Modeling Microstructure Development upon Continuous Cooling of 42CrMo4 Steel Grade for Large-Size Components
Abstract
1. Introduction
2. Experimental Section
- Optical microscopy (OM, LEICA DMI5000 M, Leica Microsystems, Wetzlar, Germany) for general observations and metallographic quantifications;
- Field-emission gun scanning electron microscopy (Zeiss Sigma 500, SEM, Carl Zeiss AG, Oberkochen, Germany) for detailed imaging of some microstructure constituents;
- Microchemical analysis by EDS energy dispersive spectroscopy (X-Max® 50 EDS analyzer, Oxford Instruments, Abingdon, UK) fully controlled by the latest AZtec software 3.0 SP2 (Oxford Instruments, Abingdon, UK). The AZtec program allows creating quantitative results through the application of the TruLine algorithm that deconvolutes the peaks in case of overlapping and removes the background automatically.
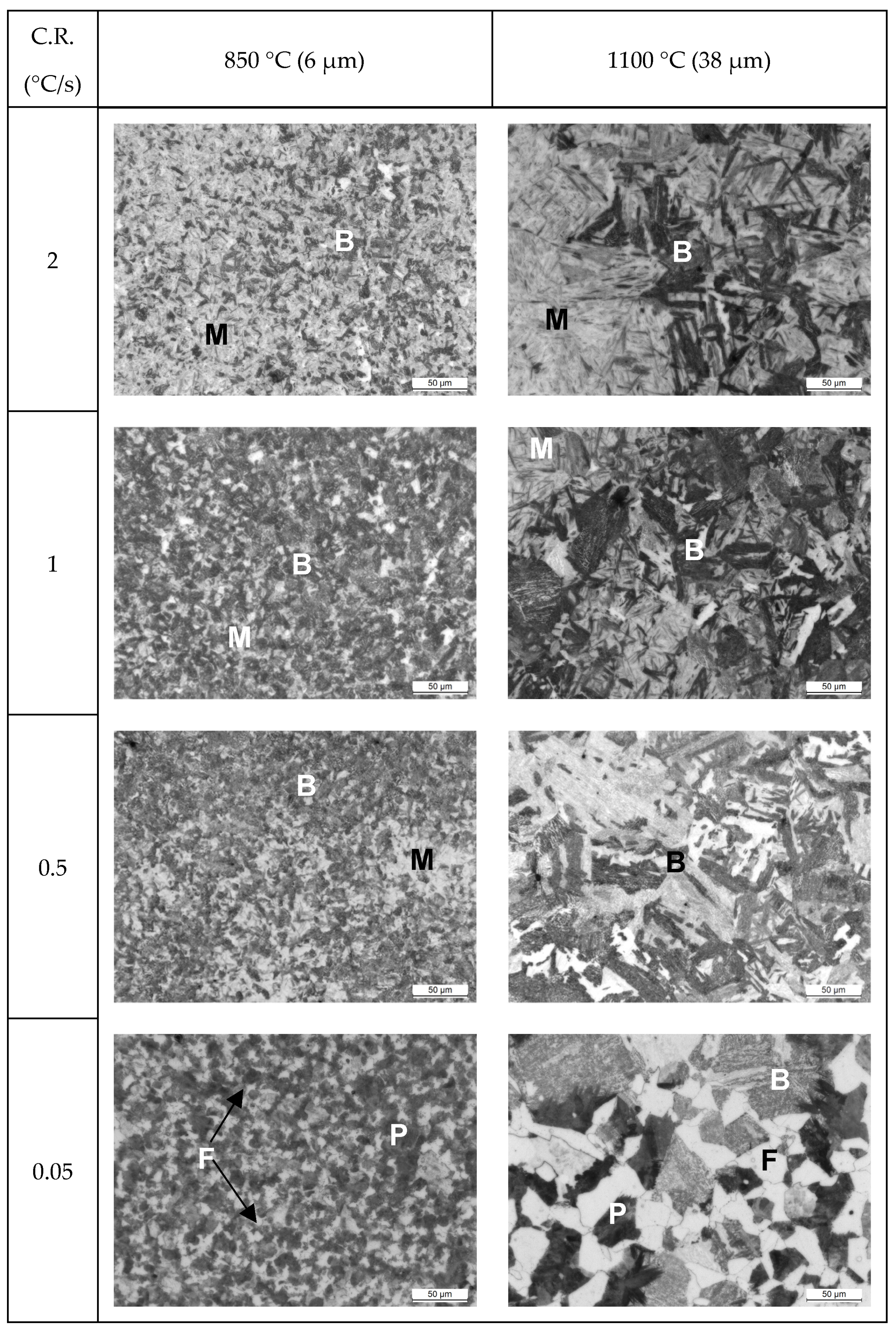
3. Results
- Austenite grain size evolution with austenization temperature;
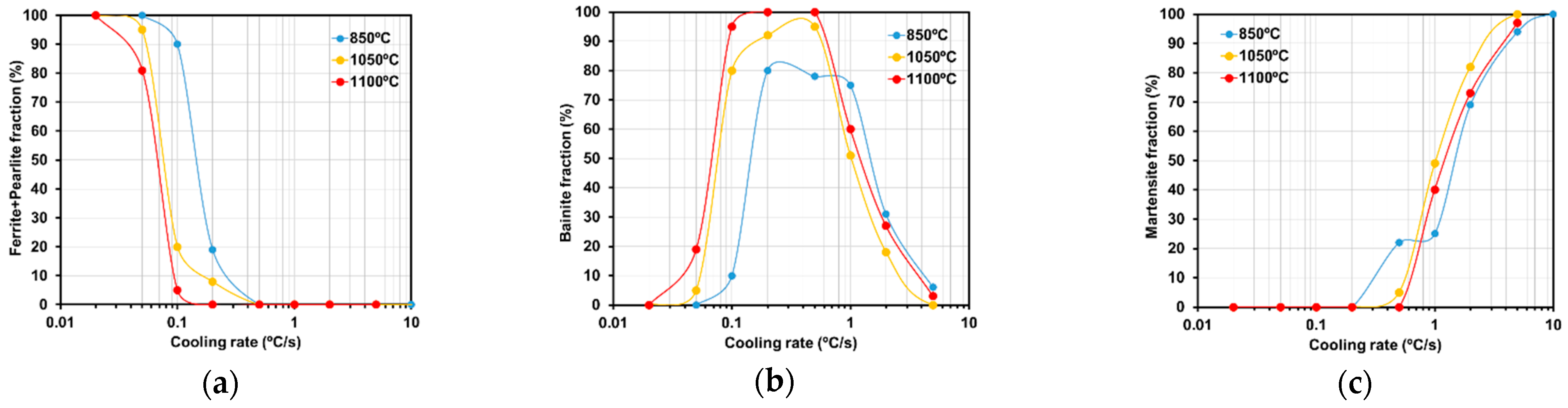
- Determination of the CCT curves for the three austenization temperatures;
- Hardness evolution with cooling rate;
- Microstructural evolution with the cooling rate;
- Application of the open-source codes and commercial simulators to the nominal composition, Table 1, for CCT determination.
