Ni-/Co-multi-platform collaborative system. Int. J. Hydrogen Energy 2021, 46, 24202–24213. [Google Scholar] [CrossRef]
- Yong, H.; Wei, X.; Wang, Y.H.; Guo, S.H.; Yuan, Z.M.; Qi, Y.; Zhao, D.L.; Zhang, Y.H. Phase evolution, thermodynamics and kinetics property of transition metal (TM = Zr, Ti, V) catalyzed Mg-Ce-Y-Ni hydrogen storage alloys. J. Phys. Chem. Solids 2020, 144, 109516. [Google Scholar] [CrossRef]
- Xie, L.S.; Li, J.S.; Zhang, T.B.; Kou, H.C. De/hydrogenation kinetics against air exposure and microstructure evolution during hydrogen absorption/desorption of Mg-Ni-Ce alloys. Renew. Energy 2017, 113, 1399–1407. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Q.F.; Li, Q.; Zhang, T.F. In-situ synchrotron X-ray diffraction investigation on hydrogen-induced decomposition of long period stacking ordered structure in Mg–Ni–Y system. Scr. Mater. 2017, 127, 102–107. [Google Scholar] [CrossRef]
- Song, W.J.; Dong, H.P.; Zhang, G.; Liu, J.; Yang, G.; Liu, Y.H.; Li, Y.Z.; Li, J.S.; Shen, J.H.; Chen, Y.X.; et al. Enhanced hydrogen absorption kinetics by introducing fine eutectic and long-period stacking ordered structure in ternary eutectic Mg-Ni-Y alloy. J. Alloys Compd. 2020, 820, 153187. [Google Scholar] [CrossRef]
- Kang, H.L.; Yong, H.; Wang, J.Z.; Xu, S.X.; Li, L.J.; Wang, S.; Hu, J.F.; Zhang, Y.H. Characterization on the kinetics and thermodynamics of Mg-based hydrogen storage alloy by the multiple alloying of Ce, Ni and Y elements. Mater. Charact. 2021, 182, 111583. [Google Scholar] [CrossRef]
- Yu, Y.C.; Ji, S.Q.; Zhang, S.H.; Wang, S.; Chen, Y.W.; Yong, H.; Liu, B.S.; Zhang, Y.H. Microstructure characteristics, hydrogen storage thermodynamic and kinetic properties of Mg–Ni–Y based hydrogen storage alloys. Int. J. Hydrogen Energy 2022, 47, 27059–27070. [Google Scholar] [CrossRef]
- Zhou, S.C.; Pan, R.K.; Luo, T.P.; Wu, D.H.; Wei, L.T.; Tang, B.Y. Ab initio study of effects of Al and Y co-doping on destabilizing of MgH2. Int. J. Hydrogen Energy 2014, 39, 9254–9261. [Google Scholar] [CrossRef]
- Tang, R.; Liu, Y.Q.; Zhu, C.C.; Zhu, J.W.; Yu, G. Effect of Al substitution for Co on the hydrogen storage characteristics of Ml0.8Mg0.2Ni3.2Co0.6−xAlx (x = 0–0.6) alloys. Intermetallics 2006, 14, 361–366. [Google Scholar] [CrossRef]
- Yang, T.; Zhai, T.T.; Yuan, Z.M.; Bu, W.G.; Xu, S.; Zhang, Y.H. Hydrogen storage properties of LaMgNi3.6M0.4 (M = Ni, Co, Mn, Cu, Al) alloys. J. Alloys Compd. 2014, 617, 29–33. [Google Scholar] [CrossRef]
- Sun, X.Z.; Pan, H.G.; Gao, M.X.; Li, R.; Lin, Y.; Ma, S. Cycling stability of La-Mg-Ni-Co type hydride electrode with Al. Trans. Nonferrous Met. Soc. China 2006, 16, s834–s838. [Google Scholar] [CrossRef]
- Ershova, O.G.; Dobrovolaky, V.D.; Solonin, Y.M.; Khyzhun, O.Y.; Koval, A.Y. The effect of Al on thermal stability and kinetics of decomposition of MgH2 prepared by mechanochemical reaction at different conditions. Mater. Chem. Phys. 2015, 162, 408–416. [Google Scholar] [CrossRef]
- Kan, H.; Zhang, N.; Wang, H. Al-Mg Alloy powders for hydrogen storage. Adv. Chem. Eng. 2012, 550–553, 497–501. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, L.L.; Zhu, Y.F.; Liu, Y.; Zhang, J.G.; Li, L.Q. Mechanism of improving hydrogenation of Mg by in-situ formation of Al* in hydriding combustion synthesis. J. Alloys Compd. 2022, 911, 164969. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Peng, W.Q.; Wei, W.L.; Xu, L.Q.; Guo, J. Preparation and hydrogen storage properties of Mg-Al-Li solid solution. Int. J. Hydrogen Energy 2016, 41, 6134–6138. [Google Scholar] [CrossRef]
- Liu, T.; Qin, C.G.; Zhu, M.; Cao, Y.R.; Shen, H.L.; Li, X.G. Synthesis and hydrogen storage properties of Mg-La-Al nanoparticles. J. Power Sources 2012, 219, 100–105. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Nobuki, T.; Kato, S.; Abe, M.; Kuji, T. Hydrogen absorption properties of the γ-Mg17Al12 phase and its Al-richer domain. J. Alloys Compd. 2007, 446–447, 157–161. [Google Scholar] [CrossRef]
