The Dependence of Electrochemical Behavior and Discharge Performance on the Zn/Gd Ratio of Mg-Li-Zn-Gd Anodes for Mg-Air Batteries
Abstract
:1. Introduction
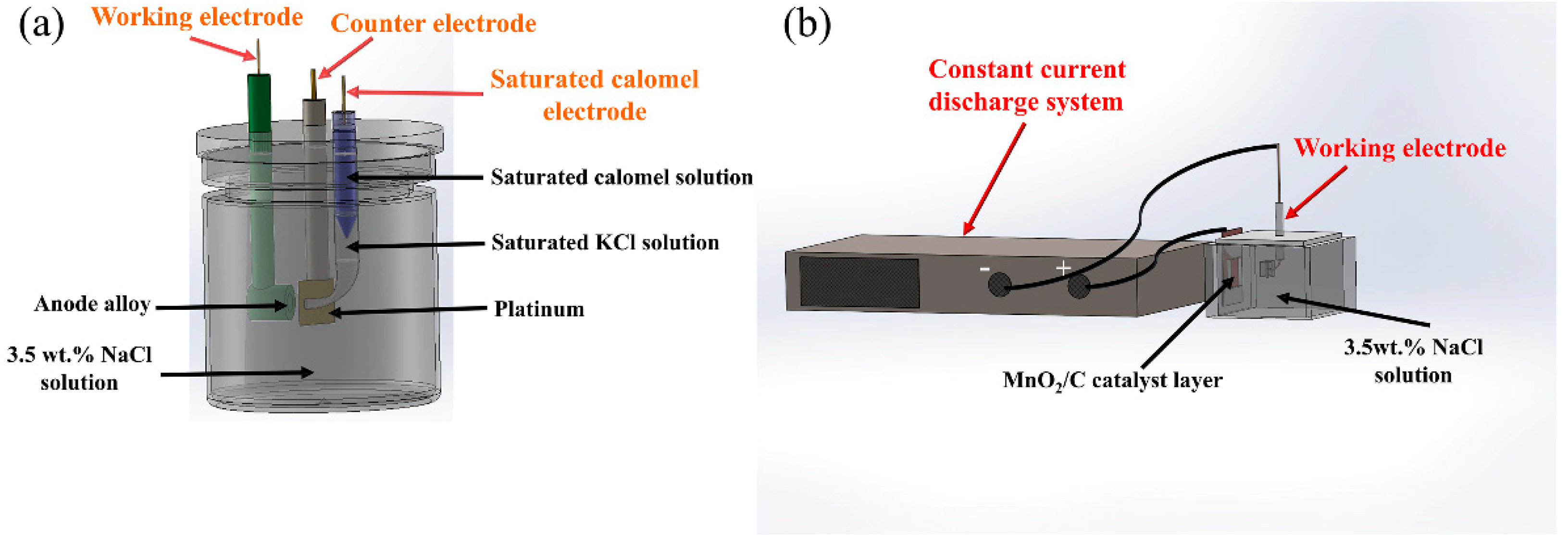
2. Materials and Methods
3. Results and Discussion
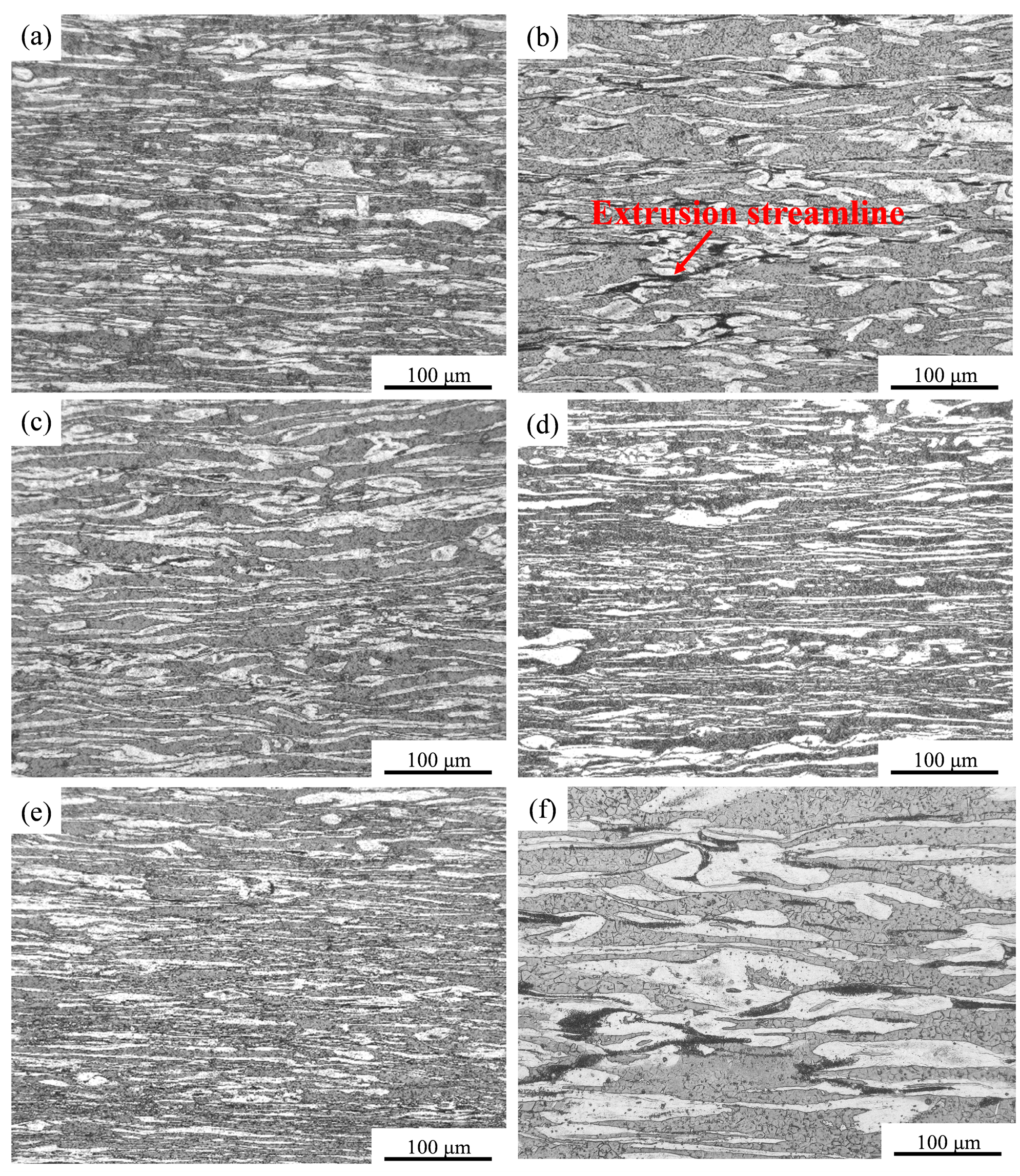
