Abstract
This study investigates the corrosion products present on TiSi, AlTi, and WTi coatings deposited onto Ti6Al4V titanium alloy substrates using the RF sputtering PVD technique. Following deposition, the coatings underwent exposure to a temperature of 600 °C for 100 h. The corroded surfaces were meticulously characterized to identify the resultant corrosion products. Utilizing scanning electron microscopy (SEM), X-ray diffraction, optical profilometry, and XPS spectroscopy, the coatings were comprehensively examined. Furthermore, Raman mapping with multivariate analysis was employed to determine the spatial distribution of oxides in the coating post-high-temperature corrosion. Additionally, XPS spectroscopy unveiled the presence of species undetected by Raman spectroscopy, such as silicon oxide SiO2, aluminum oxide Al2O3, and tungsten oxide WO2, in oxidation studies on TiSi, AlTi, and WTi coatings, corroborated by XRD analysis. The results allowed us to propose the corrosion mechanisms of these coatings and to determine that the TiSi coating exhibits a superior high-temperature corrosion response compared to the AlTi and WTi coatings. The AlTi coating experiences aluminum depletion, whereas the WTi coating shows accumulations of tungsten oxides that resemble pitting.
1. Introduction
Titanium and its alloys have become indispensable in a multitude of engineering applications, where the demand for materials with superior mechanical and chemical attributes is paramount. Renowned for their low density, remarkable mechanical strength, and exceptional corrosion resistance, titanium alloys have spurred extensive research efforts aimed at enhancing their mechanical performance for structural purposes. Originating in aerospace structures, the appeal of titanium and its alloys has transcended into diverse industrial sectors, including chemical, automotive, and biomedical fields. The exceptional properties of titanium alloys have proven instrumental in advancing technological frontiers and fueling innovation across these industries [1,2,3].
The industrial applicability of titanium is impeded by severe oxidation challenges, despite its exceptional mechanical properties. Titanium exhibits a propensity to form a diverse array of oxides, including TiO2, Magnéli phases (TinO2n−1, n ≥ 4), Ti3O5, Ti2O3, TiO, Ti2O, Ti3O, and others [4,5], with TiO2 being the most encountered, in phases such as anatase, rutile, and brookite [6]. Elevated temperatures above 500–520 °C prompt titanium to readily absorb oxygen, resulting in the formation of non-protective Rutilium (TiO2). This oxide layer facilitates oxygen diffusion, exacerbating oxidation rates and the development of fragile surface layers [7,8]. Non-stoichiometric TiO2 compounds exhibit structural defects such as oxygen vacancies and interstitial Ti cations, promoting rapid oxygen and metal ion diffusion. Increased TiO2 concentrations in surface layers render them more flexible and prone to defects [8], with a greater transformation of anatase into rutile leading to diminished corrosion resistance [9].
Oxidation effects occur predominantly on the surface of materials, and for this reason surface modification is a promising approach to improving the surface properties of titanium and its alloys [10]. Recent studies on the high-temperature behavior of titanium alloys have focused on the use of coatings deposited by the PVD technique. PVD processes provide the advantage of selecting the alloy constituents as binary or ternary compositions with controlled composition and high purity; they can also be deposited on different types of substrates [11]. The PVD technique has been used to produce thin TiSi [12,13], AlTi [14,15], and WTi [16,17] coatings and analyze their behavior when subjected to high temperatures. Research shows that the oxides formed from Si, Al, and W help to counteract the negative effects of titanium oxide by forming oxides that are stable and adherent on the coating surfaces, which causes an improvement in wear resistance and resistance to oxidation at high temperatures in these systems [18].
TiSi coatings obtained by laser alloying have shown attractive properties for industrial applications, such as improved resistance to oxidation and wear, high hardness, low relative density, and a high melting point, showing that laser alloying with Si on the surface of Ti or on titanium alloys was beneficial for substrate modification [19]. From the point of view of the stable formation of oxides on the surface of the TiSi coating, it has been inferred that titanium oxide TiO2 and silicon oxide SiO2 can form simultaneously on the exposed surface, in which this oxide (SiO2) could provide an improved corrosion resistance [20].
Aluminum is an important and effective alloying element for improving the high-temperature oxidation resistance of titanium and its alloys. At high temperature conditions, sufficient aluminum can be selectively oxidized into a stable, compact, and continuous Al2O3 aluminum oxide layer. This oxide can effectively delay the diffusion of titanium and oxygen and, therefore, improve the high temperature corrosion resistance for this type of material [21]. Dai manufactured AlTi coatings on Ti6Al4V titanium alloy fabricated by laser surface alloying. The produced coatings showed excellent oxidation resistance at 900 °C for 1000 h. [22]. In AlTi coatings, titanium oxide TiO2 and aluminum oxide Al2O3 are usually formed. The formation of other types of titanium and aluminum oxides would delay the generation of the Al2O3 protective layer, and the temperature, composition, and constituents of the high-temperature medium would affect the formation of the initial oxides and the subsequent formation of the protective layer [23]. An oxide layer with large proportions of TiO2 will have poor resistance to high-temperature corrosion; conversely, large proportions of Al2O3 oxide will offer adequate resistance to corrosion and wear [24].
WTi coatings present some notable properties, such as their electrical resistance, thermal stability, oxidation resistance, chemical stability, and good adhesion to the substrate, including a low friction coefficient and good wear resistance [25]. WTi coatings have been studied as anticorrosive protection [26]. The presence of titanium in the alloy reduces the accumulation of holes at the alloy/oxide interface, causing the modification of the microstructure of the surface oxide, improving corrosion resistance, and increasing the coating adhesion. Titanium also improves the diffusion barrier performance of tungsten due to its affinity for nitrogen and oxygen [27]. The diffusion barrier properties of the WTi coating depend principally on the concentration of titanium. The most frequently used stoichiometry is W:Ti (90:10%), where tungsten is used as a diffusion barrier because the atomic diffusivity of the metals in tungsten is low [27].
As mentioned, TiSi, AlTi, and WTi coatings produced by PVD can present improved corrosion resistances as a consequence of the effect of the alloy’s elements, which reduce the formation of non-protective titanium oxides. In this research, we wish to identify the mechanisms of high-temperature corrosion and the growth of oxides on the surface of the TiSi, AlTi, and WTi coatings produced by PVD on a Ti6Al4V substrate. Complementary surface analysis techniques are being applied, and they provide information about the predominant species and surface distribution of corrosion products.
2. Materials and Methods
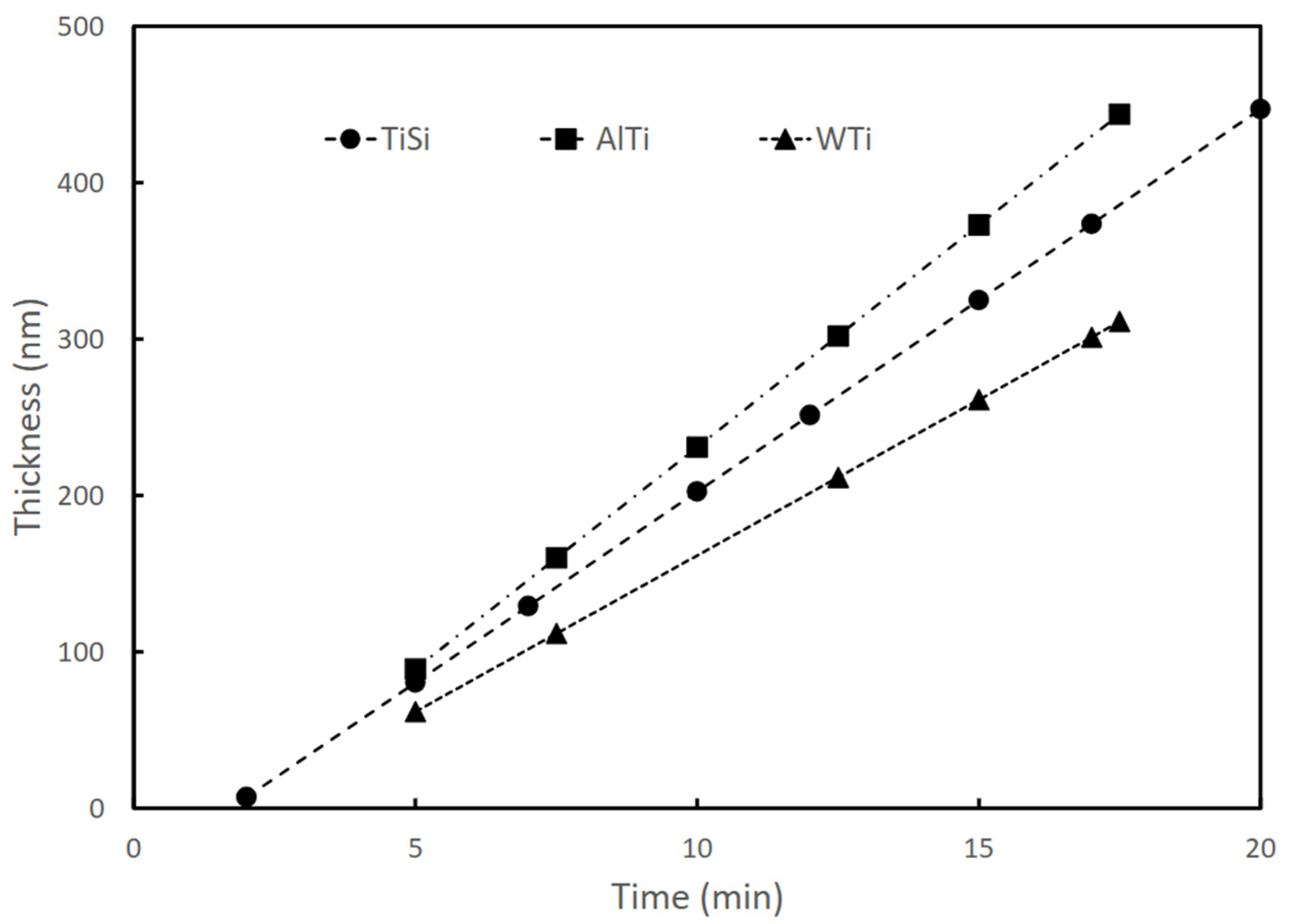
For the development of this research, samples of TiSi, TiAl, and TiW coatings deposited using an RF magnetron sputtering technique were used. The synthesis of the coatings was carried out using Alcatel type HS 2000 (Alcatel Vacuum Technology, Annecy, France) commercial equipment, following the procedure presented by Gordillo in [25]. The coatings were deposited on Titanium Aluminum Vanadium (Ti6Al4V) substrates (5.82 Al% by weight, 3.82% by weight of V, and balance of Ti). The Ti6Al4V substrates used had a size of 1.5 cm × 1.5 cm × 0.6 cm. Targets of Tungsten-titanium W:Ti (90:10%), Aluminum-Titanium Al:Ti (50:50%), and Titanium-Silicon Ti:Si (81:19%) with 99.99% purity were used. Table 1 presents the configuration parameters of the equipment for the coating’s deposition parameters. The thickness of the coatings was approximately 200 nm. The deposition rate was approximately 20 nm/min in all cases and remained constant during the sputtering process, as shown in Figure 1. The elemental composition (% by weight) was determined by energy dispersive X-ray spectroscopy (EDS) using an X-MAX 50/Oxford Instruments probe (Oxford Instruments, Abingdon, UK); the results are shown in Table 2, as reported in [25].

