Microscopic Simulation of RE3+ Migration in Ion-Type Rare Earth Ores Based on Navier–Stokes Equation—Exchange Reaction—Ion Migration Coupling
Abstract
:1. Introduction
2. Theory and Experimental Process
2.1. Theory and Mathematical Model
- Seepage control equation
- 2.
- Control equation of reaction kinetics
- 3.
- Control equation of solute transport
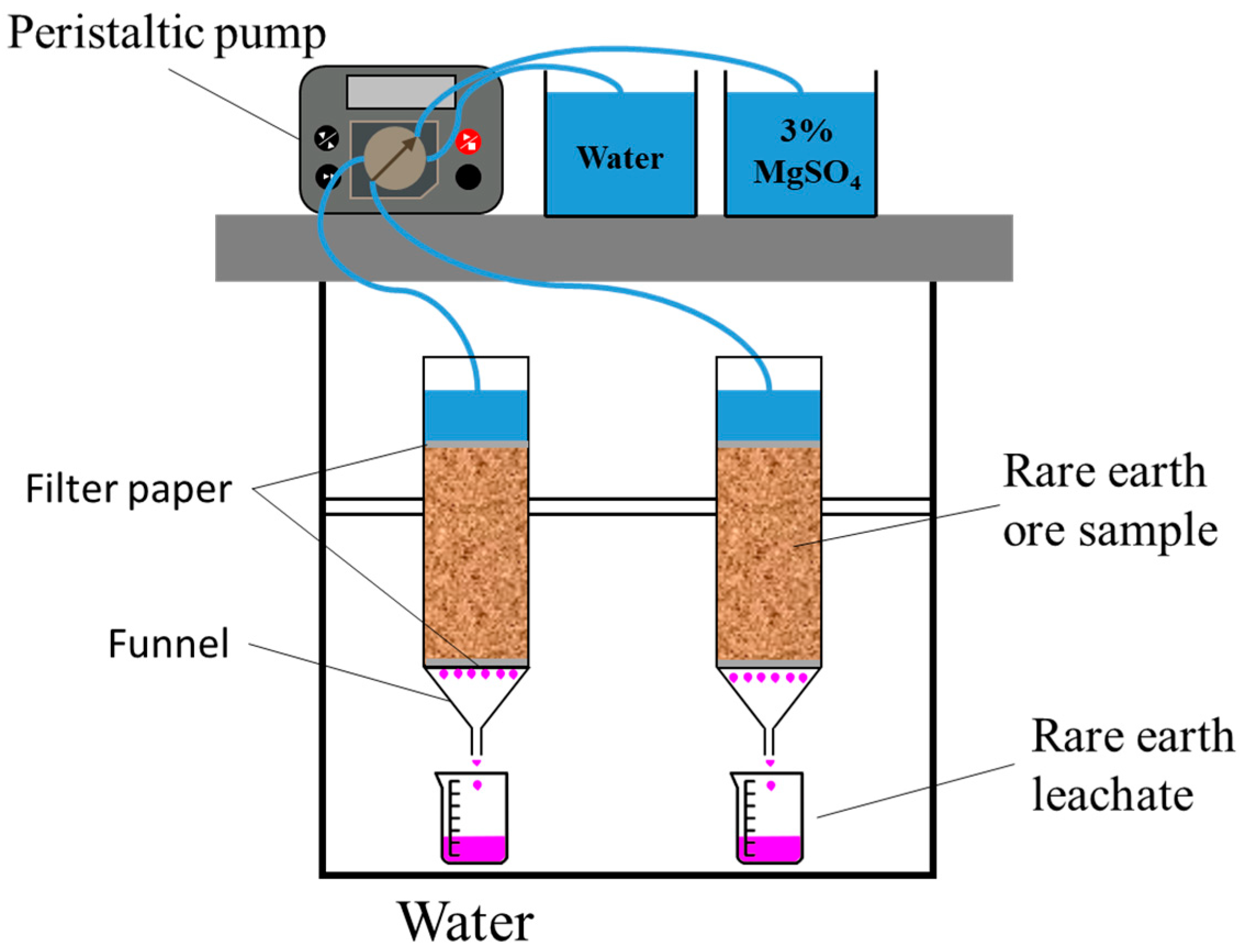
2.2. Column Leaching Experiment
2.2.1. Soil Sampling
2.2.2. Column Leaching Test
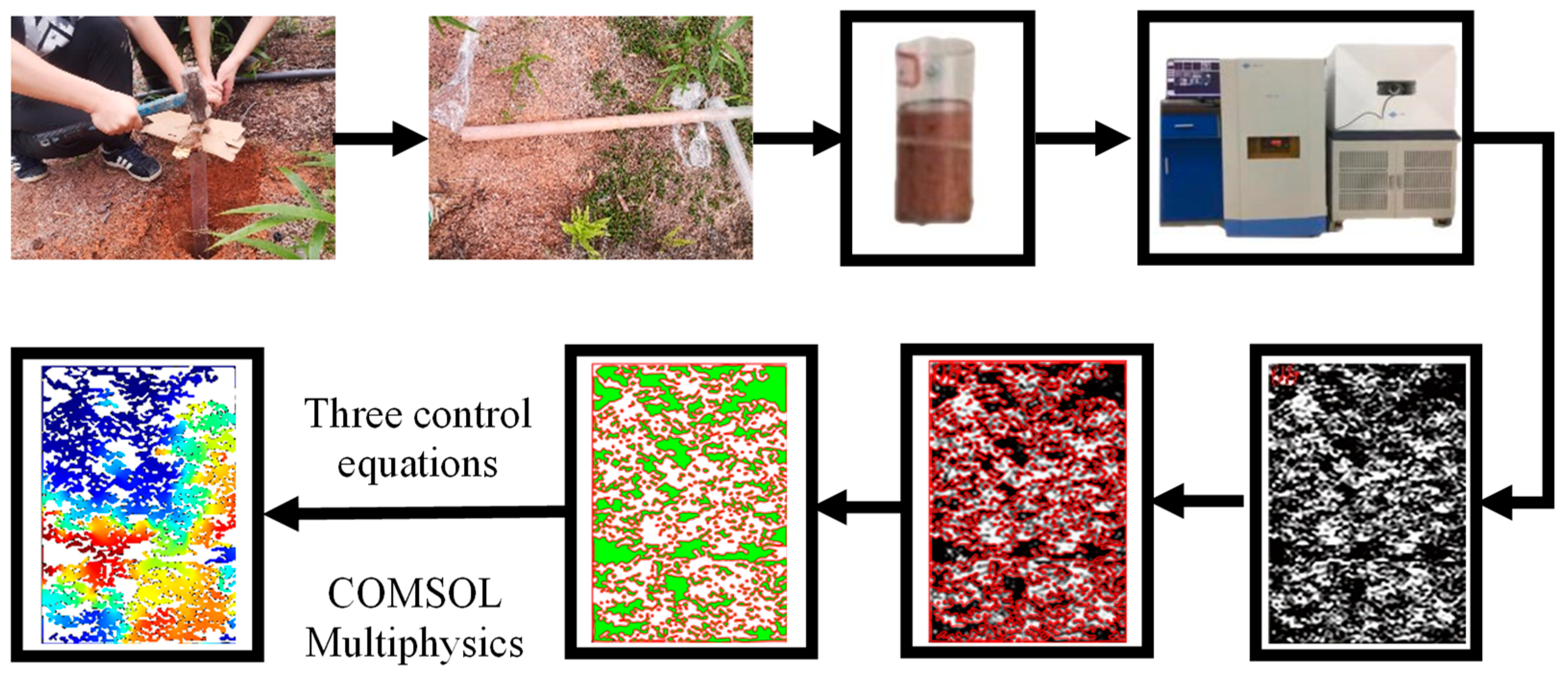
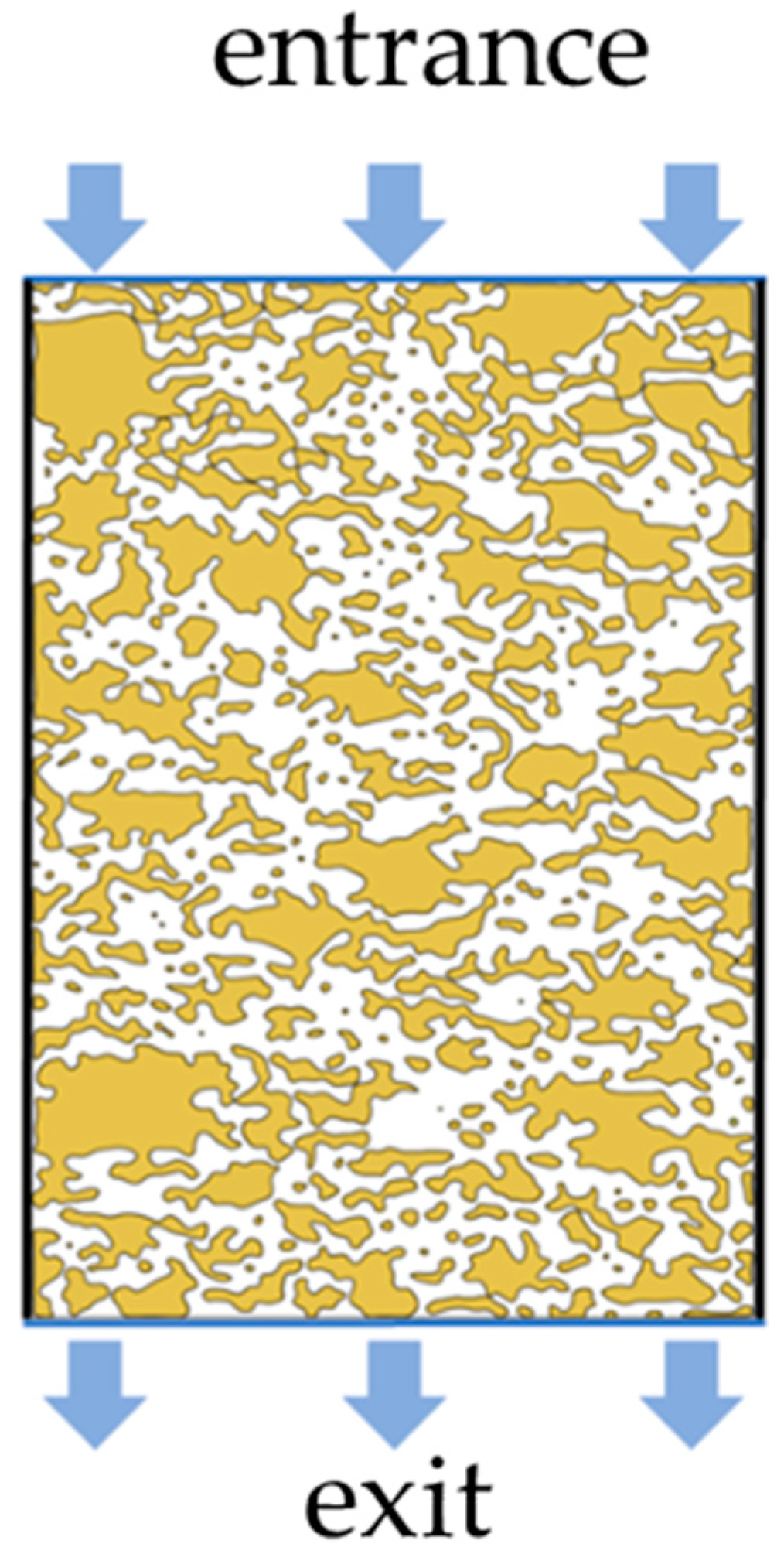
3. Model Establishment and Boundary Condition
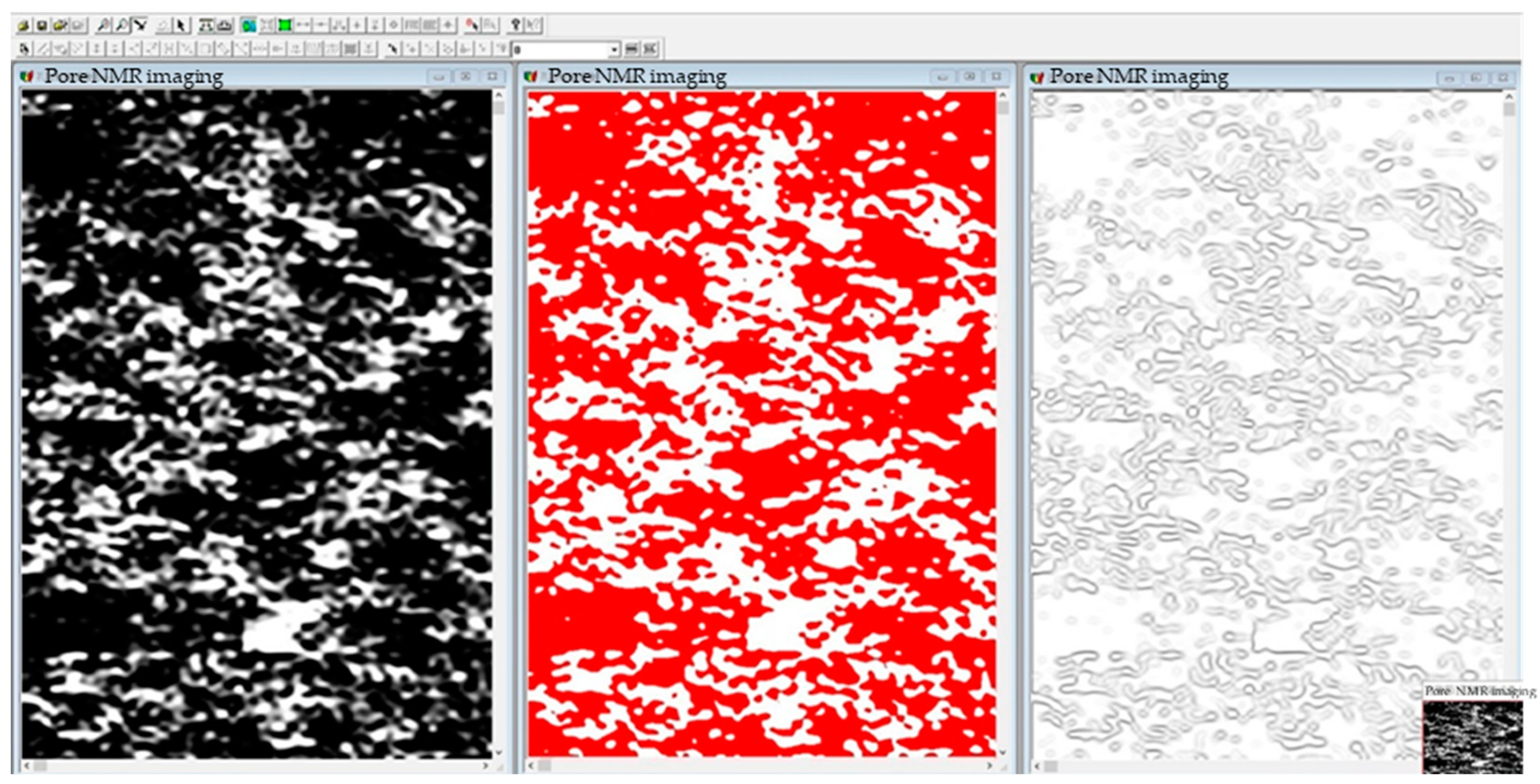
- Image binarization and sharpening. NMR images were imported into R2V software and binarized. Image binarization was the process to control the grayscale value of each pixel in each image within the range of [0, 255], while using high-pass filtering to enhance the high-frequency semaphores of the image and filter the low frequency signals to achieve the purpose of sharpening the image, so as to make the outline of the ore body particle skeleton clearer and facilitate the edge detection and contour extraction in the later stage.
- Threshold segmentation. The low threshold of the image obtained in the previous step was set to 0 and the high threshold to 56. Through the control of the high and low threshold, the area of the ore body particle skeleton located in the image was determined, which can effectively distinguish the area where the ore body particles and pores flow.
- Edge detection. The Sobel operator was used to detect the edge of the image after threshold adjustment. The Sobel operator is a filter operator based on first derivative, which can detect the target edge contour effectively by fast convolution function. The Sobel operator usually includes a set of horizontal and vertical 3 × 3 matrices. The approximate brightness difference values of the horizontal and vertical images can be obtained by convolution of the image with the plane. The matrix formula is shown as follows:
- 4.