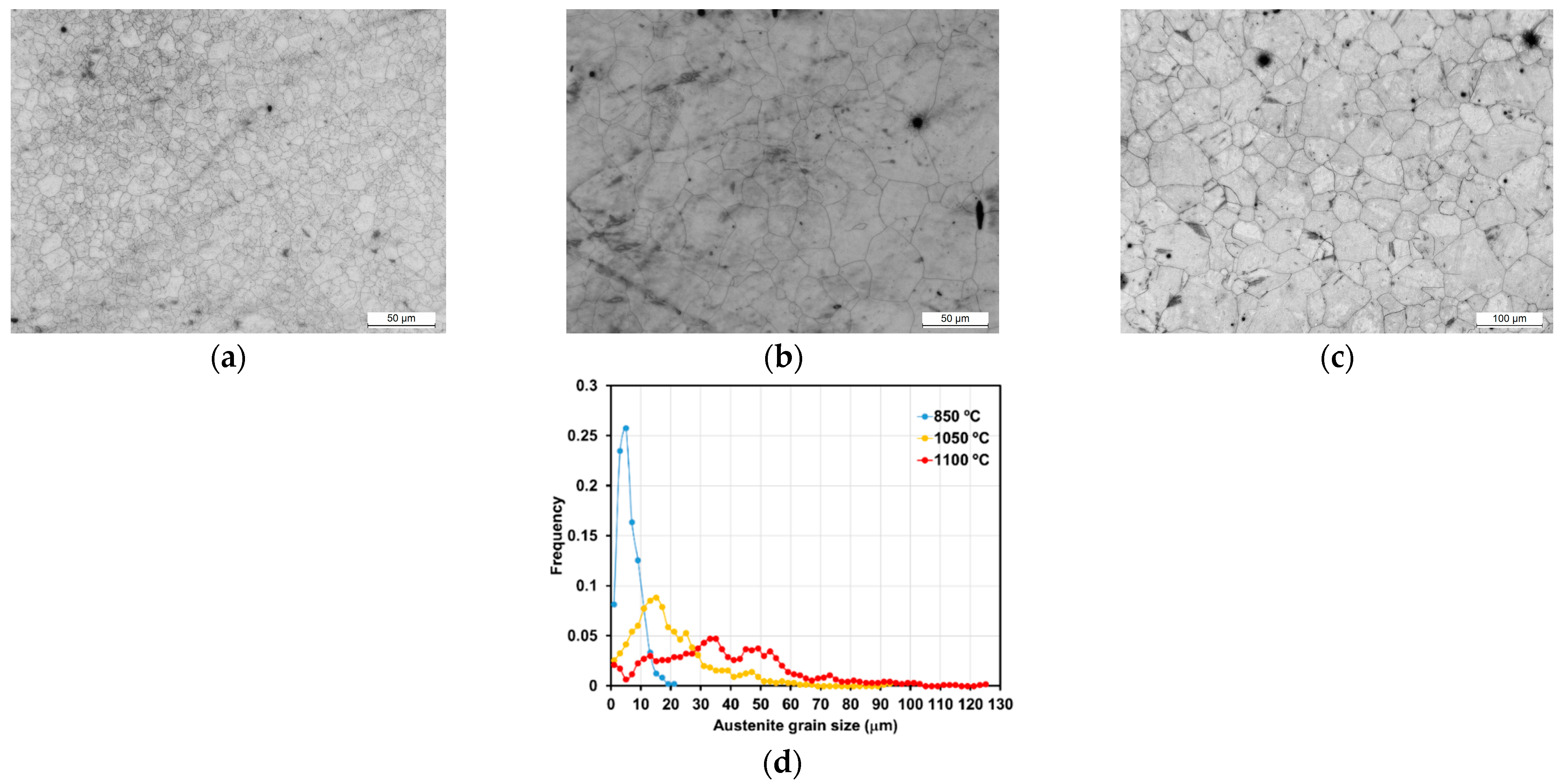
3.1. Austenite Grain Size
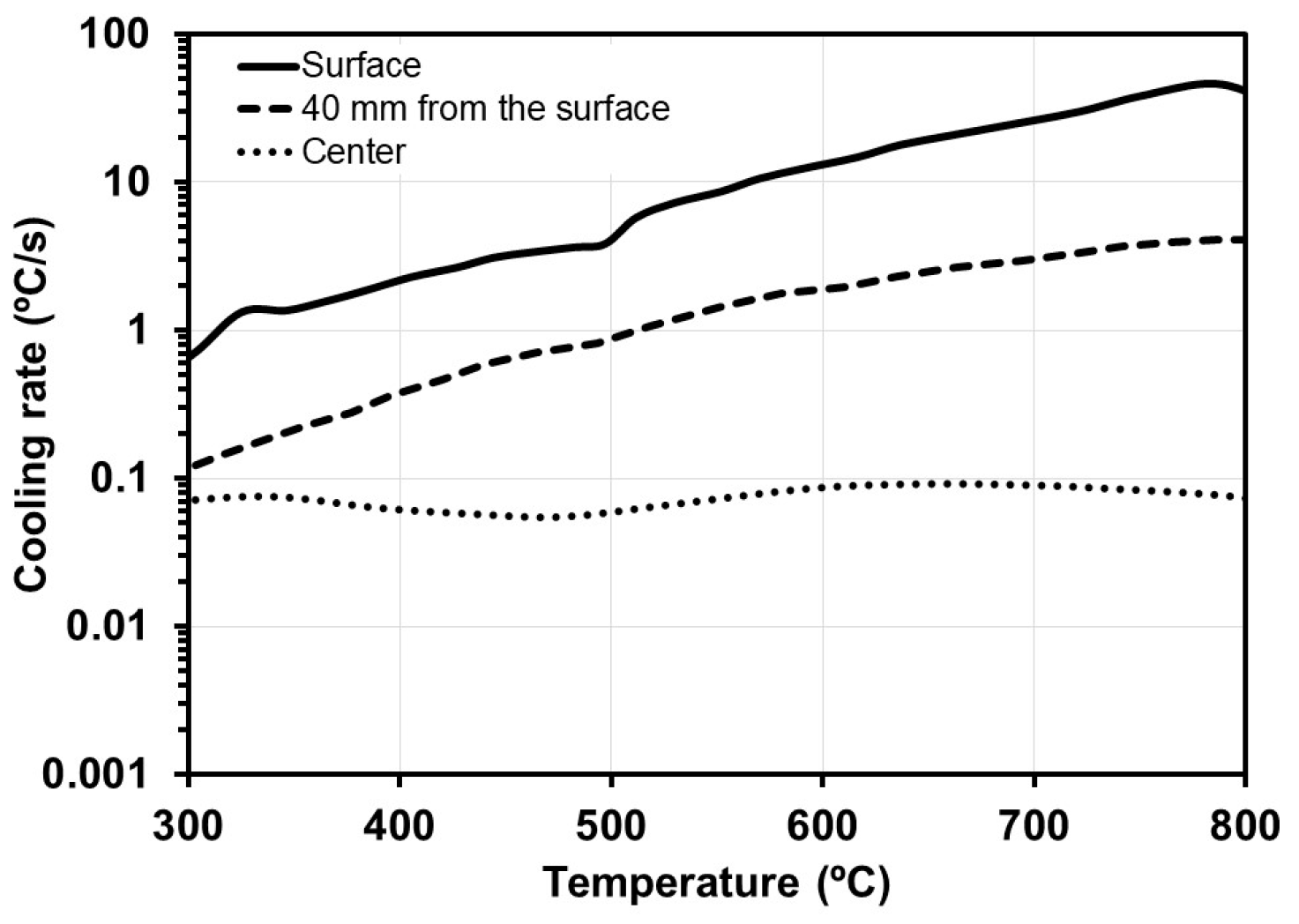
3.2. CCT Curves
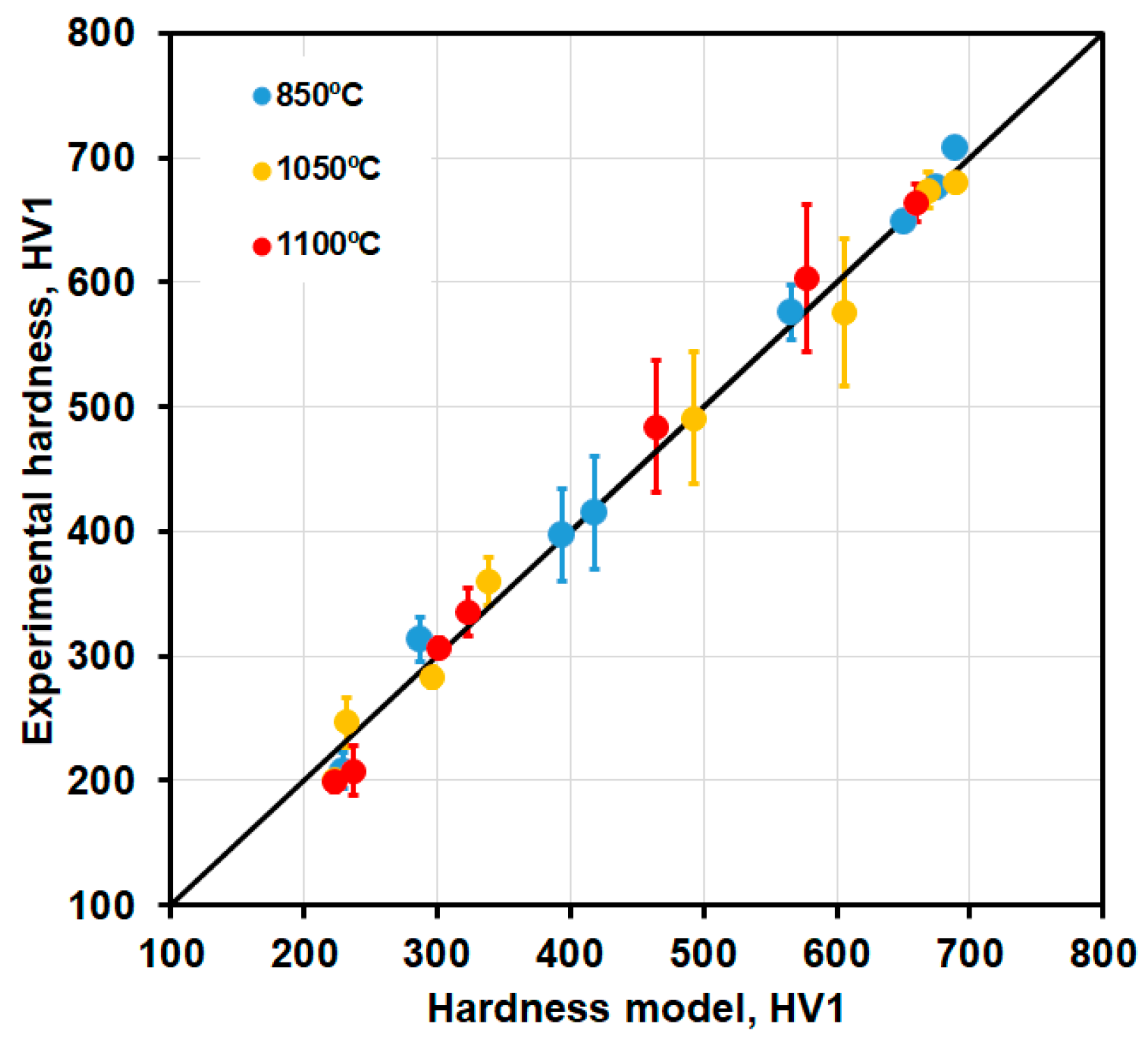
3.3. Hardness Evolution
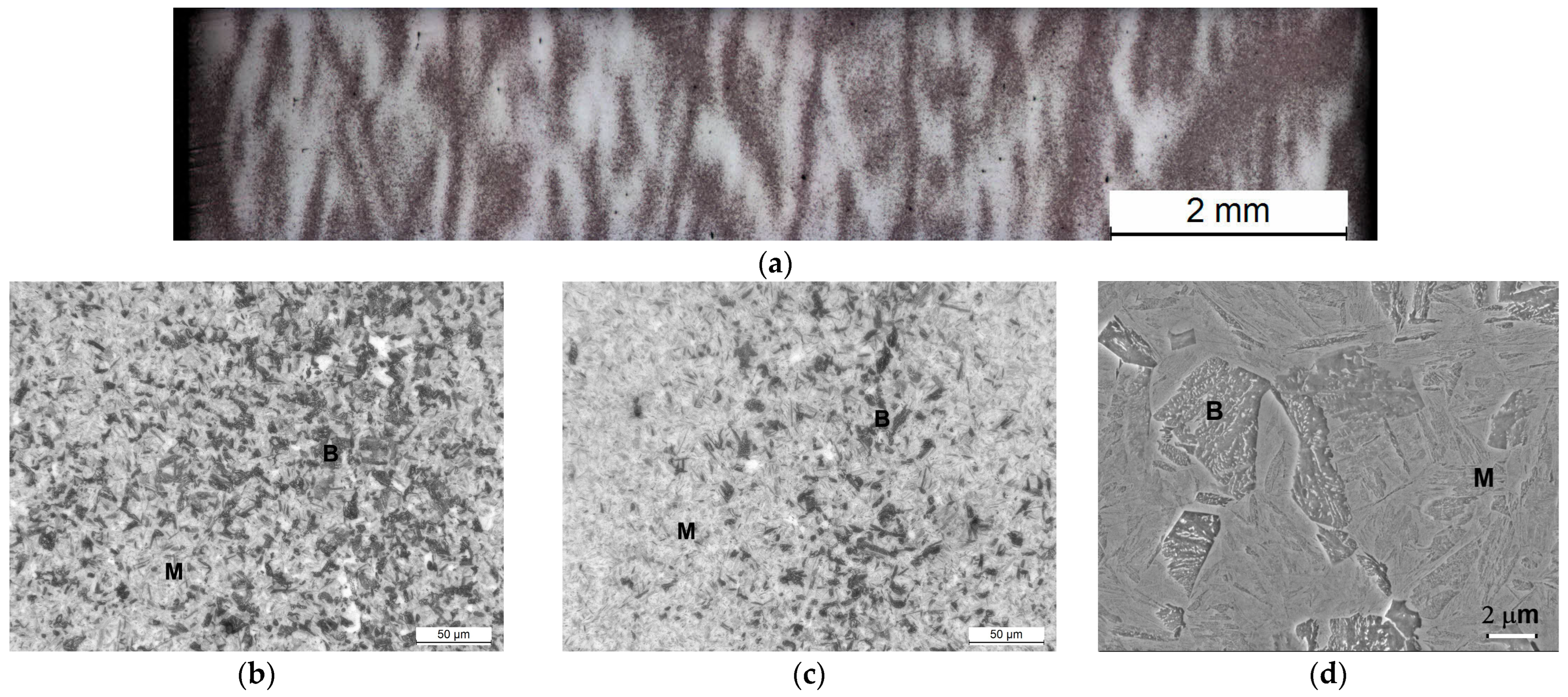
3.4. Microstructural Evolution
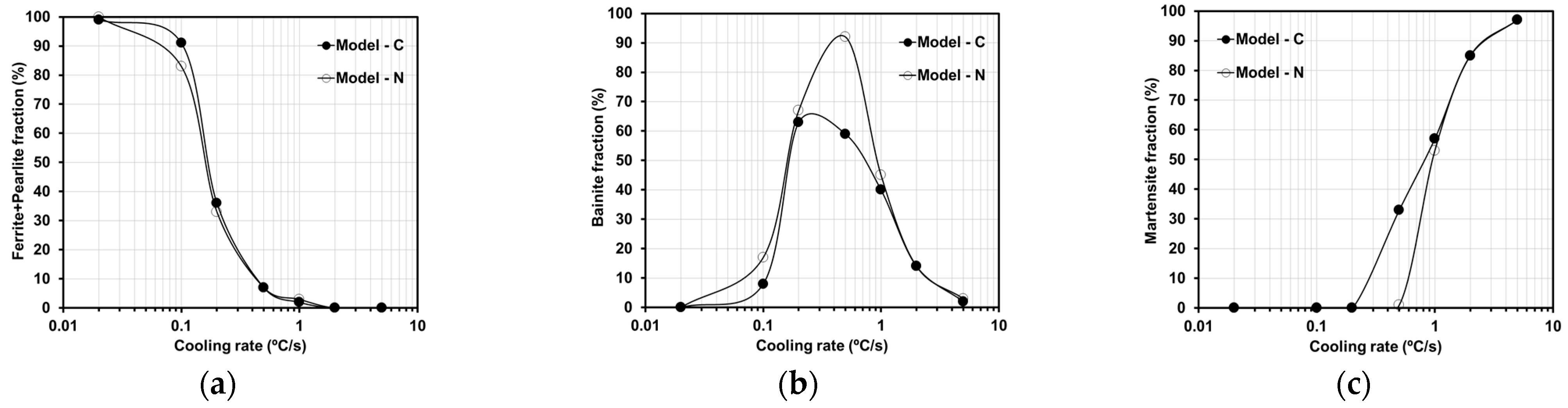
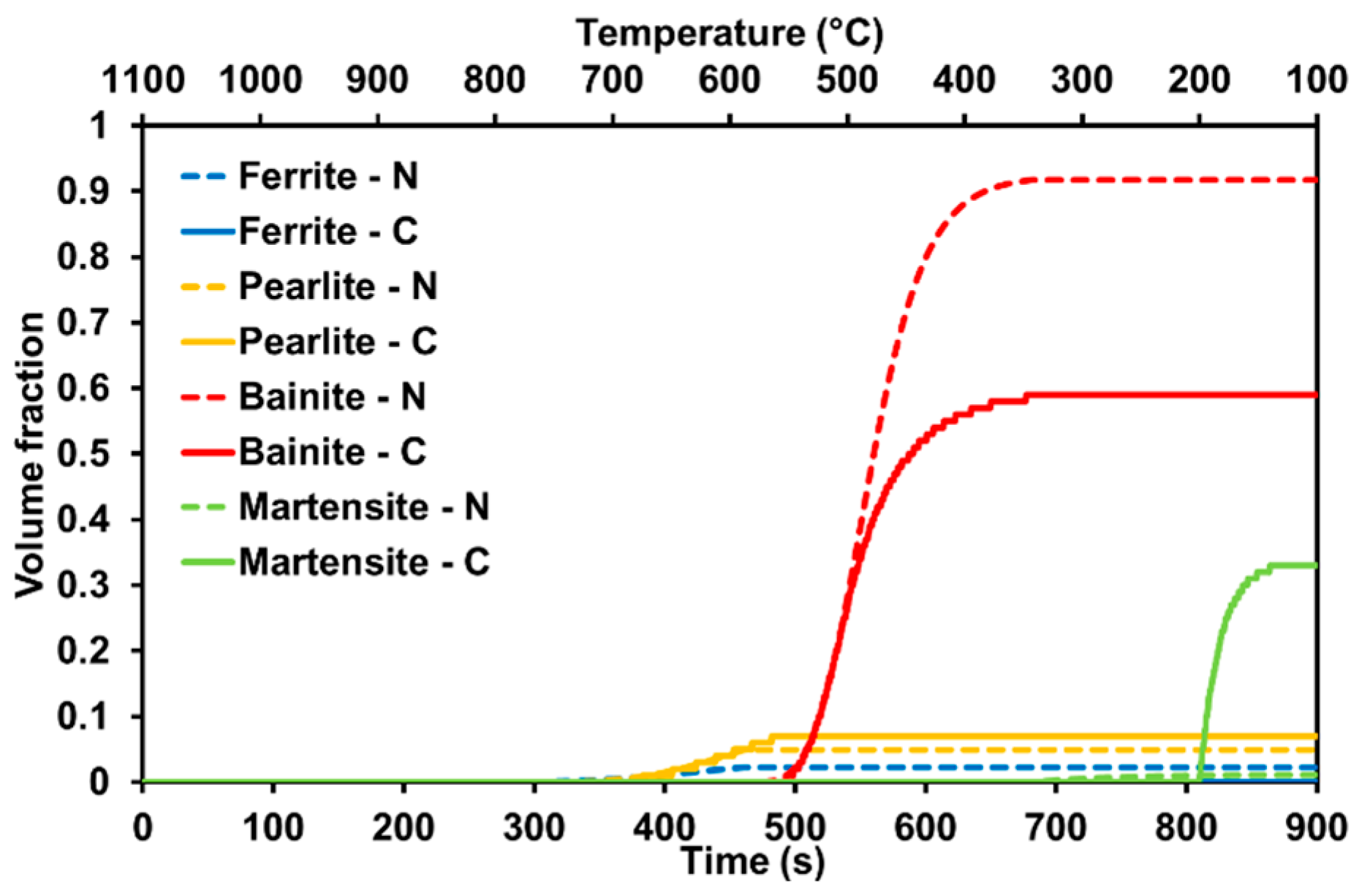
3.5. Open-Source Software: Results for the Current Steel Grade
4. Discussion
4.1. Modeling Hardness
4.2. Modeling Phase Transformation
- Significant changes in the prediction of the onset of ferrite-pearlite transformation depending on the model. Instead, the bainite and martensite start temperatures of the two models are close. In general, Nishikawa’s formulation [24] agrees better with the experimentally determined temperatures for ferrite, whereas Collins et al.’s [26] formulation excels when it comes to the bainite and martensite start temperature.