Table 1.
Summary of deposit conditions for coatings growth.
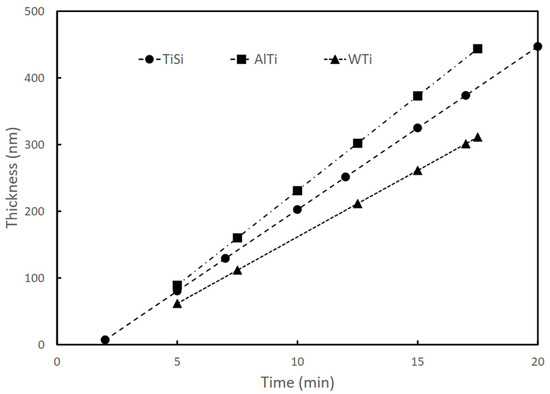
Figure 1.
Dependence of TiSi, AlTi, and WTi coating thicknesses on sputtering time (produced by RF magnetron sputtering).

Table 2.
The elemental composition (% by weight) of thin films analyzed using EDX (Energy Dispersive X-ray Spectroscopy).
The coated samples were subjected to an oxidation test in hot air at a temperature of 600 °C for a period of 100 h. The test was carried out in an EQUIFAR (Equifar, Bogotá Colombia) muffle furnace in a horizontal arrangement.
The samples were analyzed before and after the oxidation treatment. The morphology of the coatings was analyzed by scanning electron microscopy (SEM) (Tescan/Mira 3) (Tescan Mira 3, Tescan, Brno, The Czech Republic). The roughness in the samples was measured with a Bruker contour GT-K vertical scanning interferometer (Bruker Contour GTK, Bruker Nano Surfaces Division, Tucson, AZ, USA). Each sample underwent three distinct roughness measurements. Microstructural analyses were performed with X-pert Pro Panalytical X-ray (Panalytical, Almelo, The Netherlands) diffraction equipment in grazing beam mode at 5°, with the monochromatic line Kα of copper (1.540998 Å) working at 45 kV and 40 mA.
In order to carry out a semiquantitative analysis of the corrosion products on the surfaces of the coatings, Raman spectroscopy analyses were performed on the oxidized samples. A Horiba Scientific LabRAM HR Evolution Raman microscope (Horiba Jobin Yvon, Piscataway, NJ, USA) with an Ar+ laser with a wavelength of 514 nm was used. The microscope mapping tool was used to scan the surface in an array of 100 analysis points for the TiSi/AlTi coatings and 350 points for the WTi coating, in an a × b array. The Raman spectra obtained were in the range [50–1200 cm−1] (Vis NIR). In order to determine the presence of oxides on the surface of the coatings, a semiquantitative analysis methodology was applied. Each spectrum obtained was considered a response vector, and all spectra were organized in a matrix. Reference Raman spectra of the oxides TiO2 anatase phase, TiO2 rutilium phase, and tungsten oxide WO3 were obtained from the RRUFF database and organized in a reference matrix Y. The response and reference matrices were processed applying the analysis method multivariate MCR-ALS “multivariate resolution of alternating least squares curves” according to the methodology proposed in the literature [28] and using the software “MatLab R 2017b, MCR-ALS toolbox 2” (MathWorks, Inc., Natick, MA, USA). “Non-negativity” was used as an input restriction for concentrations and for constitutive spectra. From the analysis, a vector was obtained, the values of which are an indicator of the presence and concentration of each of the oxides detected by this technique. The values were plotted on a surface diagram that allows for identifying the presence of oxides.
Additionally, the oxidized coatings were analyzed using the X-ray photoelectron spectroscopy technique (XPS). Kratos Axis Ultra equipment (Kratos. Analytical, Manchester, UK) with an X-ray source of 1486.5 eV, 150 W, and a measurement resolution of 0.58 eV was used for the development of the tests. A scan of each of the samples was performed in survey mode, followed by localized scans in the W4f, Si2p, and Ti2p regions, corresponding to the locations of the tungsten, silicon, and titanium peaks. CasaXPS software (Casa Software Ltd., Wilmslow, Cheshire, UK) was used to analyze the spectra obtained.
3. Results and Discussion
3.1. Microstructural and Morphologycal Analyses before and after Corrossion Treatment
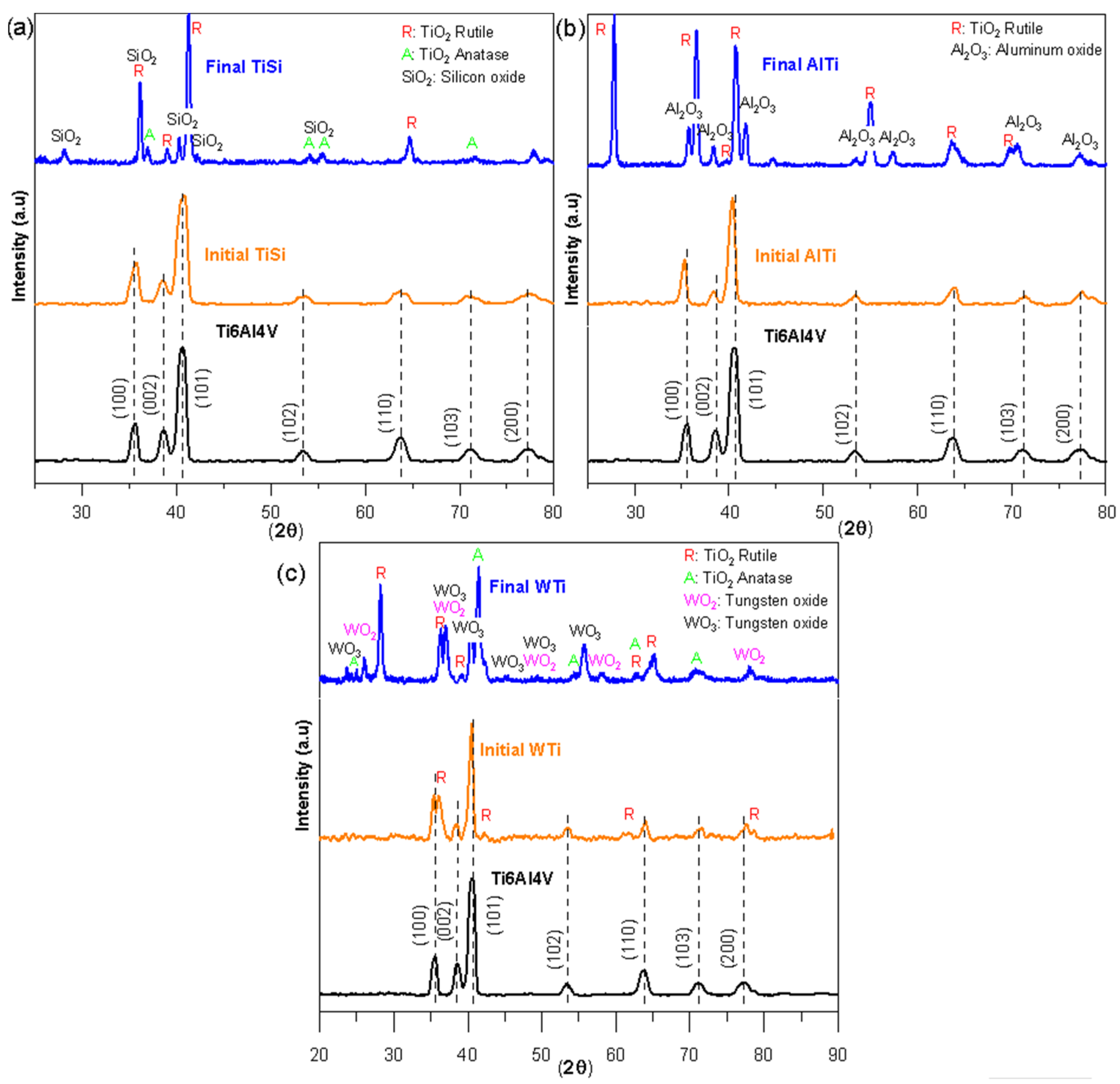
Figure 2 shows the diffractograms of the three coatings before and after the oxidation test; the diffractogram of the Ti6Al4V substrate is also included. The diffractograms of the TiSi (Figure 2a), AlTi (Figure 2b), and WTi (Figure 2c) coatings were identified, and the substrate peaks were identified as Ti6Al4V. However, in the case of the WTi coating, in addition to the characteristic peaks of the Ti6Al4V substrate, four peaks corresponding to the rutile phase of titanium oxide were identified. The analyses carried out on the oxidized samples allowed us to observe the presence of crystalline oxides. On the oxidized surface of the TiSi coating (Figure 2a), the presence of titanium oxides TiO2 rutile phase, anatase phase, and silicon oxide SiO2 was evidenced. For the oxidized coating AlTi (Figure 2b), the results showed high-intensity peaks for the rutile phase of titanium oxide TiO2 and a relatively high intensity for aluminum oxide Al2O3. The diffractograms for the oxidized WTi coating (Figure 2c) reveal the presence of the rutile phase TiO2 titanium oxide, the anatase phase of TiO2 titanium oxide, and oxides of tungsten WO2 and WO3.
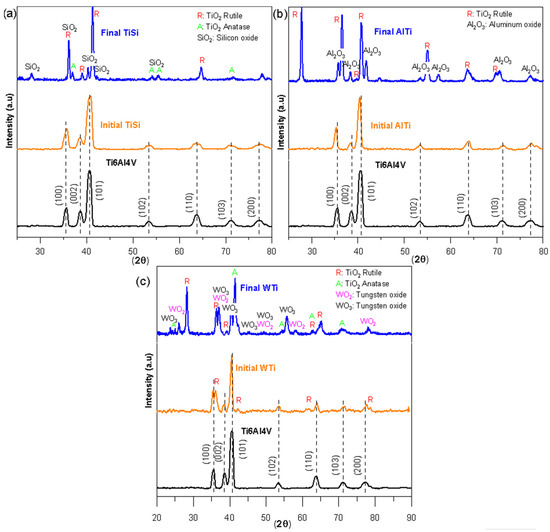
Figure 2.
X-ray diffraction patterns of thin films before and after the corrosion process: (a) TiSi coating, (b) AlTi coating, and (c) WTi coating.
