The Effect of Cold Rolling on the Corrosion Behaviour of 5083 Aluminium Alloys
Abstract
1. Introduction
2. Materials and Methods
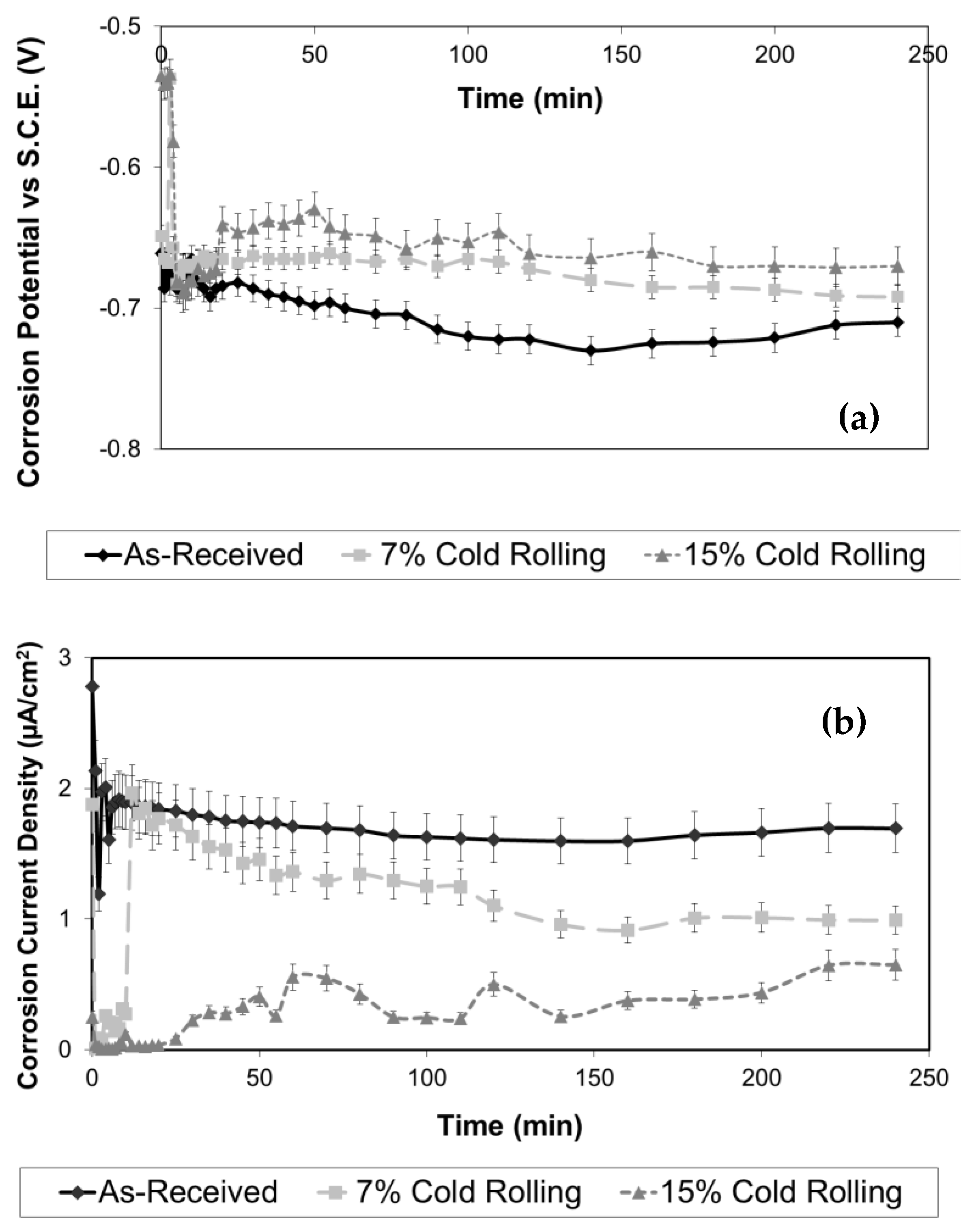
3. Results and Discussion
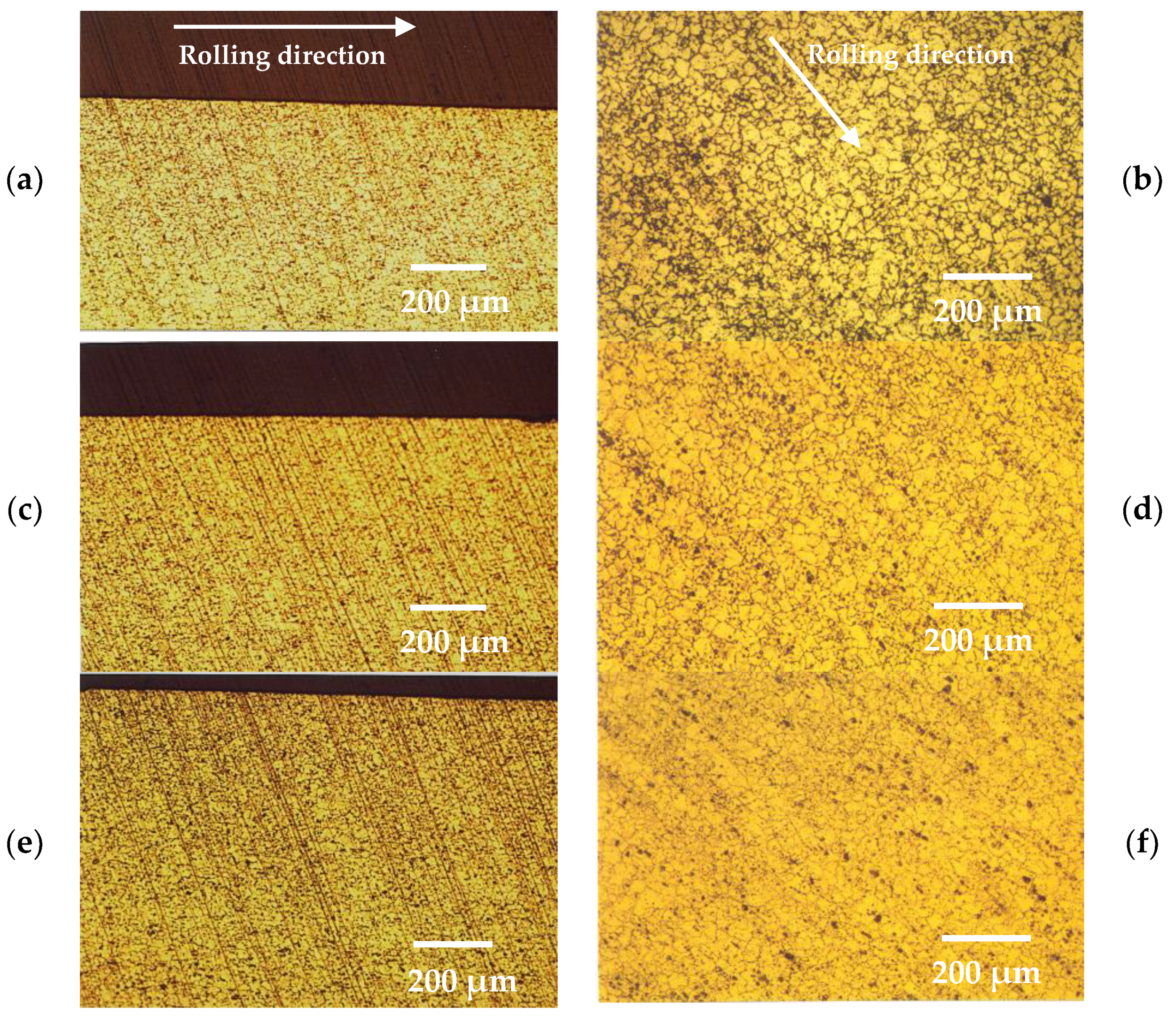
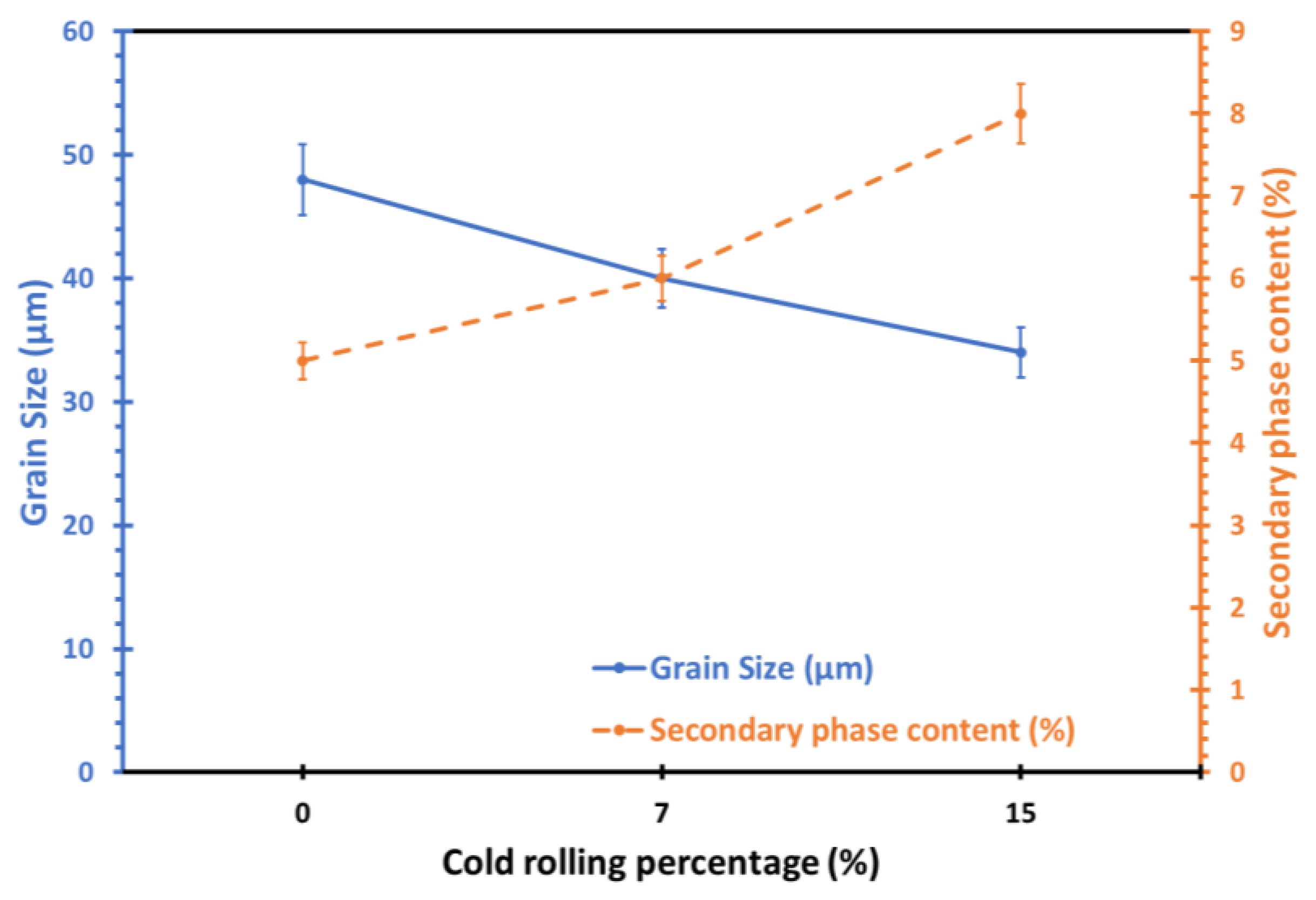
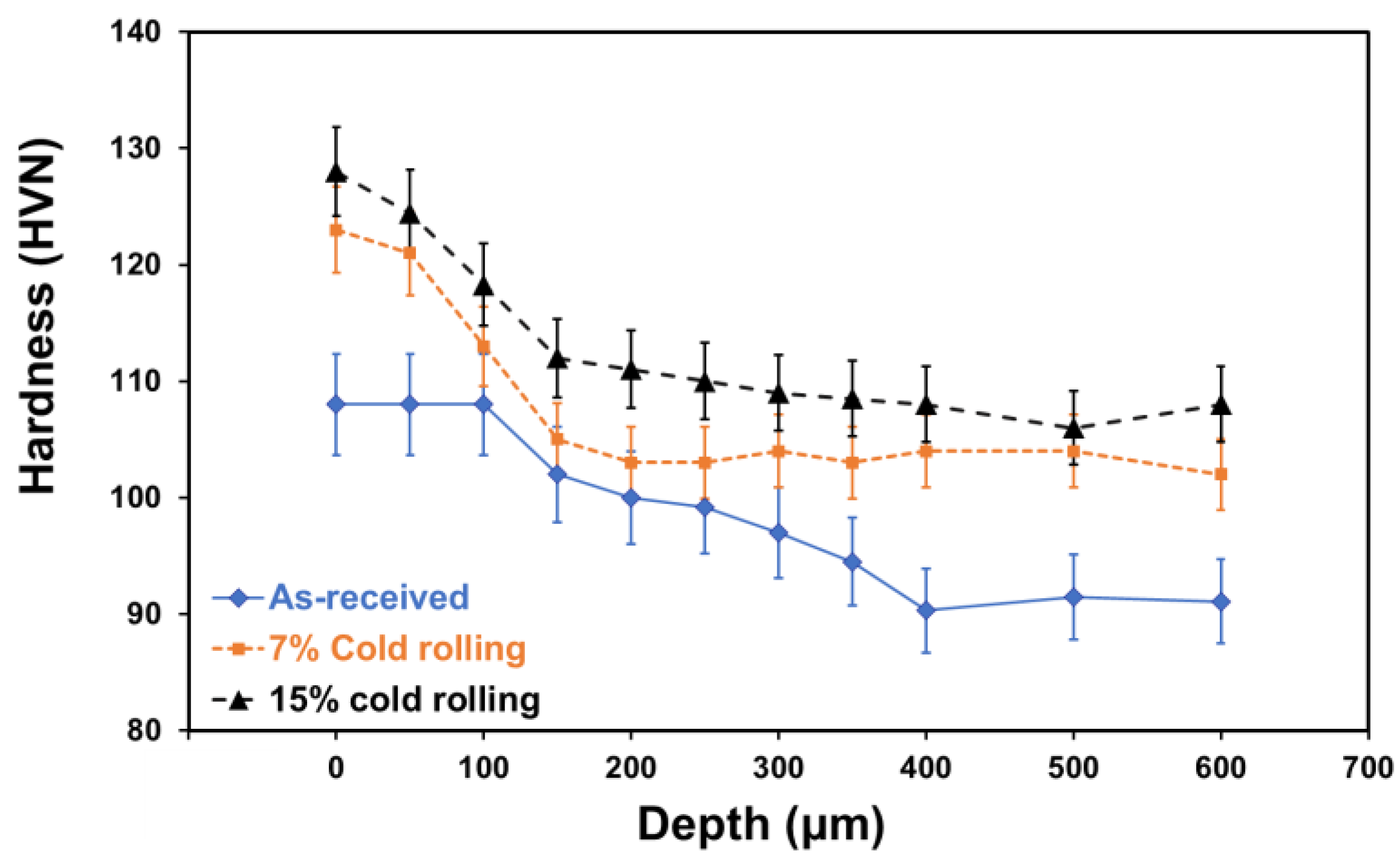