- However, looking at the phase fractions, the predictions largely deviate from the experimentally measured fractions of the different microconstituents regardless of the approach undertaken.

- ➢
- Individual models;
- ➢
- Overall transformation model;
- ➢
- Introduction of the compositional heterogeneity in the model.
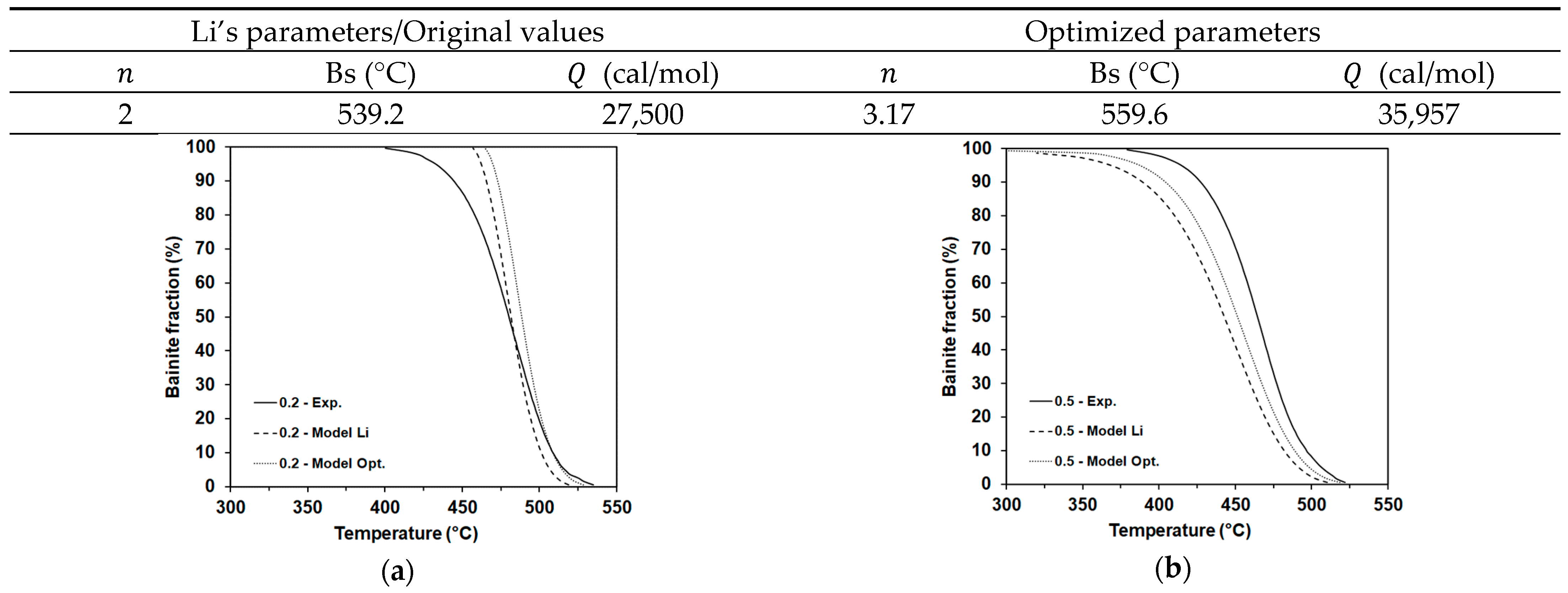
4.2.1. Improvement of the Transformation Models for Each Product Phase: Application to the Bainite Transformation Model
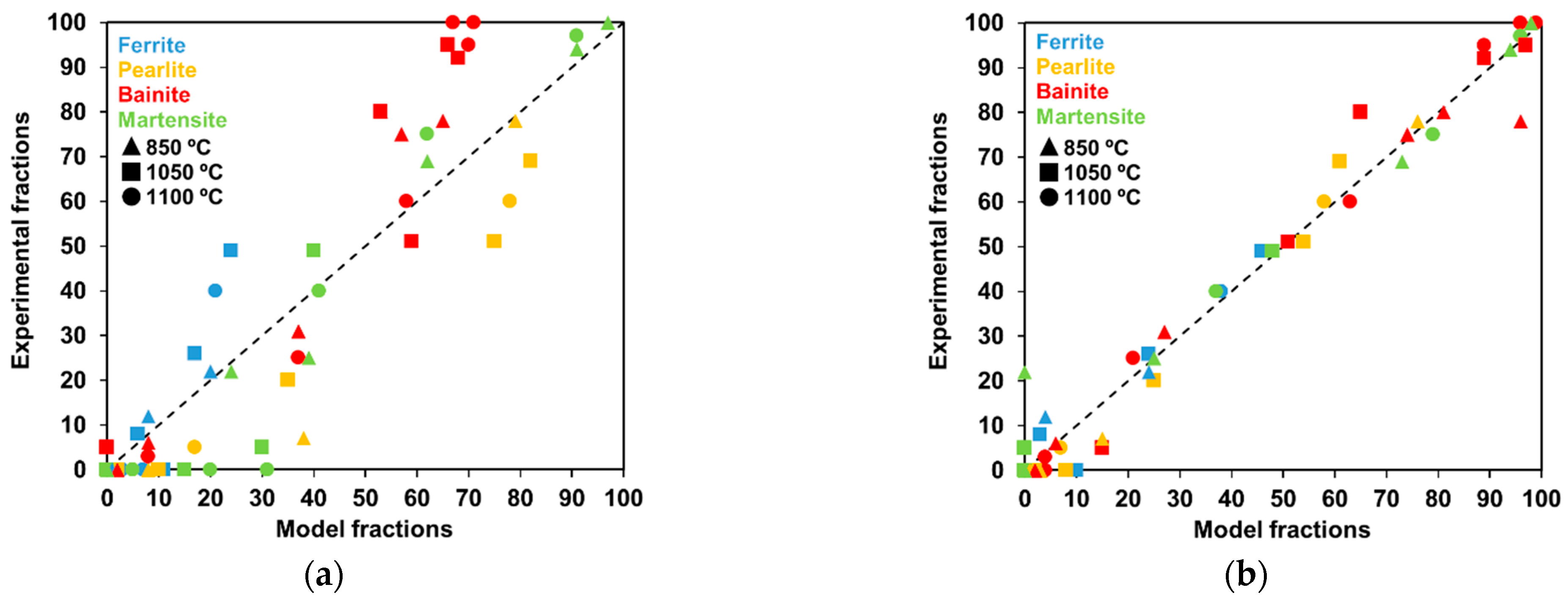
4.2.2. Improvements in the Overall Open-Source Models
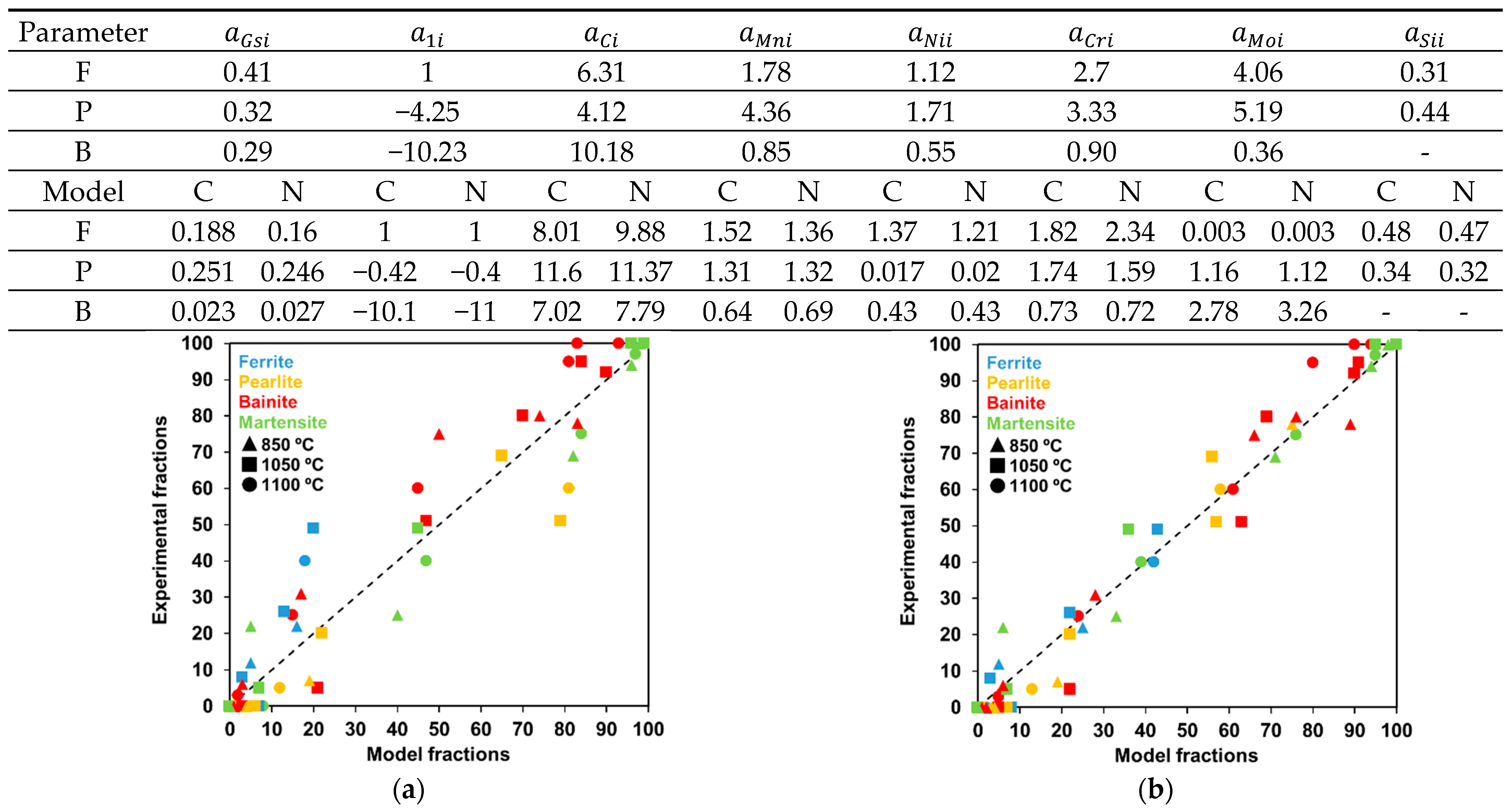
4.2.3. Sensitivity to the Chemical Composition: Introduction of the Heterogeneity in the Modeling of the Phase Transformation of 42CrMo4 Steel
4.3. Limitations
- The formulation for the transformation onset (Ae1, Ae3, Acm, Bs, etc.) of the different austenite products can strongly affect the predicted volume fractions.
- The strategy applied to integrate the full transformation model may lead to significant scatter in the results. It must be recalled that the model has to deal with conditions under which multiple transformation products are formed. From the results above, the introduction of some physical considerations (Collins et al.) or the simple possibility to transform austenite into more than one microconstituent at every integration step (Nishikawa) in the models bring about variations in the optimized parameter values. This is clearly proven in Table 3, where the values are significantly different for each model.