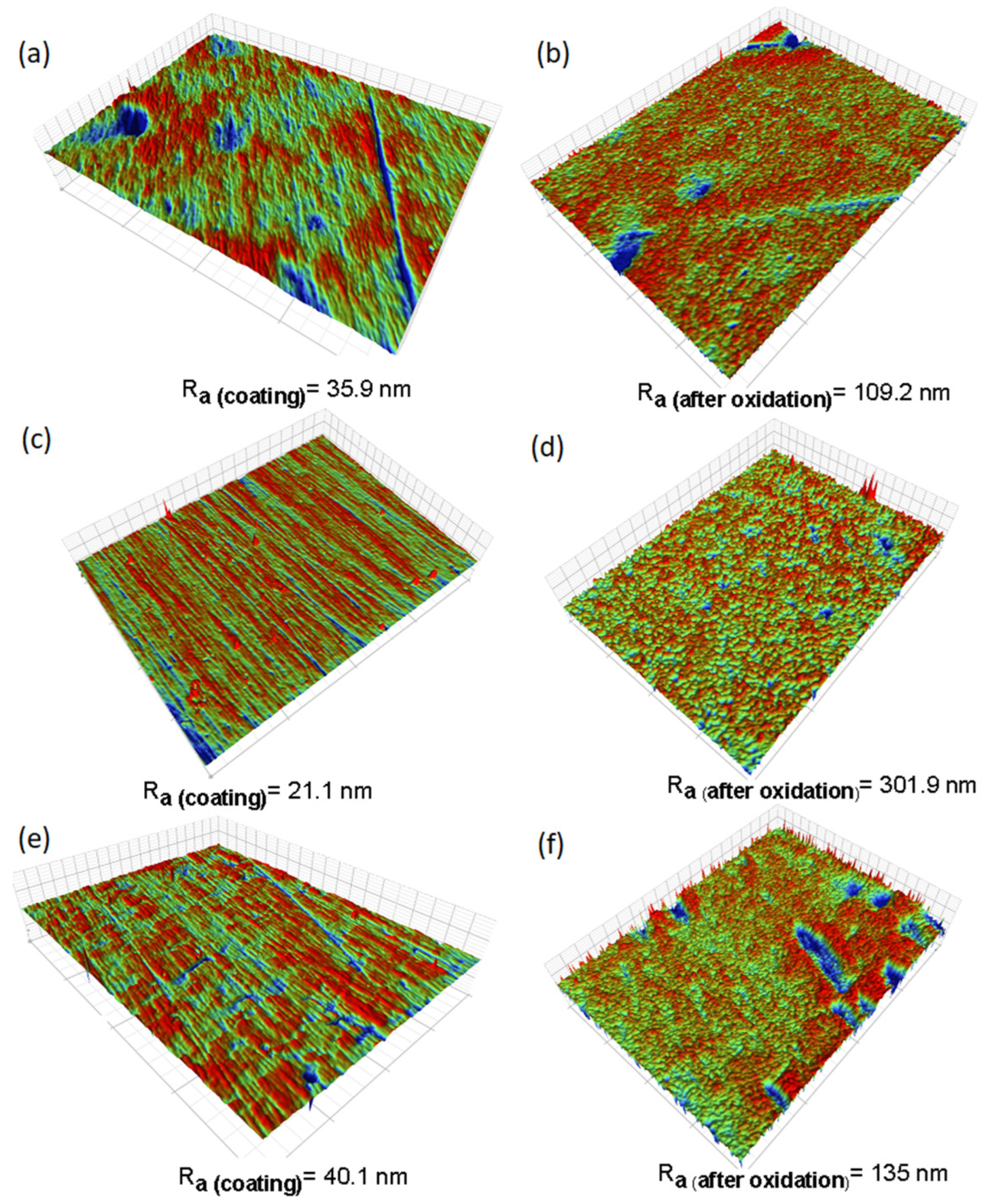
Figure 3 depicts profilometry images capturing the surface conditions before and after corrosion. These images illustrate noticeable changes in surface topography, accompanied by roughness evaluations. Table 3 shows the roughness results measured by interferometry on the samples. The TiSi and WTi coatings obtained initial roughnesses of 35.9 nm and 40.1 nm, respectively, which increased about three times after high-temperature corrosion treatment. In the case of the AlTi coating, the initial roughness was 21.1 nm and increased about 14 times at the end of the test. According to the above, we can conclude that the surface roughness of all the coatings increased drastically with the increase in temperature during the high-temperature corrosion process.
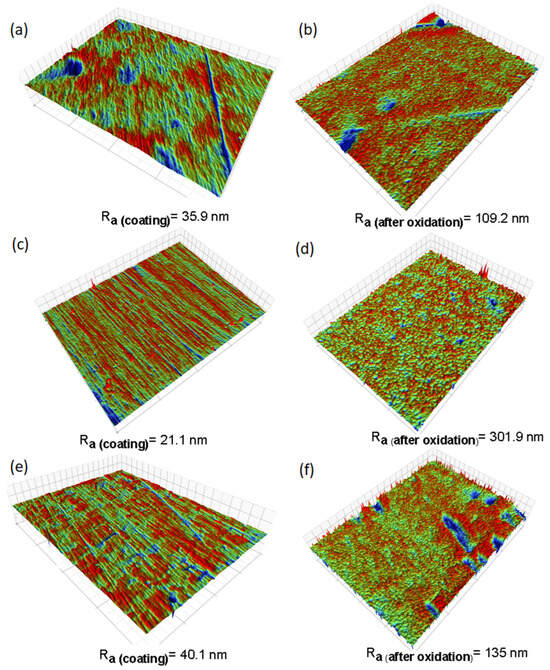
Figure 3.
Surface images of thin films before and after high-temperature corrosion treatment, captured via profilometry: (a) TiSi before corrosion, (b) TiSi after corrosion, (c) AlTi before corrosion, (d) AlTi after corrosion, (e) WTi before corrosion, and (f) WTi after corrosion.

Table 3.
Roughness of coatings TiSi, AlTi, and WTi before and after high-temperature corrosion treatment.
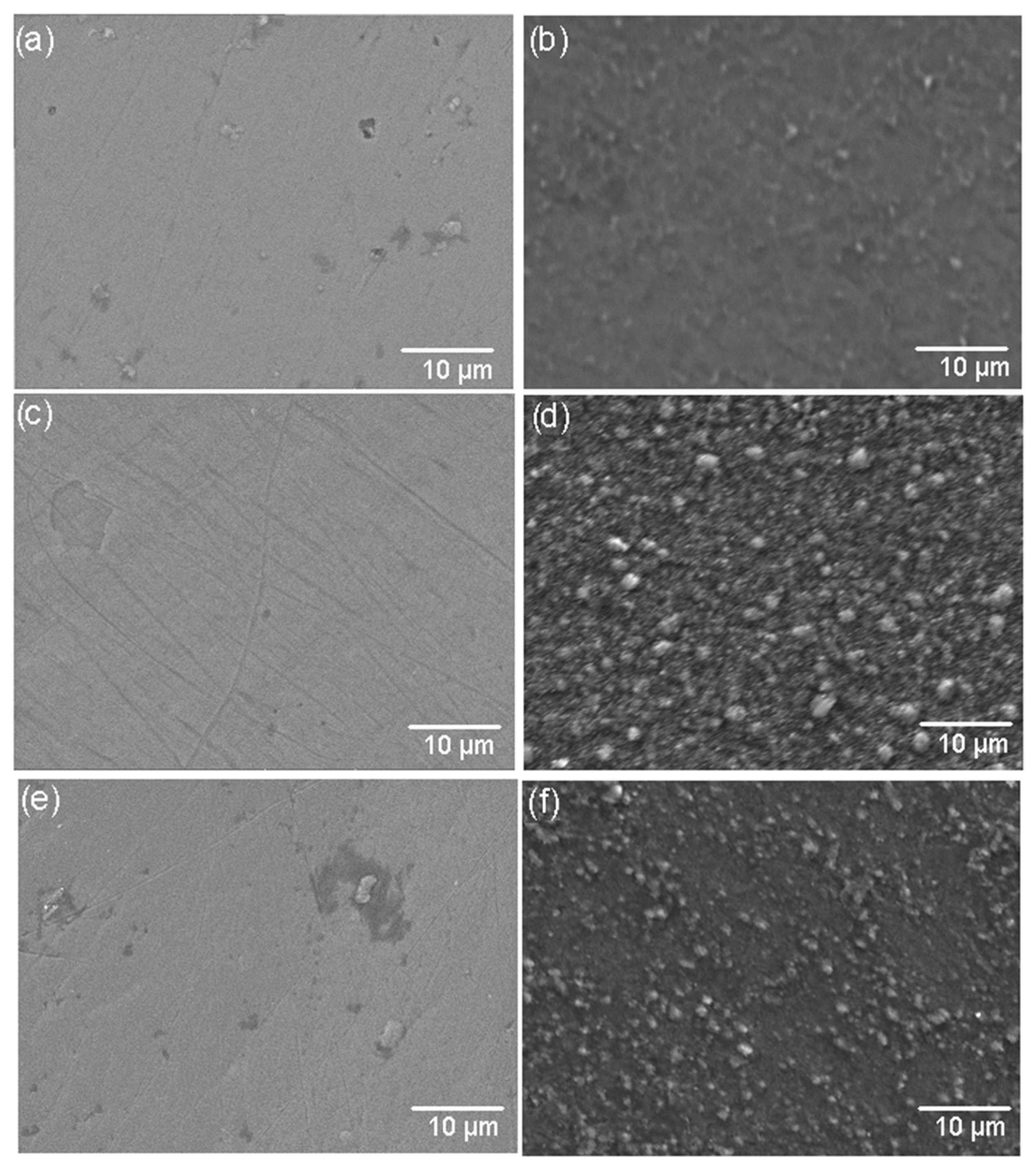
Figure 4 shows the surface morphology of the TiSi, AlTi, and WTi coatings before and after the high-temperature corrosion treatment. Micrographs (b), (d), and (f) of Figure 4 clearly show the presence of globular morphology structures on the surfaces of all the coatings, probably oxides of the coating components. In the AlTi coating in Figure 4d, it is possible to observe large oxide agglomerations extended over the entire area of the coating, which agree with the highest roughness values presented in Table 1, changing their appearance and initial roughness, as shown in Figure 4c/Table 1. For the TiW coating in Figure 4f, small agglomerated oxides can be seen, especially in islands on the surface of the coating (different from the initial morphology; see Figure 4e), which correspond to the intermediate values of roughness in Table 3. On the surface of the TiSi coating, a dense and fine structure of oxides different from the initial morphology shown in Figure 4a can be observed in Figure 4b, which agrees with the lowest roughness values presented in the corroded coatings and that, in principle, would provide us with an indication of their greater resistance to corrosion at high temperatures.
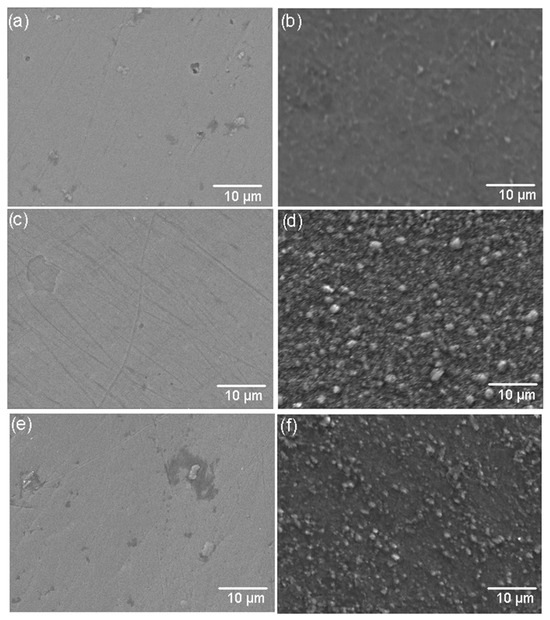
Figure 4.