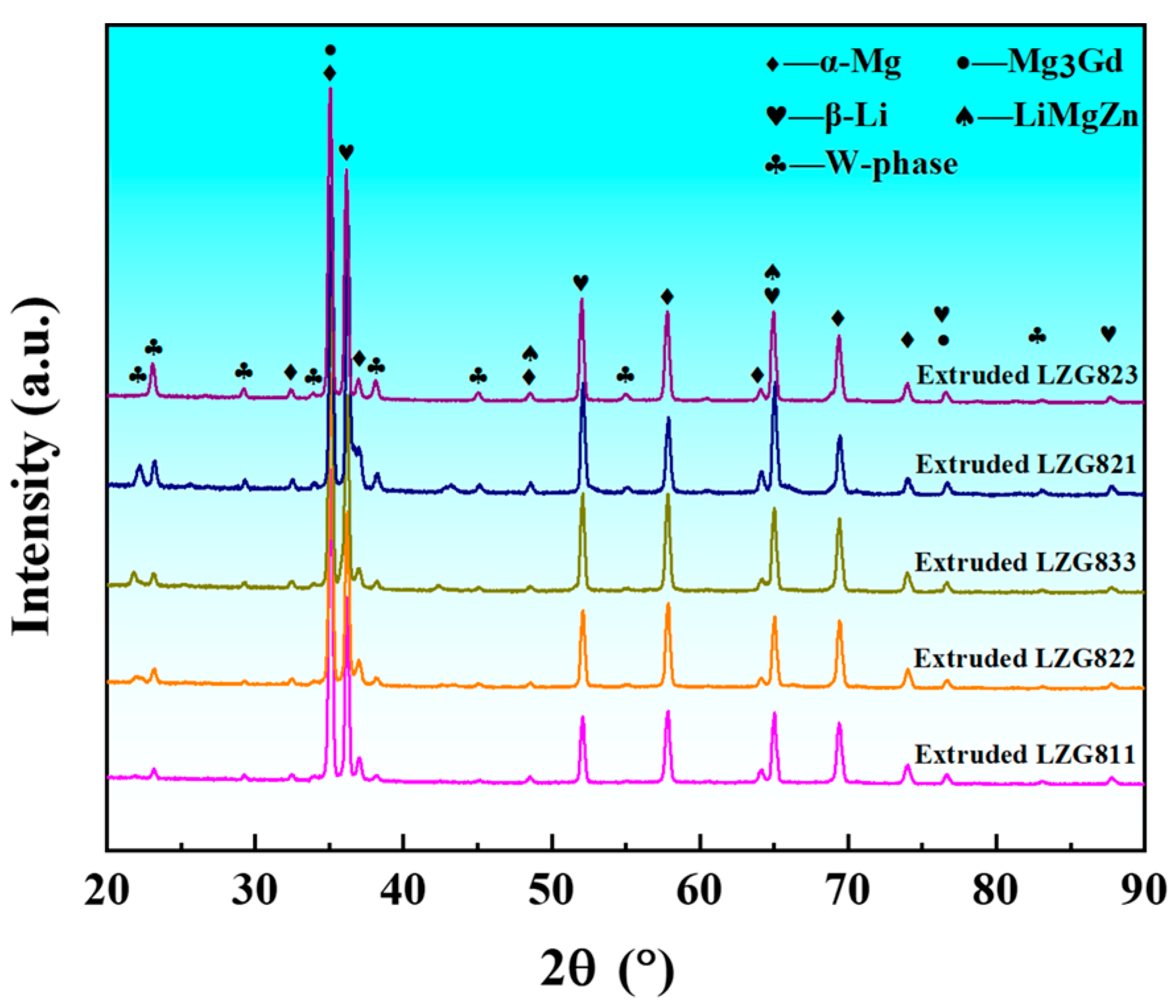
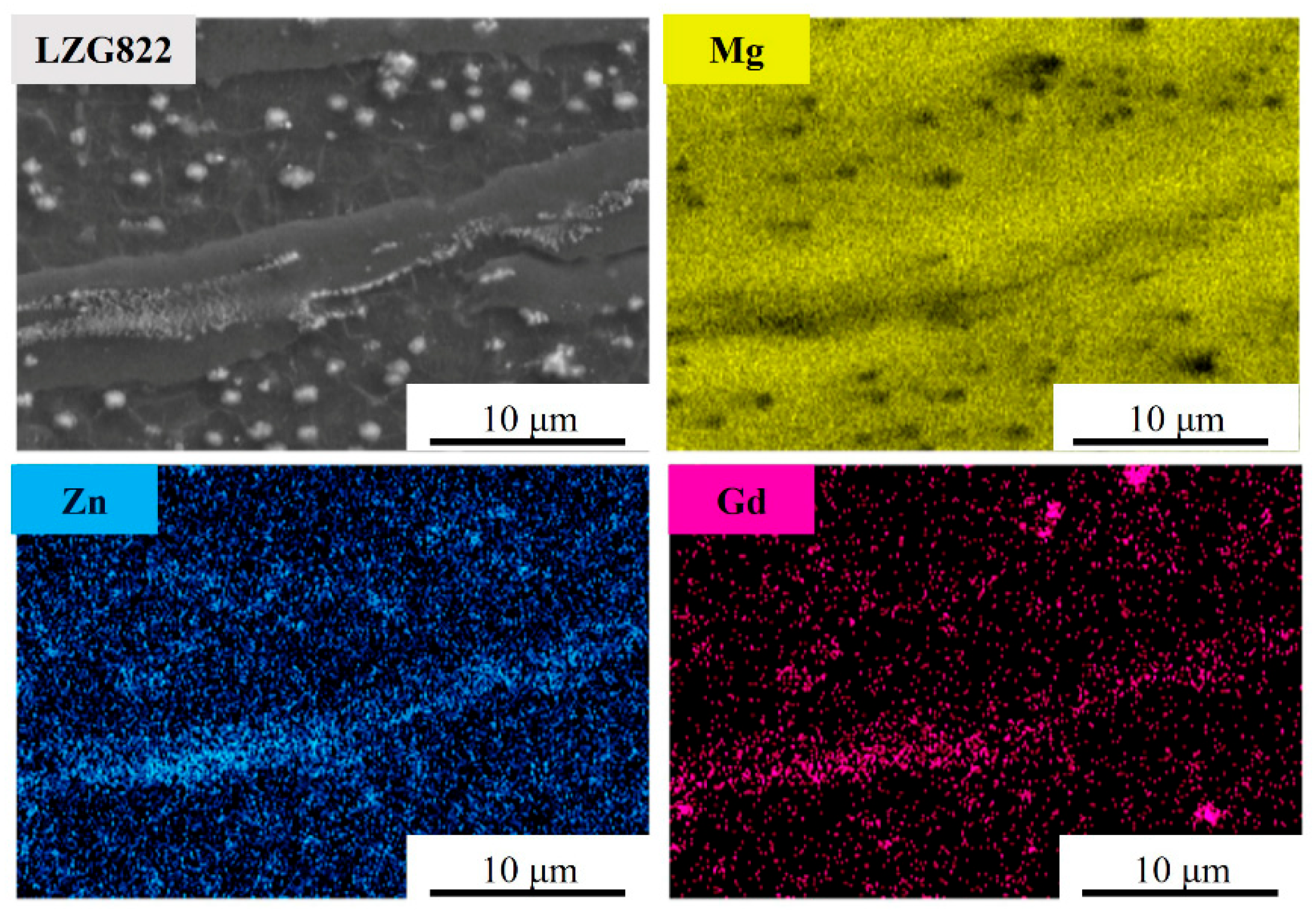
3.1. Microstructure Analysis of Extruded Mg-8Li-xZn-yGd Alloys
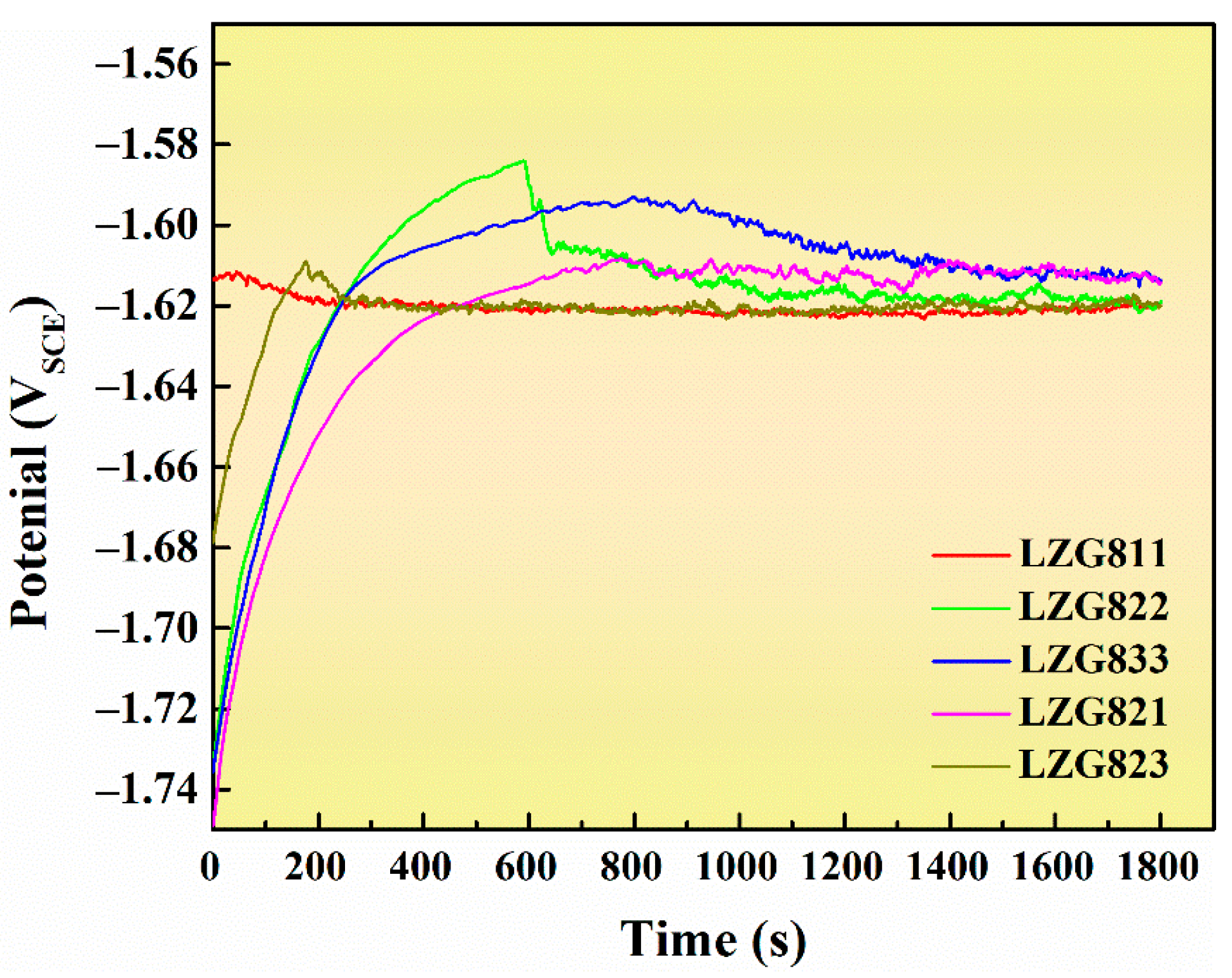
3.2. Corrosion Resistance of Extruded Mg-8Li-xZn-yGd Mg-Air Anode Alloys
3.2.1. Analysis of Static Corrosion Behavior
