Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterisation
2.2.1. Chemical Composition of the Copper Slag
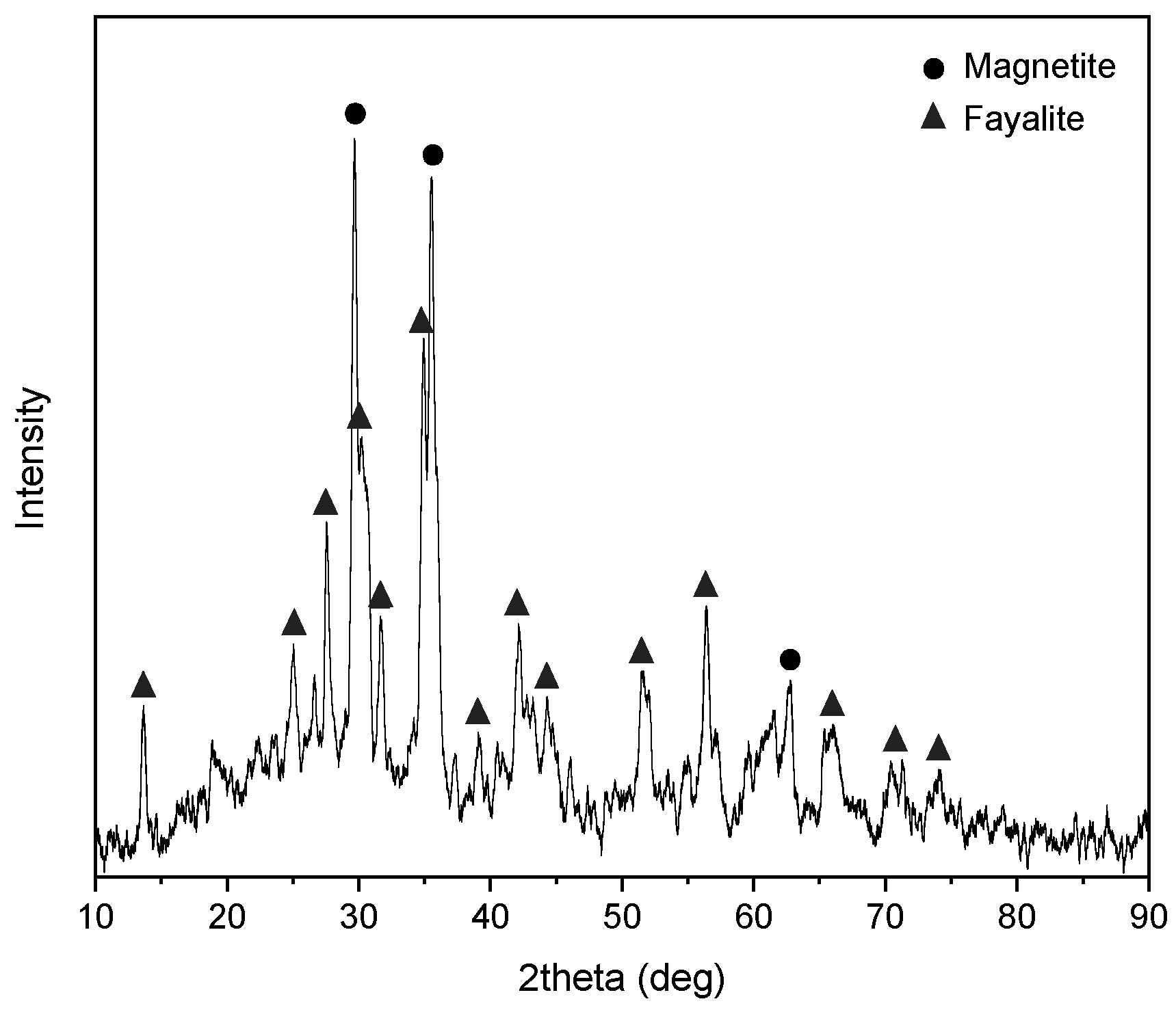
2.2.2. XRD Analysis of Copper Slag
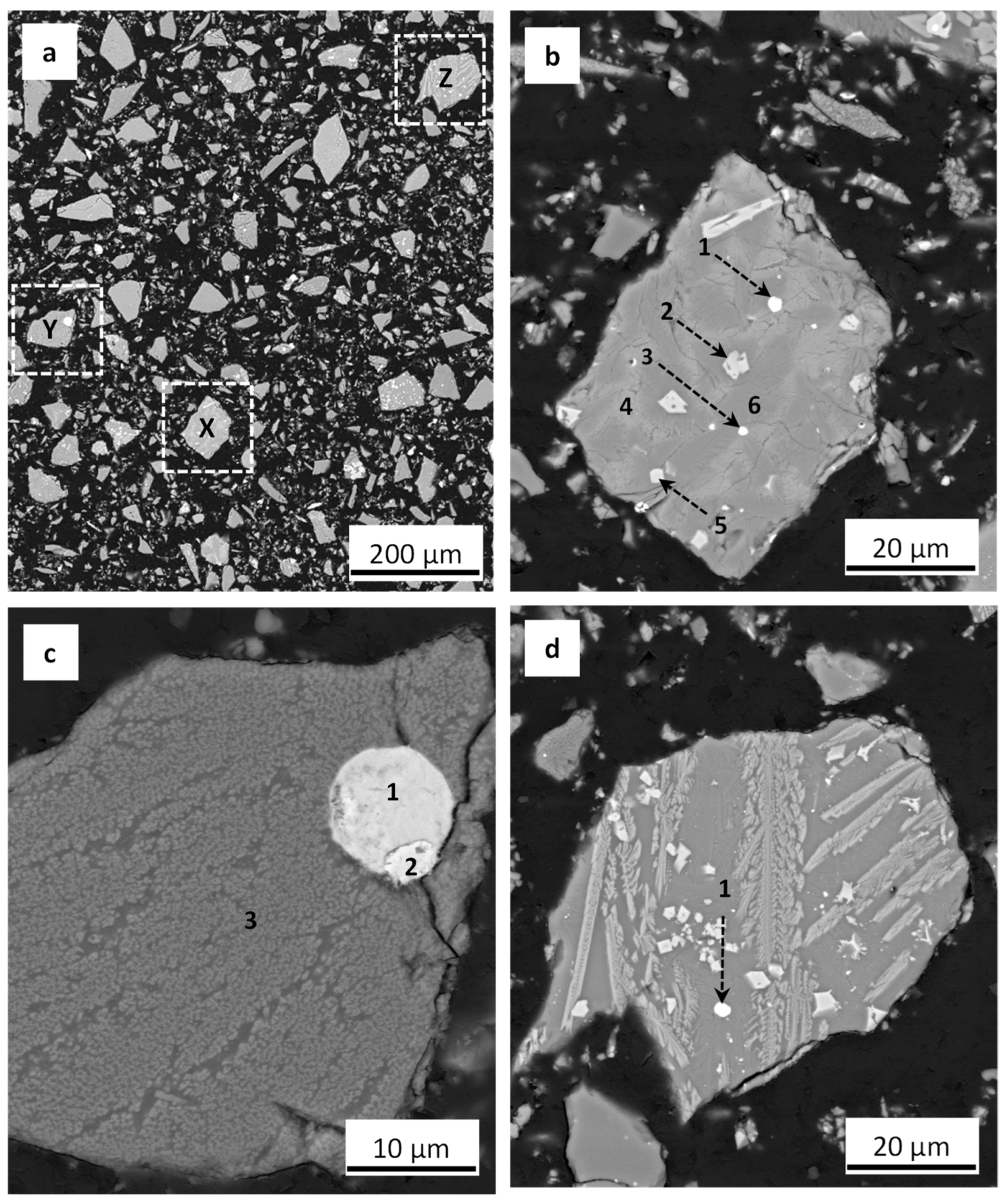
2.2.3. FESEM-EDS Analyses of Copper Slag and Roasting Product
2.3. Treatment Methodology
2.3.1. Carbocatalytic Reduction of Copper Slag
2.3.2. Sulphuric Acid Leaching of the Feed Sample and Reduction Product
3. Results and Discussions
3.1. Morphological Characteristics and Phase Composition of Copper Slag
3.2. Embedment of Metals in Mineral Phases in the Copper Slag
3.2.1. Copper Phase Occurrence and Metal Embedment in Copper Slag
3.2.2. Magnetite Phase Occurrence and Metal Embedment in Copper Slag
3.2.3. Silicate Phase Occurrence and Metal Embedment in Copper Slag
3.2.4. Distribution of Cu, Co, and Fe in the Main Phases of Copper Slag
3.3. Evaluation of Processing Technology Parameters
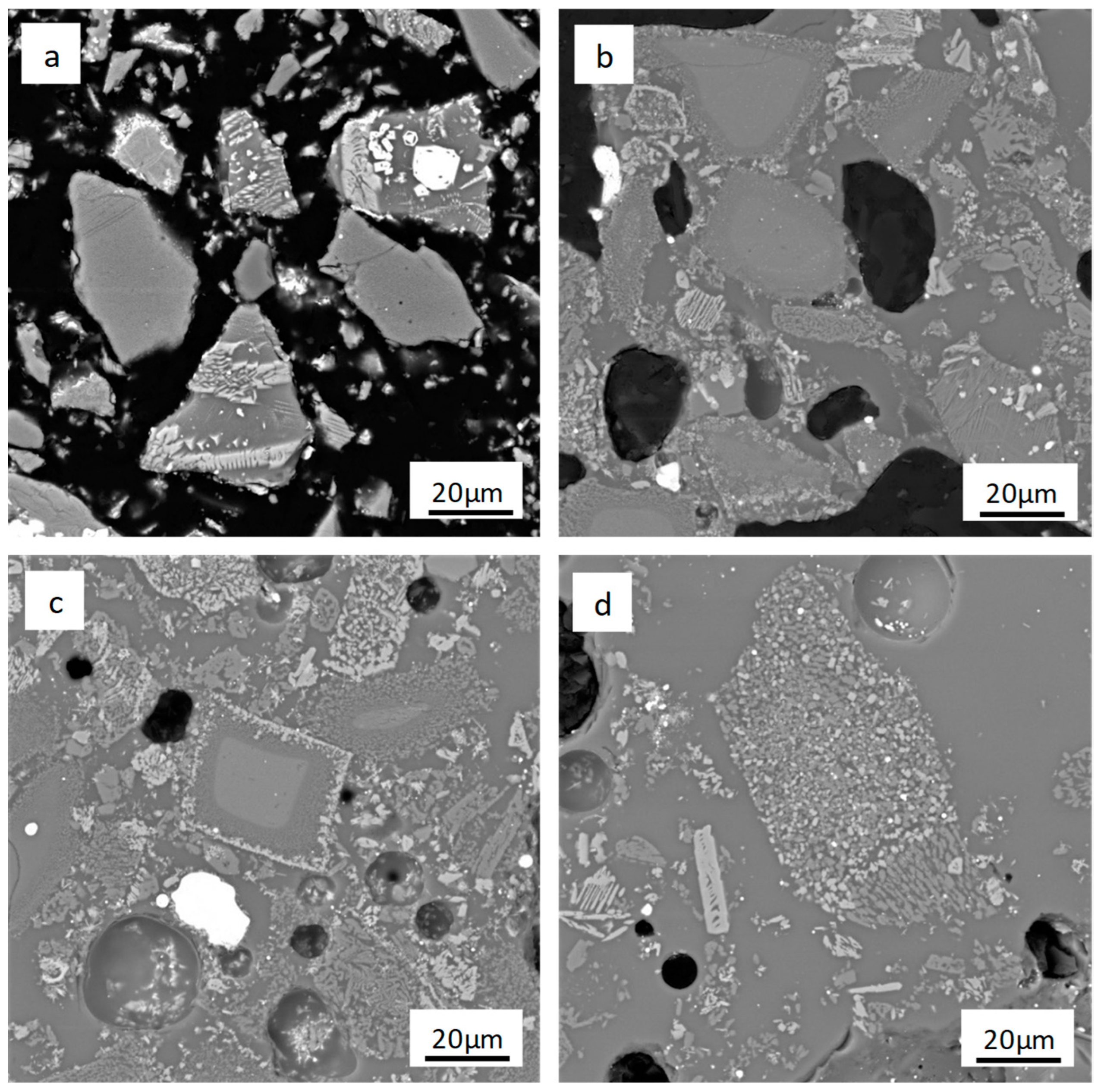