SEM images of coating surfaces before and after corrosion treatment. (a) TiSi before corrosion, (b) TiSi after corrosion, (c) AlTi before corrosion, (d) AlTi after corrosion, (e) WTi before corrosion, and (f) WTi after corrosion.
3.2. Raman Analyses of Corrosion Products on Coatings
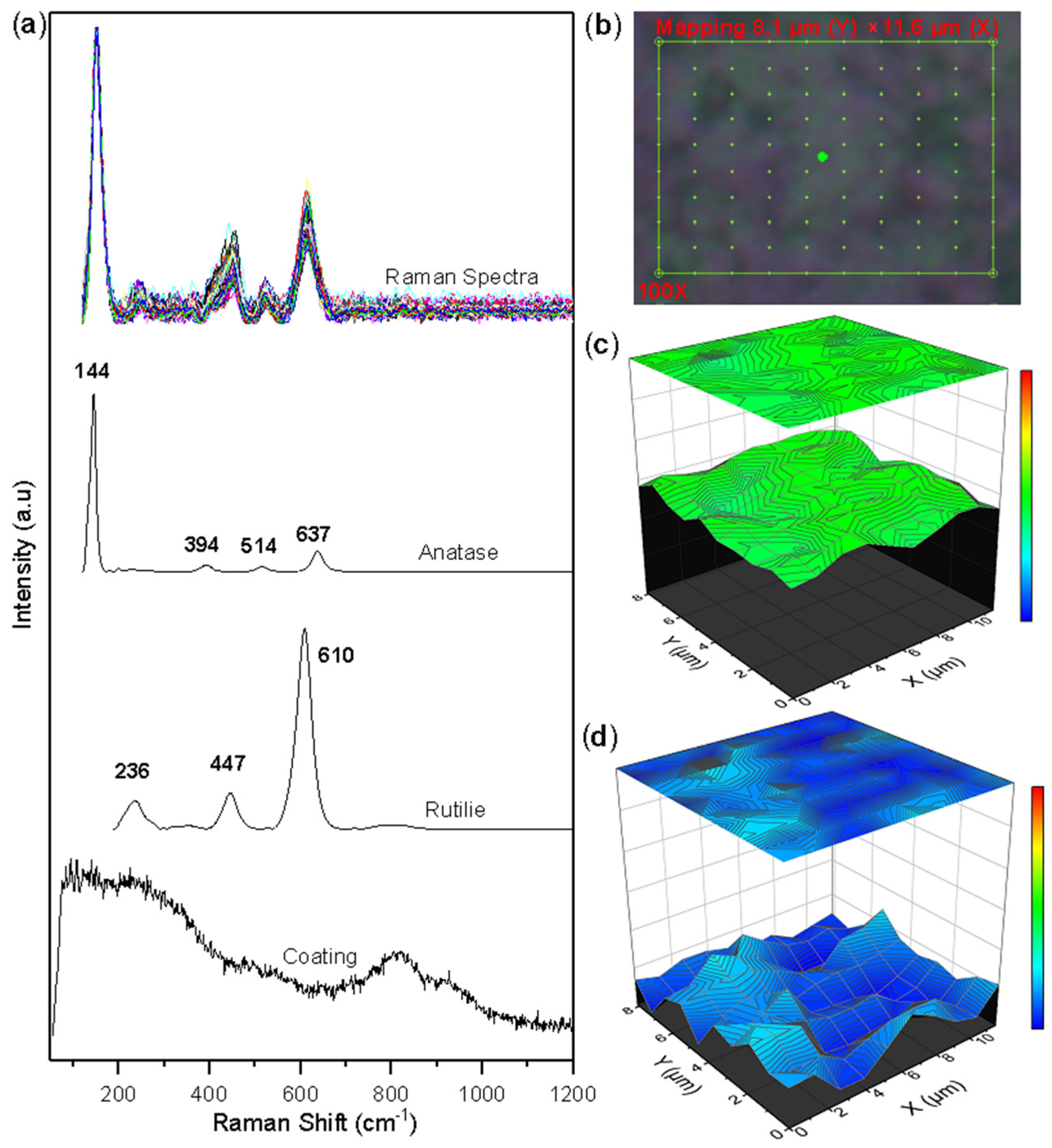
Figure 5 shows the Raman mapping, the collected spectra, and the semi-quantitative images built with the concentration vector of the corroded surface of the TiSi coating. Ordered point mapping by Raman spectroscopy was performed over a characteristic area on the 8.14 µm × 11.65 µm TiSi coating (see Figure 5b). The selected area consists of 100 points, each point with a Raman spectrum of the surface. Figure 5a allows us to observe that in the Raman spectra collected, two compounds are preferentially present, as evidenced in the characteristic peaks of the anatase and rutile phases of the TiO2 oxide. The multivariate processing method called MCR-ALS [14] was used to determine the concentration values of each of the Raman mapping points. The results are presented in Figure 5c,d. From the images, it can be seen how the anatase and rutilium phases are uniformly distributed throughout the selected area in the anatase phase and with small agglomerations of the rutilium phase. The greater presence of the anatase phase (green color on the color scale) over the rutilium phase (blue color) is confirmed.
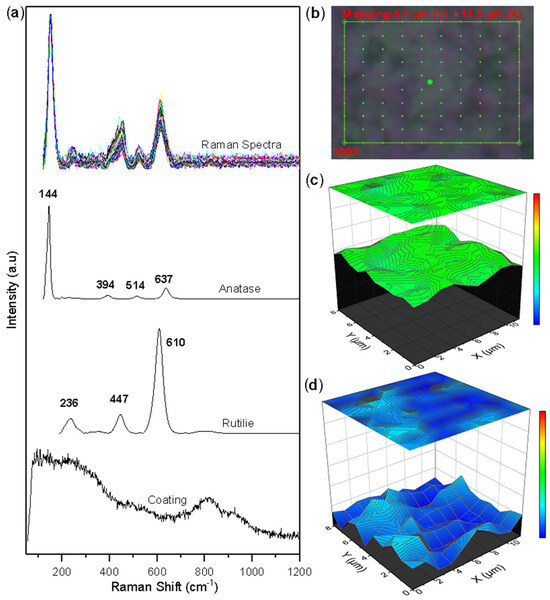
Figure 5.
Raman mapping of corroded TiSi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Anatase concentration; (d) Rutile concentration.
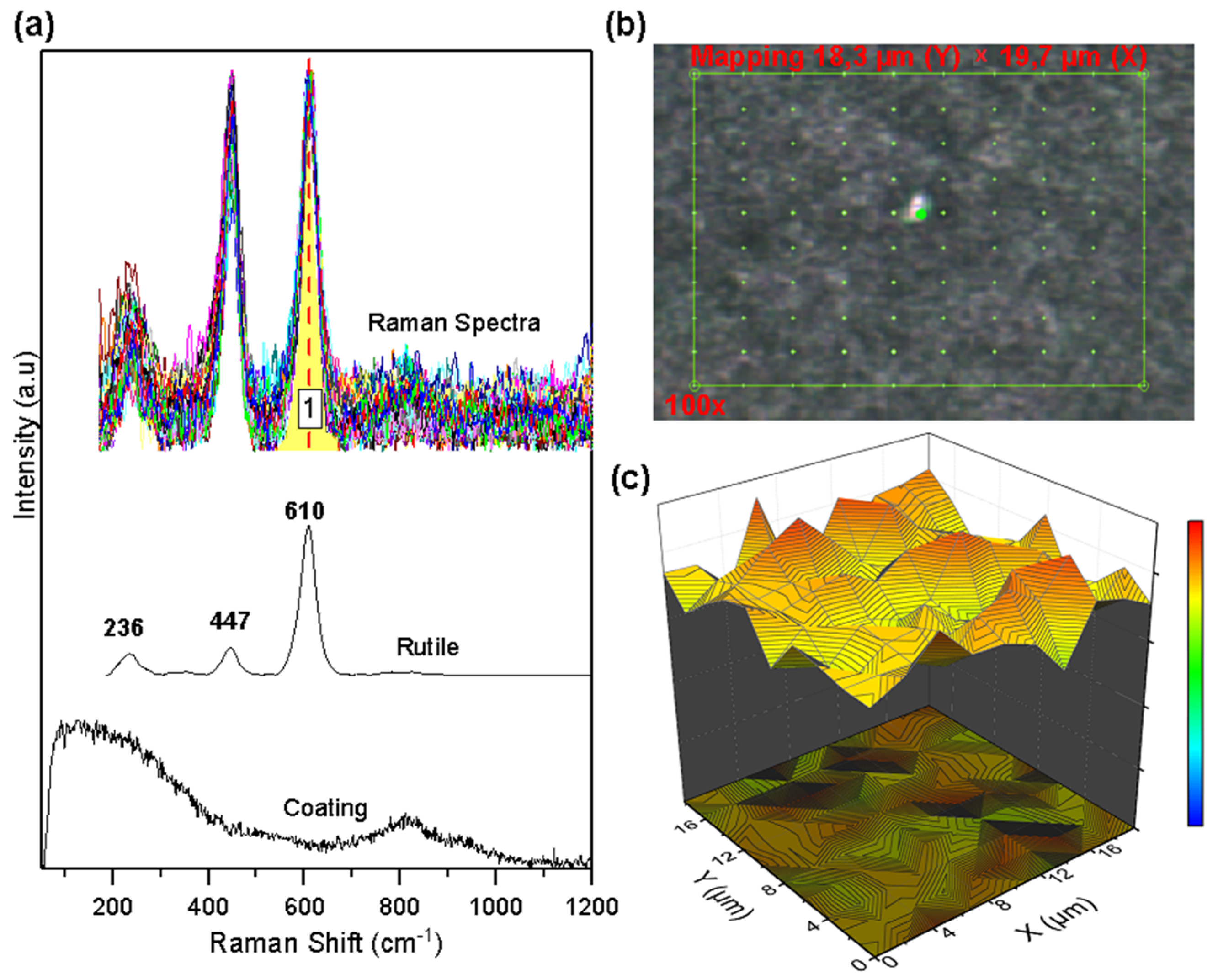
Figure 6 shows the Raman mapping, the collected spectra, and the semi-quantitative graph of the oxide concentration on the AlTi coating. Mapping was performed in an area of 18.38 µm × 19.71 µm point to point, as shown in Figure 6b. The analyzed area consists of 100 points, each point with a Raman spectrum of the surface. Figure 6a allows us to appreciate the rutile phase of TiO2 oxide as the only constituent of the collected spectra. The univariate processing method called band integral [29] was used to determine the spatial distribution of the oxide on the coating (qualitative image) through the band marked as 1 (area under the curve) in Figure 6a. This image shows the presence of the Rutile phase over the entire selected area, showing oxide groupings in some sectors but with a high distribution over the entire area selected for analysis. A semi-quantitative image was not generated since the MCR-ALS method requires that there be at least two different types of oxides in the analyzed area to carry out data processing.