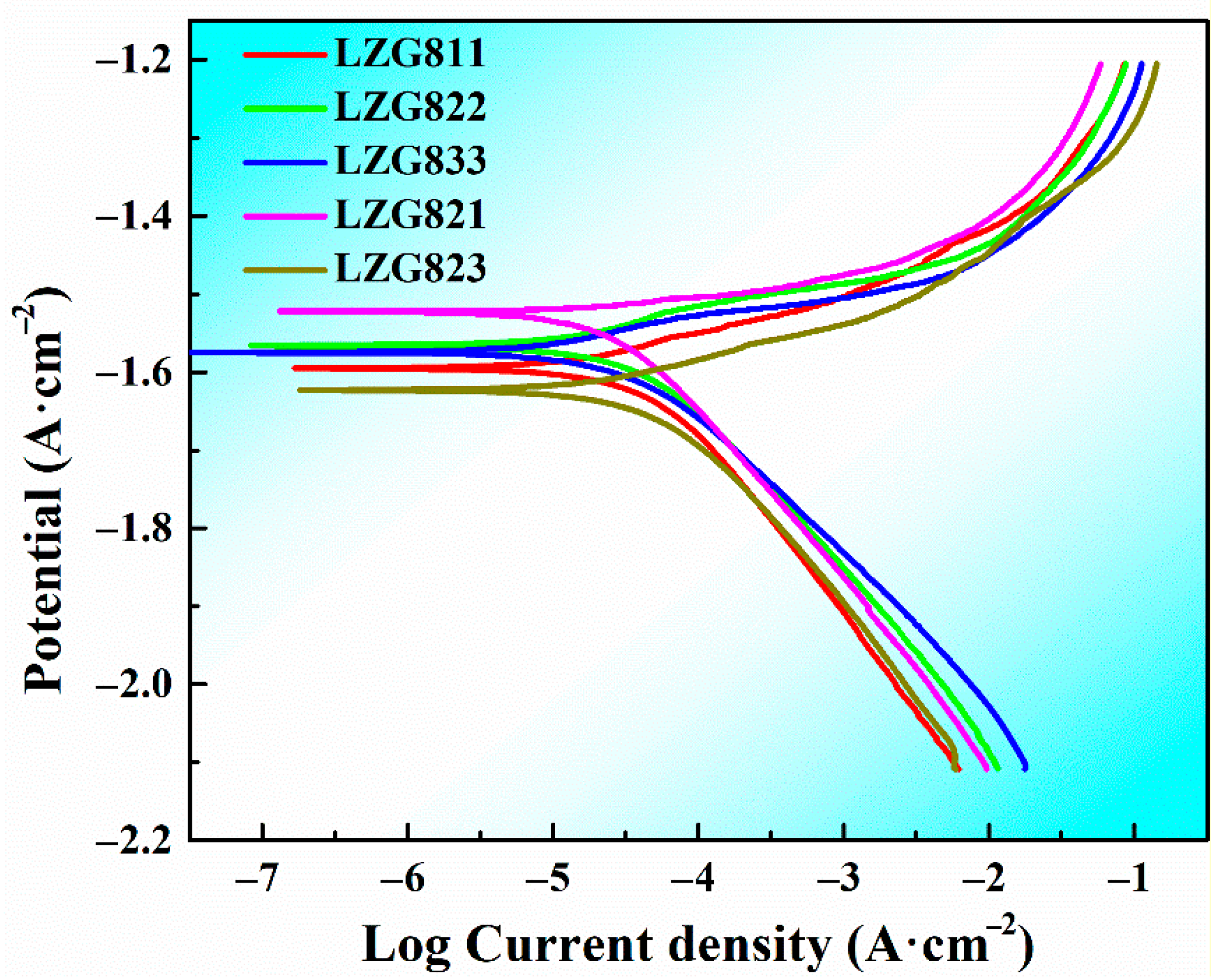
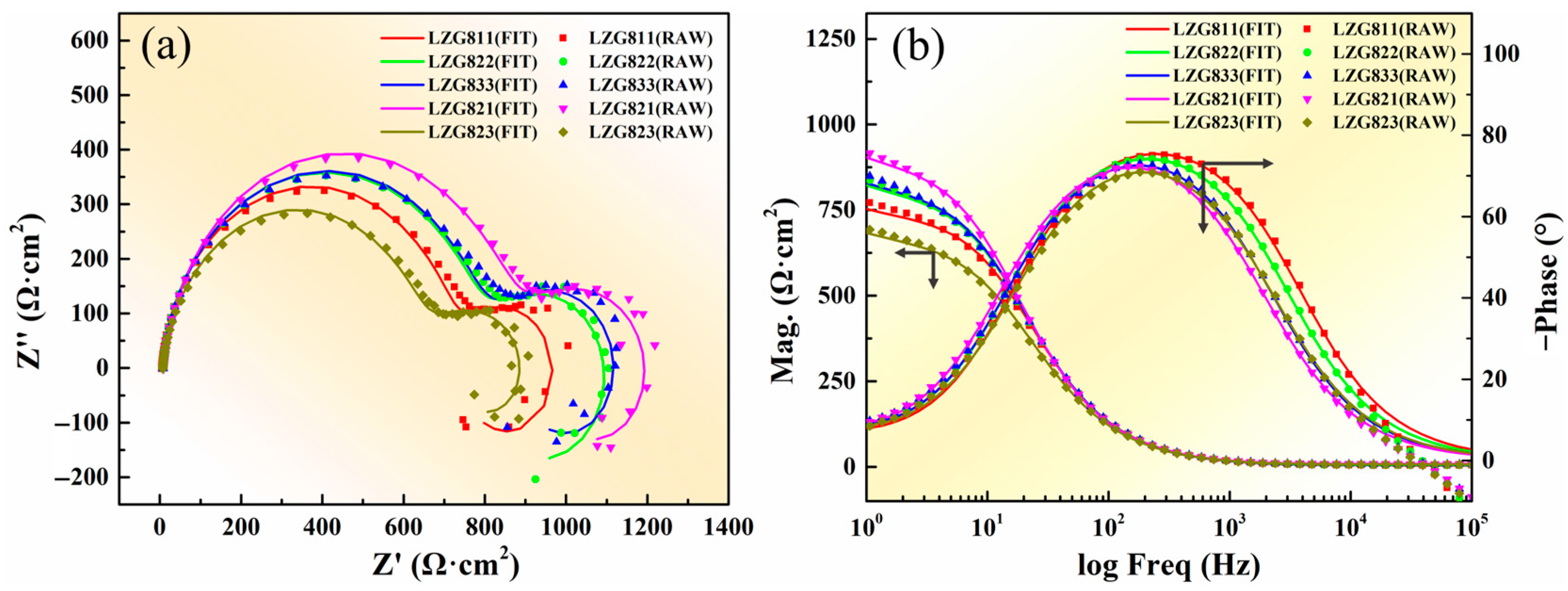
3.2.2. Analysis of Electrochemical Corrosion Behavior
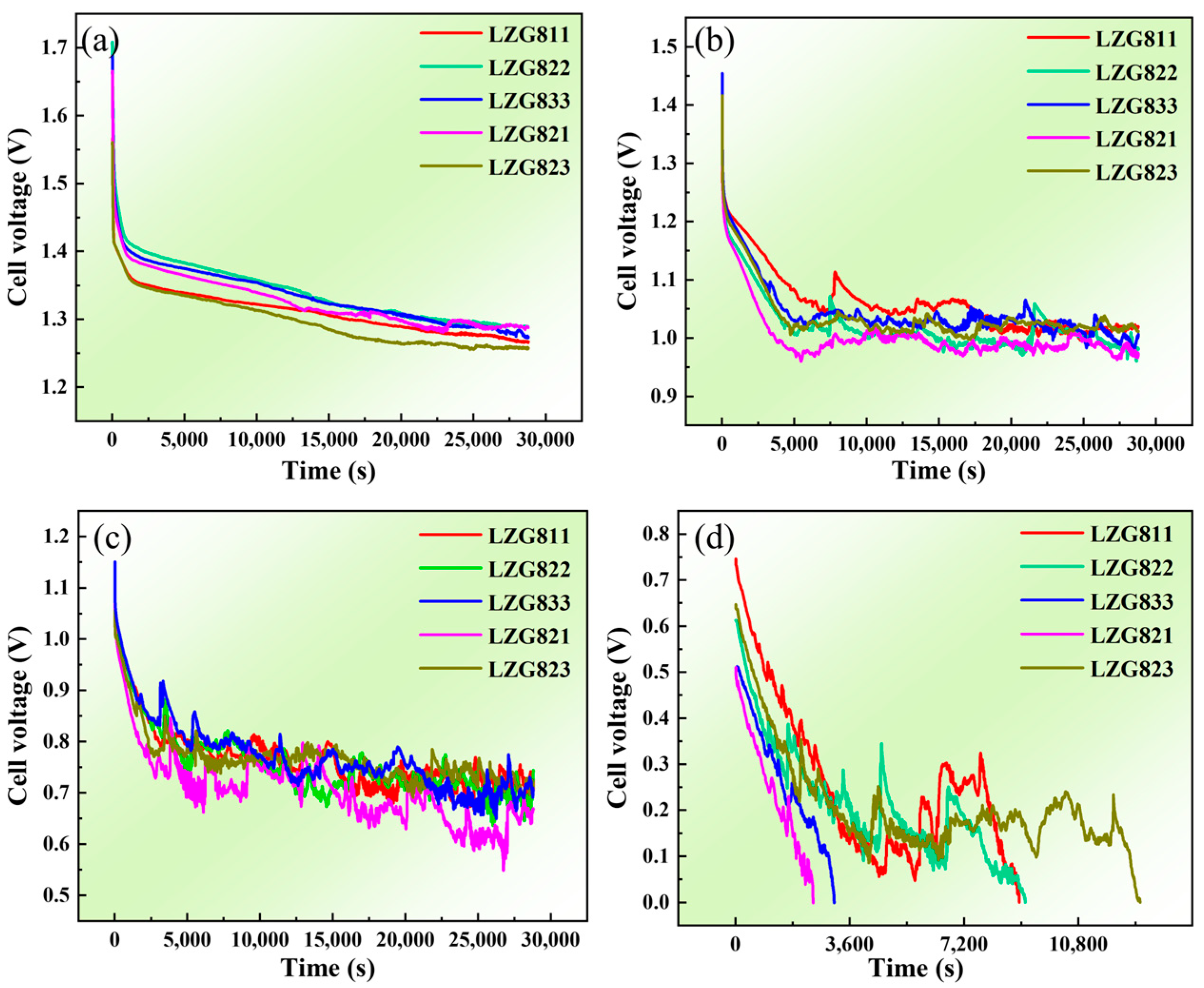
3.3. Discharge Properties of Extruded Mg-8Li-xZn-yGd Mg-Air Anode Alloys
3.3.1. Constant Current Discharge Performance Analysis
