Corrosion Behavior of Titanium Alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with Anodized and Exposed in NaCl and H2SO4 Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodized Treatment
2.3. Microstructural Characterization
2.4. Corrosion Measurements
3. Results
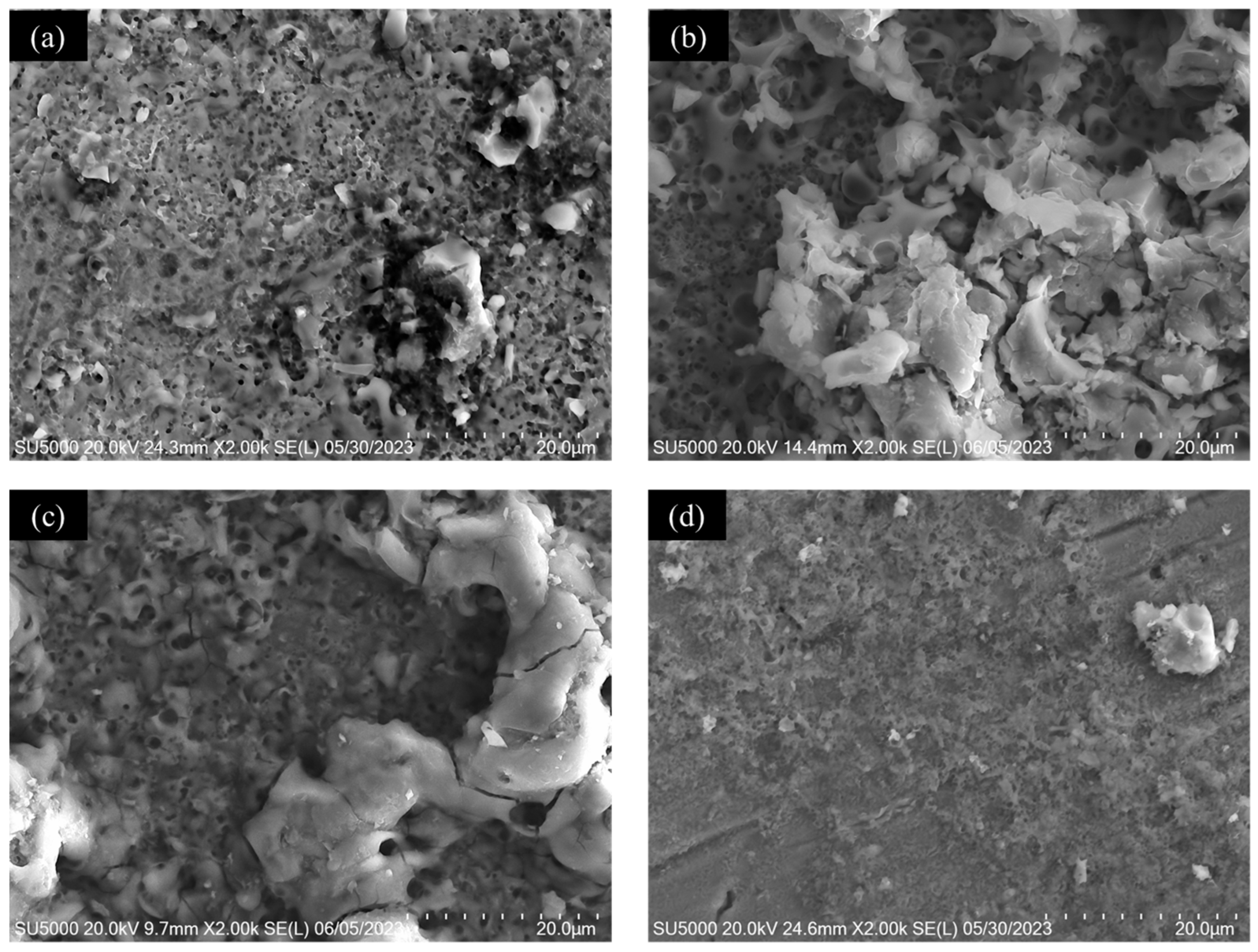
3.1. Microscopic Characterization
3.2. Electrochemical Noise
3.2.1. Hilbert–Huang Transform (HHT)
3.2.2. Recurrence Plots Analysis (RP)
3.3. Electrochemical Impedance Spectroscopy
4. Discussion
5. Conclusions
- The results indicated that the anodizing treatment on titanium alloys had a better effect when NaOH baths were used due to increased impedance, meaning greater corrosion resistance.