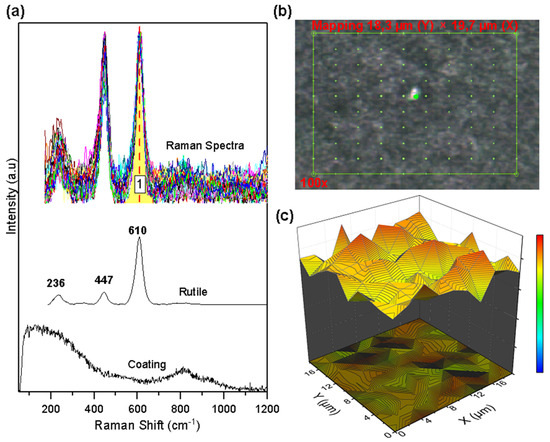
Figure 6.
Raman mapping of corroded AlTi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Rutile concentration.
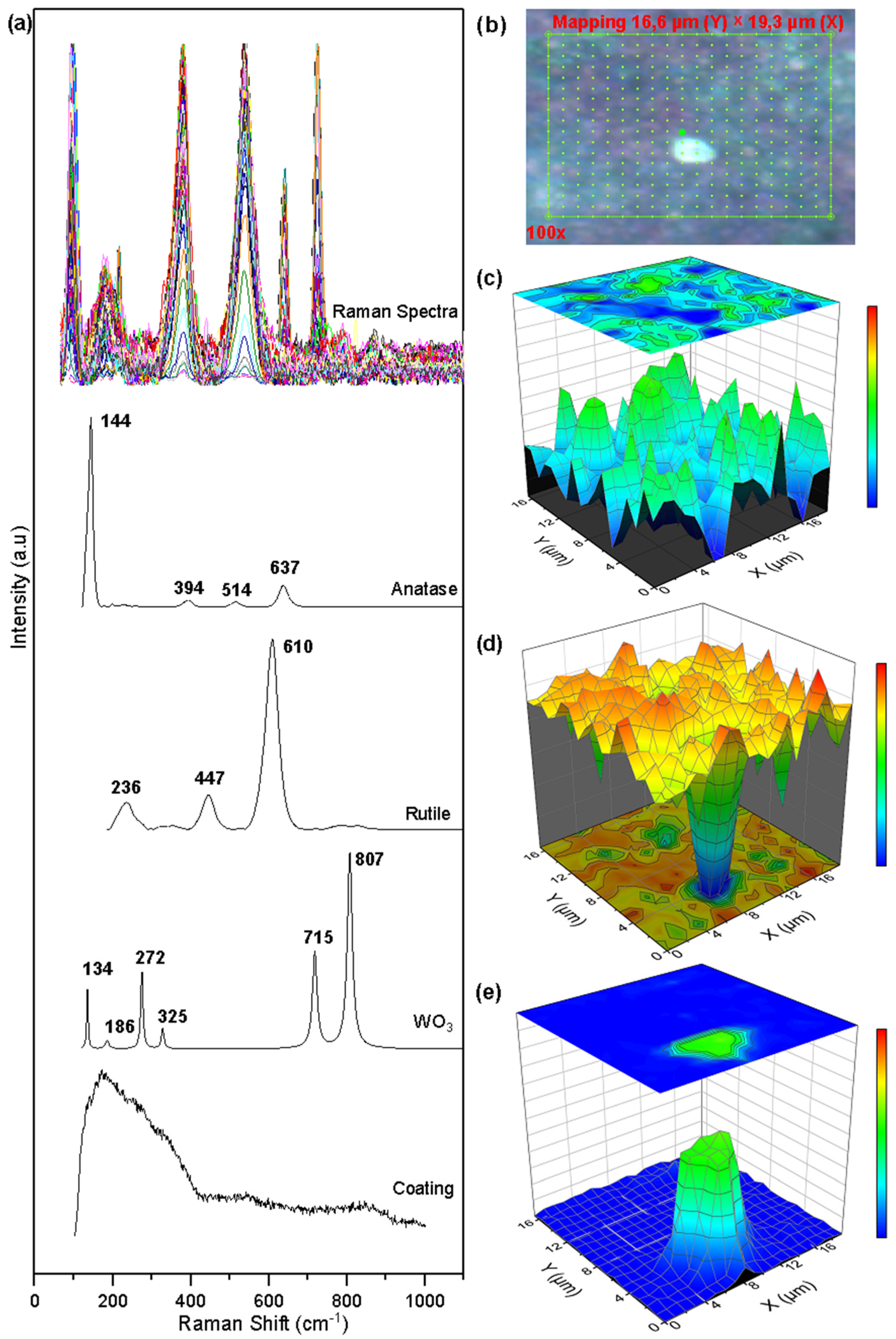
Figure 7 shows the collected spectra, the Raman mapping, and the semi-quantitative images built with the concentration vector of the corroded surface of the WTi coating. The Raman mapping was generated over an area of 16.67 µm × 19.30 µm made up of 360 points (see Figure 8b). In Figure 7a, the collected Raman spectra on the analyzed points and the characteristic peaks of the anatase phase, the rutile phase of titanium oxide TiO2, and tungsten oxide WO3 can be identified. As a method of processing the collected spectra, the MCR-ALS method [30] was again used as a means to determine the concentration values at each of the mapping points in the coating. The results of the multivariate analysis are presented in Figure 7c–e. Small agglomerations of the anatase and rutile phase TiO2 oxide can be seen, together with a uniform distribution of these over the entire analysis area. The images confirm the greater presence of the rutile phase (orange color on the color scale) over the anatase phase (blue color). The distribution of tungsten oxide WO3 is concentrated in a region of the surface of the selected area, altering the uniform distribution of titanium oxides and producing a pitting.
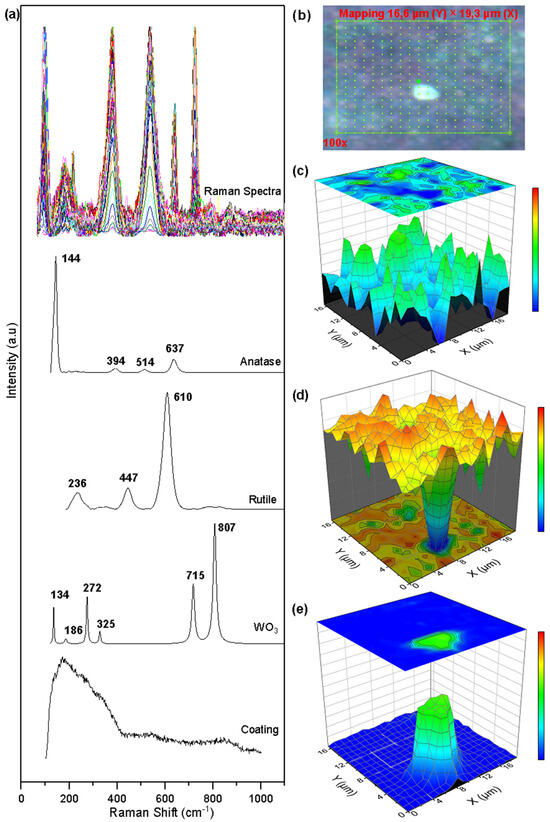
Figure 7.
Raman mapping of corroded WTi coatings: (a) Measured spectra and reference spectra; (b) Analyzed area; (c) Anatase concentration; (d) Rutile concentration; (e) Tungsten oxide.
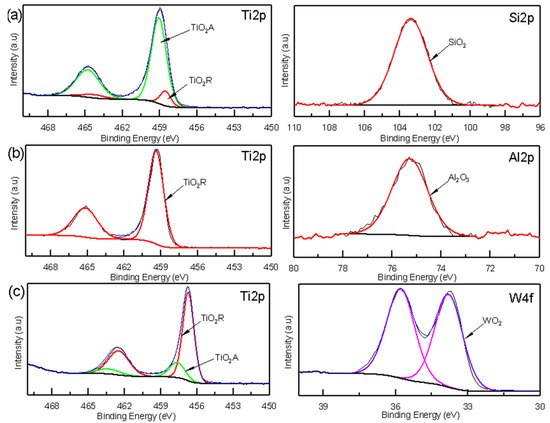
Figure 8.
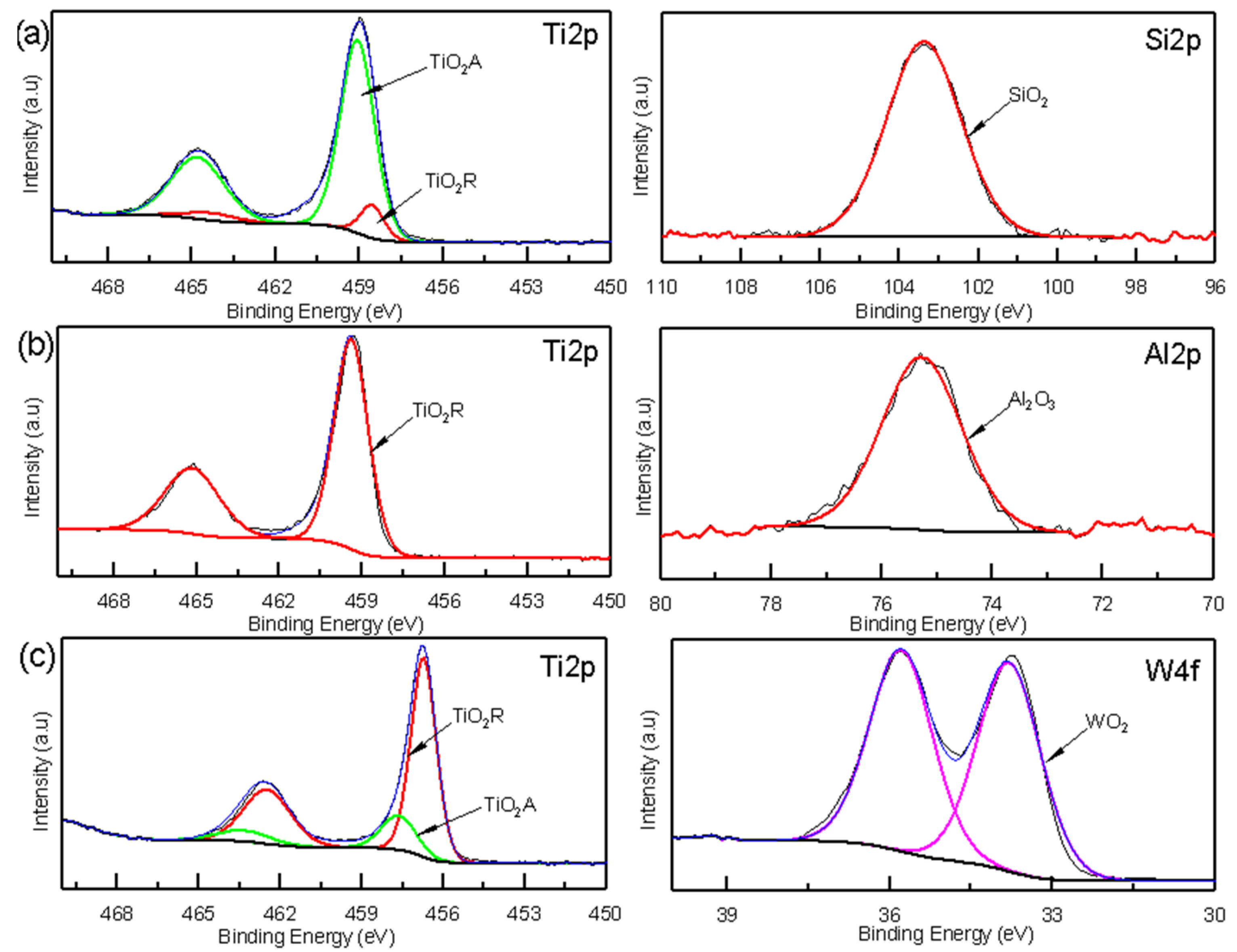
XPS spectra of the oxide coatings: (a) TiSi coating, (b) AlTi coating, and (c) WTi coating.