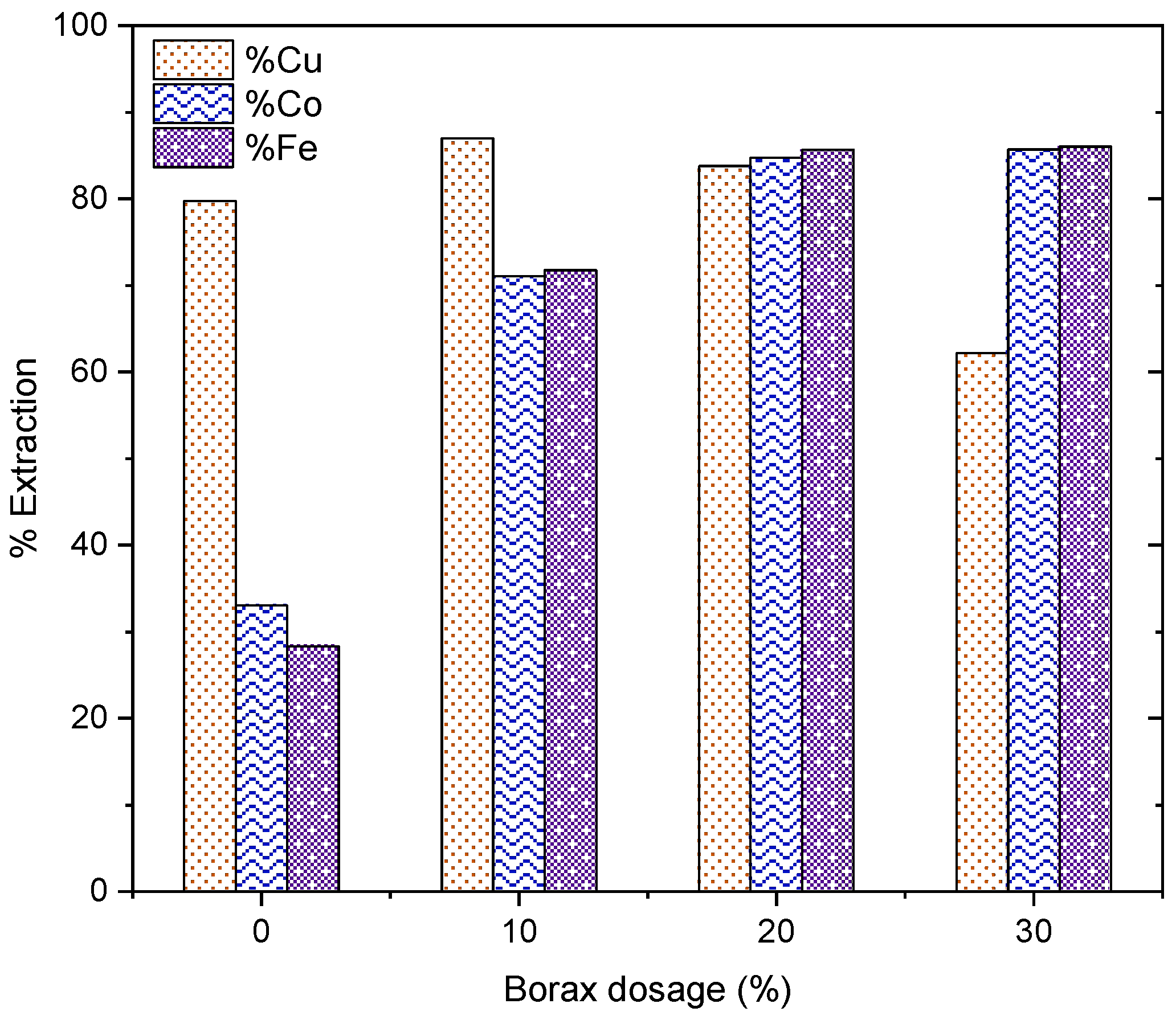
3.3.1. Effect of Borax Addition on Morphological Transformation
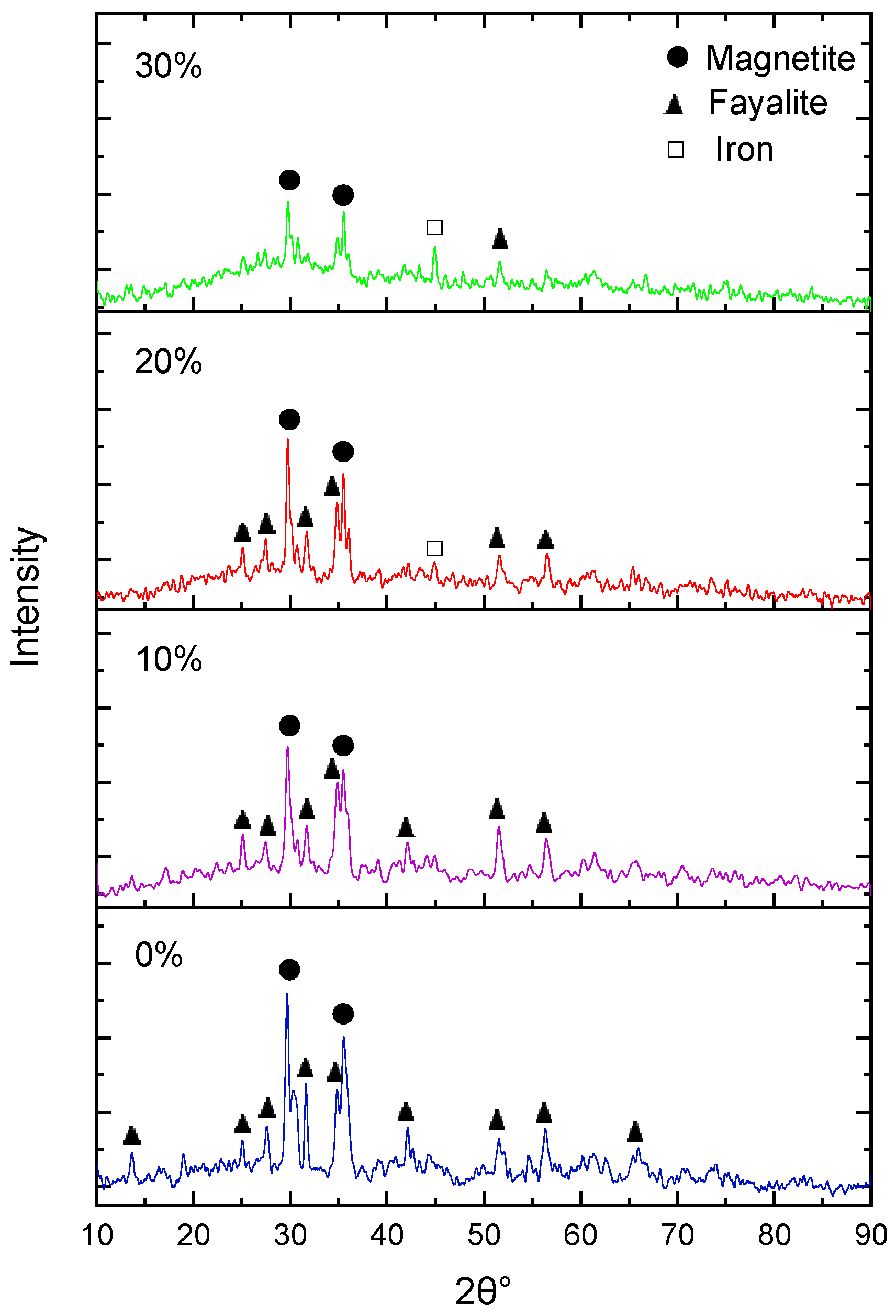
3.3.2. Effect of Borax Addition on Phase Transformation
3.3.3. Effect of Reduction Temperature on Phase Transformation
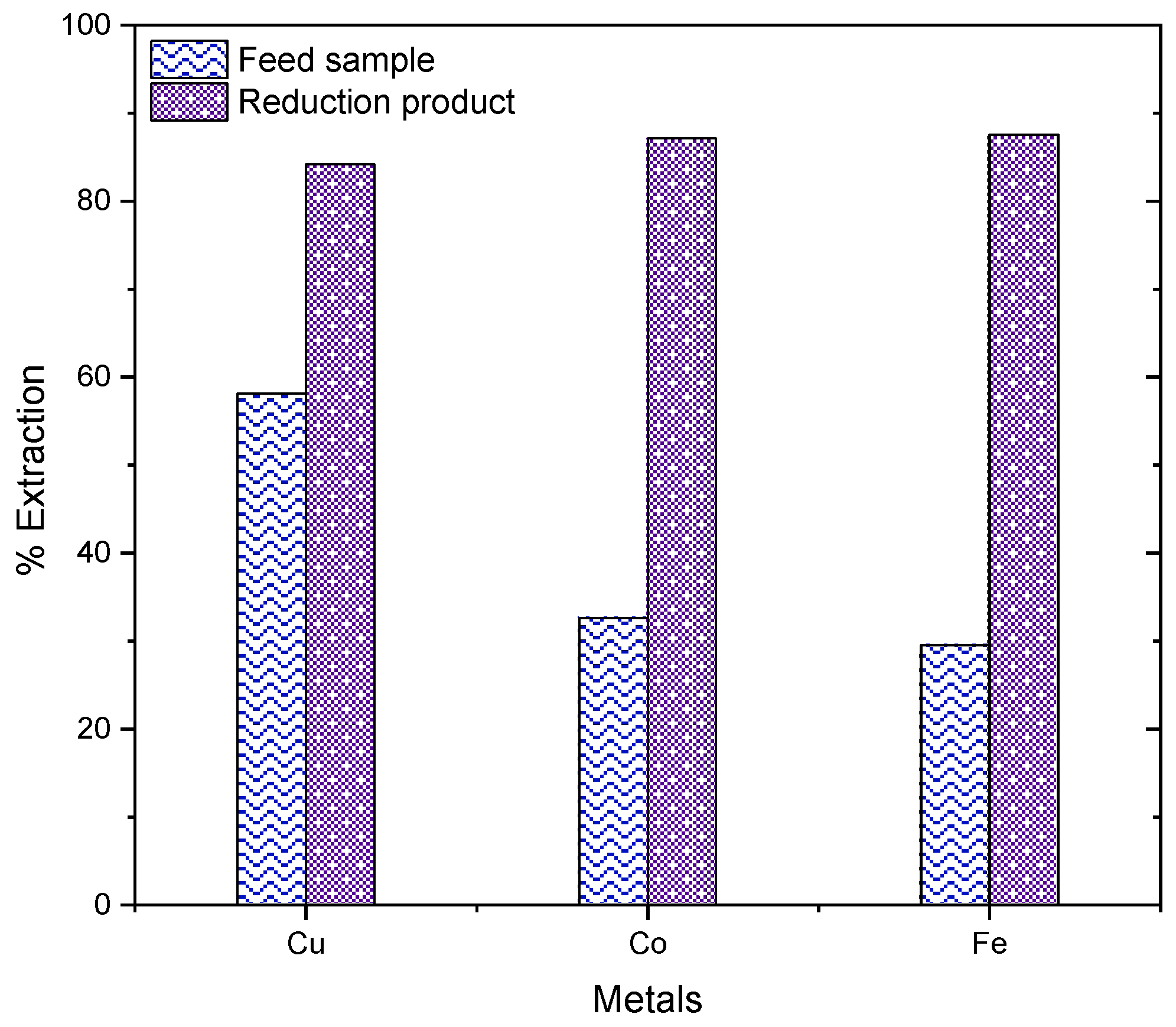
3.3.4. Comparison of Sulphuric Acid Leaching of the Feed Sample and Reduction Product
3.3.5. Effect of Carbocatalytic Reduction on Metal Extraction by Leaching
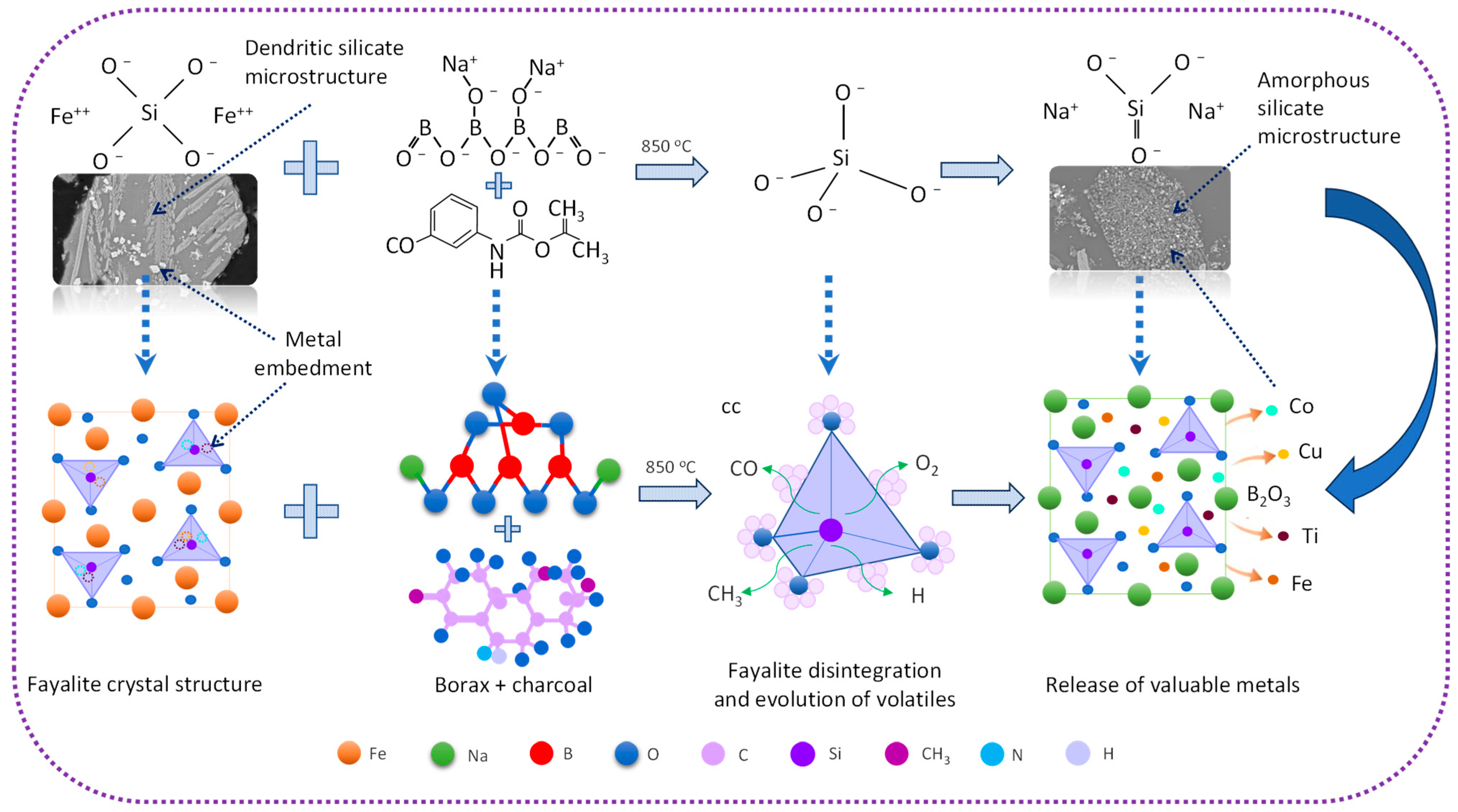
4. Theoretical Basis for the Development of Carbocatalytic Reduction Technology for the Processing of Copper Slag
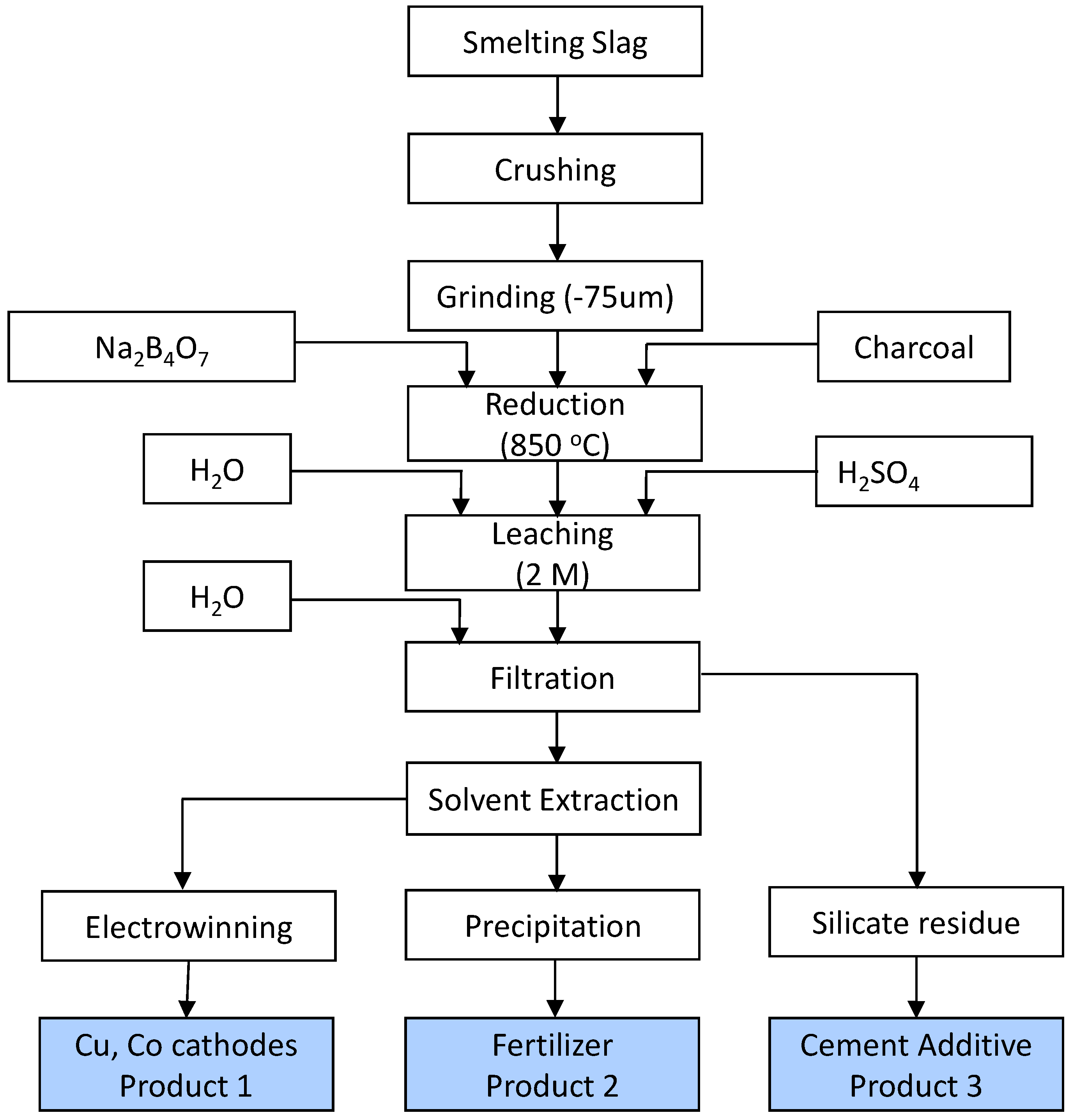
5. A Conceptual Flowsheet for Processing of Copper Smelting Slag
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review—Part I. Miner. Eng. 2022, 180, 107474. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slags: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review—Part II. Miner. Eng. 2021, 172, 107151. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhao, Z.W.; Lin, Z.; Liang, Y.J.; Kang, L.; Peng, B. Interaction mechanism between arsenate and fayalite-type copper slag at high temperatures. Trans. Nonferrous Met. Soc. China 2022, 32, 709–720. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, N.; Shen, W.; Wei, X.; Li, F.; Ma, W.; Mao, F.; Wu, P. Relationship between mineralogical phase and bound heavy metals in copper smelting slags. Resour. Conserv. Recycl. 2022, 178, 106098. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Yotamu, S.; Hara, Y.S.; Siame, J.; Nikoloski, A.N. Carbothermic Reduction of Copper Slag in the Presence of Borax. Miner. Process. Extr. Metall. Rev. 2023, 1–12. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Li, Y.; Perederity, L.; Papangelakis, V.G. Cleaning of waste smelter slag and recovery of valuable metals by presure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.; Gao, W.; Lu, M. Recovery of Iron from Copper Slag Using Coal-Based Direct Reduction: Reduction Characteristics and Kinetics. Minerals 2020, 10, 973. [Google Scholar] [CrossRef]
- Lan, C.; Zhang, S.; Liu, R.; Lyu, Q.; Yan, G.; Wang, B. Thermodynamic and kinetic behaviours of copper slag carbothermal reduction process. Ironmak. Steelmak. 2022, 50, 123–133. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, F.; Jiang, T.; Chen, F.; Wang, S.; Qiu, G. Effects of Borax on the Reduction of Pre-oxidized Panzhihua Ilmenite. JOM 2017, 70, 2018. [Google Scholar]
- Gorai, B.; Jana, R.K. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Vítková, M.; Ettler, V.; Johan, Z.; Kribek, B.; Sebek, O.; Mihaljevic, M. Primary and secondary phases in copper-cobalt smelting slags from the Copperbelt Province. Zamb. Min. Mag. 2010, 74, 581–600. [Google Scholar] [CrossRef]
- Wu, L.; Wang, H.; Dong, K. Effect of Sulfur Content on Copper Recovery in the Reduction Smelting Process. Metals 2022, 12, 857. [Google Scholar] [CrossRef]