- The linear approaches to the composition-dependent term do not reflect likely higher-order correlations or some synergistic effects on phase transformations, which are usually reported in the literature [35].
- Lastly, the chemical characterization at the micro/mesoscale of the samples is of crucial significance since it is the base for properly describing the behavior of the phase transformations while being closer to the actual observed phenomena.
5. Conclusions
5.1. Experimental Characterization
5.2. Modeling Phase Transformation upon Continuous Cooling
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Description of the Chemical Heterogeneity
| Microscopy Conditions | Acquisition Conditions | ||
|---|---|---|---|
| Potential (kV) | 20 | Step/total line length (μm) | 3–5/1400–2800 |
| Dwell time (ms) | 1500 | ||
| Aperture (μm) | 60 (High Current) | Analyzed elements | Fe-Cr-Mn-Si-Ni-Cu |
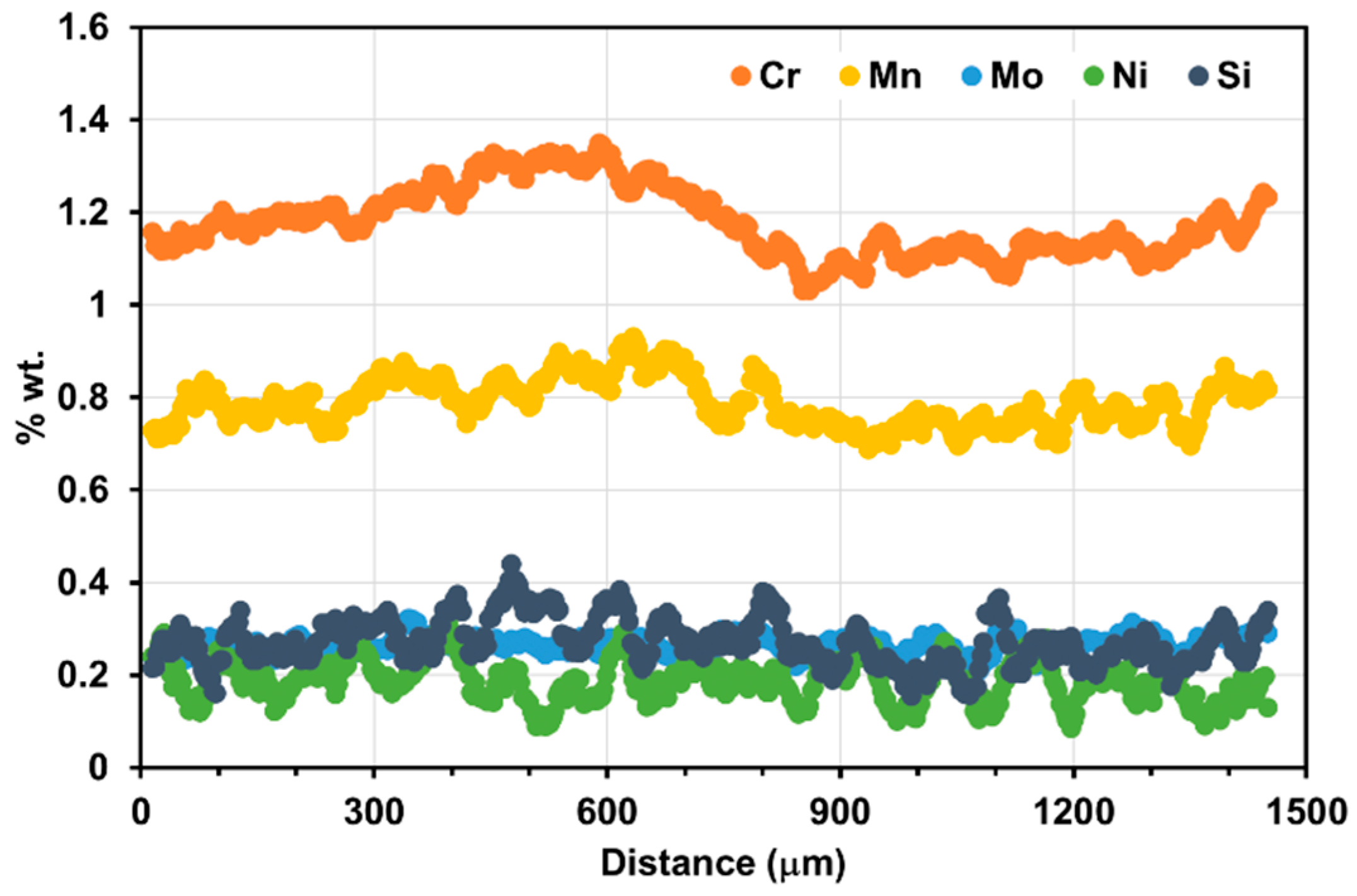
| Element | C | Mn | Si | Cr | Mo | Ni |
|---|---|---|---|---|---|---|
| Chemical composition (AES) | 0.44 | 0.74 | 0.22 | 1.02 | 0.24 | 0.14 |
| Mean chemical composition based on all EDS spectra (point-to-point) | Unknown | 0.82 | 0.26 | 1.17 | 0.28 | 0.16 |
| Amplitude EDS per element based on all EDS spectra (95% data interval, point-to-point) | Unknown | 0.08 | 0.06 | 0.12 | 0.08 | 0.07 |
| Amplitude of mean EDS among all lines (Max-Min)/2 (line-to-line) | Unknown | 0.06 | 0.01 | 0.03 | 0.03 | 0.04 |
- C distributes homogeneously (constant value of 0.44%).
- C distributes as if the material was held for a very long time (half a day) at a temperature of 1250 °C (simulating the solution heat treatment before high temperature thermomechanical processing).