3.3. XPS Analyses of Corrosion Products on Coatings
XPS spectroscopy was used to obtain information about the corrosion products based on the chemical state of their components. The XPS analyses of the TiSi, AlTi, and WiTi coatings revealed the presence of oxide species not identified by Raman spectroscopy, such as silicon oxide SiO2, aluminum oxide Al2O3, and tungsten oxide WO2. The high-resolution XPS spectra of the Ti2p and Si2p states are presented in Figure 8a. The Ti2p can be adjusted in four peaks located at 458.55, 464.32 EV (corresponding to Ti2p3/2 and Ti2p1/2 Rutilium TiO2 phase), 459.11, and 464.83 EV (corresponding to Ti2p3/2 and Ti2p1/2 anatase TiO2 phase) [31]. The Si2p can be adjusted to a localized peak at 103.38 EV, corresponding to the silicon oxide SiO2 [32]. Figure 8b presents the high-resolution XPS spectra to detect the chemical states of Ti2p and Al2p. The binding energy of Ti2p3/2 is 458.55 and Ti2p1/2 is 464.32 EV, which indicates the presence of Rutile phase TiO2 [31] in the oxide film on the AlTi coating. It fit a peak to the Al2p state located at a binding energy of 75.3 Ev, corresponding to the aluminum oxide Al2O3 [33]. Figure 8c represents the high-resolution spectra for the W4f state, showing binding energies that were fitted to two peaks located at 33.74 and 35.83 Ev, which correspond to tungsten oxide WO2 [34]. For the Ti2p state, four peaks were adjusted to binding energy values of 458.51, 464.28 EV (corresponding to Ti2p3/2 and Ti2p1/2 Rutilio TiO2 phase), 459.11, and 464.83 EV (corresponding to Ti2p3/2 and Ti 2p1/2 phase anatase TiO2) [31].
3.4. Mechanism of Corrosion on Coatings
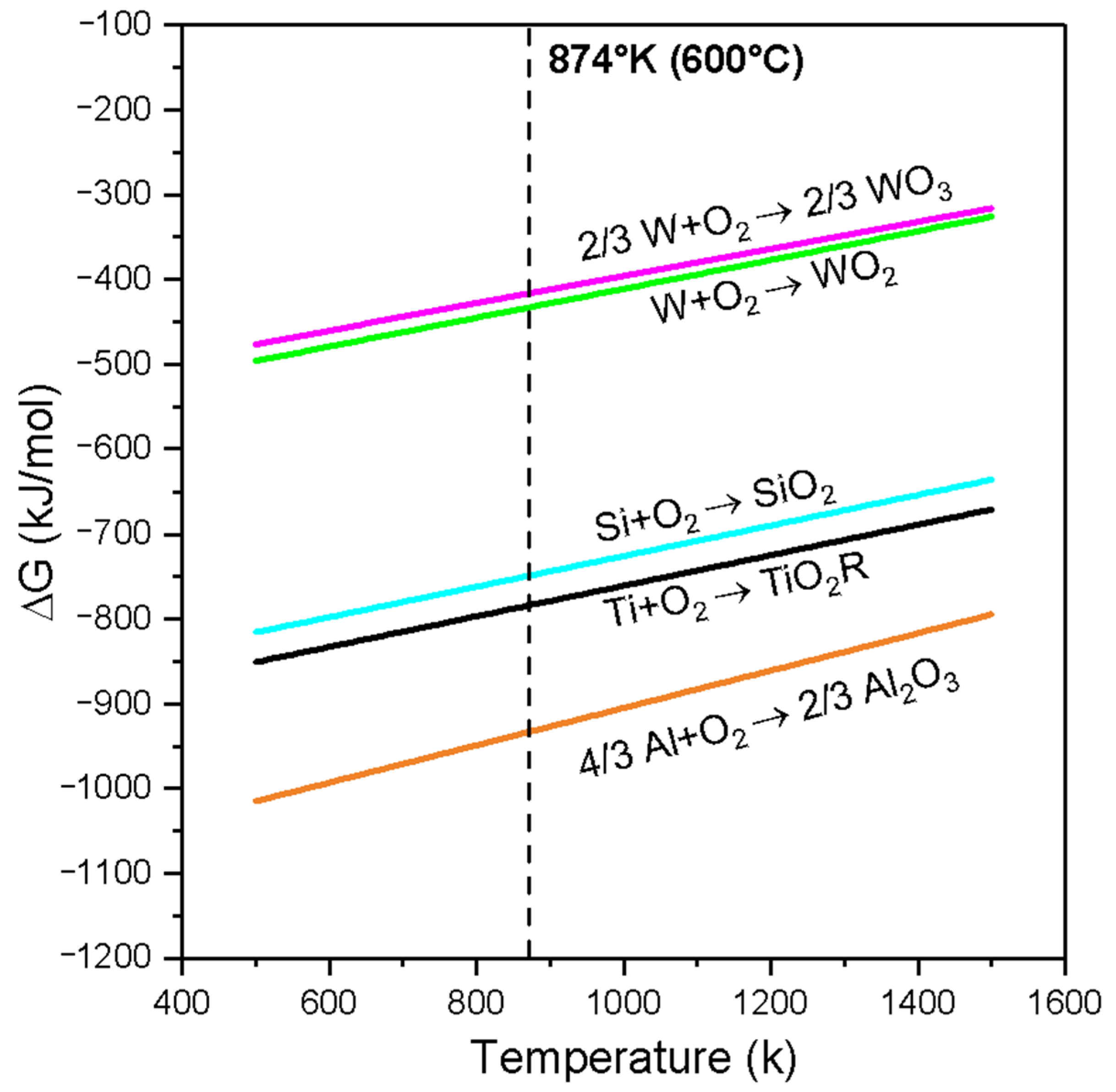
Figure 9 shows the behavior of the Gibbs free energy (∆G) of the oxides of WO3, WO2, SIO2, TIO2, and Al2O3 versus temperature (Ellingham diagram). As the free energy values become more negative, the oxides are more stable. From Figure 9, it can be concluded that the free energy of formation of the oxide Al2O3 (∆fG1) is the most negative and therefore more stable than that of the oxides TiO2 (∆fG2), SiO2 (∆fG3), WO2 (∆ fG4), and WO3 (∆fG5) at a temperature of 600 °C. The relationship that guides the oxidation process in the materials analyzed in the coatings can be seen from the following proposition: (∆fG1) < (∆fG2) < (∆fG3) < (∆fG4) < (∆fG5) [T:600 °C].
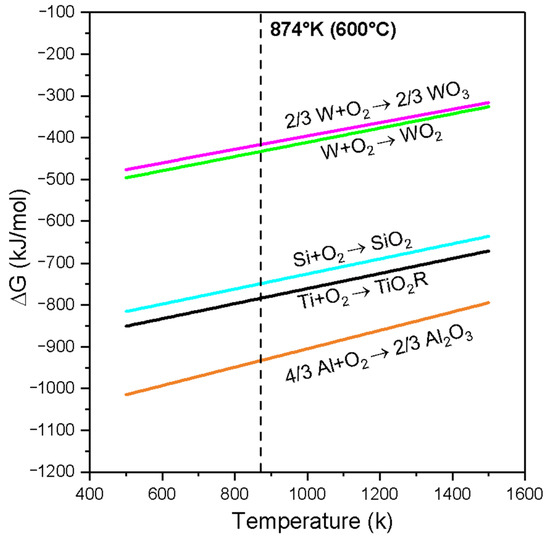
Figure 9.
Ellingham diagram for WO₃, WO₂, SiO₂, TiO₂, and Al₂O₃.
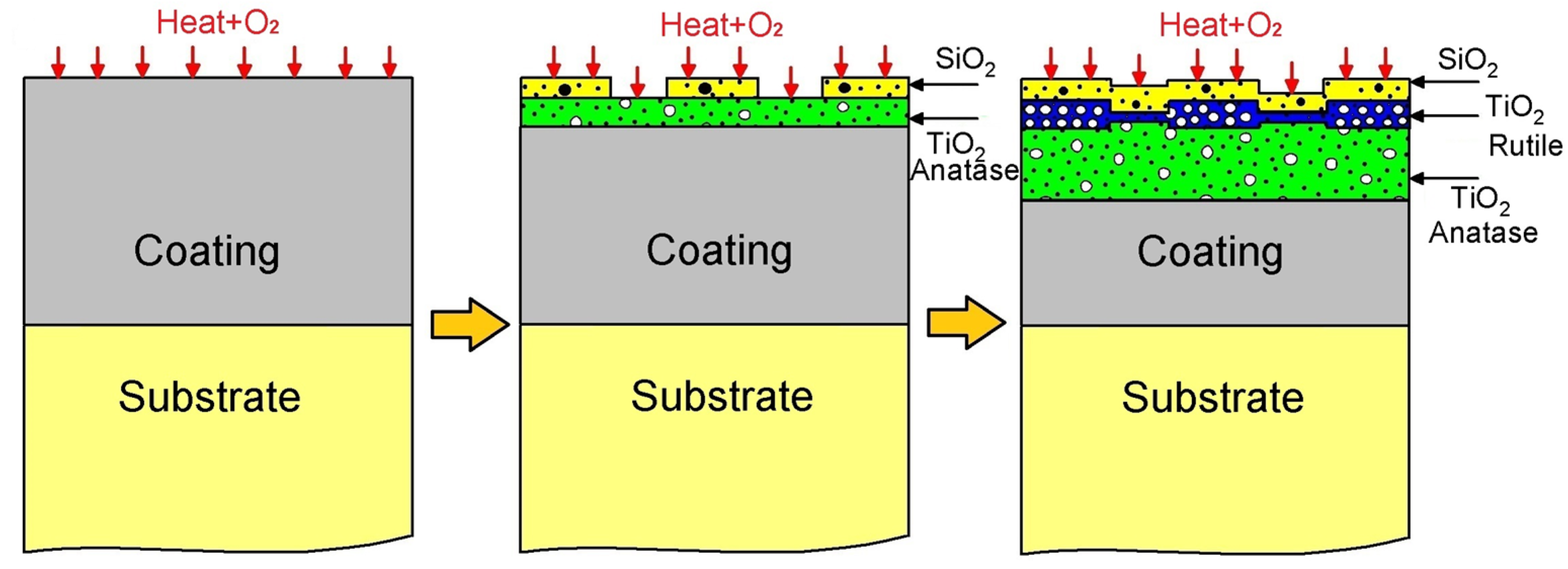
Drawing upon the insights provided by Figure 9 and the characterization of corrosion products, we propose the following oxidation mechanism for the TiSi coating, as illustrated in Figure 10. Initially, an TiSi coating is applied to a Ti6Al4V alloy substrate, subsequently subjected to oxidation at 600 °C. Upon exposure to oxygen, the corrosion mechanism initiates, closely linked by the Gibbs free energy. Notably, the more negative formation energy of TiO2 compared to SiO2 (as depicted in Figure 9) suggests a preferential formation of TiO2 oxide over SiO2 oxide, a trend validated by XPS spectroscopy, where high-resolution spectrum values of TiO2 surpass those of SiO2. The commencement of the degradation stage, facilitated by electron diffusion through the oxide layer, is particularly accelerated for TiSi due to the presence of SiO2, possessing a considerably lower diffusion coefficient than TiO2 [35], thereby enhancing resistance to oxygen penetration. Considering defects, the potential solubility of silicon ions in the TiO2 structure, either through substitution or interstitially, diminishes the concentration of defects in TiO2 [36], consequently bolstering oxidation resistance. This observation is supported by Raman spectroscopy results, indicating a 3:1 proportion of the TiO2 anatase phase to Rutile. Microscopic and optical profilometry analyses affirm the absence of cracks or fractures in the TiSi coating, mitigating the risk of failure or the growth and subsequent detachment of the TiO2 Rutile phase oxide.