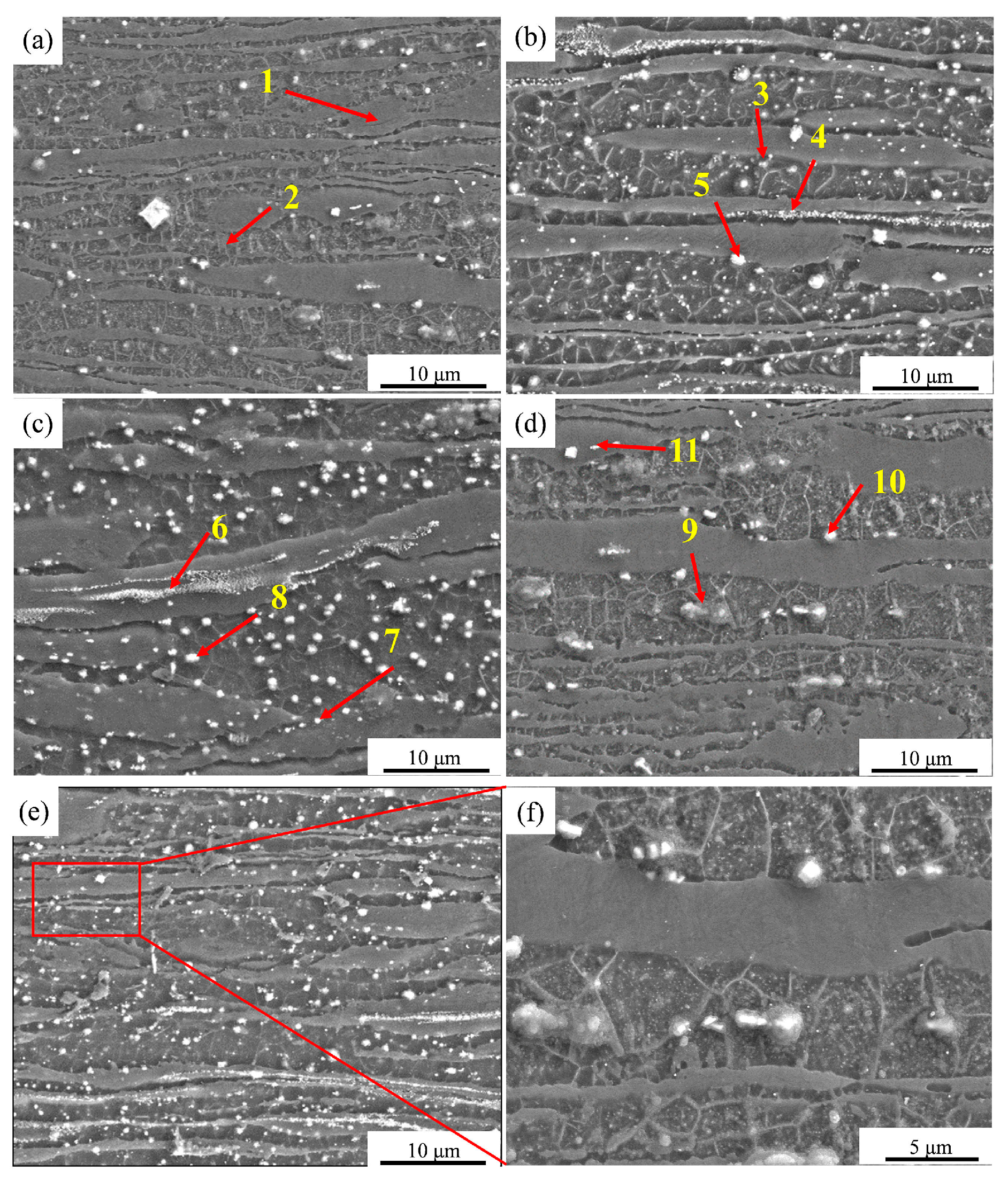
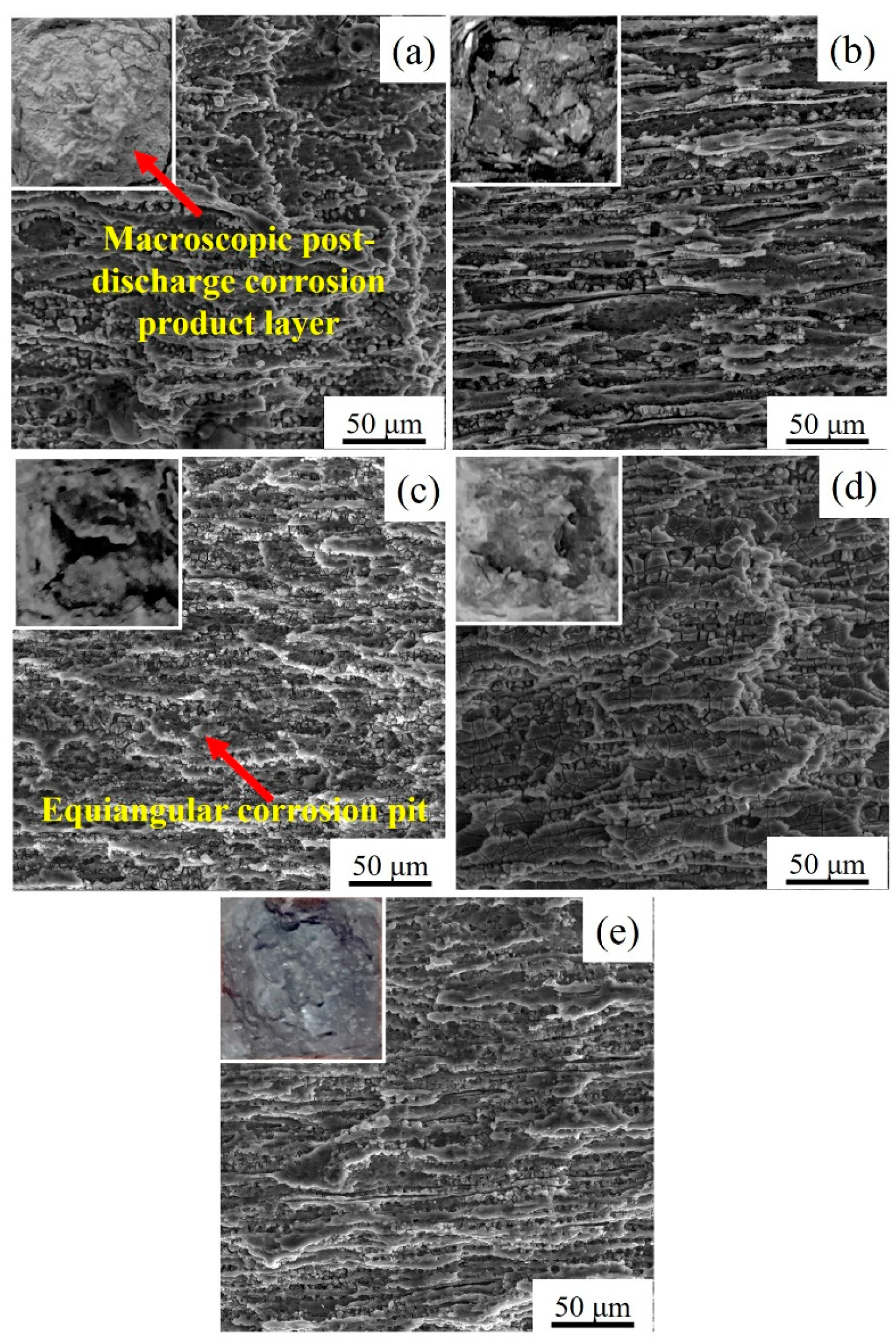
3.3.2. Surface Morphology of Extruded Alloys after Discharge
4. Conclusions
- (1)
- The microstructures of the experimental alloys are composed of an α-Mg and β-Li dual matrix, with W-Mg3Gd2Zn3, Mg3Gd, and MgLiZn second phases. The second phases show no change compared with the cast condition. Meanwhile, extrusion deformation promotes the recrystallization process through the particle-induced nucleation mechanism. When the Zn/Gd ratio is 1, the W-Mg3Gd2Zn3 phase content is increased by the further addition of Zn and Gd.