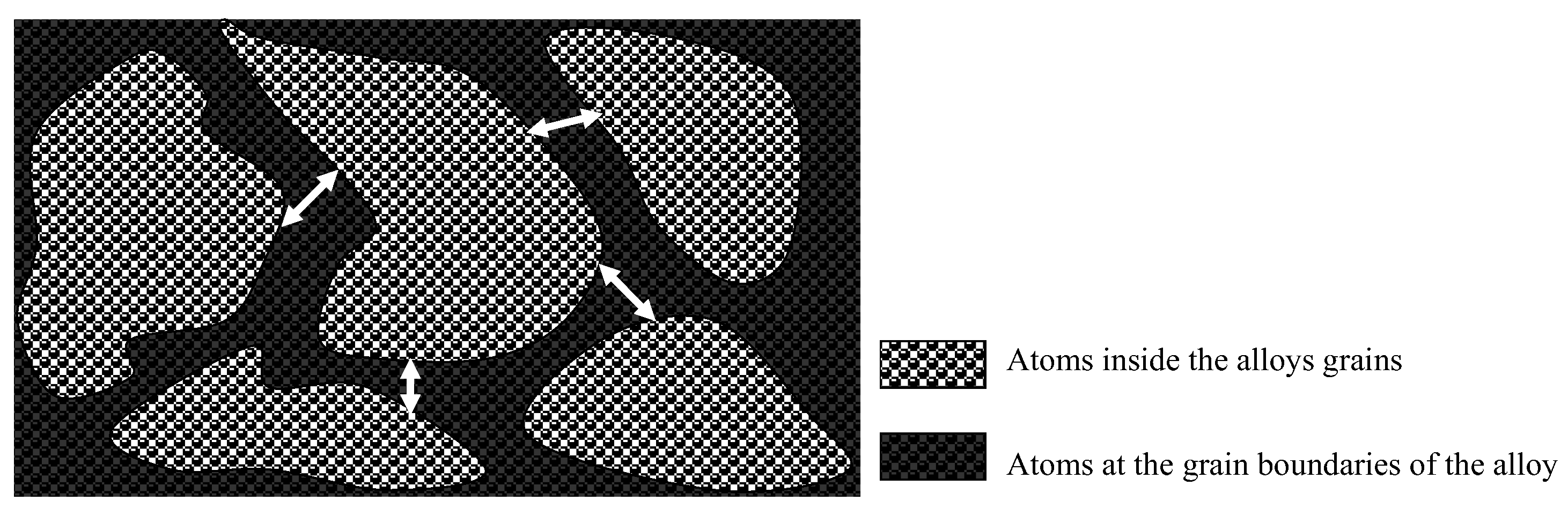
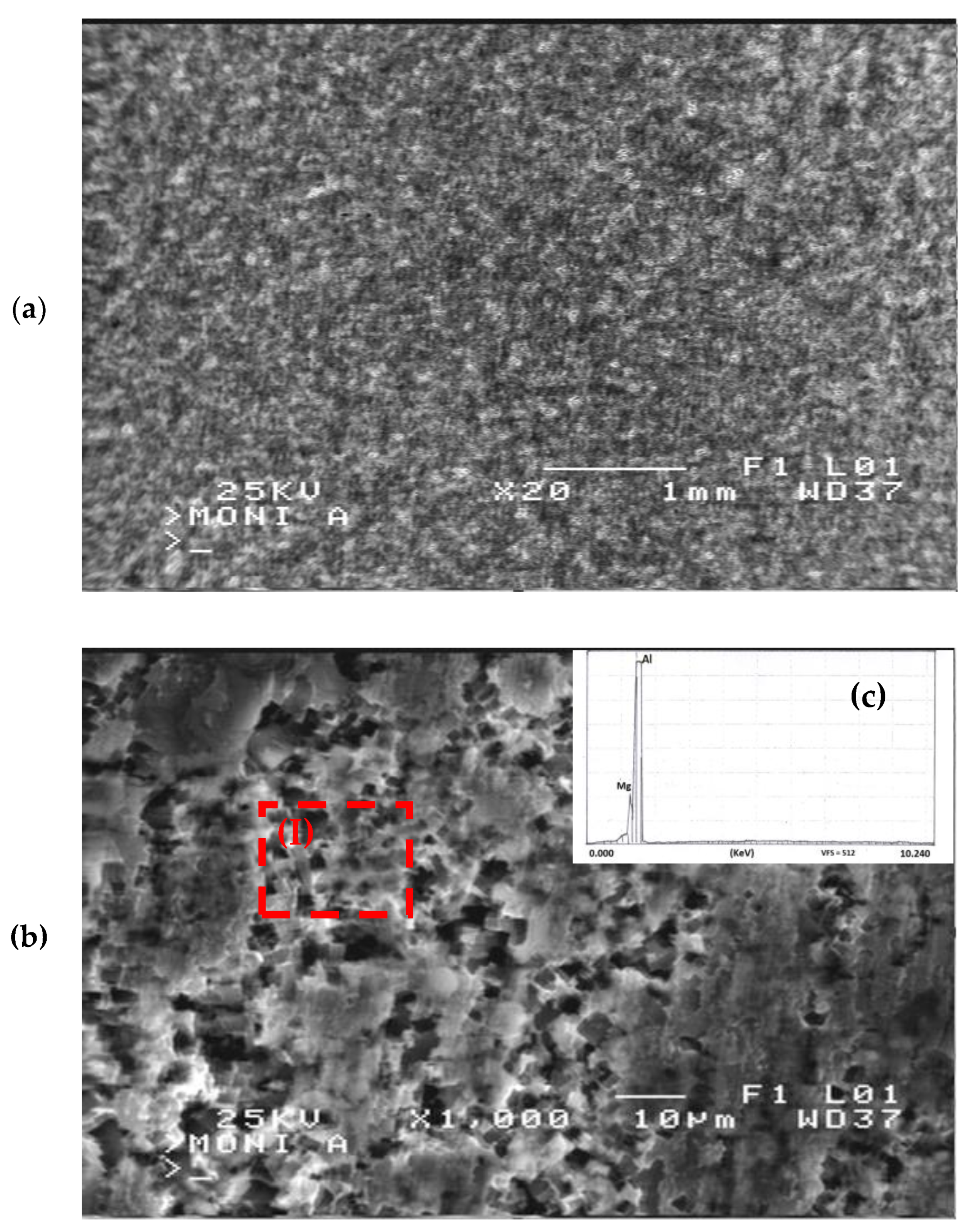
- Grain refinement of the alloy. The grain boundaries in metallic materials are believed to play a crucial role in the corrosion process, acting as ‘easy paths’ for the diffusion of halide ions from the corrosive solution to the surface layers and bulk material. In this case, the cold-rolling process contributed to a decrease in the average grain size of the material (as shown in Figure 4). As the average grain size increases, the grain boundaries become enlarged, as indicated in Figure 7. According to Wagner’s metal oxidation theory [34], the oxidation/corrosion process is determined by diffusion mechanisms that take place in the oxide phase (passivating film) and along the grain boundaries of the bulk material. If the grains are enlarged, more ions can diffuse over time. Moreover, a larger grain size results in the extension of individual grain boundaries deeper into the surface layers of the bulk material. Thus, this enlargement of the grain boundaries is thought to accelerate the corrosion process, as halide ions (Cl-) can diffuse more easily from the corrosive solution deeper into the surface layers of the material.
- 2.
