Heat Dissipation of Open-Cell-Type Aluminum Foams Manufactured by Replication-Casting Process
Abstract
:1. Introduction
2. Experimental Procedure
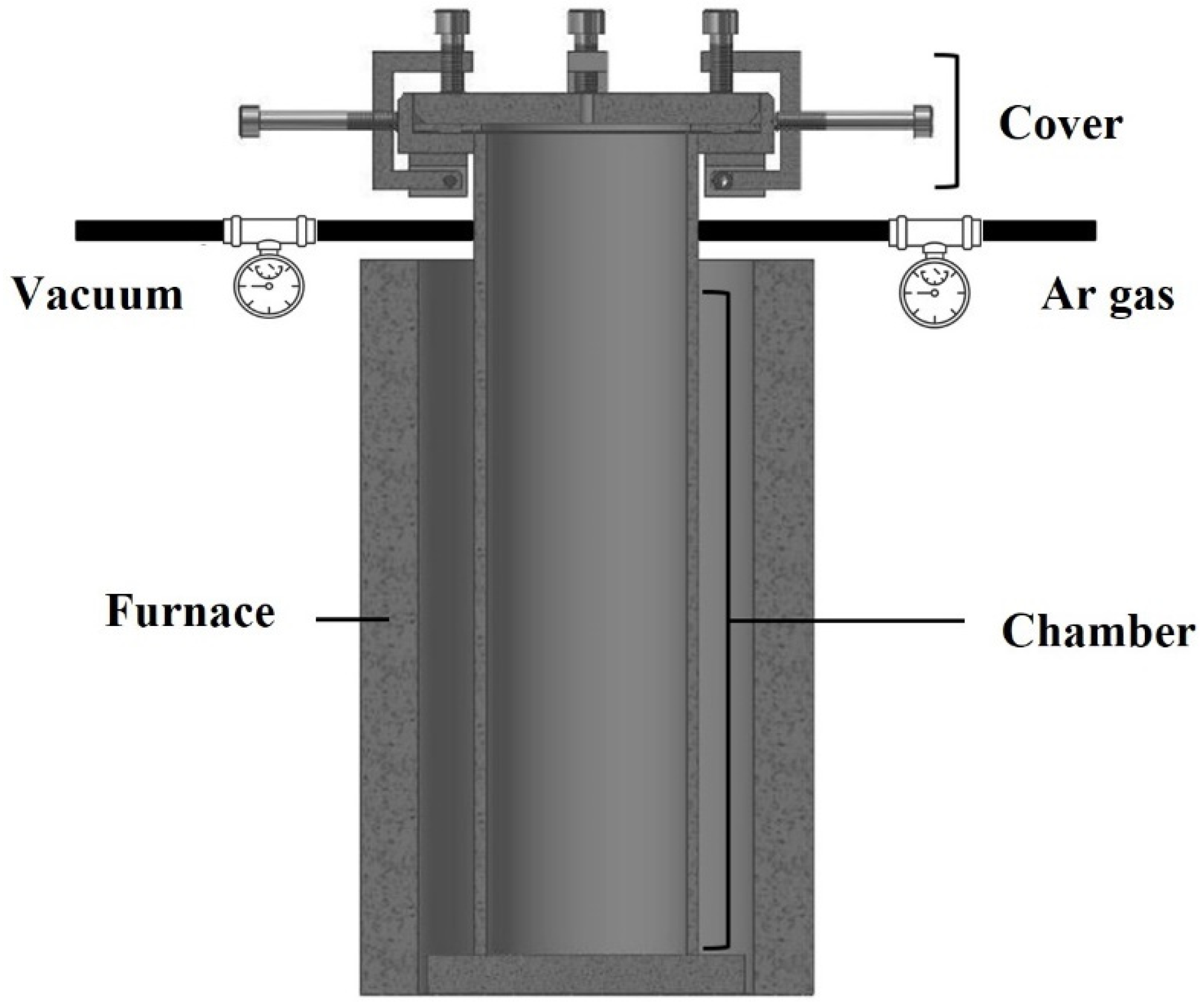
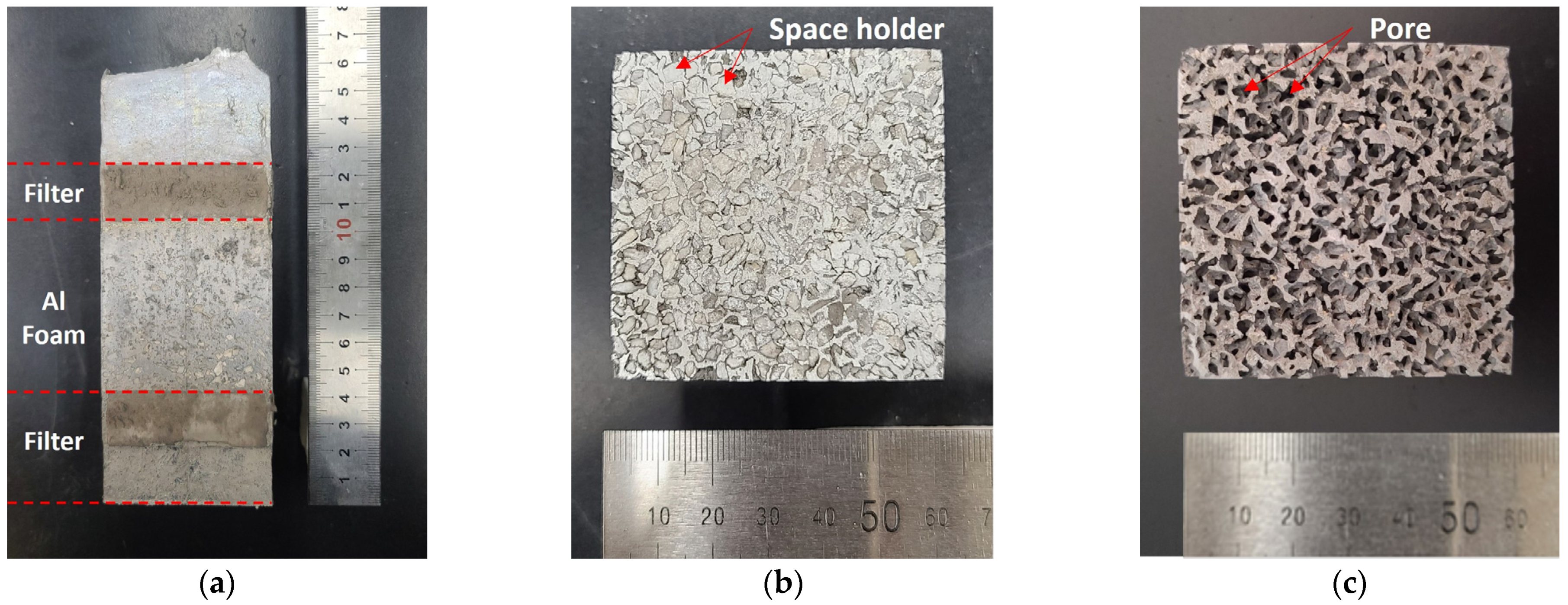
2.1. Manufacturing Method of Open-Cell-Type Aluminum Foam by Replication Casting
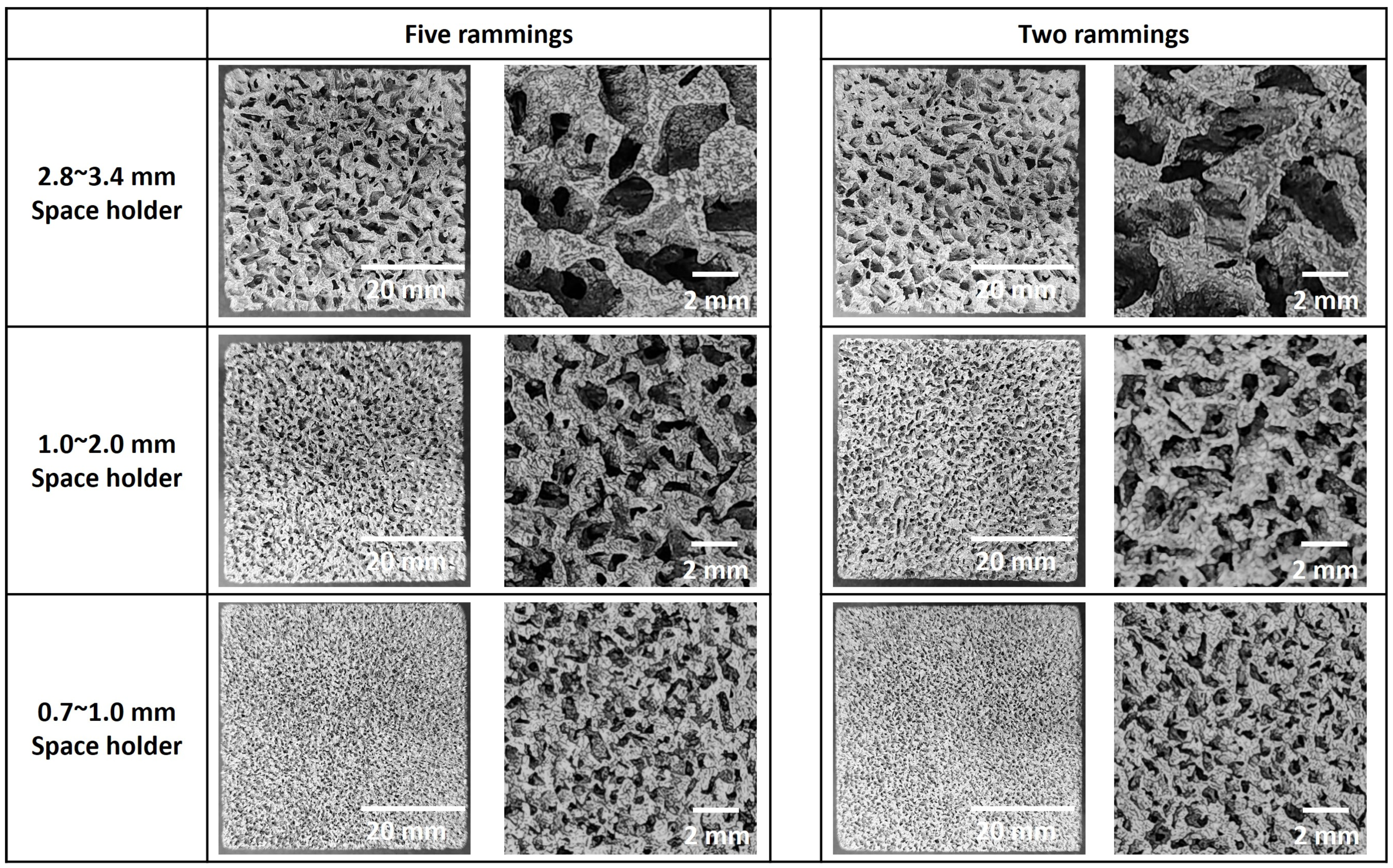
2.1.1. Preparation and Ramming of the Space Holder
2.1.2. Infiltration of Al Melts
2.1.3. Dissolution
2.2. Measurement Method
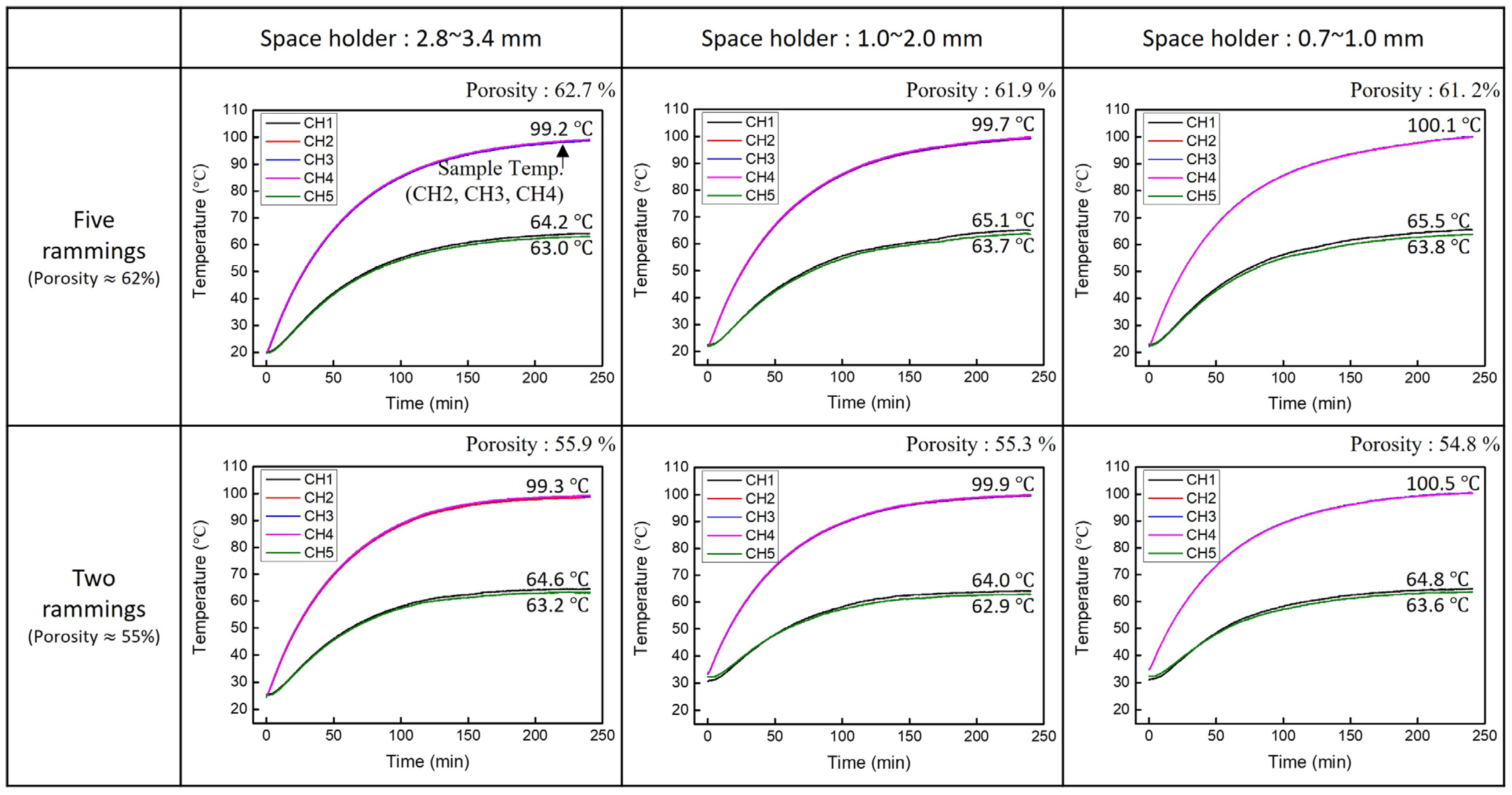