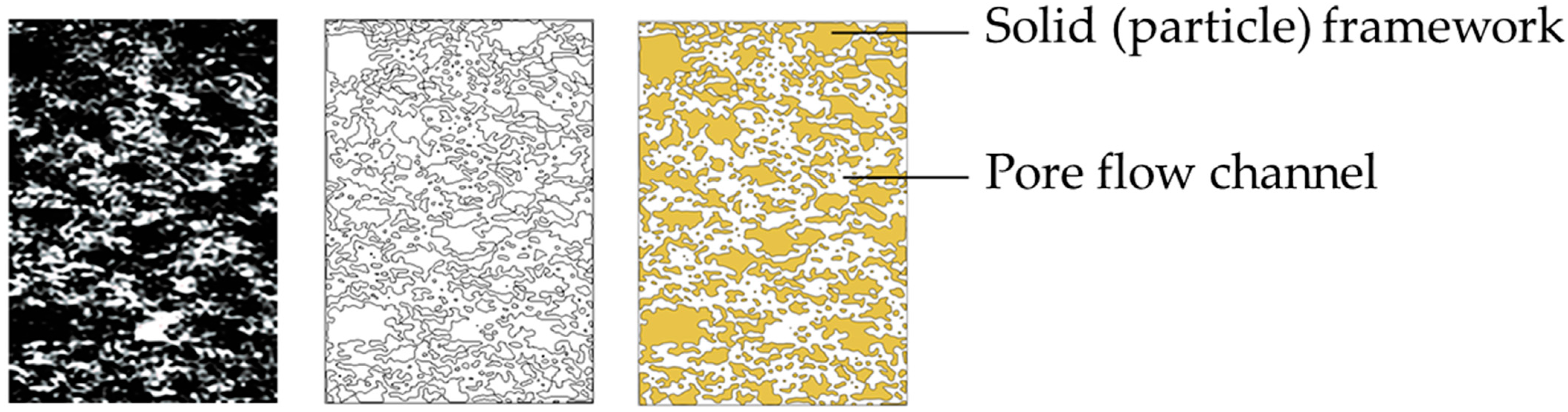
- Contour extraction and edge processing. The frame edge of ore body particles detected by Sobel operator was vectorized by raster editing, and the edge contour vector of ore body particles was generated. The Sobel operator has a positive effect on the edge detection of the target, and the image noise after sharpening can be effectively suppressed, as shown in Figure 7.
- 5.
- Pore channel connectivity processing. Since the NMR image is a two-dimensional image of the longitudinal section of the pillar, the vertical flow of the leaching solution can be reflected in the NMR image, while the seepage channel in the horizontal direction cannot be clearly reflected only through the image, and it is regarded as the particle part of the ore body.
4. Results and Discussion
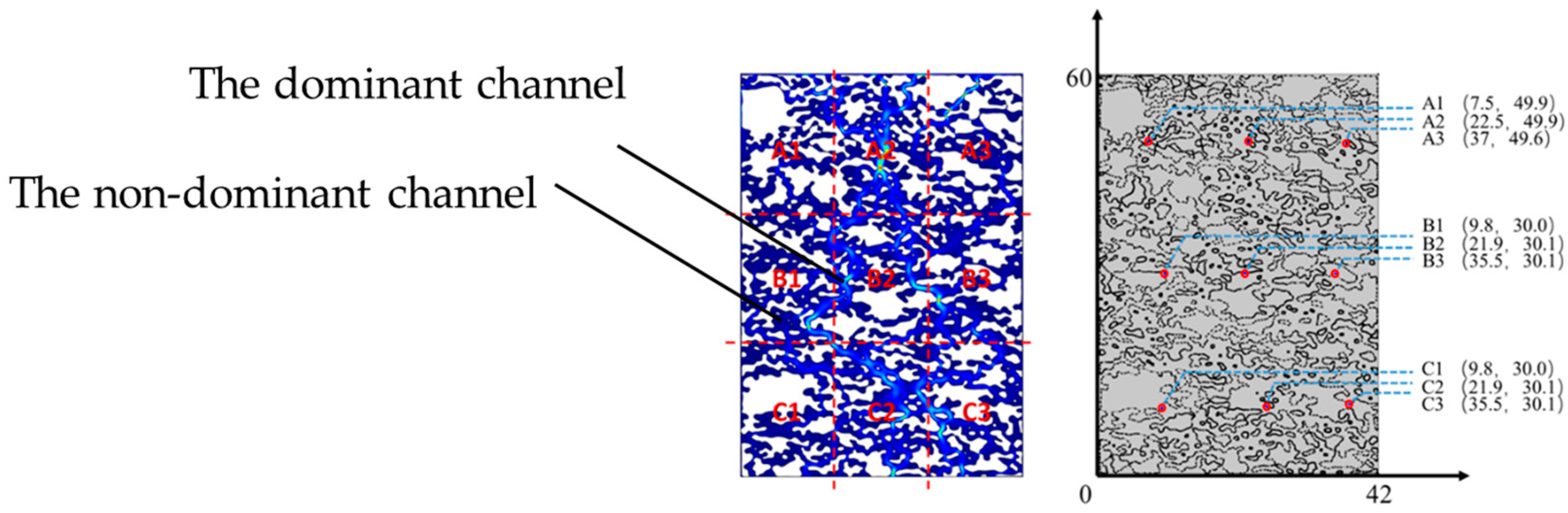
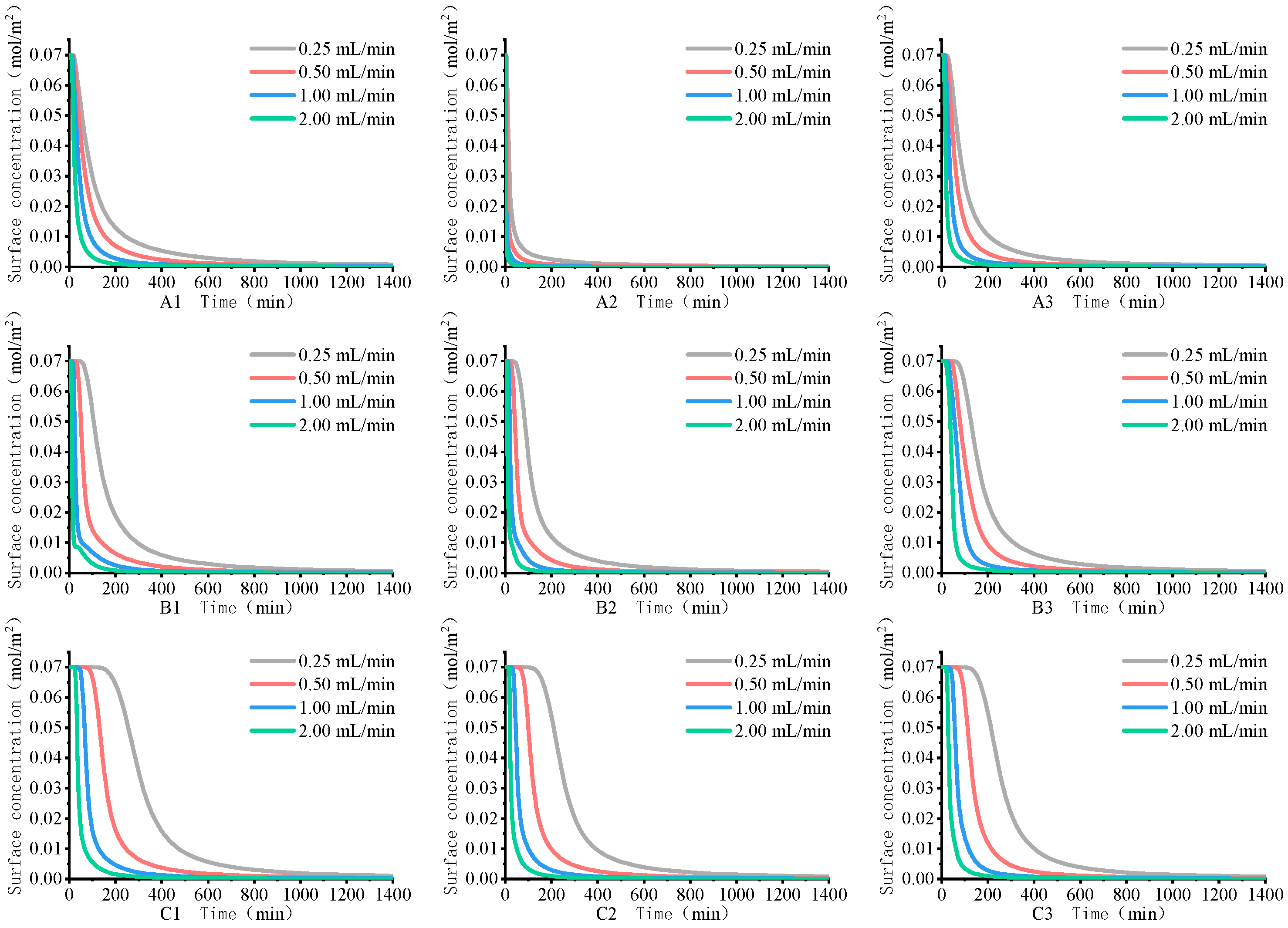
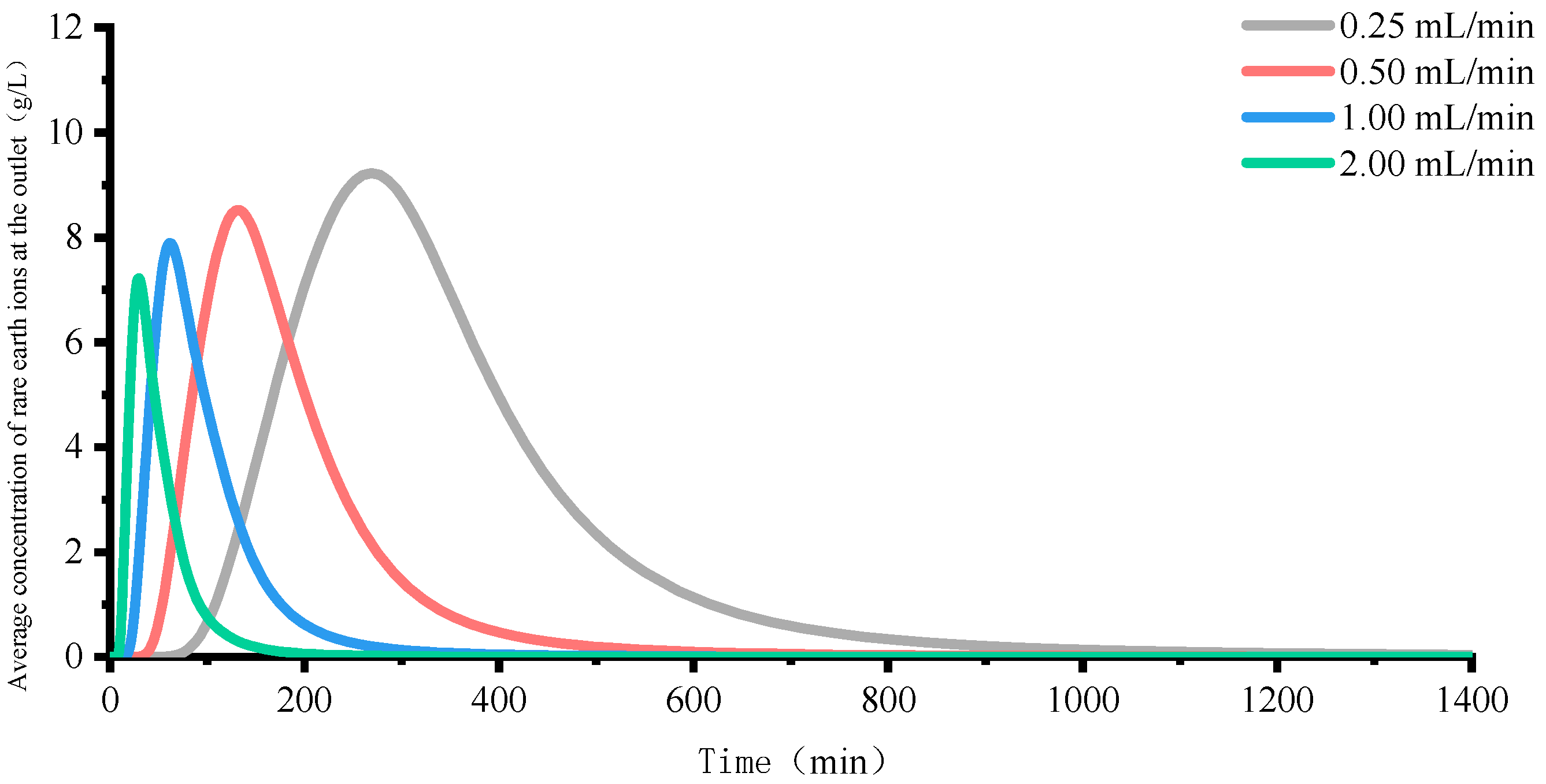
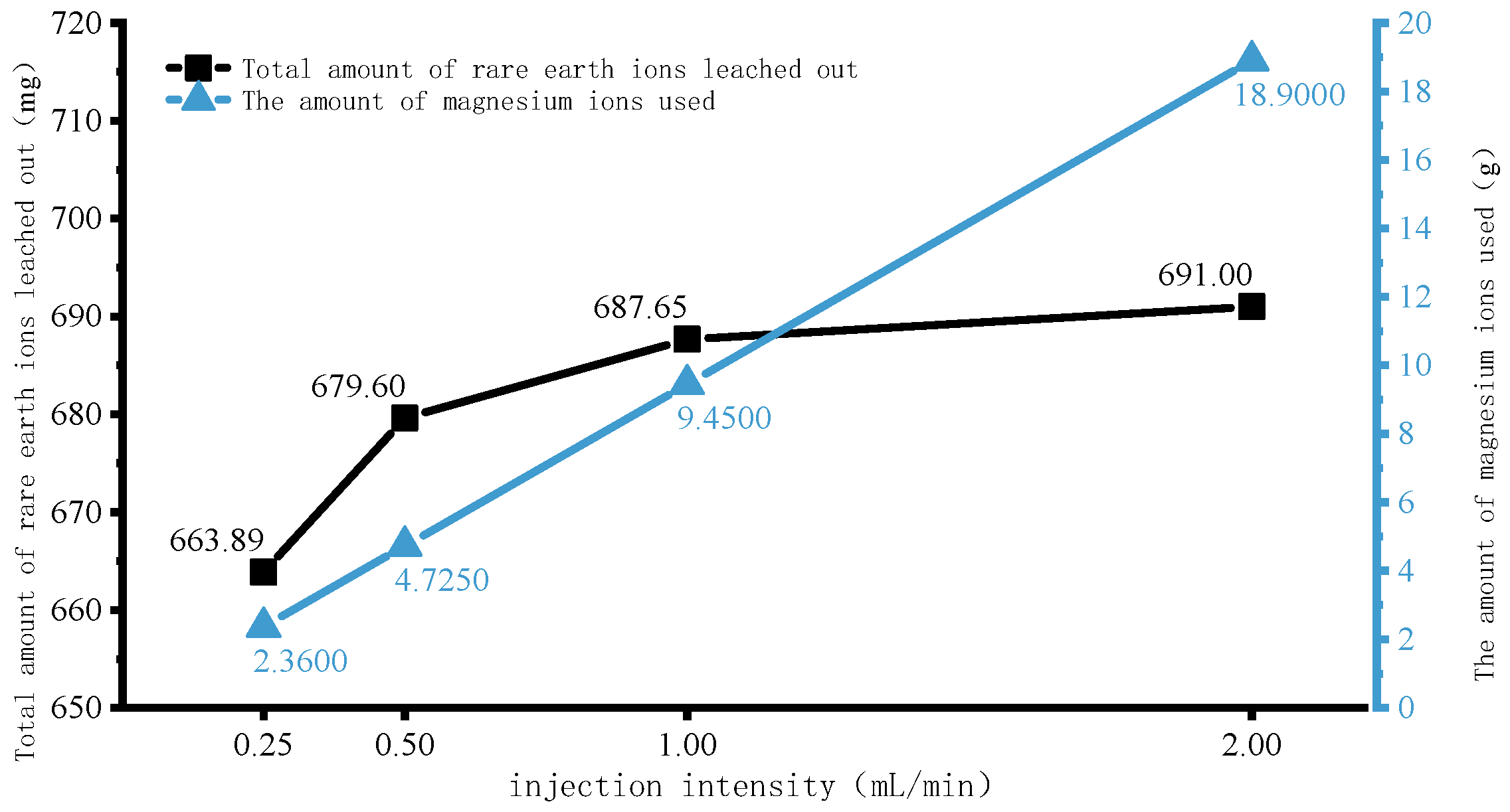
4.1. Rare Earth Ion Exchange and Migration Regular Patterns under Different Injection Intensity
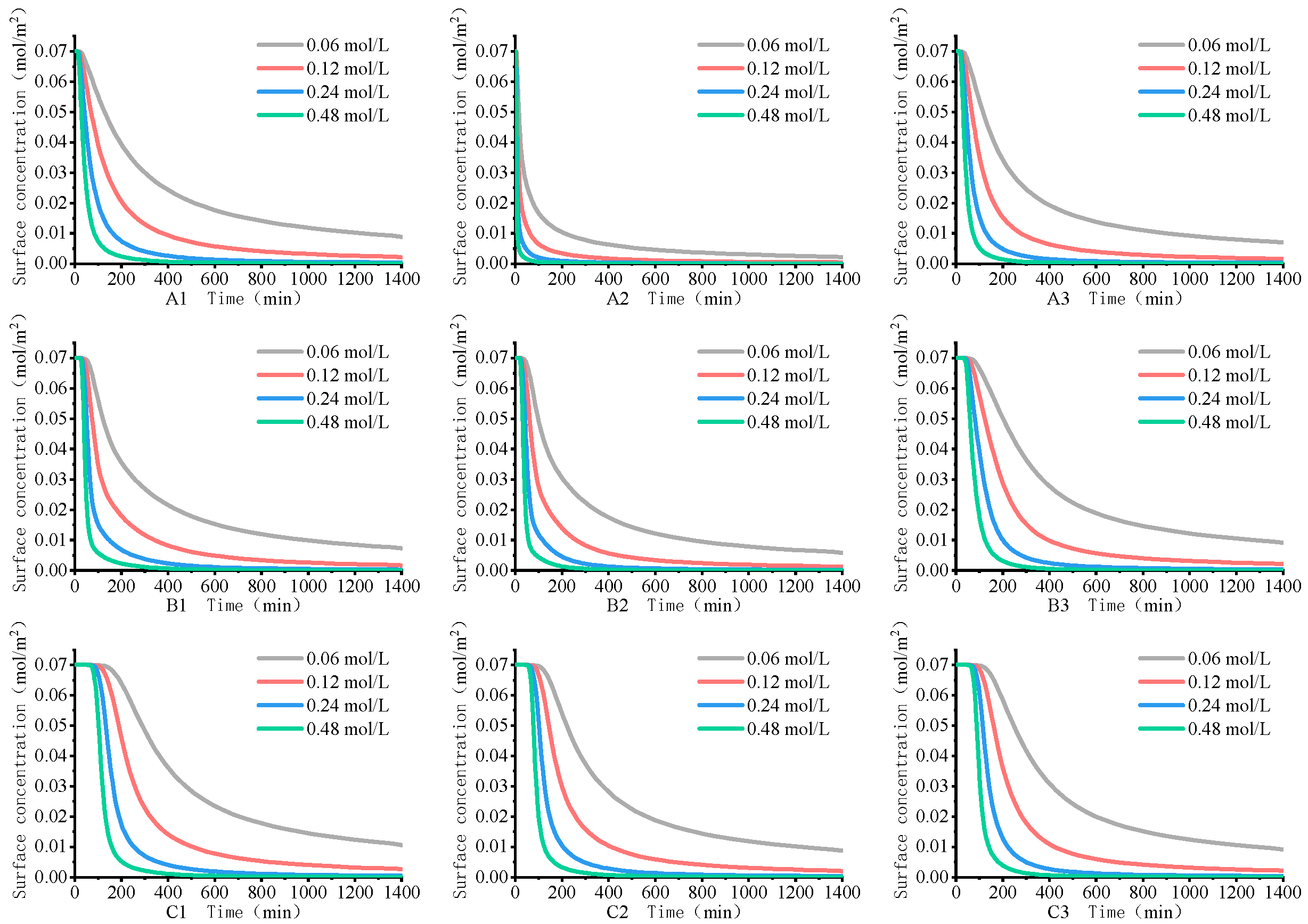
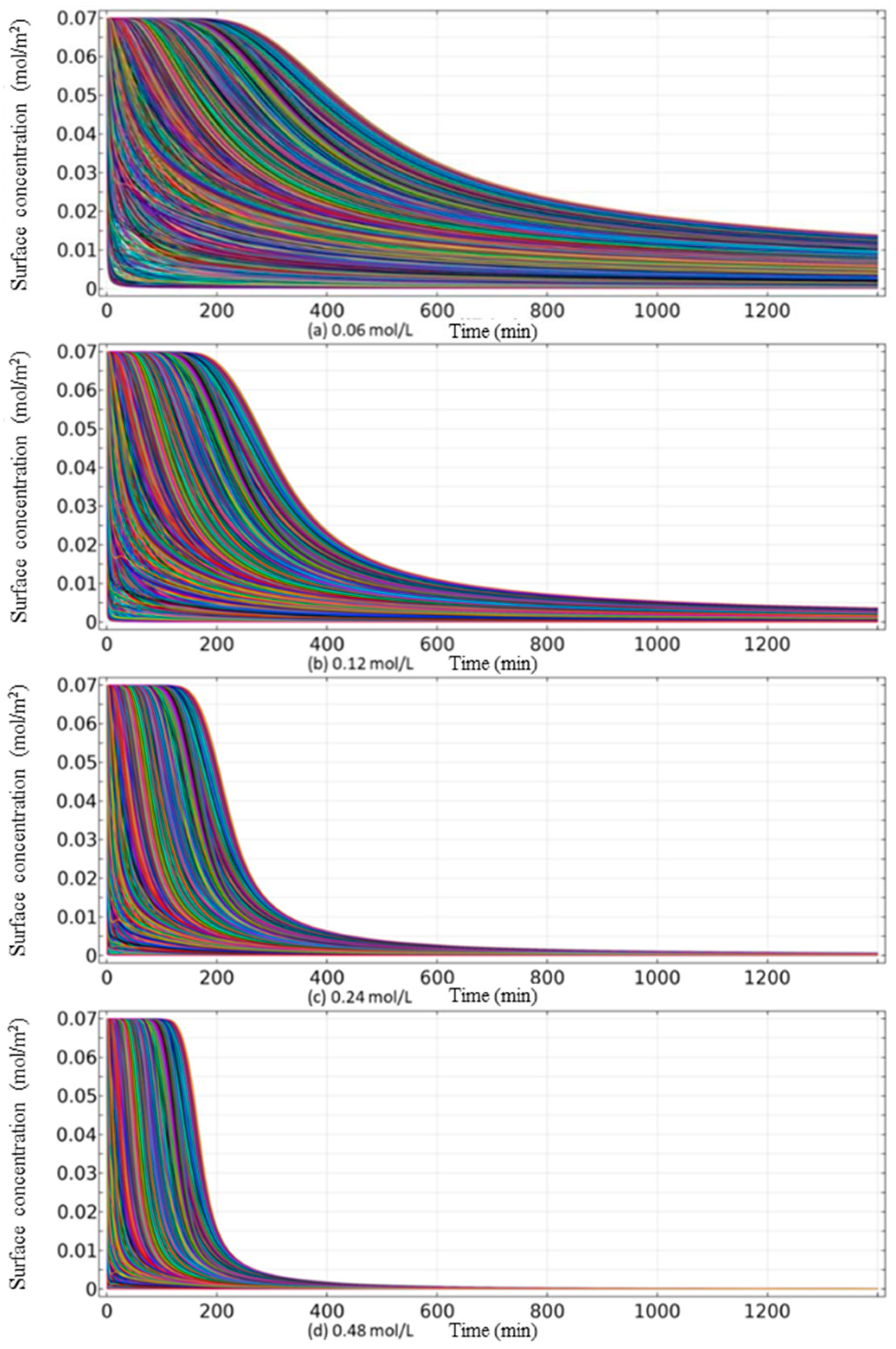
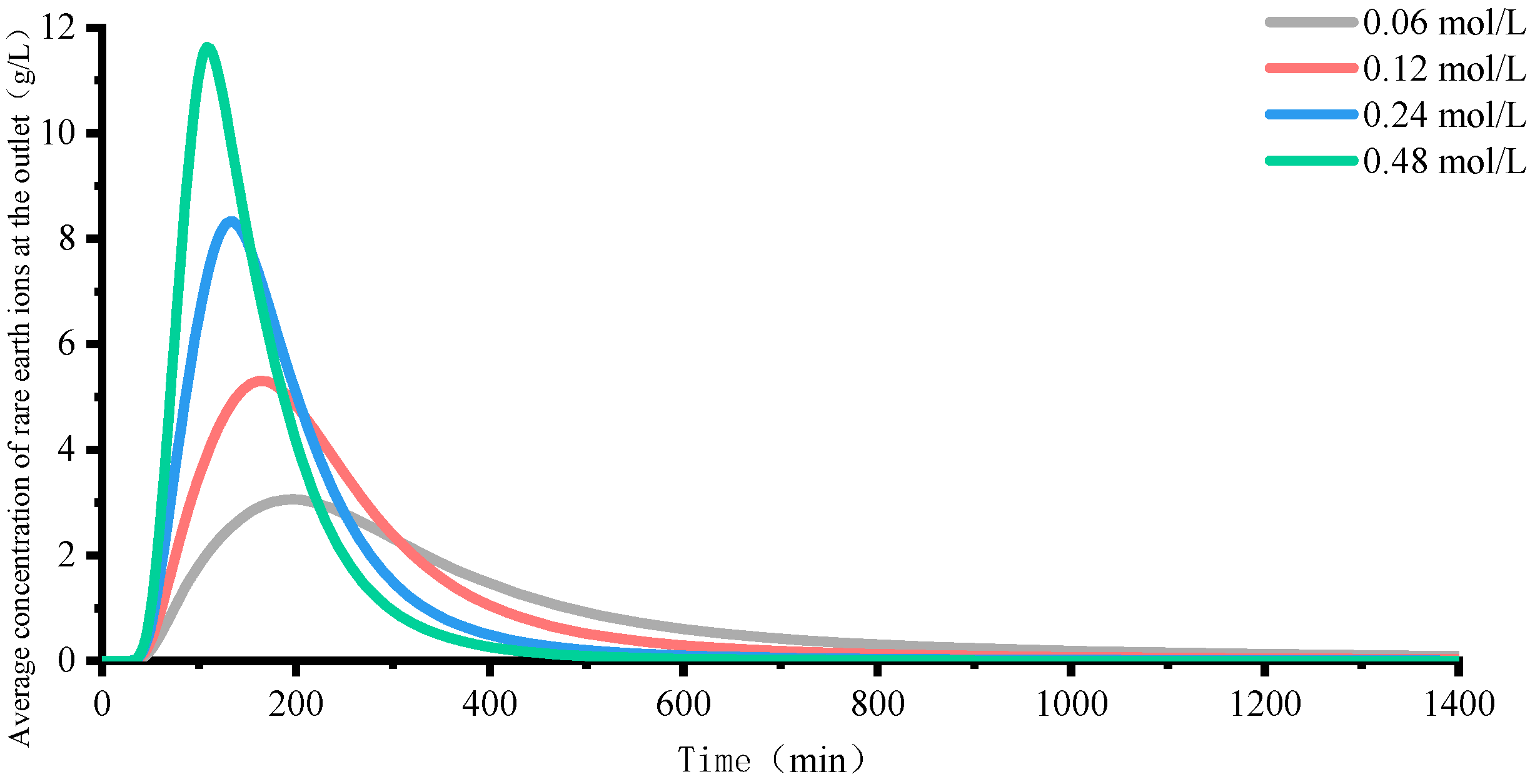
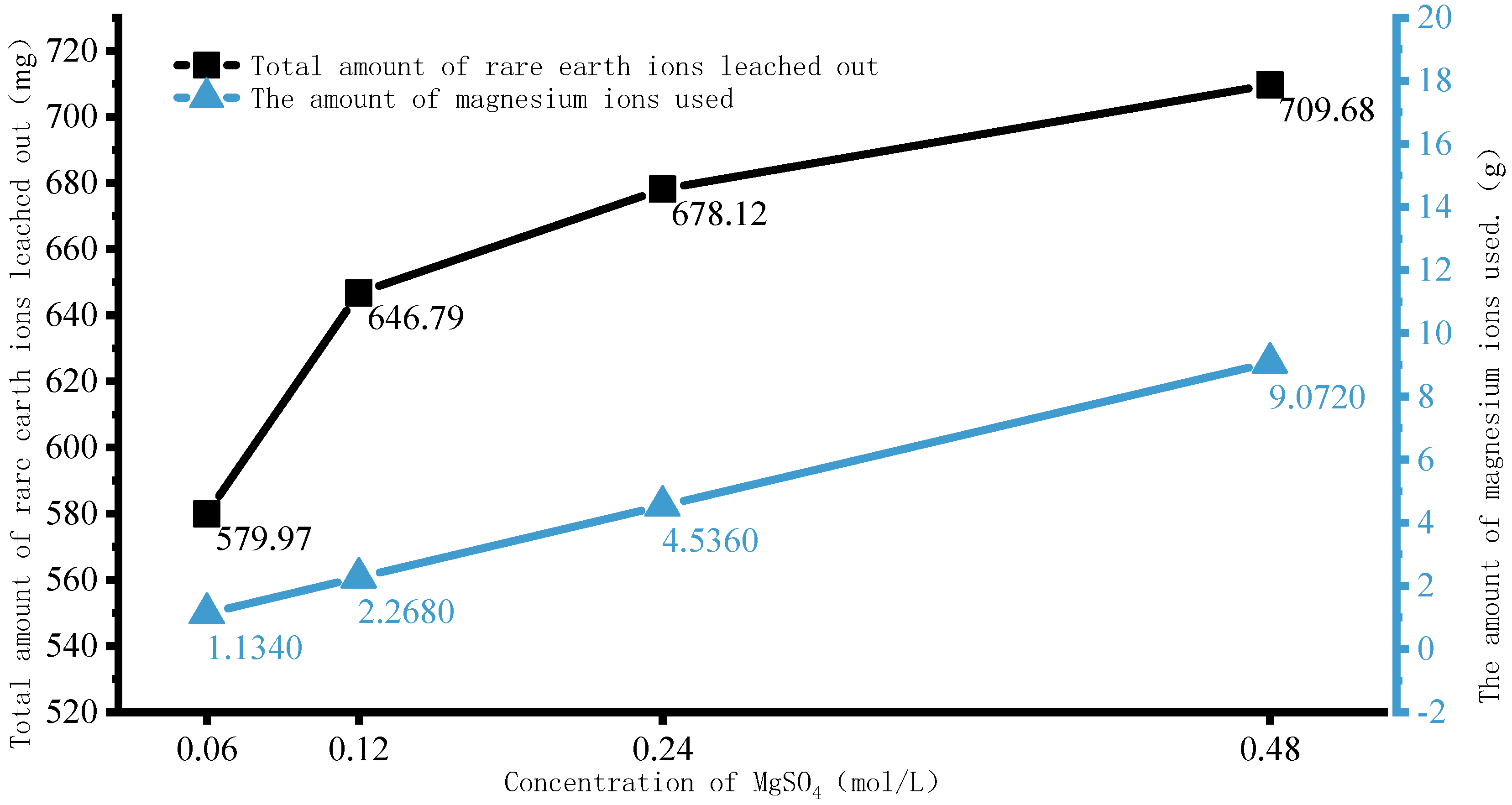
4.2. Rare Earth Ion Exchange and Migration Regular Patterns under Different Injection Concentrations
5. Conclusions
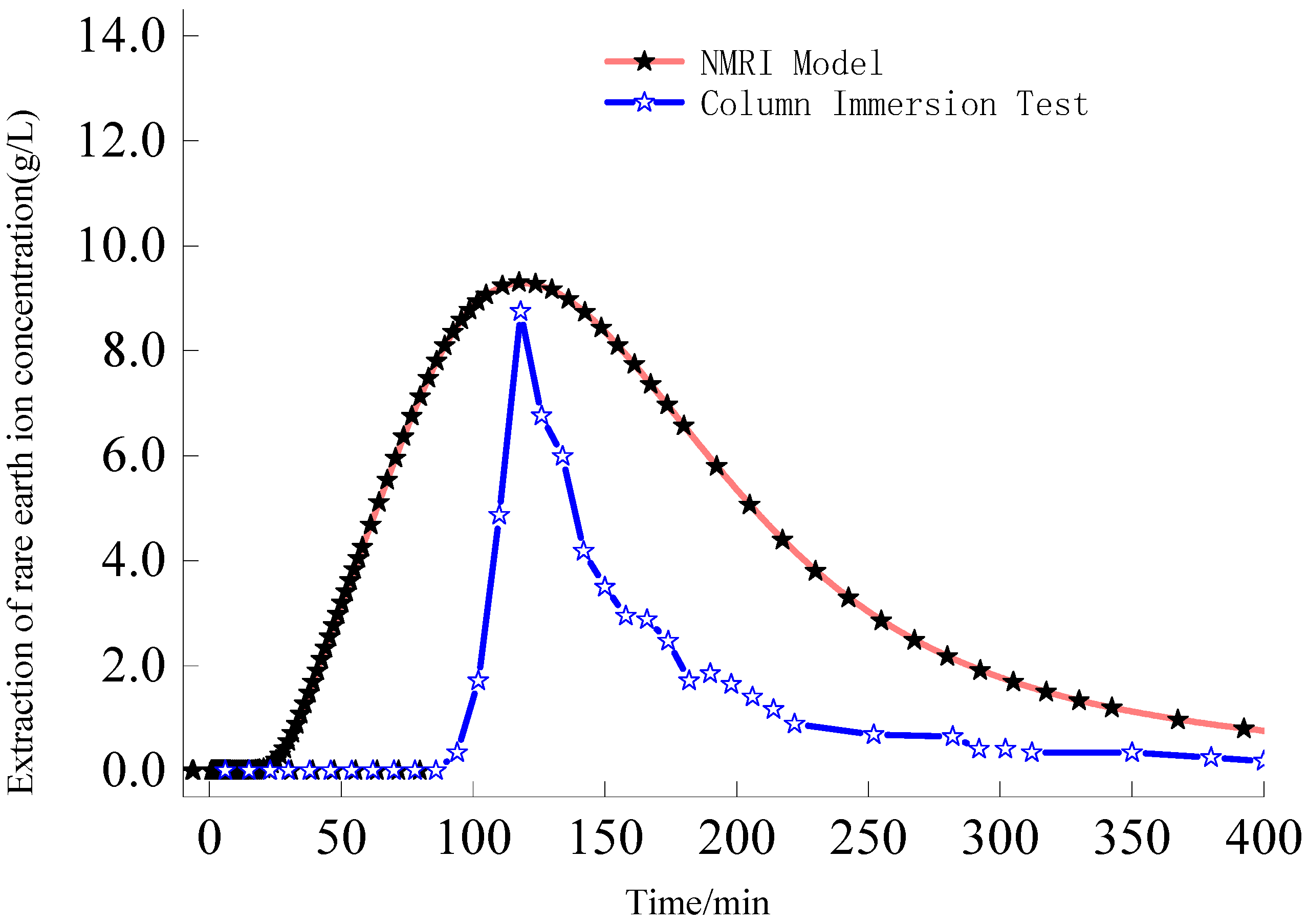
- The samples were scanned by NMR, the scanned NMR images were vectorized by image processing technology, and a two-dimensional pore percolation channel geometric model of ionic rare earth ore was established, and combined with control equations of ionic rare earth pore percolation–exchange–migration to simulate the meso-leaching-process of rare earth ions.
- Increasing the injection intensity can facilitate rare earth ions to leach out earlier and outflow from the ore body, thereby shortening the leaching period and expediting the leaching process. However, it can also lead to a decrease in the peak concentration of rare earth ions in the leaching process. For this mining area, it is recommended to control the injection intensity between 0.50 mL/min and 1.00 mL/min.