- The chemical composition of alloys is vital to generate a good, anodized alloy. The alloys with more β elements presented problems in generating a uniform oxide layer.
- Ti-6Al-4V presented more problems when generating a homogenous oxide layer due to the presence of V in the alloy. The generation of vanadium oxide made the anodized alloy susceptible to pitting attacks because the vanadium oxide has more interstitial spaces.
- The anodized alloys with fewer β elements (Ti CP2 and Ti-6Al-2Sn-4Zr-2Mo) presented higher Rpor (Rct) resistance when anodized on NaOH. On the other hand, alloys with a high presence of β elements presented more anodized Rpor (Rct) on KOH.
- Ti-6Al-4V presented a lower thickness, at 25 nm, when it was anodized in an NaOH bath.
- Ti Beta-C presented better activity against corrosion due to the presence of Cr in the alloy.
- The EN technique analyzed by HHT and RP helps determine the type of corrosion process that predominates in the electrochemical system. In this type of case, the use of EN is helpful for observing the behavior of the localization process due to the limitation of other techniques in evaluating systems that do not present uniform corrosion processes.
- Recurrence plots analysis showed that anodized alloys are more susceptible to pitting when exposed to NaCl media due to the vertical and horizontal reaction distribution. On the other hand, when exposed to H2SO4, the anodized alloys presented behavior related to passive and uniform systems.
- The EIS results indicated that Ti Beta-C presented better resistance against corrosion for obtained values of 9.7 × 107 Ω·cm2. Also, the anodized KOH presented more impedance.
- The values of CPE for the anodized alloy in KOH are in the order of 10−7 and 10−6 (F/cm2), indicating low capacitance and a better performance against ionic transference.
- The H2SO4 made the anodized alloys susceptible to present diffusion. However, NaCl can present more damage due to the generation of hydroxides and salts on the metal–coating interface.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys; Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030244392. [Google Scholar]
- Mouritz, A.P. Introduction to Aerospace Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9781855739468. [Google Scholar]
- Giurgiutiu, V. Introduction. In Structural Health Monitoring of Aerospace Composites; Elsevier: Boston, MA, USA, 2016; pp. 1–23. [Google Scholar] [CrossRef]
- Schweitzer, P.E. PA Corrosion Engineering Handbook—3 Volume Set; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429188084. [Google Scholar]
- Hebert, K.; Alkire, R. Dissolved Metal Species Mechanism for Initiation of Crevice Corrosion of Aluminum: II. Mathematical Model. J. Electrochem. Soc. 1983, 130, 1007–1014. [Google Scholar] [CrossRef]
- Rashidi, N.; Alavi-Soltani, S.R.; Asmatulu, R. Crevice Corrosion Theory, Mechanisms and Prevention Methods; SOAR: Cambridge, UK, 2007. [Google Scholar]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In Vivo Severe Corrosion and Hydrogen Embrittlement of Retrieved Modular Body Titanium Alloy Hip-Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef]
- Zieliński, A.; Sobieszczyk, S. Corrosion of Titanium Biomaterials, Mechanisms, Effects and Modelisation. Corros. Rev. 2008, 26, 1–22. [Google Scholar] [CrossRef]