2.2.1. Porosity
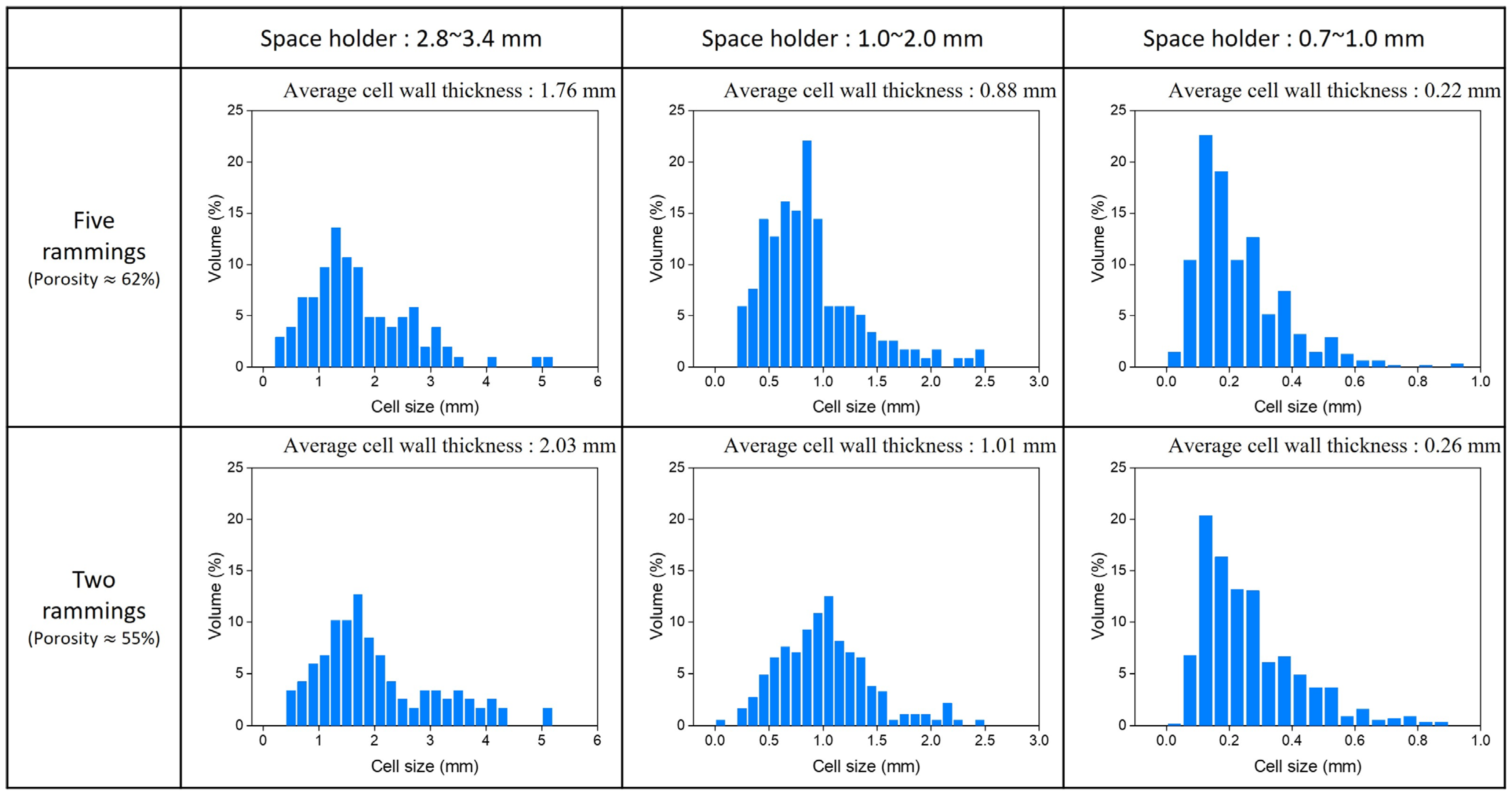
2.2.2. Cell Wall Thickness
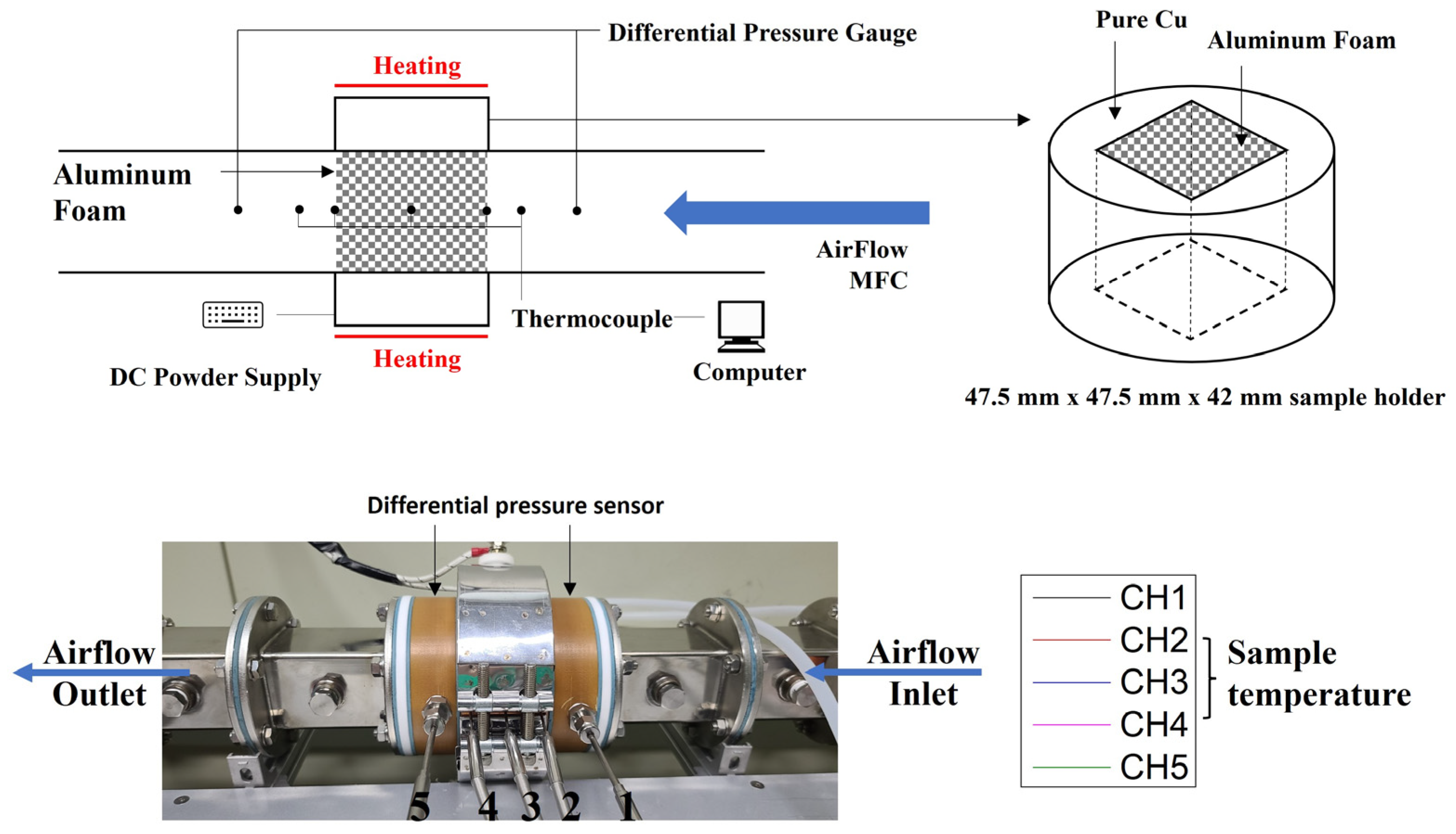
2.3. Thermal Properties Evaluation: Air-Cooling System
3. Results and Discussion
3.1. Porosity
3.2. Cell Wall Thickness
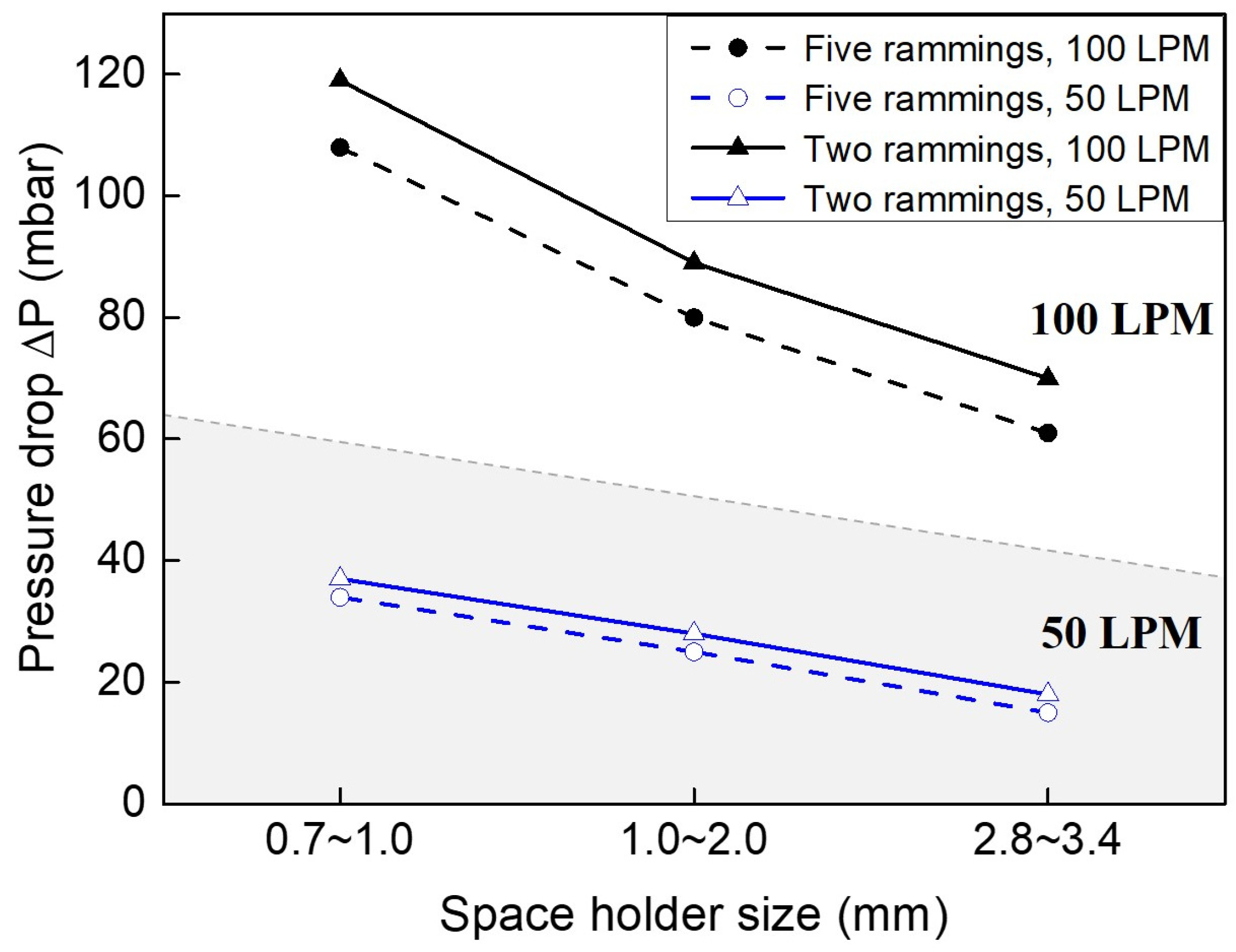
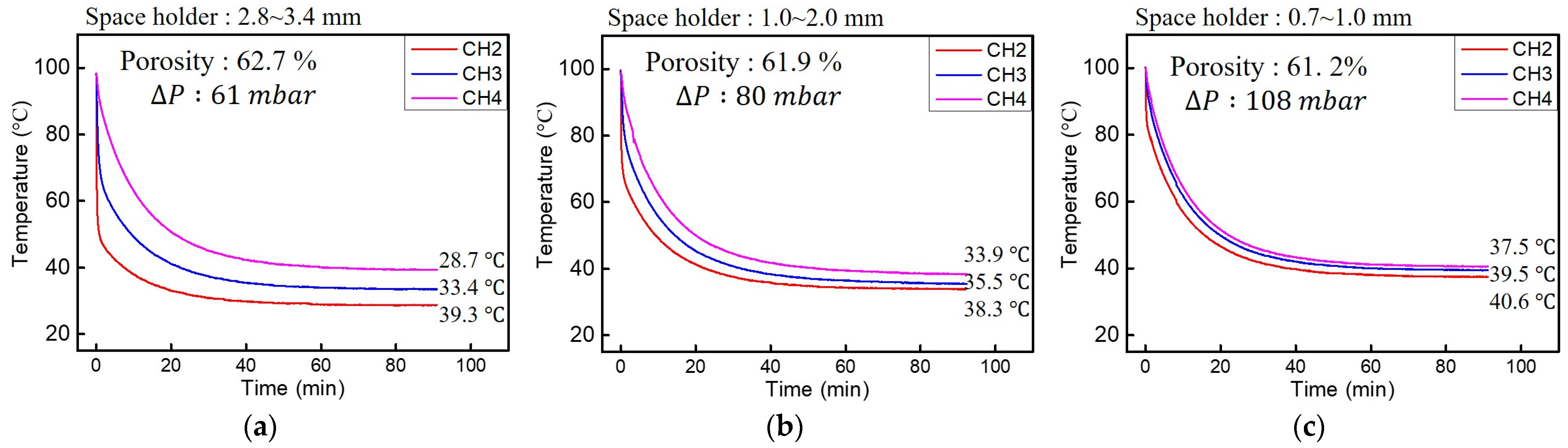
3.3. Pressure Drop
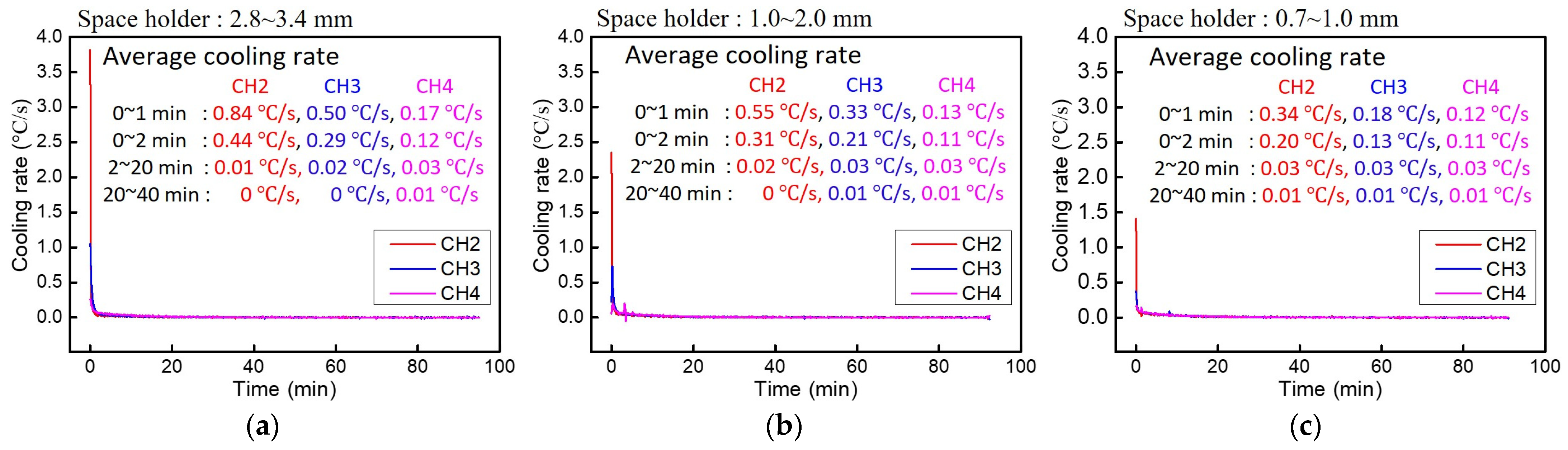
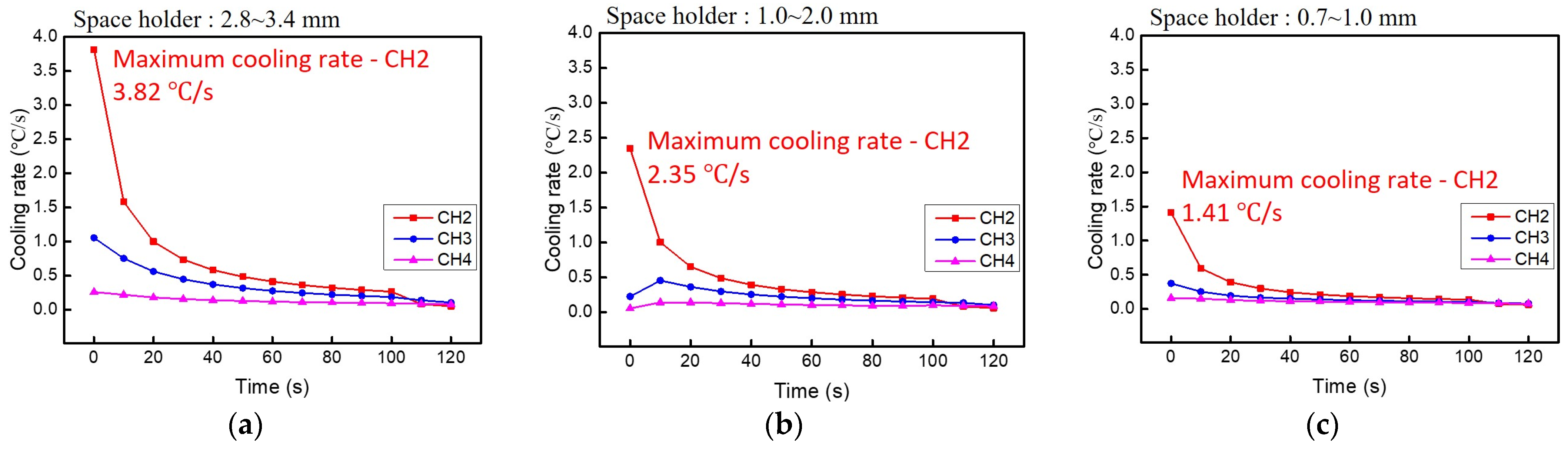
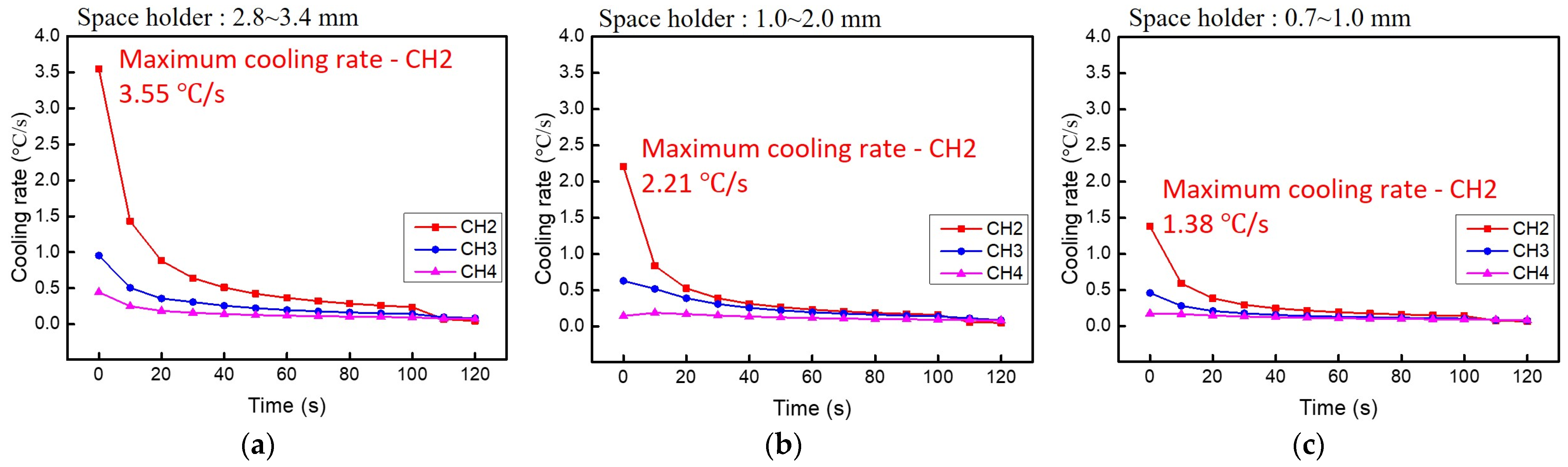
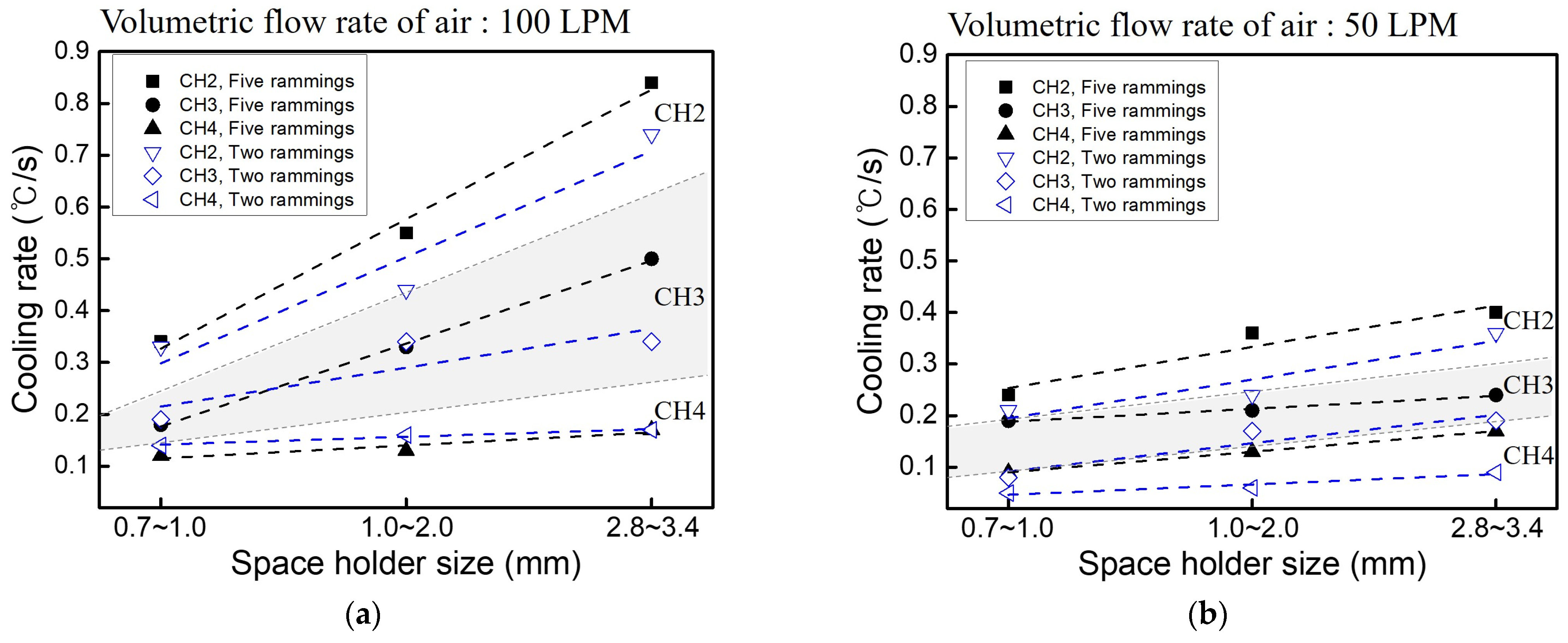