- Increasing the concentration of leaching solution accelerates the forward exchange rate of leaching agent cations and rare earth ions, thereby enhancing the leaching efficiency of rare earth ions. The greater the concentration gradient of rare earth ions in the leaching solution, the more significant the molecular diffusion effect and the faster the ion migration rate. Although the high concentration leaching solution is conducive to the leaching of rare earth ions, once the rare earth ions are essentially leached from the ore body, on the one hand, the exchange competition between rare earth and impurity ions is weakened, and impurity ions are leached out under better exchange conditions. On the other hand, the continuous injection of leaching solution causes a waste of resources. For this mining area, it is recommended to control the concentration of leaching solution between 0.12 mol/L and 0.24 mol/L.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Rao, Y.Z.; Tian, C.S.; Huang, T.; Lu, J.C.; Zhang, M.D.; Han, M. Adsorption Performance of La(III) and Y(III) on Orange Peel: Impact of Experimental Variables, Isotherms, and Kinetics. Adsorpt. Sci. Technol. 2021, 2021, 7189639. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.X.; Liu, Q.; Huang, S.H.; Wu, X.Y.; Wang, Z.W.; Zhang, L.S.; Chi, R.A. Modified tailings of weathered crust elution-deposited rare earth ores as adsorbents for recovery of rare earth ions from solutions: Kinetics and thermodynamics studies. Miner. Eng. 2023, 191, 107937. [Google Scholar] [CrossRef]
- He, Z.Y.; Zhang, R.; Sha, A.Y.; Zuo, Q.; Xu, Z.G.; Wu, M.; Chi, R.A. Anti-swelling mechanism of DMDACC on weathered crust elution-deposited rare earth ore. J. Rare Earths 2022, 40, 1803–1811. [Google Scholar] [CrossRef]
- Han, M.; Wang, D.; Rao, Y.; Xu, W.; Nie, W. An Experimental Study on the Kinetics of Leaching Ion-Adsorbed REE Deposits with Different Concentrations of Magnesium Sulfate. Metals 2023, 13, 1906. [Google Scholar] [CrossRef]