- Pourbaix, M.; Burbank, J. Atlas D-Equilibres Electrochimiques. J. Electrochem. Soc. 1964, 111, 14C. [Google Scholar] [CrossRef]
- Ono, S.; Ichinose, H.; Kawaguchi, T.; Masuko, N. The Observation of Anodic Oxide Films on Aluminum by High Resolution Electron Microscopy. Corros. Sci. 1990, 31, 249–254. [Google Scholar] [CrossRef]
- Palibroda, E.; Marginean, P. Considerations on the Adsorbed Water Concentration of Sulfuric Porous Aluminium Oxide. Thin Solid Film. 1994, 240, 73–75. [Google Scholar] [CrossRef]
- Voon, C.H.; Derman, M.N.; Hashim, U.; Ahmad, K.R.; Foo, K.L. Effect of Temperature of Oxalic Acid on the Fabrication of Porous Anodic Alumina from Al-Mn Alloys. J. Nanomater. 2013, 2013, 167047. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Anodization of Magnesium (Mg) Alloys to Improve Corrosion Resistance. Corros. Prev. Magnes. Alloys 2013, 8, 197–231. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A Review of Growth Mechanism, Structure and Crystallinity of Anodized TiO2 Nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Hugot-Le Goff, A. Structure of Very Thin TiO2 Films Studied by Raman Spectroscopy with Interference Enhancement. Thin Solid Film. 1986, 142, 193–197. [Google Scholar] [CrossRef]
- Afshar, A.; Vaezi, M.R. Anodizing of Titanium in NaOH Solution and Its Corrosion Resistance in PBS Physiologic Solution. Sci. Iran. 2003, 10, 361–366. [Google Scholar]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.M.; Delgado, A.D.; Flores-De Los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- AMS2487B; Anodic Treatment of Titanium and Titanium Alloys Solution PH 12.4 Maximum. SAE: Warrendale, PA, USA, 2018; pp. 1–7.
- AMS2488E; Anodic Treatment-Titanium and Titanium Alloys, Solution PH 13 or Higher. SAE International: Warrendale, PA, USA, 2019. Available online: https://www.sae.org/standards/content/ams2488e/?src=ams2487b (accessed on 23 June 2023).
- ASTM E407-07; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM G106-03; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2020.
- Bertocci, U.; Huet, F. Noise Analysis Applied to Electrochemical Systems. Corrosion 1995, 51, 131–144. [Google Scholar] [CrossRef]
- Coakley, J.; Vorontsov, V.A.; Littrell, K.C.; Heenan, R.K.; Ohnuma, M.; Jones, N.G.; Dye, D. Nanoprecipitation in a Beta-Titanium Alloy. J. Alloys Compd. 2015, 623, 146–156. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Cao, F.; Gao, Z.; Zhang, J.; Cao, C. Analysis of Pitting Corrosion Behavior of Pure Al in Sodium Chloride Solution with the Wavelet Technique. J. Electroanal. Chem. 2005, 578, 143–150. [Google Scholar] [CrossRef]
- Hai, L.; Guo-qiang, X.; Pan, Z.; Hua-sen, Z.; Khan, M.Y. The Hilbert–Huang Transform-Based Denoising Method for the TEM Response of a PRBS Source Signal. Pure Appl. Geophys. 2016, 173, 2777–2789. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Bai, P.; Li, X.; Liu, B.; Tan, J.; Wu, X. In-Situ Monitoring of Pitting Corrosion of AZ31 Magnesium Alloy by Combining Electrochemical Noise and Acoustic Emission Techniques. J. Alloys Compd. 2021, 878, 160334. [Google Scholar] [CrossRef]