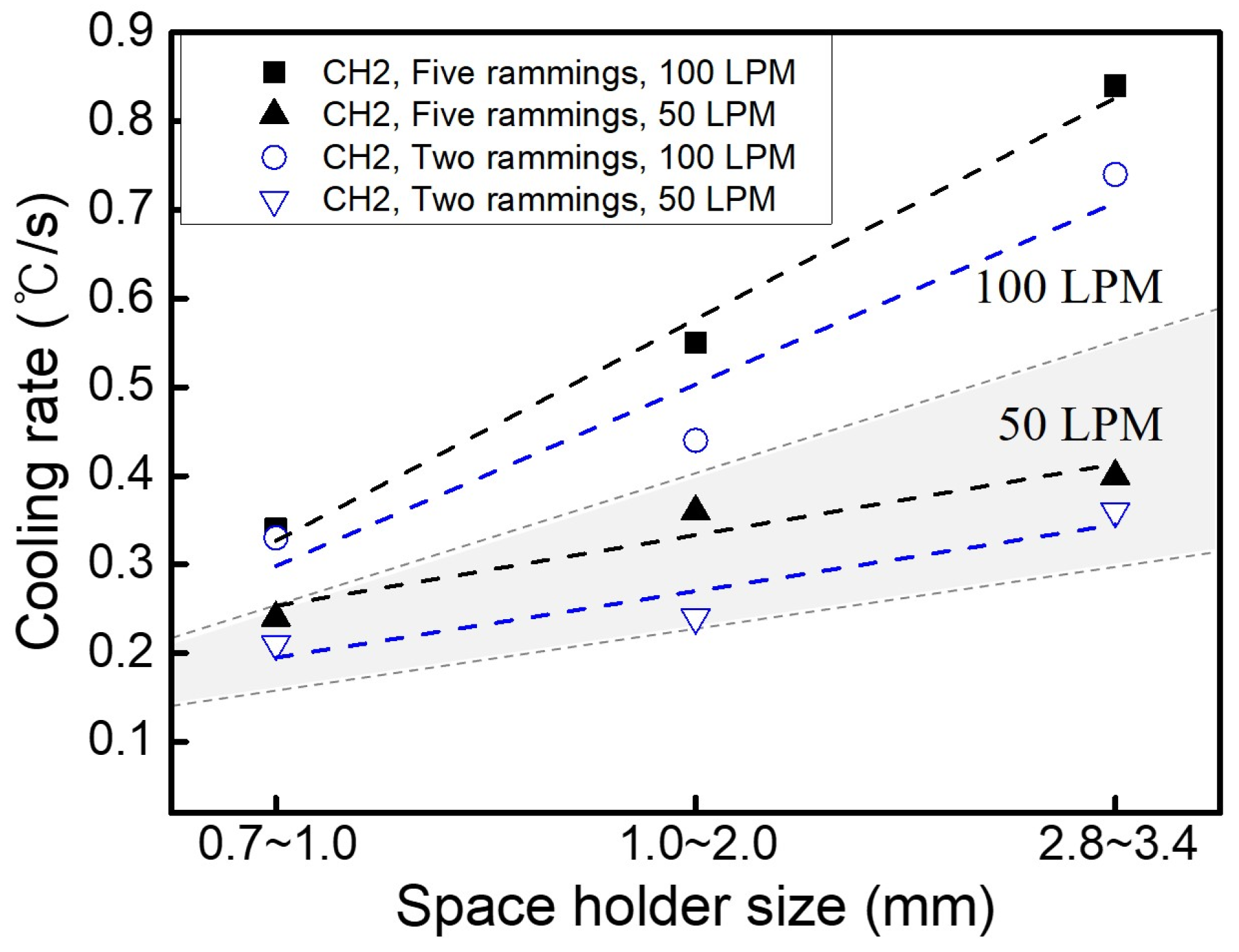
3.4. Heat Dissipation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; An, Y.; Ma, H. Research Progress in the Preparation of Aluminum Foam Composite Structures. Metals 2022, 12, 2047. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Liu, Y.; Zhou, C.; Chen, X.; Li, Y. Fabrication, Properties, and applications of open-cell aluminum foams: A review. J. Mater. Sci. Technol 2021, 62, 11–24. [Google Scholar] [CrossRef]
- Dharmasena, K.P.; Wadley, H.N.G. Electrical Conductivity of Open-cell Metal Foams. J. Mater. Res. 2002, 17, 625–631. [Google Scholar] [CrossRef]