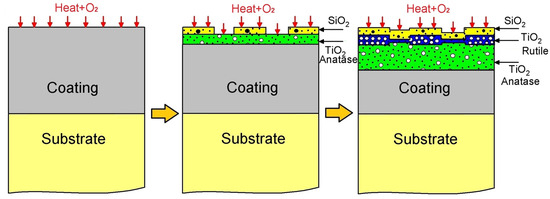
Figure 10.
Diagram of mechanism of corrosion of TiSi coating.
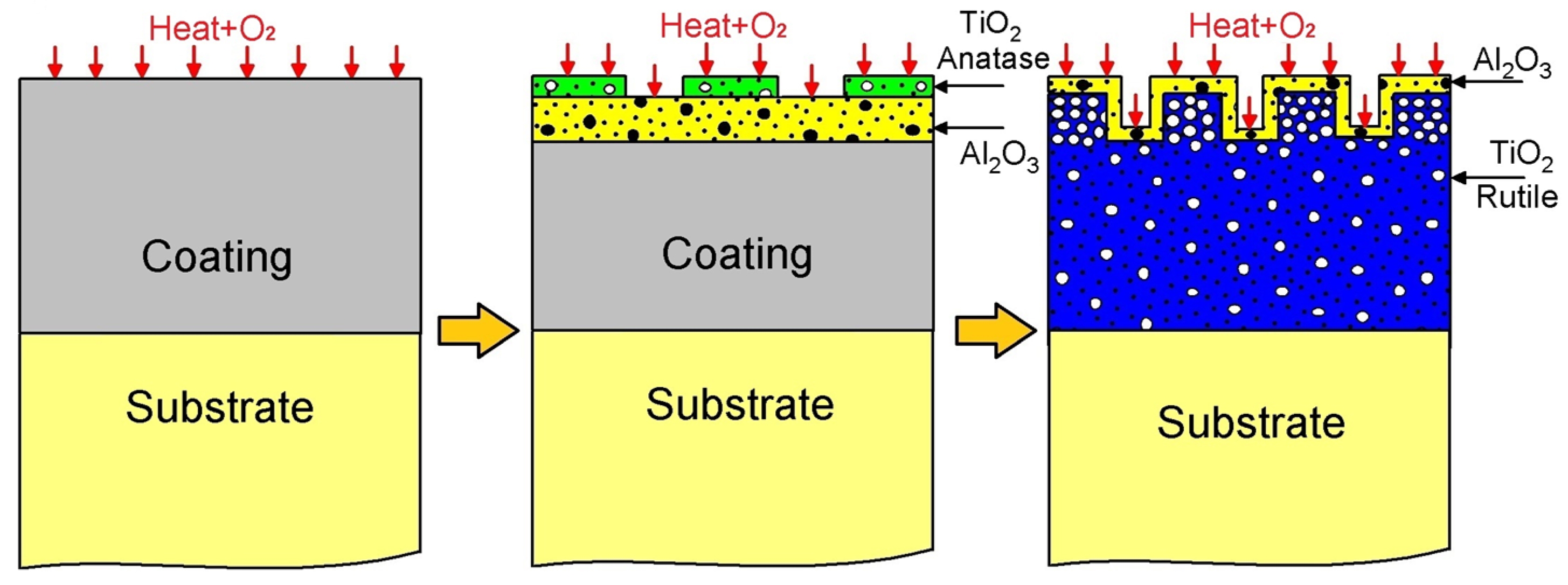
The oxidation mechanism governing the AlTi coating is proposed in Figure 11. Initially, AlTi is deposited onto the Ti6Al4V substrate, subsequently undergoing oxidation at 600 °C. Upon exposure to oxygen, TiO2 and Al2O3 oxides form. According to Gibbs, the more negative formation energy of Al2O3 compared to TiO2 suggests a preference for Al2O3 formation, indicating that Al atoms tend to create a protective Al2O3 layer, impeding the diffusion process within the coating. However, contrary to expectations, XPS spectroscopy reveals a higher proportion of TiO2 compared to Al2O3 oxide (refer to Figure 8). The degradation stage of the AlTi coating is attributed to aluminum depletion caused by oxide layer formation on the coating surface and subsequent diffusion between the coating and substrate [37]. Throughout the oxidation process, the presence of the TiO2 oxide Rutile phase within the coating contributes to degradation, as it constitutes a non-protective oxide offering vacancies that facilitate oxygen ingress to the substrate via short diffusion paths. Consequently, internal oxidation reaches the coating/substrate interface, inducing stresses that lead to cracking and the eventual detachment of the coating layer prior to complete failure. Upon complete failure, the substrate becomes exposed to oxygen, initiating a reaction with the titanium of the substrate and forming the TiO2 oxide layer Rutile phase, as evidenced by Raman spectroscopy, XPS, and X-ray diffraction analyses.
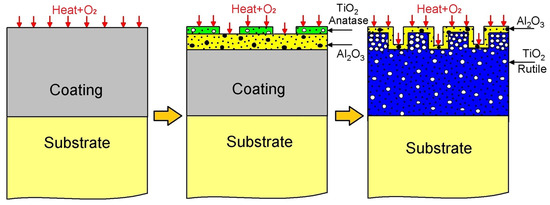
Figure 11.
Diagram of mechanism of corrosion of AlTi coating.
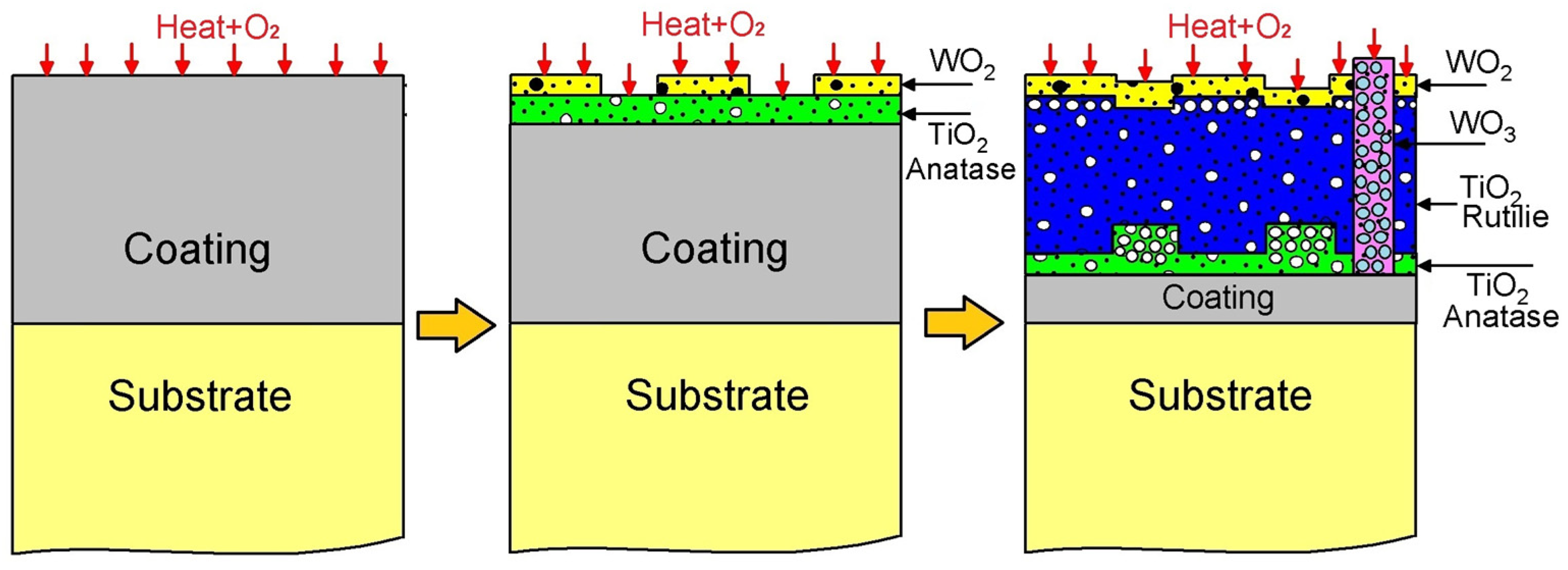
The oxidation mechanism governing the WTi coating is proposed in Figure 12. Initially, an WTi coating is applied to a Ti6Al4V titanium alloy substrate, followed by oxidation at 600 °C, resulting in the formation of titanium oxides TiO2 and tungsten oxides WO2/WO3, as confirmed by Raman, XPS, and X-ray diffraction (XRD) analyses. Figure 9 displays the Ellingham diagram depicting Gibbs energies as a function of temperature for titanium oxides TiO2 and tungsten, highlighting their respective affinities with oxygen. This concept of oxygen affinity could elucidate the onset of the oxidation mechanism [26,27]. Specifically, titanium exhibits a higher affinity, leading to the preferential formation of titanium oxide TiO2 over tungsten oxides. This characteristic of the oxide layer in the WTi coating is verified through qualitative and semi-quantitative analyses using XPS, XRD, and Raman spectroscopy. Tungsten oxides are detected in smaller proportions compared to the rutile phase, likely constrained by their Gibbs energies for oxide formation, resulting in low intensities in the XPS spectra of tungsten oxide WO2 and the presence of WO3 islands on the coating surface, as confirmed by Raman spectroscopy. Notably, reports in the literature indicate transformations of titanium oxide from the anatase phase to the rutile phase and tungsten oxide from WO2 to WO3 during oxidation processes of titanium and pure tungsten. Raman spectroscopy corroborates the transformation of the TiO2 oxide from the anatase phase to the rutile phase on the WTi coating surface, initiating the coating’s degradation stage. The diffusion channels left by TiO2 oxide accelerate titanium outward diffusion, resulting in increased non-protective oxide proportions, consequently diminishing oxidation resistance.