- Homborg, A.M.; Tinga, T.; Van Westing, E.P.M.; Zhang, X.; Ferrari, G.M.; De Wit, J.H.W.; Mol, J.M.C. A Critical Appraisal of the Interpretation of Electrochemical Noise for Corrosion Studies. Corrosion 2014, 70, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Marwan, N.; Carmen Romano, M.; Thiel, M.; Kurths, J. Recurrence Plots for the Analysis of Complex Systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Acuña-González, N.; García-Ochoa, E.; González-Sánchez, J. Assessment of the Dynamics of Corrosion Fatigue Crack Initiation Applying Recurrence Plots to the Analysis of Electrochemical Noise Data. Int. J. Fatigue 2008, 30, 1211–1219. [Google Scholar] [CrossRef]
- Mayorga-Cruz, D.; Sarmiento-Martinez, O.; Menchaca Campos, C.; Uruchurtu, J. Analysis of Michelson Optical Interferometry Using Recurrence Plots During Corrosion of Aluminium in NaCl Solution. ECS Trans. 2009, 20, 433–446. [Google Scholar] [CrossRef]
- Jirón-Lazos, U.; Corvo, F.; De la Rosa, S.C.; García-Ochoa, E.M.; Bastidas, D.M.; Bastidas, J.M. Localized Corrosion of Aluminum Alloy 6061 in the Presence of Aspergillus Niger. Int. Biodeterior. Biodegrad. 2018, 133, 17–25. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.; Liao, B.; Guo, X. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, Y.; Chen, Y.; Zhang, L.; Sheng, X. Triple-function smart anticorrosion composite coating based on graphene and ZIF-8 with excellent pH-responsive self-healing and in vitro antimicrobial properties. Prog. Org. Coat. 2024, 186, 108007. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Constant-Phase Elements. In Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2017; pp. 395–419. [Google Scholar]
- Harrington, D.A.; Van Den Driessche, P. Mechanism and Equivalent Circuits in Electrochemical Impedance Spectroscopy. Electrochim. Acta 2011, 56, 8005–8013. [Google Scholar] [CrossRef]
- Peixoto Barbosa, D.; Knörnschild, G. Anodization of Mg-Alloy AZ91 in NaOH Solutions. Surf. Coat. Technol. 2009, 203, 1629–1636. [Google Scholar] [CrossRef]
- Burleigh, T.D.; Dotson, T.C.; Dotson, K.T.; Gabay, S.J.; Sloan, T.B.; Ferrell, S.G. Anodizing Steel in KOH and NaOH Solutions. J. Electrochem. Soc. 2007, 154, C579. [Google Scholar] [CrossRef]
- Hsu, H.C.; Hsu, S.K.; Wu, S.C.; Hung, Y.H.; Ho, W.F. Surface Modification of Nanotubular Anodized Ti–7.5Mo Alloy Using NaOH Treatment for Biomedical Application. Thin Solid Film. 2020, 710, 138273. [Google Scholar] [CrossRef]
- Laurindo, C.A.H.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on Anodized Titanium Surface: Effect of High Current Density. Mater. Sci. Eng. C 2014, 37, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, H.; Ding, X.; Yan, Q.; Wang, L.; Ma, W. Simulation of Anodizing Current-Time Curves and Morphology Evolution of TiO2 Nanotubes Anodized in Electrolytes with Different NH4F Concentrations. Electrochim. Acta 2015, 176, 1083–1091. [Google Scholar] [CrossRef]
- Mazzarolo, A.; Curioni, M.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Anodic Growth of Titanium Oxide: Electrochemical Behaviour and Morphological Evolution. Electrochim. Acta 2012, 75, 288–295. [Google Scholar] [CrossRef]