- Luo, X.P.; Yuan, X.Q.; He, K.Z.; Zhang, Y.B.; Luo, C.G.; Liu, Z.S.; Zhou, H.P. Precipitation process for combined impurity removal from a magnesium sulphate-based leachate of ionic rare earth ore. Miner. Eng. 2022, 189, 107911. [Google Scholar] [CrossRef]
- Li, L.Q.; Liu, C.H.; Zhang, H.P.; Huang, B.; Luo, B.; Bie, C.; Sun, X.Q. The enrichment of rare earth from magnesium salt leaching solution of ion-adsorbed type deposit: A waste-free process for removing impurities. J. Environ. Manag. 2022, 310, 114743. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhou, F.; Liu, C.F. Effect of polyacrylamide on the process of removing impurities in the rare earth leachate. Physicochem. Probl. Miner. Process. 2021, 57, 182–191. [Google Scholar] [CrossRef]
- Bezaatpour, J.; Fatehifar, E.; Rasoulzadeh, A. Coarse-grained geological porous media structure modeling using heuristic algorithm and evaluation of porosity, hydraulic conductivity, and pressure drop with experimental results. Environ. Earth Sci. 2021, 80, 1–14. [Google Scholar] [CrossRef]
- Zhao, K.; Zhuo, Y.L.; Wang, X.J.; Zhong, W. Aggregate Evolution Mechanism during Ion-Adsorption Rare Earth Ore Leaching. Adv. Mater. Sci. Eng. 2018, 2018, 4206836. [Google Scholar] [CrossRef]
- Wang, S.F.; Lv, L.; Xue, K.X.; Zhang, D.J.; Li, M.D.; Li, D.W.; Yuan, C. Micropore Structure and Fractal Characteristics of Clays Due to Freeze-Thaw and Compression Based on Mercury Intrusion Porosimetry. Front. Earth Sci. 2022, 10, 851673. [Google Scholar] [CrossRef]
- Gong, L.X.; Nie, L.; Xu, Y.; Ji, X.K.; Liu, B.M. Characterization of Micro-Scale Pore Structure and Permeability Simulation of Peat Soil Based on 2D/3D X-ray Computed Tomography Images. Eurasian Soil Sci. 2022, 55, 790–801. [Google Scholar] [CrossRef]
- Yin, S.H.; Chen, X.; Yan, R.F.; Wang, L.M. Pore structure characterization of undisturbed weathered crust elution-deposited rare earth ore based on X-ray micro-CT scanning. Minerals 2021, 11, 236. [Google Scholar] [CrossRef]
- Kihm, J.H.; Hwang, G. Numerical Simulation of Water Table Drawdown due to Groundwater Pumping in a Contaminated Aquifer System at a Shooting Test Site, Pocheon, Korea. Econ. Environ. Geol. 2021, 54, 247–257. [Google Scholar] [CrossRef]