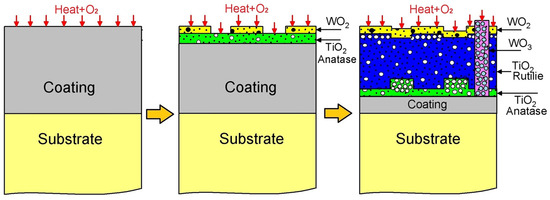
Figure 12.
Diagram of mechanism of corrosion of WTi coating.
4. Conclusions
This study investigated the corrosion products of TiSi, AlTi, and WTi coatings deposited on Ti6Al4V titanium alloy substrates. The coatings were applied using RF magnetron sputtering, and XRD analysis revealed their crystalline nature. Subsequently, the samples were exposed to an air atmosphere at a temperature of 600 °C for a duration of 100 h.
Corroded TiSi (86:14 wt.%) coating samples revealed the formation of globular structures on the surface, supported by an increase in roughness. Rutile, anatase, and silicon oxide were identified, and semiquantitative mapping Raman analyses revealed the presence of anatase (predominantly) and rutile, uniformly distributed on the surface. The presence of silicon oxide favors the formation of a protective anatase-rutile layer, preventing any evident formation of cracks or fractures in the coating.
The corrosion process resulted in the formation of globular structures on the surface of AlTi (13:87 wt.%) coatings, accompanied by a more significant increase in roughness compared to other coatings. Mapping Raman analyses revealed a uniformly distributed rutile layer across the entire surface. Probably, an aluminum oxide passive layer formed, but aluminum depletion facilitated the formation of a non-protective rutile phase. This triggered the oxidation of titanium within both the coating and substrate.
WTi (21:79 wt.%) coatings also demonstrated alterations in surface morphology, marked by a significant increase in roughness and the development of globular structures. Raman mapping revealed a concentrated distribution of Rutile and Anatase across the surface, while WO3 was found localized in specific regions, contributing to the formation of pitting.
The results indicate that, comparatively, TiSi (86:14 wt.%) exhibited a better high-temperature corrosion response compared to AlTi (13:87 wt.%) and WTi (21:79 wt.%) coatings. The surface of the AlTi coating was influenced by aluminum depletion, leading to the formation of non-protective titanium oxides. Furthermore, the accumulation of tungsten oxides, morphologically resembling pitting, could be observed.
Author Contributions
Conceptualization, O.P. and J.O.; methodology, O.P. and O.G.; validation, O.G., O.P. and J.O.; data curation, formal analysis, software, writing—original draft preparation, O.G.; investigation, O.G., W.S.H., J.E.A. and G.C.; resources, V.T.-A.; writing—review and editing, O.P., J.O. and V.T.-A.; visualization, O.G.; and supervision, O.P., J.O., W.S.H., J.E.A., G.C. and V.T.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
Acknowledgments
To the Universidad Nacional de Colombia and the Instituto Nacional de Pesquisas Espaciais (Associated Laboratory of Sensors and Materials) in Brazil for the use of their laboratories and facilities in the development of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saurabh, A.; Meghana, C.M.; Singh, P.K.; Verma, P.C. Titanium-based materials: Synthesis, properties, and applications. Mater. Today Proc. 2022, 56, 412–419. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti–6Al–4V alloy by thermal oxidation. Surf. Coat. Technol. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Shvets, P.; Maksimova, K.; Demin, M.; Dikaya, O.; Goikhman, A. Cathodic arc sputtering of functional titanium oxide thin films, demonstrating resistive switching. Phys. B. Condens. Matter 2017, 513, 15–20. [Google Scholar] [CrossRef]
- Duarte, L.T.; Bolfarini, C.; Biaggio, S.R.; Rocha-Filho, R.C.; Nascente, P.A. Growth of aluminum-free porous oxide layers on titanium and its alloys Ti-6Al-4V and Ti-6Al-7Nb by micro-arc oxidation. Mater. Sci. Eng. C 2014, 41, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.P.; Casagrande, A.; Sambogna, G. Effect of Ni, Si and Cr in the structural formation of diffusion aluminide coatings on commercial-purity titanium. Surf. Coat. Technol. 2006, 201, 230–242. [Google Scholar] [CrossRef]
- Ebach-Stahl, A.; Eilers, C.; Laska, N.; Braun, R. Cyclic oxidation behaviour of the titanium alloys Ti-6242 and Ti-17 with Ti–Al–Cr–Y coatings at 600 and 700 °C in air. Surf. Coat. Technol. 2013, 223, 24–31. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Hu, T.; Leicht, P.; Liu, Y. Oxidation resistance and thermal stability of Ti (C, N) and Ti (C, N, O) coatings deposited by chemical vapor deposition. Int. J. Refract. Met. Hard Mater. 2016, 54, 295–303. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation mechanism of biomedical titanium alloy surface and experiment. Int. J. Corros. 2020, 1678615. [Google Scholar] [CrossRef]
- Gudla, V.C.; Bordo, K.; Engberg, S.; Rechendorff, K.; Ambat, R. High frequency pulse anodising of magnetron sputtered Al–Zr and Al–Ti Coatings. Mater. Des. 2016, 95, 340–347. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Godlewska, E. Silicide coatings on Ti-6Al-1Mn (at.%) alloy and their oxidation resistance. Surf. Coat. Technol. 2018, 334, 491–499. [Google Scholar] [CrossRef]
- Bouzakis, K.D.; Bouzakis, E.; Kombogiannis, S.; Paraskevopoulou, R.; Skordaris, G.; Makrimallakis, S.; Andersson, J.M. Effect of silicon content on PVD film mechanical properties and cutting performance of coated cemented carbide inserts. Surf. Coat. Technol. 2013, 237, 379–389. [Google Scholar] [CrossRef]
- Lauwerens, W.; De Boeck, A.; Thijs, M.; Claessens, S.; Van Stappen, M.; Steenackers, P. PVD Al–Ti and Al–Mn coatings for high temperature corrosion protection of sheet steel. Surf. Coat. Technol. 2001, 146, 27–32. [Google Scholar] [CrossRef]
- Sanchette, F.; Loi, T.H.; Billard, A.; Frantz, C. Structure—Properties relationship of metastable Al-Cr and Al-Ti alloys deposited by rf magnetron sputtering: Role of nitrogen. Surf. Coat. Technol. 1995, 74, 903–909. [Google Scholar] [CrossRef]
- Louro, C.; Cavaleiro, A. Thermal Oxidation of Tungsten-Based Sputtered Coatings. J. Electrochem. Soc. 1997, 144, 259–266. [Google Scholar] [CrossRef]
- Arvizua, M.A.; Triana, C.A.; Stefanov, B.I.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in sputter-deposited W–Ti oxide films: Durability enhancement due to Ti. Sol. Energy Mater. Sol. Cells 2014, 125, 184–189. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lee, J.-W. Microstructure, corrosion and tribological behaviors of TiAlSiN coatings deposited by cathodic arc plasma deposition. Thin Solid Film. 2009, 517, 5231–5236. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, A.H.; Zhang, Z.; Zheng, R.R.; Xia, H.B.; Wang, Y.N. Laser alloying of Ti–Si compound coating on Ti–6Al–4V alloy for the improvement of bioactivity. Appl. Surf. Sci. 2014, 305, 16–23. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Effect of silicon on corrosion resistance of Ti–Si alloys. Mater. Sci. Eng. B 2011, 176, 79–86. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys—A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, F.; Wang, A.; Yu, H.; Chen, C. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Garbacz, H.; Pouquet, J.M.; García-Lecina, E.; Díaz-Fuentes, M.; Wieciński, P.; Martin, R.H.; Kurzydlowski, K.J. Microstructure, fatigue and corrosion properties of the Ti–Al intermetallic layers. Surf. Coat. Technol. 2011, 205, 4433–4440. [Google Scholar] [CrossRef]
- Du, H.L.; Datta, P.K.; Hu, D.; Wu, X. High temperature corrosion mechanisms of certain new TiAl-based inter-metallic alloys in an aggressive H2/H2O/H2S environment at 850 °C. Corros. Sci. 2007, 49, 2406–2420. [Google Scholar] [CrossRef]
- Gordillo, O.; Hincapie, W.; Piamba, O.; Olaya, J.; Trava-Airoldi, V. Study of Adhesive Wear Test on TiSi, AlTi, and WTi Coatings. Coatings 2022, 12, 1370. [Google Scholar] [CrossRef]
- Petrović, S.; Peruško, D.; Gaković, B.; Mitrić, M.; Kovač, J.; Zalar, A.; Milosavljević, M. Effects of thermal annealing on structural and electrical properties of sputtered W–Ti thin films. Surf. Coat. Technol. 2010, 204, 2099–2102. [Google Scholar] [CrossRef]
- Petrović, S.; Gaković, B.; Peruško, D.; Trtica, M.; Radak, B.; Panjan, P.; Miljanić, Š. Surface modification of a WTi thin film on Si substrate by nanosecond laser pulses. Appl. Surf. Sci. 2008, 254, 4013–4017. [Google Scholar] [CrossRef]
- Sacré, P.Y.; De Bleye, C.; Chavez, P.F.; Netchacovitch, L.; Hubert, P.; Ziemons, E. Data processing of vibrational chemical imaging for pharmaceutical applications. J. Pharm. Biomed. Anal. 2014, 101, 123–140. [Google Scholar] [CrossRef]
- Smith, G.P.; McGoverin, C.M.; Fraser, S.J.; Gordon, K.C. Raman imaging of drug delivery systems. Adv. Drug Deliv. Rev. 2015, 89, 21–41. [Google Scholar] [CrossRef]
- Azzouz, T.; Tauler, R. Application of multivariate curve resolution alternating least squares (MCR-ALS) to the quantitative analysis of pharmaceutical and agricultural samples. Talanta 2008, 74, 1201–1210. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Hsiao, C.Y. High temperature oxidation resistance of multicomponent Cr–Ti–Al–Si–N coatings. Surf. Coat. Technol. 2009, 204, 992–996. [Google Scholar] [CrossRef]
- Luthin, J.; Linsmeier, C. Influence of oxygen on the carbide formation on tungsten. J. Nucl. Mater. 2001, 290, 121–125. [Google Scholar] [CrossRef]
- Libardi, J.; Grigorov, K.G.; Massi, M.; da Silva Sobrinho, A.S.; Pessoa, R.S.; Sismanoglu, B. Diffusion of silicon in titanium dioxide thin films with different degree of crystallinity: Efficiency of TiO2 and TiN barrier layers. Vacuum 2016, 128, 178–185. [Google Scholar] [CrossRef]
- Crespo-Villegas, J.; Cavarroc, M.; Knittel, S.; Martinu, L.; Klemberg-Sapieha, J.E. Protective TixSiy coatings for enhanced oxidation resistance of the γ-TiAl alloy at 900 C. Surf. Coat. Technol. 2022, 430, 127963. [Google Scholar] [CrossRef]
- Arunchandran Chenan, J.; Jyothymol, J.; Abirami, S.; Bonu, V.; Barshilia, H.C. Comprehensive Electrochemical Studies on Nanolayered Multilayered Ti/TiN and TiAl/TiAlN Coatings Deposited on Ti6Al4V Substrates. Corrosion 2023, 79, 134–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).