- Lee, C.C.; Mansfeld, F. Analysis of Electrochemical Noise Data for a Passive System in the Frequency Domain. Corros. Sci. 1998, 40, 959–962. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Behnamian, Y. Detection of Corrosion Degradation Using Electrochemical Noise (EN): Review of Signal Processing Methods for Identifying Corrosion Forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Xia, D.H.; Qin, Z.; Song, S.; Macdonald, D.; Luo, J.L. Combating Marine Corrosion on Engineered Oxide Surface by Repelling, Blocking and Capturing Cl−: A Mini Review. Corros. Commun. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Pan, C.; Wang, X.; Behnamian, Y.; Wu, Z.; Qin, Z.; Xia, D.H.; Hu, W. Monododecyl Phosphate Film on LY12 Aluminum Alloy: PH-Controlled Self-Assembly and Corrosion Resistance. J. Electrochem. Soc. 2020, 167, 161510. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, M.K.; Jung, G.C.; Vang, M.S.; Park, Y.J. The Effects of Spark Anodizing Treatment of Pure Titanium Metals and Titanium Alloys on Corrosion Characteristics. Surf. Coat. Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Fouda, M.E.; Allagui, A.; Elwakil, A.S.; Das, S.; Psychalinos, C.; Radwan, A.G. Non-linear Charge-Voltage Relationship in Constant Phase Element. AEU Int. J. Electron. Commun. 2020, 117, 153104. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the Corrosion Mechanism of Mg Investigated by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Gateman, S.M.; Gharbi, O.; Gomes de Melo, H.; Ngo, K.; Turmine, M.; Vivier, V. On the Use of a Constant Phase Element (CPE) in Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 36, p. 101133. [Google Scholar]
- Martínez-Aparicio, B.; Martínez-Bastidas, D.; Gaona-Tiburcio, C.; Martin, U.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Localized corrosion of 15–5 PH and 17–4 PH stainless steel in NaCl solution. J. Solid State Electrochem. 2023, 27, 2993–3001. [Google Scholar] [CrossRef]
- Rajan, S.T.; VV, A.T.; Terada-Nakaishi, M.; Chen, P.; Hanawa, T.; Nandakumar, A.K.; Subramanian, B. Zirconium-Based Metallic Glass and Zirconia Coatings to Inhibit Bone Formation on Titanium. Biomed. Mater. 2020, 15, 065019. [Google Scholar] [CrossRef]
- Radovanović, M.B.; Tasić, Ž.Z.; Simonović, A.T.; Petrović Mihajlović, M.B.; Antonijević, M.M. Corrosion Behavior of Titanium in Simulated Body Solutions with the Addition of Biomolecules. ACS Omega 2020, 5, 12768–12776. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olgui-Coca, J.; Lopez-Leon, L.D.; Estupiñán-López, F.; Lira-Martínez, A.; Gaona Tiburcio, C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals 2023, 13, 1510. [Google Scholar] [CrossRef]
- Dubent, S.; Mazard, A. Characterization and corrosion behaviour of grade 2 titanium used in electrolyzers for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 15622–15633. [Google Scholar] [CrossRef]
- Escriva-Cerdán, C.; Blasco-Tamarit, E.; García-García, D.M.; Akid, R.; Walton, J. Effect of temperature on passive film formation of UNS N08031 Cr-Ni alloy in phosphoric acid contaminated with different aggressive anions. Electrochem. Acta 2013, 111, 552–561. [Google Scholar] [CrossRef]
- Liu, C.; Leyland, A.; Bi, Q.; Matthews, A. Corrosion resistance of multi-layerded plasma-assisted physical vapour deposition TiN and CrN coatings. Surf. Coat. Technol. 2001, 141, 164–173. [Google Scholar] [CrossRef]