- Mackwell, S.J. Oxidation Kinetics of Fayalite (Fe2SiO4). Phys. Chem. Miner. 1992, 19, 220–228. [Google Scholar] [CrossRef]
- Đorđević, T.; Tasev, G.; Aicher, C.; Potysz, A.; Nagl, P.; Lengauer, C.L.; Pędziwiatr, A.; Serafimovski, T.; Boev, I.; Boev, B. Mineralogy and environmental stability of metallurgical slags from the Euronickel smelter, Vozarci, North Macedonia. Appl. Geochem. 2024, 170, 106068. [Google Scholar] [CrossRef]
- Hu, J.H.; Wang, H.; Zhao, L.M.; Li, L.; Liu, H.L. Characterization of copper slag from impoverishment. J. Saf. Environ. 2011, 11, 90–93. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Peng, N.; Peng, B.; Liang, Y.; Qu, S.; Dong, Z.; Zeng, W. Copper behavior and fayalite microstructure changes influenced by Cu2O dissolution. JOM 2019, 71, 2891–2898. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Effect of Iron Phase Evolution on Copper Separation from Slag Via Coal-Based Reduction. Metall. Mater. Trans. B 2018, 49, 3086–3096. [Google Scholar] [CrossRef]
- Hannon, A.C.; Vaishnav, S.; Alderman, O.L.G.; Bingham, P.A. The structure of sodium silicate glass from neutron diffraction and modeling of oxygen-oxygen correlations. J. Am. Ceram. Soc. 2021, 104, 6155–6171. [Google Scholar] [CrossRef]
- Shi, G.; Liao, Y.; Su, B.; Zhang, Y.; Wang, W.; Xi, J. Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid. Sep. Purif. Technol. 2020, 241, 116699. [Google Scholar] [CrossRef]
- Kobylin, P.; Furta, L.; Vilaev, D. HSC Chemistry 10; Equilibrium Module; Metso Outotec: Espoo, Finland, 2021. [Google Scholar]


| Cu | Co | Fe | Al | Mg | Mn | Ca | Pb | Zn | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 1.04 | 0.79 | 25.63 | 11.05 | 3.50 | 0.66 | 7.15 | 0.03 | 0.06 | 0.05 |
| Mineral Phase | Point | Co | Cu | S | Fe | Si | O | Ca | Al | Mg | Ti | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper matte | 1 | 0.21 | 65.57 | 18.58 | 2.98 | 0.47 | 0.72 | 0.14 | ||||
| Magnetite | 2 | 1.36 | 58.26 | 0.24 | 21.75 | 0.24 | 1.81 | 0.35 | 0.97 | |||