| Chemical Element | Cr | ||||
|---|---|---|---|---|---|
| Cr | 1.000 | Mn | |||
| Mn | 0.701 | 1.000 | Mo | ||
| Mo | 0.655 | 0.618 | 1.000 | Ni | |
| Ni | 0.048 | 0.122 | 0.032 | 1.000 | Si |
| Si | 0.204 | 0.515 | 0.132 | −0.009 | 1.000 |
Appendix B. Description Particle Swarm Approach
References
- Bhagyalaxmi; Sharma, S.; Jayashree, P.K.; Hegde, A. Vegetable oil quench effect on impact toughness and hardness of 42CrMo4 steel. Mater. Today Proc. 2022, 63, 113–116. [Google Scholar] [CrossRef]
- Abdul Khadeer, S.; Ramesh Babu, P.; Ravi Kumar, B.; Seshu Kumar, A. Evaluation of friction welded dissimilar pipe joints between AISI 4140 and ASTM A 106 Grade B steels used in deep exploration drilling. J. Manuf. Process. 2020, 56 Pt A, 197–205. [Google Scholar] [CrossRef]
- Costa, L.L.; Brito, A.M.G.; Rosiak, A.; Schaeffer, L. Microstructure evolution of 42CrMo4 during hot forging process of hollow shafts for wind turbines. Int. J. Adv. Manuf. Technol. 2020, 106, 511–517. [Google Scholar] [CrossRef]
- Gramlich, A.; Hinrichs, T.; Springer, H.; Krupp, U. Recycling-Induced Copper Contamination of a 42CrMo4 Quench and Tempering Steel: Alterations in Transformation Behavior and Mechanical Properties. Steel Res. Int. 2023, 94, 2200623. [Google Scholar] [CrossRef]
- Smokvina Hanza, S.; Stic, L.; Liveri, L.; Spada, V. Corrosion behaviour of tempered 42CrMo4 steel. Mater. Technol. 2021, 55, 427–433. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Lu, H.; Liu, J.; Cai, G. Surface Integrity and Corrosion Resistance of 42CrMo4 High-Strength Steel Strengthened by Hard Turning. Materials 2021, 14, 6995. [Google Scholar] [CrossRef]
- Behrens, B.A.; Brunotte, K.; Petersen, T.; Diefenbach, J. Mechanical and Thermal Influences on Microstructural and Mechanical Properties during Process-Integrated Thermomechanically Controlled Forging of Tempering Steel AISI 4140. Materials 2020, 13, 5772. [Google Scholar] [CrossRef]
- Zhu, J.G.; Sun, X.; Barber, G.C.; Han, X.; Qin, H. Bainite Transformation-Kinetics-Microstructure Characterization of Austempered 4140 Steel. Metals 2020, 10, 236. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.W.; Lee, H.W.; Han, K.B.; Lee, J.M.; Kang, J.H. Hardness Prediction of Wind Turbine Components Considering the Tempering Effect. Int. J. Eng. Trends Technol. 2022, 70, 57–62. [Google Scholar] [CrossRef]
- Nabil, M.; Taha, O.; Elbitar, T.; El Mahallawi, I. Thermomechanical processing of 42CrMoS4 steel. Int. Heat Treat. Surf. Eng. 2013, 4, 87. [Google Scholar] [CrossRef]
- Hunkel, M. Segregations in Steels during Heat Treatment—A Consideration along the Process Chain. HTM J. Heat Treat. Mater. 2021, 76, 79–104. [Google Scholar] [CrossRef]
- Gutman, L.; Kennedy, J.R.; Roch, F.; Marceaux dit Clément, A.; Salsi, L.; Cauzid, J.; Rouat, B.; Combeau, H.; Založnik, M.; Zollinger, J. Characterisation of mesosegregations in large steel ingots. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1274, 012049. [Google Scholar] [CrossRef]
- Krauss, G. Solidification, segregation, and banding in carbon and alloy steels. Metall. Mater. Trans. B 2003, 34, 781. [Google Scholar] [CrossRef]
- Abraham Mathews, J.; Sietsma, J.; Petrov, R.H.; Santofimia, M.J. Austenite formation from a steel microstructure containing martensite/austenite and bainite bands. J. Mater. Res. Technol. 2023, 25, 5325. [Google Scholar] [CrossRef]
- Kuziak, R.; Pidvysots’kyy, V.; Pernach, M.; Rauch, Ł.; Zygmunt, T.; Pietrzyk, M. Selection of the best phase transformation model for optimization of manufacturing processes of pearlitic steel rails. Arch. Civ. Mech. Eng. 2019, 19, 535. [Google Scholar] [CrossRef]
- Vasilyev, A.A.; Sokolov, D.F.; Kolbasnikov, N.G.; Sokolov, S.F. Modeling of the γ→α Transformation in Steels. Phys. Solid State 2012, 54, 1669. [Google Scholar] [CrossRef]
- Kumar, M.; Sasikumar, R.; Kesevan Nair, P. Competition between nucleation and early growth from austenite- Studies using cellular automaton simulations. Acta Mater. 1998, 46, 6291. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sommer, F.; Mittemeijer, E.J. Isothermal austenite–ferrite transformation of Fe–0.04at.% C alloy: Dilatometric measurement and kinetic analysis. Acta Mater. 2008, 15, 3833. [Google Scholar] [CrossRef]
- Erişir, E.; Bilir, O.G. Phase Field Modeling of Microstructure Evolution during Intermediate Quenching and Intermediate Annealing of Medium-Carbon Dual-Phase Steel. In Proceedings of the Supplemental Proceedings 144th Annual Meeting & Exhibition TMS, Orlando, FL, USA, 15–19 March 2015; pp. 1433–1440. [Google Scholar]
- Iung, T.; Kandel, M.; Quidort, D.; de Lassat, Y. Physical modelling of phase transformations in high strength steels. Metall. Res. Technol. 2003, 100, 173–181. [Google Scholar] [CrossRef]
- Retz, P.; Shan, Y.V.; Sobotka, E.; Vogric, M.; Wei, W.; Povoden-Karadeniz, E.; Kozeschnik, E. Progress of Physics-based Mean-field Modeling and Simulation of Steel. Berg Huettenmaenn Monatshefte 2022, 167, 15–22. [Google Scholar] [CrossRef]
- Geng, X.; Wang, H.; Xue, W.; Xiang, S.; Huang, H.; Meng, L.; Ma, G. Modeling of CCT diagrams for tool steels using different machine learning techniques. Comput. Mater. Sci. 2020, 171, 109235. [Google Scholar] [CrossRef]
- Li, M.V.; Niebuhr, D.V.; Meekisho, L.L.; Atteridge, D.G. A Computational Model for the Prediction of Steel Hardenability. Metall. Mater. Trans. B 1998, 29, 661–672. [Google Scholar] [CrossRef]
- Nishikawa, A. Transformations Diagrams. 2020. Available online: https://github.com/arthursn/transformation-diagrams (accessed on 18 July 2023).
- Collins, J.; Piemonte, M.; Taylor, M.; Fellowes, J.; Pickering, E. A Rapid, Open-Source CCT Predictor for Low-Alloy Steels, and Its Application to Compositionally Heterogeneous Material. Metals 2023, 13, 1168. [Google Scholar] [CrossRef]
- Collins, J. Low Alloy Steel CCT Predictor. 2023. Available online: https://zenodo.org/record/776762 (accessed on 19 June 2023).
- Bach, F.W.; Schaper, M.; Yu, Z.; Nürnberger, F.; Gretzki, T.; Rodman, D.; Springer, R. Computation of isothermal transformation diagrams of 42CrMo4 steel from dilatometer measurements with continuous cooling. Int. Heat Treat. Surf. Eng. 2010, 4, 171–175. [Google Scholar] [CrossRef]
- Maynier, P.; Dollet, J.; Bastien, P. Prediction of Microstructure via Empirical Formulae Based on CCT Diagrams, Hardenability Concepts With Applications to Steel; The Metallurgical Society of AIME: San Ramon, CA, USA, 1978; pp. 163–178. [Google Scholar]
- Grange, R.A. Estimating Critical Ranges in Heat Treatment of Steels. Met. Prog. 1961, 79, 73–75. [Google Scholar]
- Kung, C.Y.; Rayment, J.J. An Examination of the Validity of Existing Empirical Formulae for the Calculation of Ms Temperature. Metall. Trans. A 1982, 13A, 328–331. [Google Scholar] [CrossRef]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721. [Google Scholar]
- van Bohemen, S.M.C. Bainite and martensite start temperature calculated with exponential carbon dependence. Mater. Sci. Technol. 2012, 28, 487–495. [Google Scholar] [CrossRef]
- Rauch, L.; Bachniak, D.; Kuziak, R.; Kusiak, J.; Pietrzyk, M. Problem of Identification of Phase Transformation Models Used in Simulations of Steels Processing. J. Mater. Eng. Perform. 2018, 27, 5725–5735. [Google Scholar] [CrossRef]
- Miranda, L.J. Pyswarms: A Research Toolkit for Particle Swarm Optimization. Available online: https://github.com/ljvmiranda921/pyswarms (accessed on 17 May 2024).