- (2)
- The corrosion resistance is improved with an increasing Zn/Gd ratio, and the extruded Mg-8Li-2Zn-1Gd (LZG821) alloy has the optimum corrosion resistance, with a corrosion rate of 0.493 mm·year−1, while the extruded LZG823 alloy has the best electrochemical activity. Dynamic recrystallization occurs during extrusion deformation, to reduce the effective corrosion area, improve the density of corrosion products, and enhance the corrosion resistance property.
- (3)
- The electrical properties of the extruded Mg-8Li-2Zn-1Gd (LZG821) and Mg-8Li-2Zn-3Gd (LZG823) alloys decrease at high current densities. The extruded Mg-8Li-1Zn-1Gd (LZG811) alloy has the optimal discharge performance, with a discharge specific capacity of 1371.04 mA·g−1 at a current density of 40 mA∙cm−2, and the anode efficiency reaches nearly 70%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Xiong, W.; Yu, S.; Liu, Y.; Li, J.; Liu, L.; Bi, X.; Zhu, G.; Liu, E.; Zhao, Y.; et al. Effect of Gd content on the discharge and electrochemical behaviors of the magnesium alloy AZ31 as an anode for Mg-air battery. J. Mater. Sci. 2021, 56, 12789–12802. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Peñuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 01, 4–17. [Google Scholar] [CrossRef]
- Taniguchi, A.; Fujioka, N.; Ikoma, M.; Ohta, A. Development of nickel/metal-hydride batteries for EVs and HEVs. J. Power Sources 2001, 100, 117–124. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by side battery technologies with lithium-ion based batteries. Adv. Energy Mater. 2020, 10, 89–110. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Li, H.B.; Yoo, H.D.; Chi, X.; An, Q.; Liu, J.; Yu, M.; Wang, W.; Yao, Y. Mixed-phase mullite electrocatalyst for pH-neutral oxygen reduction in magnesium-air batteries. Nano Energy 2016, 27, 8–16. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Xue, C.; Yang, X.; Wang, S.; Su, H. Ultra-light Mg–Li alloy by design to achieve unprecedented high stiffness using the CALPHAD approach. Calphad 2023, 81, 102556. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Wang, H.; Tong, X.; Zhang, M.; Zhang, Z. Texture evolution and their effects on the mechanical properties of duplex Mg-Li alloy. J. Alloys Compd. 2016, 669, 72–78. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Cui, L.Y.; Chen, X.B.; Zeng, R.C.; Guan, S.K.; Li, S.Q.; Zhang, F.; Zou, Y.H.; Liu, Q.Y. In vitro corrosion of micro-arc oxidation coating on Mg-1Li-1Ca alloy-The influence of intermetallic compound Mg2Ca. J. Alloys Compd. 2018, 764, 250–260. [Google Scholar] [CrossRef]
- Liu, W.; Feng, S.; Li, A.; Zhao, J.; Wu, G.; Wang, X.; Xiao, L.; Ding, W. Effect of rolling strain on microstructure and tensile properties of dual-phase Mg–8Li–3Al–2Zn–0.5Y alloy. J. Mater. Sci. Technol. 2018, 34, 2256–2262. [Google Scholar] [CrossRef]
- Chen, X.B.; Li, C.; Xu, D. Biodegradation of Mg-14Li alloy in simulated body fluid: A proof-of-concept study. Bioact. Mater. 2017, 3, 110–117. [Google Scholar] [CrossRef]
- Zeng, R.C.; Sun, L.; Zheng, Y.F.; Cui, H.Z.; Han, E.H. Corrosion and characterization of dual phase Mg-Li-Ca alloy in Hank’s solution: The influence of microstructural features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, L.; Chen, G.; Wang, L.; Liang, W. Influence of Ca addition on microstructural evolution and mechanical properties of near-eutectic Mg-Li alloys by copper-mold suction casting. J. Alloys Compd. 2016, 664, 85–91. [Google Scholar] [CrossRef]
- Jang, H.S.; Seol, D.; Lee, J. Modified embedded-atom method interatomic potentials for Mg–Al–Ca and Mg–Al–Zn ternary systems. J. Magnes. Alloys 2021, 9, 317–335. [Google Scholar] [CrossRef]
- He, F.Y.; Hu, W.X.; Liu, L.J.; Ma, S.B.; He, W. Effect of Zn addition on microstructure and mechanical properties of Mg-3Y-2Nd-0.5Zr alloy. China Foundry 2023, 20, 299–306. [Google Scholar] [CrossRef]
- Gong, Y.; Wei, K.; Jiang, W.; Xiang, C.; Ding, H.; Wang, Z. Effect of Zn Addition on the Microstructure and Discharge Performance of Mg-Al-Mn-Ca Alloys for Magnesium-Air Batteries. Metals 2024, 14, 1014. [Google Scholar] [CrossRef]
- Gong, C.; He, X.; Fang, D.; Liu, B.; Yan, X. Effect of second phases on discharge properties and corrosion behaviors of the Mg-Ca-Zn anodes for primary Mg-air batteries. J. Alloys Compd. 2020, 861, 158493. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Bao, R.; Wu, R.; Zhang, H. Achieving high strength in a Mg–Li–Zn–Y alloy by α-Mg precipitation. Mater. Sci. Eng. A 2022, 846, 143272. [Google Scholar] [CrossRef]
- Okafor, C.; Datye, A.; Zhang, S.; Schwarz, U.D.; Cai, Y.; Munroe, N. Development and biomaterial characterization of Mg-Li-Zn-Ca alloys. Mater. Today Commun. 2022, 33, 104999. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Wu, R.; An, D.; Wei, Z.; Wang, J.; Feng, J.; Zhang, J.; Hou, L.; Liu, M. Simultaneous achievement of high electromagnetic shielding effectiveness (X-band) and strength in Mg-Li-Zn-Gd/MWCNTs composite. J. Alloys Compd. 2021, 882, 160524. [Google Scholar] [CrossRef]
- Yin, S.; Duan, W.; Liu, W.; Wu, L.; Bao, J.; Yu, J.; Li, L.; Zhao, Z.; Cui, J.; Zhang, Z. Improving the corrosion resistance of MgZn1.2GdxZr0.18 (x=0, 0.8, 1.4, 2.0) alloys via Gd additions. Corros. Sci. 2020, 177, 108962. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Renganathan, N.G.; Gopukumar, S. Performance of a magnesium-lithium alloy as an anode for magnesium batteries. J. Appl. Electrochem. 2004, 34, 1135–1139. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Xue, J. Discharge performance of the magnesium anodes with different phase constitutions for Mg-air batteries. J. Power Sources 2018, 396, 667–674. [Google Scholar] [CrossRef]
- ASTM G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–9.