- Balla, A.; Marcu, C.; Axante, D.; Borodi, G.; Lazar, D. Catalytic reduction of sulfuric acid to sulfur dioxide. Cent. Eur. J. Chem. 2012, 10, 1817–1823. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Moreno, J.M.C.; Prodana, M.; Ionita, D.; Demetrescu, I.; Marcu, M.; Popovici, I.A.; Vasilescu, E. Microstructure, Surface Characterization, and Electrochemical Behavior of New Ti-Zr-Ta-Ag Alloy in Simulated Human Electrolyte. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 513–523. [Google Scholar] [CrossRef]
- Munirathinam, B.; Neelakantan, L. Titania Nanotubes from Weak Organic Acid Electrolyte: Fabrication, Characterization and Oxide Film Properties. Mater. Sci. Eng. C 2015, 49, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.F.; Jin, L.; Zhou, L. Surface Characteristics and Electrochemical Corrosion Behavior of a Pre-Anodized Microarc Oxidation Coating on Titanium Alloy. Mater. Sci. Eng. C 2013, 33, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y. Unique Constant Phase Element Behavior of the Electrolyte–Graphene Interface. Nanomaterials 2019, 9, 923. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Pedeferri, M.P.; Ormellese, M. Pitting Corrosion on Anodized Titanium: Effect of Halides. Mater. Corros. 2018, 69, 1441–1446. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Attarzadeh, F.R.; Vakili-Azghandi, M. Effect of Multi-Pass Friction Stir Processing on the Electrochemical and Corrosion Behavior of Pure Titanium in Strongly Acidic Solutions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 403–411. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Zheng, H.; Latham, K.; Wlodarski, W.; Kalantar-Zadeh, K. Anodization of Ti Thin Film Deposited on ITO. Langmuir 2009, 25, 509–514. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of Tapered, Conical-Shaped Titania Nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Galván-Martínez, R.; Cabrera-de la Cruz, D.; Contreras, A.; Orozco-Cruz, R. A Novel Experimental Arrangement for Corrosion Study of X60 Pipeline Steel Weldments at Turbulent Flow Conditions. Corros. Eng. Sci. Technol. 2016, 51, 400–407. [Google Scholar] [CrossRef]
- Galvan-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion Study of the X52 Steel Immersed in Seawater with a Corrosion Inhibitor Using a Rotating Cylinder Electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS Studies to Account for the Passive Behavior of the Alloy Ti-6Al-4V in Hank’s Solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Luo, J.-L.; Huet, F. Review—Electrochemical Noise Applied in Corrosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Scharifker, B.K.; Monstany, J.; Palomar-Pardave, M.; González, I. On the Theory of the Potentiostatic Current Transient for Diffusion-Controlled Three-Dimensional Electrocrystallization Processes. J. Electrochem. Soc. 1999, 146, 1005. [Google Scholar] [CrossRef]
- Gil, A.; Galicia, L.; González, I. Diffusion coefficients and electrode kinetic parameters of different Fe(III)-sulfate complexes. J. Electroanal. Chem. 1996, 417, 129–134. [Google Scholar] [CrossRef]
- Cabrera-Sierra, R.; Hallen, J.M.; Vazquez-Arenas, J.; Vázquez, G.; González, I. EIS characterization of tantalum and niobium oxide films based on a modification of the point defect model. J. ELectroanal. Chem. 2010, 638, 51–58. [Google Scholar] [CrossRef]