- Lu, W.C.; Ou, S.F.; Lin, M.H.; Wong, M.F. Hydrogen absorption/desorption performance of Mg-Al alloys synthesized by reactive mechanical milling and hydrogen pulverization. J. Alloys Compd. 2016, 682, 318–325. [Google Scholar] [CrossRef]
- Zhong, H.C.; Wang, H.; Ouyang, L.Z. Improving the hydrogen storage properties of MgH2 by reversibly forming Mg-Al solid solution alloys. Int. J. Hydrogen Energy 2014, 39, 3320–3326. [Google Scholar] [CrossRef]
- Cao, W.C.; Ding, X.; Zhang, Y.; Zhang, J.X.; Chen, R.R.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Formation of AlNi phase and its influence on hydrogen absorption kinetics of Mg77Ni23-xAlx alloys at intermediate temperatures. Int. J. Hydrogen Energy 2022, 47, 25733–25744. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.H.; Lin, X.; Ding, W.J.; Zou, J.X. Promoting hydrogen industry with high-capacity Mg-based solid-state hydrogen storage materials and systems. Front. Energy 2023, 17, 320–323. [Google Scholar] [CrossRef]
- Wang, H.G.; Liu, Y.F.; Zhang, J. Hydrogen purification by Mg alloy hydrogen adsorbent. Adsorption 2022, 28, 85–95. [Google Scholar] [CrossRef]
- Saville, A.I.; Creuziger, A.; Mitchell, E.B.; Vogel, S.C.; Benzing, J.T.; Klemm-Toole, J.; Clarke, K.D.; Clarke, A.J. MAUD Rietveld Refinement Software for Neutron Diffraction Texture Studies of Single and Dual-Phase Materials. Integr. Mater. Manuf. Innov. 2021, 10, 36936346. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.A.; Paskevicius, M.; Javadian, P.; Davies, I.J.; Buckley, C.E. Methods for accurate high-temperature Sieverts-type hydrogen measurements of metal hydrides. J. Alloys Compd. 2019, 787, 1225–1237. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Morton, A.J.; Nie, J.F. The 18R and 14H long-period stacking ordered structures in Mg–Y–Zn alloys. Acta Mater. 2010, 58, 2936–2947. [Google Scholar] [CrossRef]
- Asadi, A.H.; Kalayeh, P.M.; Mirzadeh, H.; Malekan, M.; Emamy, M. Precipitation kinetics and mechanical properties of Mg–Y–Zn and Mg–Y–Ni alloys containing long-period stacking ordered (LPSO) structures. J. Mater. Res. Technol. 2023, 24, 9513–9522. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, S.; Bi, Y.D.; Li, Y.P.; Qin, G.W. Phase equilibria of the long-period stacking ordered phase in the Mg–Ni–Y system. Intermetallics 2015, 57, 127–132. [Google Scholar] [CrossRef]
- Lu, R.P.; Jiao, K.; Li, N.T.; Hou, H.; Wang, J.F.; Zhao, Y.H. Microstructure and damping properties of LPSO phase dominant Mg-Ni-Y and Mg-Zn-Ni-Y alloys. J. Alloys Compd. 2022, 250543791. [Google Scholar] [CrossRef]
- Song, W.J.; Ma, W.H.; He, S.; Chen, W.; Shen, J.H.; Sun, D.L.; Wei, Q.M.; Yu, X.B. TiO2@C catalyzed hydrogen storage performance of Mg-Ni-Y alloy with LPSO and ternary eutectic structure. J. Alloys Compd. 2023, 258666888. [Google Scholar] [CrossRef]
- Friedrichs, O.; Sánchez-López, J.C.; López-Cartes, C.; Dornheim, M.; Klasssen, T.; Bormann, R.; Fernández, A. Chemical and microstructural study of the oxygen passivation behaviour of nanocrystalline Mg and MgH2. Appl. Surf. Sci. 2006, 252, 2334–2345. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Xu, E.; You, X.M.; Bu, C.; Zhang, L.F.; Wang, Q.; Zhao, Z.G. Influence of micro-amount O2 or N2 on the hydrogenation/dehydrogenation kinetics of hydrogen-storage material MgH2. Int. J. Hydrogen Energy 2017, 42, 8057–8062. [Google Scholar] [CrossRef]
- Hou, Q.H.; Zhang, J.Q.; Guo, X.T.; Xu, G.Z.; Yang, X.L. Synthesis of low-cost biomass charcoal-based Ni nanocatalyst and evaluation of their kinetic enhancement of MgH2. Int. J. Hydrogen Energy 2022, 47, 15209–15223. [Google Scholar] [CrossRef]
- Dong, J.J.; Panda, S.; Zhu, W.; Zou, J.X.; Ding, W.J. Enhanced hydrogen sorption properties of MgH2 when doped with mechanically alloyed amorphous Zr0.67Ni0.33 particles. Int. J. Hydrogen Energy 2020, 45, 28144–28153. [Google Scholar] [CrossRef]
- Aono, K.; Orimo, S.; Fujii, H. Structural and hydriding properties of MgYNi4:: A new intermetallic compound with C15b-type Laves phase structure. J. Alloys Compd. 2000, 309, L1–L4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).