- Martin, H.; Amoako-Yirenkyi, P.; Pohjonen, A.; Frempong, N.K.; Komi, J.; Somani, M. Statistical Modeling for Prediction of CCT Diagrams of Steels Involving Interaction of Alloying Elements. Metall. Mater. Trans. B 2021, 52, 223–235. [Google Scholar] [CrossRef]
- Pouchou, J.; Pichoir, F. X-Ray Optics and Microanalysis; Brown, J., Packwood, R., Eds.; University of Western Ontario: London, ON, USA, 1987; p. 249. [Google Scholar]
- Ramesh Babu, S.; Ivanov, I.; Porter, D. Influence of Microsegregation on the Onset of the Martensitic Transformation. ISIJ Int. 2019, 59, 169. [Google Scholar] [CrossRef]
- Eberhart, R.C.; Yuhui, S. Particle Swarm optimization: Developments, Applications and Resources. In Proceedings of the 2001 Congress on Evolutionary Computation, Seoul, Republic of Korea, 27–30 May 2001; pp. 81–86. [Google Scholar]
- Meissner, M.; Schmuker, M.; Schneider, G. Optimized Particle Swarm Optimization (OPSO) and its application to artificial neural network training. BMC Bioinform. 2006, 7, 125. [Google Scholar] [CrossRef]
- Pedersen, M.E.H.; Chipperfield, A.J. Simplifying particle swarm optimization. Appl. Soft Comput. 2010, 10, 618. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, Y.; Zhao, J. Meta-learning-based multi-objective PSO model for dynamic scheduling optimization. Energy Rep. 2023, 9, 1227. [Google Scholar] [CrossRef]

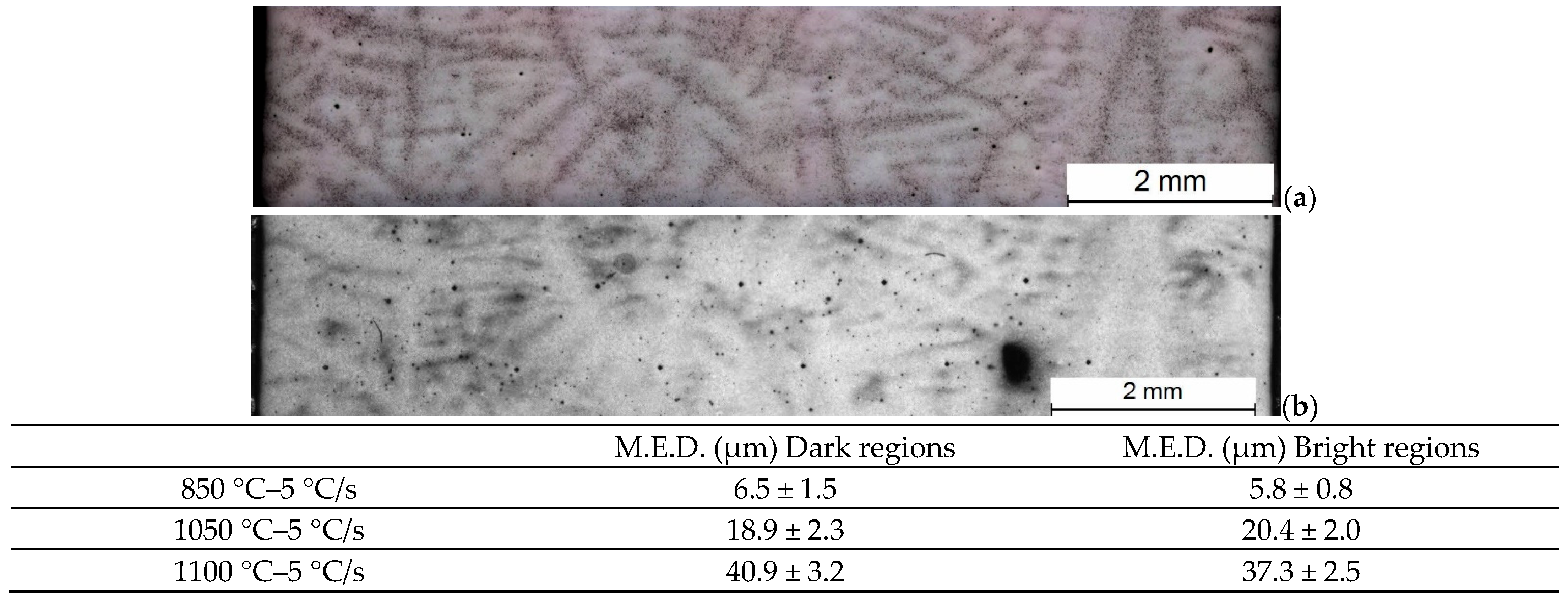
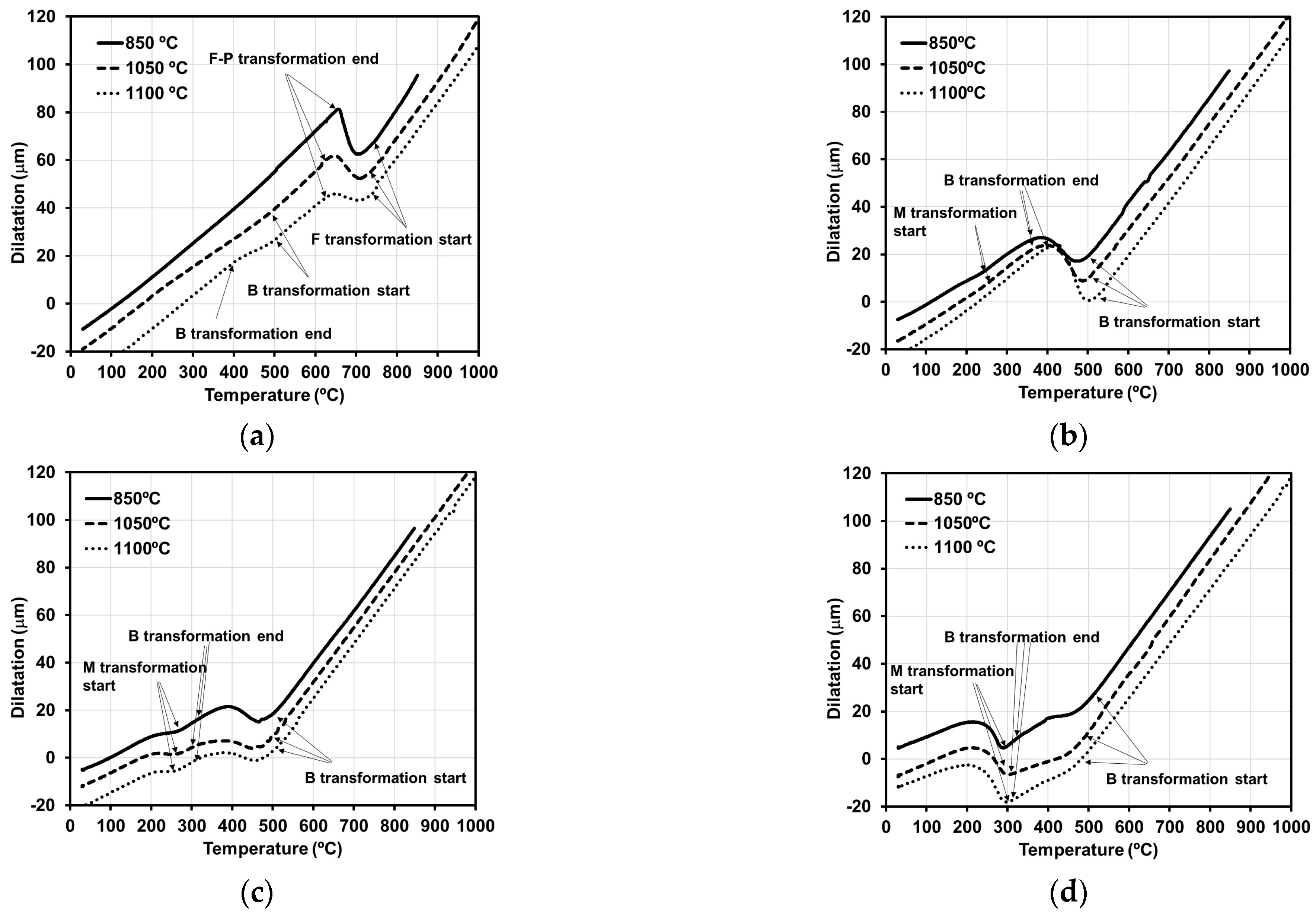
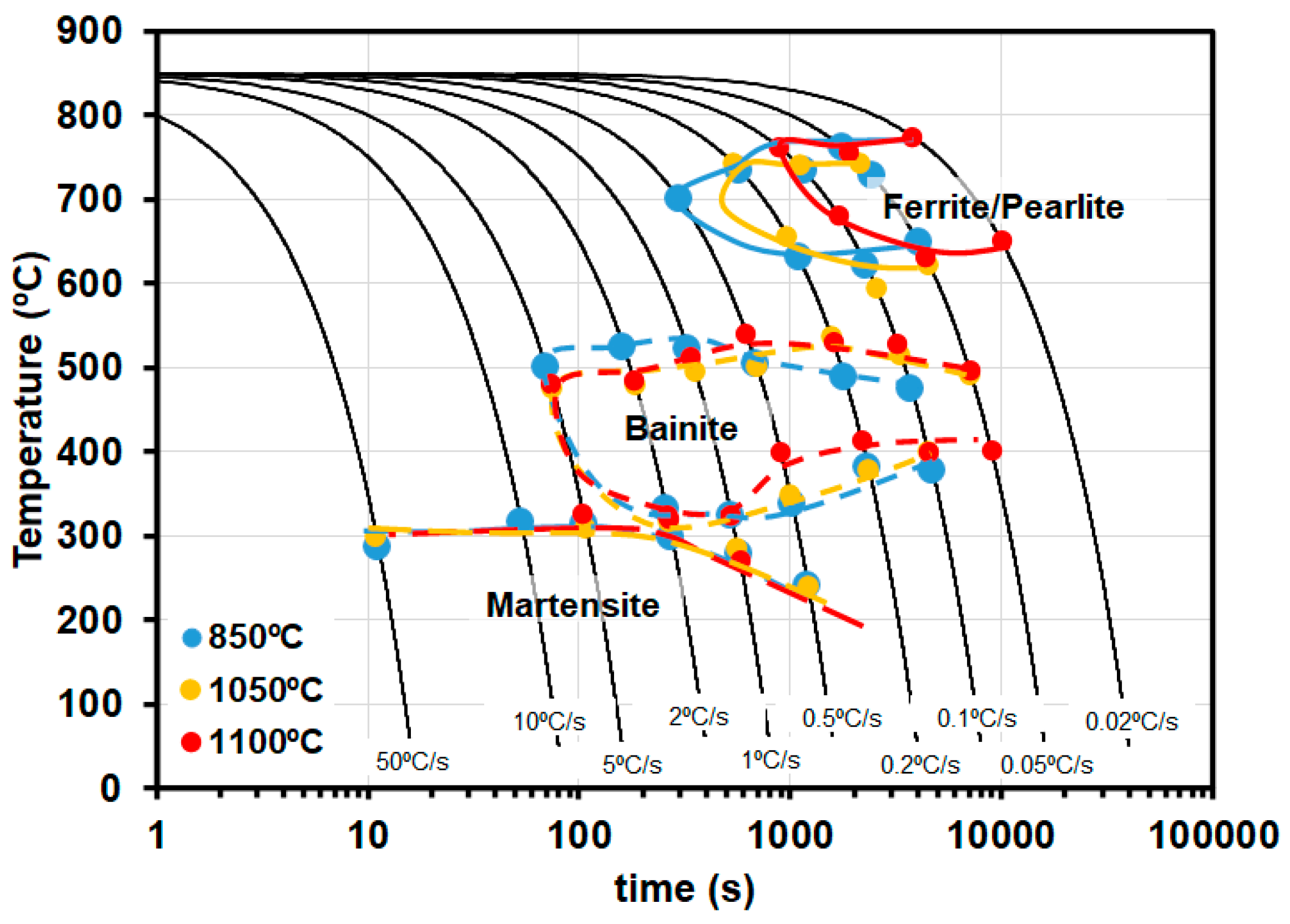
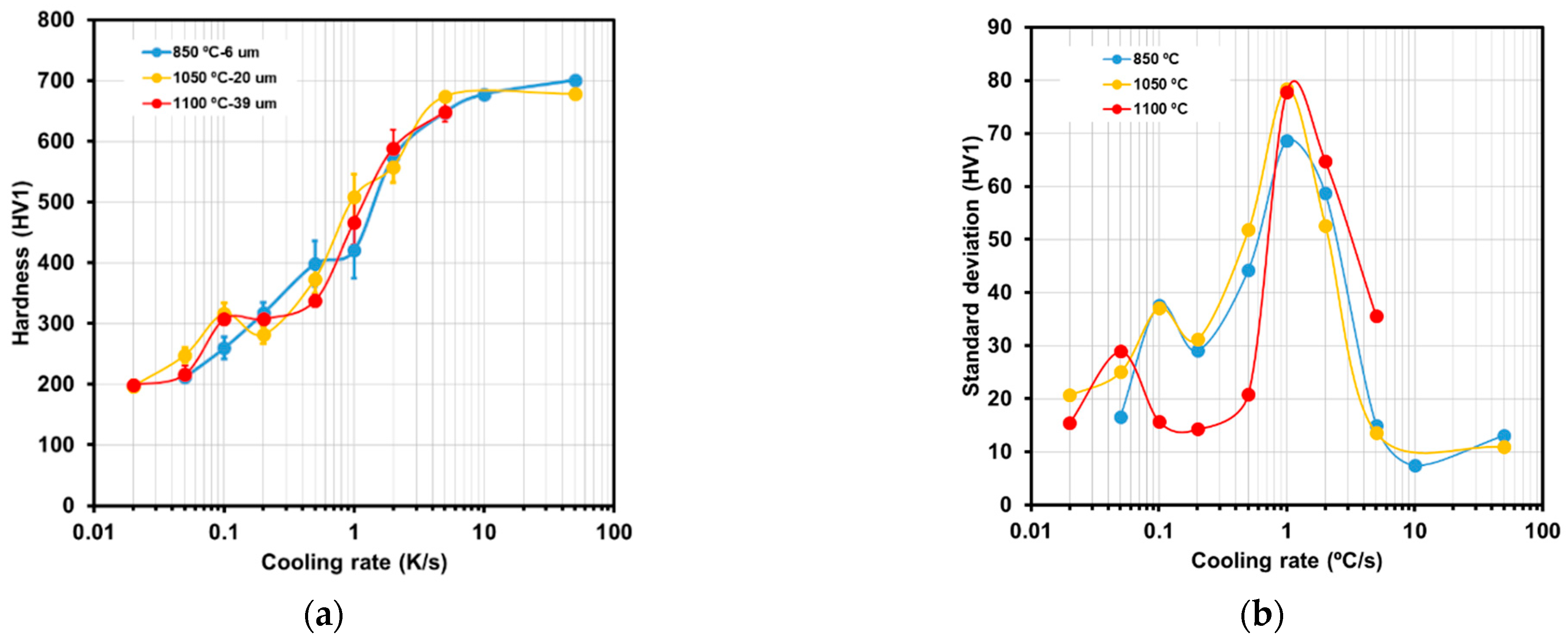
| C | Mn | Si | Cr | Mo | Ni |
|---|---|---|---|---|---|
| 0.44 | 0.74 | 0.22 | 1.02 | 0.24 | 0.14 |
| Open-Source SW | Included Features (Integration Scheme) | Omitted Features | Common Features | ||||
|---|---|---|---|---|---|---|---|
| Ae3 | Ae1 | Bs | Ms | Transformation Kinetics | |||
| J. Collins [26] |
|