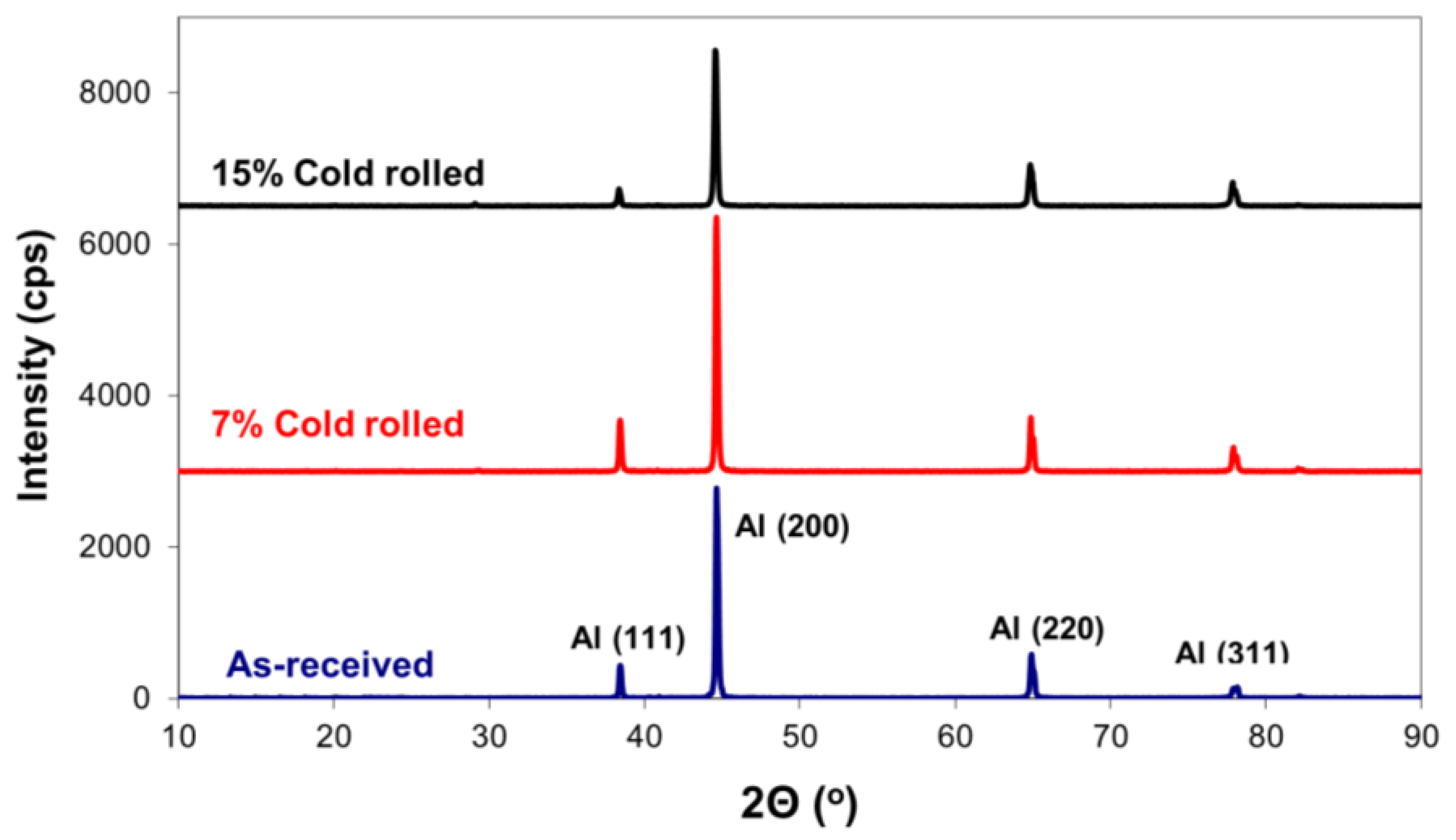
- Formation of corrosion products. Another aspect to consider is the generation of corrosion products on the alloy’s surface during corrosion. By utilizing X-ray diffraction (XRD), a structural analysis of the corroded surface layers in both the as-received and cold-rolled specimens was performed and is shown in Figure 8. The primary corrosion product identified was aluminium oxide (Al2O3). Peaks from the aluminium solid solution substrate (Mg dissolved in the Al matrix) were also noticeable. However, a comparison of X-ray diffraction patterns in Figure 8 reveals an increase in aluminium oxide peaks with a higher cold-rolling rate. The formation of aluminium oxides on the alloy’s surface is believed to hinder the diffusion of halide ions and, consequently, the corrosion process.
- 3.