- Moghanni-Bavil-Olyaei, H.; Arjomandi, J. Performance of Al-1Mg-1Zn-0.1Bi-0.02In as anode for the Al-AgO battery. RSC Adv. 2015, 5, 91273–91279. [Google Scholar] [CrossRef]
- Liang, F.; Lu, H.; Jing, L.; Sun, Z. Performance of Al-0.6Mg-0.05Ga-0.1Sn-0.1In as anode for Al-air battery in KOH electrolytes. J. Electrochem. Soc. 2015, 162, A2623–A2627. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Zhang, S.; Zhang, X.; Lu, C.; Ding, W. Effects of Zn/Gd ratio and content of Zn, Gd on phase constitutions of Mg alloys. Mater. Trans. 2008, 49, 941–944. [Google Scholar] [CrossRef]
- Chang, T.C.; Wang, J.Y.; Chu, C.L.; Lee, S. Mechanical properties and microstructures of various Mg-Li alloys. Mater. Lett. 2006, 60, 3272–3276. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, S.; Tang, Y.; Zhao, D. Quasicrystals in as-cast Mg-Zn-RE alloys. Scr. Metall. Mater. 1993, 28, 1513–1518. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Feng, Y.; Peng, C. Effect of hot rolling on the microstructure and discharge properties of Mg-1.6 wt.% Hg-2 wt.% Ga alloy anodes. J. Alloys Compd. 2018, 765, 736–746. [Google Scholar] [CrossRef]
- Xie, J.S.; Wang, L.L.; Zhang, J.H.; Lu, L.W.; Zhang, Z.; He, Y.Y.; Wu, R.Z. Developing new Mg alloy as potential bone repair material via constructing weak anode nano-lamellar structure. J. Magnes. Alloys 2023, 11, 154–175. [Google Scholar] [CrossRef]
- Geng, X.; Dong, Q.; Zhang, X. Improved corrosion properties of Mg-Gd-Zn-Zr alloy by micro-arc oxidation. Metals 2024, 14, 236. [Google Scholar] [CrossRef]
- Wan, D.Q.; Sun, Y.M.; Xue, Y.D.; Dong, S.Y.; Han, G.L.; Wang, Y.; Yang, F.; Tang, H.; Wang, Y.Y. Improvement in corrosion resistance of Mg97Zn1Y2 alloy by Zr addition. China Foundry 2024. [Google Scholar] [CrossRef]
- Geng, X.; Jiang, J.; Zhang, X. Corrosion Behavior of Mg-xGd-1Zn-0.4Zr Alloys with Different Gd Additions for Biomedical Application. Metals 2022, 12, 1763. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Lai, Y.; Wen, L.; Ai, Y.; Ren, X.; Zhang, W. Study on the Microstructure and Properties of Mg-Gd-Ni-Y Alloy Containing LPSO Phase. Metals 2023, 13, 1989. [Google Scholar] [CrossRef]
- Wan, D.Q.; Xue, Y.D.; Hu, J.J.; Wang, H.B.; Hou, W. Corrosion and chemical behavior of Mg97Zn1Y2-1wt.%SiC under different corrosion solutions. China Foundry 2021, 18, 68–74. [Google Scholar] [CrossRef]
- Bian, J.C.; Yu, B.Y.; Hao, J.F.; Zhu, H.W.; Wu, H.S.; Chen, B.; Li, W.R.; Li, Y.F.; Zheng, L.; Li, R.X. Improvement of microstructure, mechanical properties, and corrosion resistance of WE43 alloy by squeeze casting. China Foundry 2022, 19, 419–426. [Google Scholar] [CrossRef]
- Wang, N.; Li, W.; Huang, Y.; Wu, G.; Hu, M.; Li, G.; Shi, Z. Wrought Mg-Al-Pb-RE alloy strips as the anodes for Mg-air batteries. J. Power Sources 2019, 436, 226855. [Google Scholar] [CrossRef]
- Wang, N.; Mu, Y.; Li, Q.; Shi, Z. Discharge and corrosion behaviour of AP65 magnesium anode plates with different rolling reductions. RSC Adv. 2017, 7, 53226–53235. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wang, C.F.; Yin, S.Q.; Teng, D.; Guan, R.G. Development of low-carbon energy storage material: Electrochemical behavior and discharge properties of iron-bearing Al–Li-based alloys as Al–air battery anodes. J. Power Sources 2023, 585, 233654. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.J.; Xue, J.L. The role of micro-naoscale AlSb precipitates in improving the discharge performance of Al-Sb alloy anodes for Al-air batteries. J. Power Sources 2019, 425, 186–194. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Q.; Le, Q.; Zou, Q.; Wang, H.; Atrens, A. The influence of samarium (Sm) on the discharge and electrochemical behaviors of the magnesium alloy AZ80 as an anode for the Mg-air battery. Electrochim. Acta 2020, 348, 136315. [Google Scholar] [CrossRef]