- Rivero, E.P.; Rodríguez, F.A.; Cruz-Díaz, M.R.; Gónzalez, I. Reactive diffusion migration layer and mass transfer wall function to model active chlorine generation in a filter press type electrochemical reactor for organic pollutant degradation. Chem. Eng. Resear. Des. 2018, 138, 533–545. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2018; pp. 175–478. [Google Scholar]
- Macdonald, J.R. Frequency response of inifield dielectric and conductive systems involving an exponetial distribution of activation energy. J. Appl. Phys. 1985, 58, 1955. [Google Scholar] [CrossRef]
- Macdonald, J.R. Generalizations of “universal dielectric response” and a general distribution-of-activation-energies model for dielectric and conductive systems. J. App. Phys. 1985, 58, 1971. [Google Scholar] [CrossRef]
- Mulder, W.H.; Sluyters, J.H.; Pajkossy, T.; Nyikos, L. Tafel current at fractal electrodes: Connection with admittance spectra. J. Electroanal. Chem. 1990, 285, 103. [Google Scholar] [CrossRef]
- Kim, C.; Pyun, S.; Kim, J. An investigation of the capacitance dispersion on the fractal carbon electrode with edge and basal orientations. Electrochim. Act. 2003, 48, 3455. [Google Scholar] [CrossRef]
- Schiller, C.A.; Strunz, W. The evaluation of experimental dielectric data of barrier coatings by means of different models. Electrochim. Act. 2001, 46, 3619. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between constant-phase element (CPE) parameters and physical properties of films with a distributed resistivity. Electrochim. Act. 2017, 225, 592. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; De Los Rios, J.P.F.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Du, X.Q.; Yang, Q.S.; Chen, Y.; Yang, Y.; Zhang, Z. Galvanic Corrosion Behavior of Copper/Titanium Galvanic Couple in Artificial Seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, M.F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Ramirez, A.; Gonzalez-Rodriguez, J.; Campillo, B. An Electrochemical Study of the Corrosion Behavior of a Dual Phase Steel in 0.5 m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786. [Google Scholar]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]















| Elements | Ti CP2 | Alloys | ||
|---|---|---|---|---|
| Ti-6Al-2Sn-4Zr-2Mo | Ti-6Al-4V | Ti Beta-C | ||
| Fe | 0.038 ± 0.005 | – | 0.21 ± 0.01 | 0.08 ± 01 |
| Al | – | 6.75 ± 0.20 | 7.14 ± 0.37 | 4.2 ± 0.13 |
| V | – | – | 4.03 ± 0.08 | 8.1 ± 0.07 |
| Zr | – | 4.18 ± 0.01 | – | 4.3 ± 0.01 |
| Cr | – | – | – | 3.3 ± 0.07 |
| Mo | – | 1.99 ± 0.008 | – | 3.9 ± 0.01 |
| Sn | – | 2.08 ± 0.01 | – | – |
| Ti | 99.94 ± 0.005 | 84.65 ± 0.19 | 87.71 ± 0.36 | 75.2 ± 0.14 |
| Alloy | Electrolyte | Average Thickness (μm) |
|---|---|---|
| Ti CP2 | NaOH | 0.45 |
| KOH | 2.4 | |
| Ti-6Al-2Sn-4Zr-2Mo | NaOH | 2.45 |
| KOH | 1.5 | |
| Ti-6Al-4V | NaOH | 0.024 |
| KOH | 0.2 | |
| Ti Beta-C | NaOH | 0.67 |
| KOH | 0.75 |
| Anodized | Alloy | RR | DET |
|---|---|---|---|
| NaOH | NaCl | ||
| Ti CP2 | 0.0671 | 0.6951 | |
| Ti-6Al-2Sn-4Zr-2Mo | 0.0927 | 0.827 | |
| Ti-6Al-4V | 0.0816 | 0.7368 | |
| Ti Beta-C | 0.0988 | 0.9697 | |
| H2SO4 | |||
| Ti CP2 | 0.0991 | 0.9155 | |
| Ti-6Al-2Sn-4Zr-2Mo | 0.0868 | 0.5373 | |
| Ti-6Al-4V | 0.1666 | 0.4723 | |
| Ti Beta-C | 0.1088 | 0.9001 | |
| Anodized | Alloy | RR | DET |
|---|---|---|---|
| KOH | NaCl | ||
| Ti CP2 | 0.0824 | 0.5542 | |
| Ti-6Al-2Sn-4Zr-2Mo | 0.0788 | 0.8378 | |
| Ti-6Al-4V | 0.5779 | 0.9962 | |
| Ti Beta-C | 0.3508 | 0.6901 | |