- Crystallographic texturing. The development of crystallographic texturing has been found to impact the corrosion performance of alloys, as observed in magnesium alloys where basal planes exhibit a lower corrosion rate [35]. However, in the case of 5083 aluminium alloy, findings by Li et al. [10] indicate that crystallographic orientation did not considerably influence the corrosion behaviour of this alloy.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. Metals Handbook, Properies and Selection of Non-Ferrous Alloys, 9th ed.; ASM: Metals Park, OH, USA, 1985; Volume 2. [Google Scholar]
- Barnes, A.; Raman, H.; Lowerson, A.; Edwards, D. Recent Application of Superformed 5083 Aluminium Alloy in the Aerospace Industry. Mater. Sci. Forum 2012, 735, 361–371. [Google Scholar] [CrossRef]
- Li, S.-S.; Yue, X.; Li, Q.-Y.; Peng, H.-L.; Dong, B.-X.; Liu, T.-S.; Yang, H.-Y.; Fan, J.; Shu, S.-L.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Jebaraj, A.V.; Aditya, K.V.V.; Sampath Kumar, T.; Ajaykumar, L.; Deepak, C.R. Mechanical and corrosion behaviour of aluminium alloy 5083 and its weldment for marine applications. Mater. Today Proc. 2020, 22, 1470–1478. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behaviour of aluminium alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M.; Sanchez-Amaya, J.M. Influence of the degree of polishing of alloy AA 5083 on its behaviour against localised alkaline corrosion. Corros. Sci. 2004, 46, 1909–1920. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. Wear behaviour of 5083 wrought aluminium alloy under free corrosion conditions. Tribol.-Mater. Surf. Interfaces 2007, 1, 161–164. [Google Scholar] [CrossRef]
- Zazi, N.; Chopart, J.-P.; Bouabdallah, A. Thermomechanical Treatments Effect on Corrosion Behaviour of Aluminium-magnesium Alloy AA5083–H321. Prot. Met. Phys. Chem. Surf. 2015, 51, 267–274. [Google Scholar] [CrossRef]
- Li, Z.; Yi, D.; Tan, C.; Wang, B. Investigation of the stress corrosion cracking behaviour in annealed 5083 aluminium alloy sheets with different texture types. J. Alloys Compd. 2020, 815, 152690. [Google Scholar] [CrossRef]
- Faraji, M.; Shabestari, S.G.; Razavi, S.H. The Effects of Cold Rolling and Stabilization Treatment on the Improvement of Microstructure, Mechanical Properties, and Corrosion Behaviour of AA5083 Sheet. J. Mater. Eng. Perform. 2023, 32, 2097–2108. [Google Scholar] [CrossRef]
- Abdulstaar, M.; Mhaede, M.; Wollmann, M.; Wagner, L. Investigating the effects of bulk and surface severe plastic deformation on the fatigue, corrosion behaviour and corrosion fatigue of AA5083. Surf. Coat. Technol. 2014, 254, 244–251. [Google Scholar] [CrossRef]
- Beura, V.K.; Kale, C.; Srinivasan, S.; Williams, C.L.; Solanki, K.N. Corrosion behavior of a dynamically deformed Al–Mg alloy. Electrochim. Acta 2020, 354, 136695. [Google Scholar] [CrossRef]
- Zhou, L.; Hyer, H.; Chang, J.; Mehta, A.; Huynh, T.; Yang, Y.; Sohn, Y. Microstructure, mechanical performance, and corrosion behavior of additively manufactured aluminum alloy 5083 with 0.7 and 1.0 wt% Zr addition. Mater. Sci. Eng. A 2021, 823, 141679. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, M.; Wang, H.; Gong, W.; Lai, R.; Li, Y.; Teng, J. The corrosion behavior and mechanical properties of 5083 Al-Mg alloy manufactured by additive friction stir deposition. Corros. Sci. 2023, 213, 110972. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P.; Giannakopoulos, K.I. The effect of heat treatment on the corrosion behaviour of 319 cast aluminium alloy. Mater. Corros. 2009, 60, 415–418. [Google Scholar] [CrossRef]
- Dabalá, M.; Ramous, E.; Magrini, M. Corrosion resistance of cerium-based chemical conversion coatings on AA5083 aluminium alloy. Mater. Corros. 2004, 55, 381–386. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of thermo-mechanical processing on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2002, 11, 2491–2506. [Google Scholar] [CrossRef]
- Wang, L.; Liang, J.; Li, H.; Cheng, L.; Cui, Z. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl−. Corros. Sci. 2021, 178, 109076. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Yang, W.; Liu, Y.; Terryn, H.; Cui, Z. Study on the roles of bisulfite in the stress corrosion cracking of 7050-T7451 aluminum alloy in the thin electrolyte layer environment. Corros. Sci. 2023, 215, 111030. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Zhou, X.; Hashimoto, T.; Wang, J. The corrosion behaviour of machined AA7150-T651 aluminium alloy. Corros. Sci. 2017, 126, 265–271. [Google Scholar] [CrossRef]
- Liu, Y.; Hashimoto, T.; Zhou, X.; Thompson, G.E.; Scamans, G.M.; Rainforth, W.M.; Hunter, J.A. Influence of near-surface deformed layers on filiform corrosion of AA3104 aluminium alloy. Surf. Interface Anal. 2013, 45, 1553–1557. [Google Scholar] [CrossRef]