| Battery Systems | Voltage (V) | Theoretical Energy Density (mWh·g−1) | Theoretical Specific Capacity (mAh·g−1) |
|---|---|---|---|
| Li-air | 2.91 | 13,300 | 3884 |
| Na-air | 2.33 | 2715 | 1165 |
| Zn-air | 1.65 | 1080 | 825 |
| Al-air | 2.71 | 8070 | 2978 |
| Mg-air | 3.10 | 6800 | 2233 |
| Alloy Composition | Alloy Number | Actual Element Content (wt.%) | |||
|---|---|---|---|---|---|
| Mg | Li | Zn | Gd | ||
| Mg-8Li-1Zn-1Gd | LZG811 | Bal. | 8.13 | 1.04 | 0.98 |
| Mg-8Li-2Zn-2Gd | LZG822 | Bal. | 7.91 | 1.91 | 2.06 |
| Mg-8Li-3Zn-3Gd | LZG833 | Bal. | 7.88 | 3.08 | 3.11 |
| Mg-8Li-2Zn-1Gd | LZG821 | Bal. | 8.25 | 2.10 | 1.03 |
| Mg-8Li-2Zn-3Gd | LZG823 | Bal. | 8.04 | 1.93 | 2.93 |
| Marking Point | Mg (at.%) | Zn (at.%) | Gd (at.%) | Second Phase |
|---|---|---|---|---|
| 1 | 99.51 | 0.43 | 0.06 | α-Mg |
| 2 | 99.41 | 0.38 | 0.21 | β-Li |
| 3 | 99.27 | 0.73 | \ | MgLiZn |
| 4 | 96.23 | 2.17 | 1.6 | W phase (Mg3Zn3Gd2) |
| 5 | 72.72 | 0.56 | 26.72 | Mg3Gd |
| 6 | 89.12 | 6.14 | 4.74 | W phase (Mg3Zn3Gd2) |
| 7 | 98.72 | 1.26 | 0.02 | MgLiZn |
| 8 | 76.46 | 1.14 | 22.41 | Mg3Gd |
| 9 | 98.28 | 1.72 | \ | MgLiZn |
| 10 | 80.04 | 0.64 | 19.32 | Mg3Gd |
| 11 | 87.43 | 8.61 | 3.96 | W phase (Mg3Zn3Gd2) |
| Alloy | LZG811 | LZG822 | LZG833 | LZG821 | LZG823 |
|---|---|---|---|---|---|
| OCP/V | −1.62051 ± 0.017 | −1.61893 ± 0.009 | −1.61244 ± 0.018 | −1.61234 ± 0.012 | −1.62068 ± 0.024 |
| Alloys | Ecorr/V | Icorr/mA·cm−2 | Pi/mm·year−1 |
|---|---|---|---|
| LZG811 | −1.595 | 0.03296 | 0.753 |
| LZG822 | −1.566 | 0.02964 | 0.677 |
| LZG833 | −1.575 | 0.02672 | 0.611 |
| LZG821 | −1.522 | 0.01922 | 0.439 |
| LZG823 | −1.623 | 0.03655 | 0.835 |
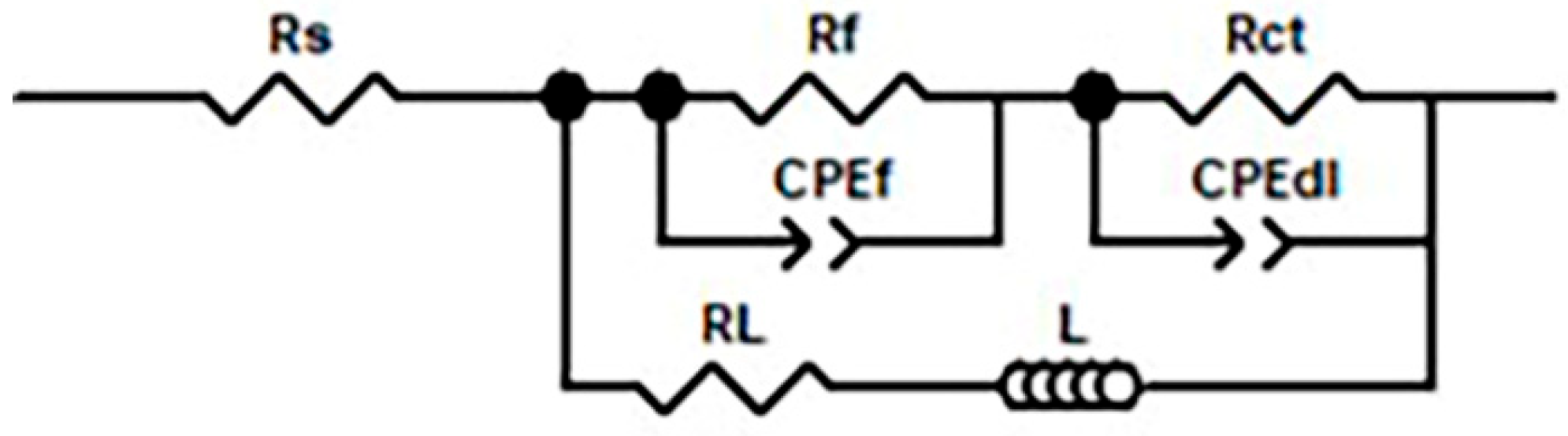
| Alloys | RS | CPEdl | Rct | CPEf | Rf | L | RL | ||
|---|---|---|---|---|---|---|---|---|---|
| Ω·cm2 | Y1/ μΩ−1·cm−2·Sn | n1 | Ω·cm2 | Y2/ μΩ−1·cm−2·Sn | n2 | Ω·cm2 | H·cm2 | Ω·cm2 | |
| LZG811 | 4.45 | 18.08 | 0.937 | 719.4 | 33.13 | 0.802 | 342.4 | 25,864 | 2417 |
| LZG822 | 5.63 | 19.02 | 0.931 | 778.6 | 26.86 | 0.791 | 379.6 | 52,325 | 2275 |
| LZG833 | 7.58 | 19.20 | 0.930 | 783.2 | 28.92 | 0.762 | 474.5 | 34,885 | 2895 |
| LZG821 | 8.66 | 20.13 | 0.925 | 860.9 | 28.11 | 0.78 | 411.4 | 63,879 | 3460 |
| LZG823 | 7.10 | 22.16 | 0.918 | 642.8 | 32.39 | 0.775 | 290.7 | 52,189 | 3172 |
| Alloys | Discharge Specific Capacity/mA·g−1 | Anode Efficiency/% | ||||
|---|---|---|---|---|---|---|
| 5 mA∙cm−2 | 20 mA∙cm−2 | 40 mA∙cm−2 | 5 mA∙cm−2 | 20 mA∙cm−2 | 40 mA∙cm−2 | |
| LZG811 | 772.20 | 1283.08 | 1371.04 | 40 | 65 | 70 |
| LZG822 | 761.90 | 1186.06 | 1330.01 | 39 | 62 | 69 |
| LZG833 | 733.95 | 1164.48 | 1286.17 | 39 | 62 | 67 |
| LZG821 | 662.25 | 1080.35 | 1148.19 | 34 | 56 | 59 |
| LZG823 | 684.93 | 1155.24 | 1229.35 | 36 | 59 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Wang, N.; Han, H.; Liu, Z.; Zhang, G.; Guan, R. The Dependence of Electrochemical Behavior and Discharge Performance on the Zn/Gd Ratio of Mg-Li-Zn-Gd Anodes for Mg-Air Batteries. Metals 2024, 14, 1202. https://doi.org/10.3390/met14111202
Yin S, Wang N, Han H, Liu Z, Zhang G, Guan R. The Dependence of Electrochemical Behavior and Discharge Performance on the Zn/Gd Ratio of Mg-Li-Zn-Gd Anodes for Mg-Air Batteries. Metals. 2024; 14(11):1202. https://doi.org/10.3390/met14111202
Chicago/Turabian StyleYin, Siqi, Ningyuan Wang, Haoxuan Han, Zichen Liu, Guangzong Zhang, and Renguo Guan. 2024. "The Dependence of Electrochemical Behavior and Discharge Performance on the Zn/Gd Ratio of Mg-Li-Zn-Gd Anodes for Mg-Air Batteries" Metals 14, no. 11: 1202. https://doi.org/10.3390/met14111202
APA StyleYin, S., Wang, N., Han, H., Liu, Z., Zhang, G., & Guan, R. (2024). The Dependence of Electrochemical Behavior and Discharge Performance on the Zn/Gd Ratio of Mg-Li-Zn-Gd Anodes for Mg-Air Batteries. Metals, 14(11), 1202. https://doi.org/10.3390/met14111202