- Lu, D.C.; Li, X.Q.; Du, X.L.; Lin, Q.T.; Gong, Q.M. Numerical simulation and analysis on the mechanical responses of the urban existing subway tunnel during the rising groundwater. Tunn. Undergr. Space Technol. Inc. Trenchless Technol. Res. 2020, 98, 103297. [Google Scholar] [CrossRef]
- Xu, J.; Lan, W.; Ren, C.; Zhou, X.G.; Wang, S.H.; Yuan, J. Modeling of coupled transfer of water, heat and solute in saline loess considering sodium sulfate crystallization. Cold Reg. Sci. Technol. 2021, 189, 103335. [Google Scholar] [CrossRef]
- Wu, Z.J.; Lu, H.; Weng, L.; Liu, Q.S.; Shen, J.Q. Study on the seepage characteristics of fractured sandstone based on real-time imaging technology of nuclear magnetic resonance. Chin. J. Rock Mech. Eng. 2021, 40, 263–275. [Google Scholar]
- Sheikhzadeh, G.A.; Mehrabian, M.A.; Mansouri, S.H.; Sarrafi, A. Computational modelling of unsaturated flow of liquid in heap leaching—Using the results of column tests to calibrate the model. Int. J. Heat Mass Transf. 2005, 48, 279–292. [Google Scholar] [CrossRef]
- Wu, C.Y. Simulation and Analysis for Percolation Leaching Process of Ionic Rare Earth Ore. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2016. [Google Scholar]
- Liu, Q.S.; Xiao, H.; Tan, C.L.; Yu, X.Y.; Wang, D.L.; Qiu, T.S. Coupled simulation of percolation-reaction-stress in leaching process of ion-type rare earth ore. J. Chin. Soc. Rare Earths 2022, 40, 880–892. [Google Scholar]
- Zeng, J. Numerical simulation of seepage characteristics and ion exchange reaction in leaching process of ionic rare earth ore. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2022. [Google Scholar]
- Wu, A.X.; Yin, S.H.; Wang, H.J.; Su, D.Y. Solutes transport mechanism and model of dump leaching. J. Cent. South Univ. 2006, 2, 385–389. [Google Scholar]
- Wu, A.X.; Liu, J.Z.; Tang, L.Y. Simulation of Coupled Flowing-Reaction-Deformation with Mass Transfer in Heap Leaching Processes. Appl. Math. Mech. 2007, 28, 327–335. [Google Scholar] [CrossRef]