| Silicate | 3 | 4.42 | 15.55 | 22.36 | 37.64 | 9.5 | 4.56 | 2.16 | 0.22 | 2.53 |
| Mineral Phase | Point | Co | Cu | S | Fe | Si | O | Ca | Al | Mg | Ti | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper matte | 1 | 0.38 | 57.56 | 20.8 | 11.01 | 2.34 | 4.76 | 0.6 | 0.64 | 0.46 | ||
| Magnetite | 2 | 1.85 | 51.33 | 3.94 | 24.87 | 1.43 | 2.49 | 0.34 | 1.14 | 1.08 | ||
| Silicate | 3 | 1.3 | 5.08 | 2.04 | 24.91 | 14.07 | 24.81 | 4.71 | 2.29 | 0.76 | 0.12 | 1.49 |
| Silicate | 4 | 1.25 | 31.11 | 16.29 | 28.37 | 2.51 | 3.9 | 0.39 | 0.14 | 3.52 | ||
| Silicate | 5 | 1.44 | 25.24 | 12.26 | 25.9 | 6 | 2.07 | 1.8 | 0.43 | 0.91 | ||
| Silicate | 6 | 1.32 | 20.9 | 19.44 | 31.46 | 7.98 | 3.43 | 2.41 | 0.16 | 1.18 |
| Mineral Phase | Point | Co | Cu | S | Fe | Si | O | Ca | Al | Mg | Ti | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper matte | 1 | 0.11 | 73.52 | 18.36 | 2.5 | 0.08 | 0.67 | |||||
| Copper metallic | 2 | 0.14 | 89.62 | 0.12 | 3.17 | 0.37 | 1.38 | 0.08 | ||||
| Silicate | 3 | 1.05 | 0.54 | 22.4 | 23.25 | 40.9 | 1.73 | 5.17 | 2.25 | 0.45 | 2.82 |
| Material Condition | Cu | Co | Fe |
|---|---|---|---|
| Feed | 1.04 | 0.79 | 25.63 |
| Leachate | 0.87 | 0.67 | 21.96 |
| Leach residue | 0.17 | 0.12 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiri, T.C.; Singh, P.; Nikoloski, A.N. Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals 2024, 14, 1119. https://doi.org/10.3390/met14101119
Phiri TC, Singh P, Nikoloski AN. Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals. 2024; 14(10):1119. https://doi.org/10.3390/met14101119
Chicago/Turabian StylePhiri, Tina Chanda, Pritam Singh, and Aleksandar N. Nikoloski. 2024. "Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt" Metals 14, no. 10: 1119. https://doi.org/10.3390/met14101119
APA StylePhiri, T. C., Singh, P., & Nikoloski, A. N. (2024). Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals, 14(10), 1119. https://doi.org/10.3390/met14101119