| H2SO4 | |||
| Ti CP2 | 0.3045 | 0.888 | |
| Ti-6Al-2Sn-4Zr-2Mo | 0.1421 | 0.9126 | |
| Ti-6Al-4V | 0.1105 | 0.4718 | |
| Ti Beta-C | 0.0829 | 0.8696 | |
| Alloy | Rs (Ω·cm2) | Rpor (Ω·cm2) | CPE-T (F/cm2) | n | R (Ω·cm2) | CPE-T2 (F/cm2) | n | W (Ω·cm2) | X2 |
|---|---|---|---|---|---|---|---|---|---|
| Ti CP2 | |||||||||
| 3.5% NaCl | |||||||||
| NaOH | 19.1 | 2.4 × 104 | 3.37 × 10−5 | 0.7 | 1.72 × 105 | 4.75 × 10−5 | 0.8 | - | 0.01 |
| KOH | 20.3 | 92.4 | 9.78 × 10−6 | 0.8 | 1.01 × 106 | 1.36 × 10−5 | 0.8 | - | 0.01 |
| 3.5% H2SO4 | |||||||||
| NaOH | 9.8 | 1530 | 4.29 × 10−5 | 0.8 | 2.41 × 106 | 2.59 × 10−5 | 0.8 | - | 5 × 10−3 |
| KOH | 9.1 | 2.65 × 107 | 8.92 × 10−6 | 0.9 | - | - | - | - | 5 × 10−2 |
| Ti-6Al-2Sn-4Zr-2Mo | |||||||||
| 3.5% NaCl | |||||||||
| NaOH | 21.3 | 20.8 × 103 | 1.47 × 10−4 | 0.6 | 18 × 103 | 2.05 × 10−4 | 0.8 | - | 7 × 10−4 |
| KOH | 21.8 | 8409 | 1.34 × 10−4 | 0.7 | 2.88 × 104 | 1.11 × 10−4 | 0.7 | - | 9 × 10−3 |
| 3.5% H2SO4 | |||||||||
| NaOH | 4.5 | 2473 | 1.96 × 10−4 | 0.8 | 3.15 × 105 | 4.06 × 10−4 | 0.8 | - | 2 × 10−3 |
| KOH | 4.6 | 83.5 | 5.64 × 10−6 | - | - | - | 1.39 × 105 | 6 × 10−3 | |
| Ti-6Al-4V | |||||||||
| 3.5% NaCl | |||||||||
| NaOH | 20.1 | 1761 | 6.55 × 10−5 | 0.7 | 7.85 × 105 | 1.27 × 10−4 | 0.3 | - | 3 × 10−4 |
| KOH | 14.8 | 1238 | 9.34 × 10−5 | 0.7 | 1.12 × 105 | 5.01 × 10−4 | 0.4 | - | 1 × 10−2 |
| 3.5% H2SO4 | |||||||||
| NaOH | 3.7 | 296 | 7.61 × 10−5 | 0.7 | - | - | - | 2.7 × 104 | 1 × 10−2 |
| KOH | 3.57 | 2158 | 4.70 × 10−4 | 0.6 | - | - | - | 3.15 × 104 | 7 × 10−3 |
| Ti Beta-C | |||||||||
| 3.5% NaCl | |||||||||
| NaOH | 66.9 | 1589 | 2.11 × 10−6 | 0.8 | 2.1 × 105 | 3.16 × 10−5 | 0.4 | - | 1 × 10−3 |
| KOH | 20.7 | 5418 | 6.23 × 10−7 | 0.9 | 2.43 × 106 | 2.17 × 10−6 | 0.7 | - | 6 × 10−3 |
| 3.5% H2SO4 | |||||||||
| NaOH | 4.1 | 284 | 6.93 × 10−6 | 0.9 | 4.4 × 104 | 8.94 × 10−5 | 0.7 | - | 1 × 10−3 |
| KOH | 3.7 | 1201 | 1.05 × 10−7 | 0.9 | 8.54 × 105 | 4.31 × 10−6 | 0.6 | - | 1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaona-Tiburcio, C.; Jáquez-Muñoz, J.M.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Lara-Banda, M.; Lira-Martinez, M.A.; Reyes-Blas, H.; Baltazar-Zamora, M.Á.; Landa-Ruiz, L.; Lopez-Leon, L.D.; et al. Corrosion Behavior of Titanium Alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with Anodized and Exposed in NaCl and H2SO4 Solutions. Metals 2024, 14, 160. https://doi.org/10.3390/met14020160
Gaona-Tiburcio C, Jáquez-Muñoz JM, Nieves-Mendoza D, Maldonado-Bandala E, Lara-Banda M, Lira-Martinez MA, Reyes-Blas H, Baltazar-Zamora MÁ, Landa-Ruiz L, Lopez-Leon LD, et al. Corrosion Behavior of Titanium Alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with Anodized and Exposed in NaCl and H2SO4 Solutions. Metals. 2024; 14(2):160. https://doi.org/10.3390/met14020160
Chicago/Turabian StyleGaona-Tiburcio, Citlalli, Jesús Manuel Jáquez-Muñoz, Demetrio Nieves-Mendoza, Erick Maldonado-Bandala, Maria Lara-Banda, Manuel Alejandro Lira-Martinez, Hortensia Reyes-Blas, Miguel Ángel Baltazar-Zamora, Laura Landa-Ruiz, Luis Daimir Lopez-Leon, and et al. 2024. "Corrosion Behavior of Titanium Alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with Anodized and Exposed in NaCl and H2SO4 Solutions" Metals 14, no. 2: 160. https://doi.org/10.3390/met14020160