- Wang, D.; Wu, F.Y.; Rao, Y.Z.; Xu, W.; Han, M.; Shi, L. Simulation of an Ionic Rare Earth Leaching Process Based on the Darcy Law-Chemical Reaction Engineering-Transfer of Dilute Substance Coupling. Minerals 2022, 12, 1500. [Google Scholar] [CrossRef]
- Hu, S.L.; Cao, X.J.; Wang, G.S.; Long, P.; Zhou, X.Y. An Ion Exchange Model for Leaching Process of Weathered Crust Elution-deposited Rare Earth. Min. Metall. Eng. 2018, 38, 1–5. [Google Scholar]
- Long, P.; Wang, G.S.; Tian, J.; Hu, S.L.; Luo, S.H. Simulation of one-dimensional column leaching of weathered crust elution-deposited rare earth ore. Trans. Nonferrous Met. Soc. China 2019, 29, 625–633. [Google Scholar] [CrossRef]
- Li, Q.; Qin, L.; Wang, G.S.; Long, P.; Peng, C.L. Leaching Mechanism of Ion-Adsorption Rare Earth. J. Chin. Soc. Rare Earths 2021, 39, 543–554. [Google Scholar]
- Guo, Z.Q.; Zhao, K.; Jin, J.F.; Zhu, Z.C.; Li, G. Solute Transport Mechanism of Ion-Adsorption Type R are Earth in-situ Leaching Mining. J. Chin. Soc. Rare Earths 2019, 37, 121–128. [Google Scholar]
- Liu, J.; Brady, B.H. Evaluation of velocity-dependent in situ leaching processes: Single-porosity model. Metall. Mater. Trans. B. Process Metall. Mater. Process. Sci. 1998, 29, 1227–1234. [Google Scholar] [CrossRef]
- Phong, P.H.; Anh, P.B.V.; Ha, V.T.T.; Hung, L.Q.; Thanh, L. Simulating and Monitoring the Temporal and Spatial Transfer of NPK Fertilizer in Agricultural Soils Using a Mathematical Model and Multi-Channel Electrical Conductivity Measurement. J. Soil Sci. Plant Nutr. 2020, 21, 374–388. [Google Scholar] [CrossRef]
- Yang, Z.B.; He, Q.Q.; Wang, K.; Liu, N. Application of Portable X-ray Fluorescence Spectrometer in the Investigation of Contaminated Site. Environ. Monit. Forewarning 2023, 15, 23–26+51. [Google Scholar]
- Gao, L.H.; Xing, R.R.; Zhu, Y.R.; Zhao, W.Y. The total amount of rare earth in rare earth ferroalloy was determined by EDTA titration. Met. Funct. Mater. 2022, 29, 98–102. [Google Scholar]
- Yang, B.H.; Wu, A.X.; Miu, X.X.; Liu, J.Z. 3D characterization and analysis of pore structure of packed ore particle beds based on computed tomography images. Trans. Nonferrous Met. Soc. China 2014, 24, 833–838. [Google Scholar] [CrossRef]