- Liu, E.; Pan, Q.; Liu, B.; Ye, J.; Wang, W. Microstructure Evolution of the Near-Surface Deformed Layer and Corrosion Behavior of Hot Rolled AA7050 Aluminum Alloy. Materials 2023, 16, 4632. [Google Scholar] [CrossRef]
- Ramoul, C.E.; Ghelloudj, O.; Gharbi, A.; Tlili, S.; Beliardouh, N.E.; Chouchane, T. Plastic Deformation Effect on Wear and Corrosion resistance of Super Martensitic Stainless Steel. J. Bio-Tribo-Corros. 2021, 7, 114. [Google Scholar] [CrossRef]
- Bahmani, A.; Lotfpour, M.; Taghizadeh, M.; Kim, W.-J. Corrosion behavior of severely plastically deformed Mg and Mg alloys. J. Magnes. Alloys 2022, 10, 2607–2648. [Google Scholar] [CrossRef]
- Uddin, M.; Santifoller, R.; Hall, C.; Schlaefer, T. Effect of Combined Grinding–Burnishing Process on Surface Integrity, Tribological, and Corrosion Performance of Laser-Clad Stellite 21 Alloys. Adv. Eng. Mater. 2023, 25, 2201332. [Google Scholar] [CrossRef]
- Nah, J.J.; Kang, H.G.; Huha, M.Y.; Engler, O. Effect of strain states during cold rolling on the recrystallized grain size in an aluminium alloy. Scr. Mater. 2008, 58, 500–503. [Google Scholar] [CrossRef]
- Sezek, S.; Aksakal, B. Deformation and temperature behaviour during cold, warm and hot flat rolling of AA5454-O alloy. Mater. Des. 2009, 30, 3450–3459. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Limited: Essex, UK, 2014. [Google Scholar]
- Bérubé, L.P.; Éspérance, G.L. A quantitative method of determining the degree of texture of zinc electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy; McGraw-Hill: London, UK, 1988. [Google Scholar]
- She, X.; Jiang, X.; Zhang, R.; Wang, P.; Tang, B.; Du, W. Study on microstructure and fracture characteristics of 5083 aluminium alloy thick plate. J. Alloys Compd. 2020, 825, 153960. [Google Scholar] [CrossRef]
- Fine, M.E. Precipitation hardening of aluminium alloys. Metall. Trans. A 1975, 6, 625–630. [Google Scholar] [CrossRef]
- Zhang, X.Y. Understanding the corrosion resistance of nanocrystalline materials: The influence of grain size. In Corrosion Protection and Control Using Nanomaterials; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 34–58. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of crystallographic texture and twinning on the corrosion behaviour of Mg alloys: A review. J. Magnes. Alloys 2022, 10, 313–325. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Kenmochi, K.; Yarita, I.; Abe, H.; Fukuhara, A.; Komatu, T.; Kaito, H. Effect of micro-defects on the surface brightness of cold-rolled stainless-steel strip. J. Mater. Proc. Technol. 1998, 69, 106–111. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A review of passivity breakdown on metal surfaces: Influence of chloride- and sulfide-ion concentrations, temperature, and pH. Emergent Mater. 2021, 4, 1187–1203. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- McCafferty, E. The electrode kinetics of pit initiation on aluminium. Corros. Sci. 1995, 37, 481–492. [Google Scholar] [CrossRef]
- Marichev, V.A. Kinetics of chloride ion adsorption on stainless alloys by in situ contact electric resistance technique. Electrochim. Acta 2008, 53, 6304–6316. [Google Scholar] [CrossRef]
- Korb, L.J.; Olson, D.L. Metals Handbook, Corrosion, 9th ed.; ASM: Metals Park, OH, USA, 1987; Volume 13. [Google Scholar]
- McCafferty, E. Sequence of steps in the pitting of aluminium by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Benson, R.C. Analysis of the Gases Evolved during the Pitting Corrosion of Aluminium in Various Electrolytes. J. Electrochem. Soc. 1995, 127, 2528–2530. [Google Scholar] [CrossRef]



| Crystallographic Planes (hkl) | TC | |
|---|---|---|
| As-received | 111 | 0.218 |
| 200 | 3.026 | |
| 220 | 1.347 | |
| 311 | 0.23 | |
| 7% Cold-rolled | 111 | 0.272 |
| 200 | 2.858 | |
| 220 | 1.295 | |
| 311 | 0.416 | |
| 15% Cold-rolled | 111 | 0.133 |
| 200 | 2.786 | |
| 220 | 1.613 | |
| 311 | 0.846 |
| Cold Rolling (%) | Icorr (μA) | Ecorr (mV) |
|---|---|---|
| 0 | 4 ± 0.33 | 760 ± 36 |
| 7 | 3.5 ± 0.25 | 820 ± 28 |
| 15 | 2.6 ± 0.22 | 850 ± 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagopoulos, C.N.; Georgiou, E.P. The Effect of Cold Rolling on the Corrosion Behaviour of 5083 Aluminium Alloys. Metals 2024, 14, 159. https://doi.org/10.3390/met14020159
Panagopoulos CN, Georgiou EP. The Effect of Cold Rolling on the Corrosion Behaviour of 5083 Aluminium Alloys. Metals. 2024; 14(2):159. https://doi.org/10.3390/met14020159
Chicago/Turabian StylePanagopoulos, C. N., and E. P. Georgiou. 2024. "The Effect of Cold Rolling on the Corrosion Behaviour of 5083 Aluminium Alloys" Metals 14, no. 2: 159. https://doi.org/10.3390/met14020159
APA StylePanagopoulos, C. N., & Georgiou, E. P. (2024). The Effect of Cold Rolling on the Corrosion Behaviour of 5083 Aluminium Alloys. Metals, 14(2), 159. https://doi.org/10.3390/met14020159