| Grange [29] | Grange [29] | Li [23] | Kung-Rayment [30] | Original work by Li et al. [23] |
| A. Nishikawa [24] |
|
| Andrews [31] | Andrews [31] | Van Bohemen [32] | ||
| Taus (°C) | Ferrite (Li) | Ferrite (N/C) | Pearlite (Li) | Pearlite (N/C) | Bainite (Li) | Bainite (N/C) |
|---|---|---|---|---|---|---|
| 850 | 330 | 2463/367 | 93 | 394/280 | 0.0017 | 0.00355/0.0026 |
| 1050 | 869 | 2975/490 | 199 | 571/592 | 0.0034 | 0.00473/0.00254 |
| 1100 | 1465 | 5269/790 | 299 | 1203/882 | 0.0049 | 0.00412/0.00261 |
| Model | Type | Chemical | RMSE |
|---|---|---|---|
| N | Original | Homogeneous | 0.271 |
| C | Original | Homogeneous | 0.271 |
| N | Original | Heterogeneous | 0.263 |
| C | Original | Heterogeneous | 0.260 |
| N | Optimized | Homogeneous | 0.046 |
| C | Optimized | Homogeneous | 0.122 |
| N | Optimized | Heterogeneous | 0.054 |
| C | Optimized | Heterogeneous | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Sanchez, S.; Iza-Mendia, A.; Jorge-Badiola, D. Modeling Microstructure Development upon Continuous Cooling of 42CrMo4 Steel Grade for Large-Size Components. Metals 2024, 14, 1096. https://doi.org/10.3390/met14101096
Fernandez-Sanchez S, Iza-Mendia A, Jorge-Badiola D. Modeling Microstructure Development upon Continuous Cooling of 42CrMo4 Steel Grade for Large-Size Components. Metals. 2024; 14(10):1096. https://doi.org/10.3390/met14101096
Chicago/Turabian StyleFernandez-Sanchez, Sergio, Amaia Iza-Mendia, and Denis Jorge-Badiola. 2024. "Modeling Microstructure Development upon Continuous Cooling of 42CrMo4 Steel Grade for Large-Size Components" Metals 14, no. 10: 1096. https://doi.org/10.3390/met14101096
APA StyleFernandez-Sanchez, S., Iza-Mendia, A., & Jorge-Badiola, D. (2024). Modeling Microstructure Development upon Continuous Cooling of 42CrMo4 Steel Grade for Large-Size Components. Metals, 14(10), 1096. https://doi.org/10.3390/met14101096