- Wu, A.X.; Yang, B.H.; Liu, J.Z.; Zhang, J. Analysis of Pore Structure Evolution of Ore Granular Media during Leaching Based on X-ray Computed Tomography. Chin. J. Process Eng. 2007, 5, 960–966. [Google Scholar]
- Luo, X.P.; Zhang, Y.B.; Zhou, H.P.; He, K.Z.; Zhang, B.Y.; Zhang, D.M.; Xiao, W.J. Pore structure characterization and seepage analysis of ionic rare earth orebodies based on computed tomography images. Int. J. Min. Sci. Technol. 2022, 32, 411–421. [Google Scholar] [CrossRef]





| Soil Sample Number | Volume/cm3 | Weight/g | Natural Density/(g/cm3) | Mean Density/(g/cm3) | Porosity (n) | Void Ratio (e) |
|---|---|---|---|---|---|---|
| 1 | 50.0 | 68.51 | 1.37 | 1.38 | 0.473 | 0.898 |
| 2 | 50.0 | 67.85 | 1.36 | |||
| 3 | 50.0 | 70.38 | 1.41 |
| Particle Size | Particle Diameter/mm | |||||
|---|---|---|---|---|---|---|
| >5 | 2.5~5 | 1~2.5 | 0.5~1 | 0.075~0.5 | <0.075 | |
| Percent | 12.83% | 33.70% | 17.53% | 16.87% | 12.63% | 6.43% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wu, F.; Rao, Y.; Zhao, Z.; Xu, W.; Han, M. Microscopic Simulation of RE3+ Migration in Ion-Type Rare Earth Ores Based on Navier–Stokes Equation—Exchange Reaction—Ion Migration Coupling. Metals 2024, 14, 1130. https://doi.org/10.3390/met14101130
Wang D, Wu F, Rao Y, Zhao Z, Xu W, Han M. Microscopic Simulation of RE3+ Migration in Ion-Type Rare Earth Ores Based on Navier–Stokes Equation—Exchange Reaction—Ion Migration Coupling. Metals. 2024; 14(10):1130. https://doi.org/10.3390/met14101130
Chicago/Turabian StyleWang, Dan, Fuyu Wu, Yunzhang Rao, Zhilian Zhao, Wei Xu, and Min Han. 2024. "Microscopic Simulation of RE3+ Migration in Ion-Type Rare Earth Ores Based on Navier–Stokes Equation—Exchange Reaction—Ion Migration Coupling" Metals 14, no. 10: 1130. https://doi.org/10.3390/met14101130
APA StyleWang, D., Wu, F., Rao, Y., Zhao, Z., Xu, W., & Han, M. (2024). Microscopic Simulation of RE3+ Migration in Ion-Type Rare Earth Ores Based on Navier–Stokes Equation—Exchange Reaction—Ion Migration Coupling. Metals, 14(10), 1130. https://doi.org/10.3390/met14101130