- Monno, M.; Negri, D.; Mussi, V.; Aghaei, P.; Groppi, G.; Tronconi, E.; Strano, M. Cost-Efficient Aluminum Open-Cell Foams: Manufacture, Characterization, and Heat Transfer Measurements. Adv. Eng. Mater. 2018, 20, 1701032. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Li, X.; Lu, C.; Wang, Z.; Wang, W. Energy Absorption Performance of Open-Cell Aluminum Foam and Its Application in Landing Buffer System. J. Mater. Eng. Perform. 2021, 30, 6132–6145. [Google Scholar] [CrossRef]
- Karuppasamy, R.; Barik, D. Production methods of aluminum foam: A brief review. Mater. Today Proc. 2021, 37, 1584–1587. [Google Scholar] [CrossRef]
- Banhart, J. Aluminum foams for lighter vehicles. Int. J. Veh. Des. 2005, 37, 114–125. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Hu, H.; Lai, Z.; Weng, X.; Ding, G.; Zhuang, D. Numerical model of dehumidifying process of wet air flow in open-cell metal foam. Appl. Therm. Eng. 2017, 113, 309–321. [Google Scholar] [CrossRef]
- Hutter, C.; Büchi, D.; Zuber, V.; Rudolf von Rohr, P. Heat transfer in metal foams and designed porous media. Chem. Eng. Sci. 2011, 66, 3806–3814. [Google Scholar] [CrossRef]
- Zhao, C.Y. Review on thermal transport in high porosity cellular metal foams with open cells. Int. J. Heat Mass Transf. 2012, 55, 3618–3632. [Google Scholar] [CrossRef]
- Fischer, S.F.; Schüler, P.; Fleck, C.; Bührig-Polaczek, A. Influence of the casting and mould temperatures on the (micro)structure and compression behaviour of investment-cast open-pore aluminium foams. Acta Mater. 2013, 61, 5152–5161. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Dhakad, S.K. Preparation of metal foam by different methods: A review. Mater. Today-Proc. 2019, 26, 1784–1790. [Google Scholar] [CrossRef]
- Queheillalt, D.T.; Hass, D.D.; Sypeck, D.J.; Wadley, H.N.G. Synthesis of open-cell metal foams by templated directed vapor deposition. J. Mater. Res. 2001, 16, 1028–1036. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Wan, T.; Liu, Y.; Zhou, C.; Ding, X.; Chen, X.; Li, Y. Fabrication of high-porosity open-cell aluminum foam via high-temperature deformation of CaCl2 space-holders. Mater. Lett. 2021, 284, 129018. [Google Scholar] [CrossRef]
- Neville, B.P.; Rabiei, A. Composite metal foams processed through powder metallurgy. Mater. Des. 2008, 29, 388–396. [Google Scholar] [CrossRef]
- Sánchez, A.; Cruz, A.; Rivera, J.E.; Romero, J.A.; Suárez, M.A.; Gutiérrez, V.H. Manufacturing of Open-Cell Zn-22Al-2Cu Alloy Foams by a Centrifugal-Replication Process. Metall. Mater. Trans. A 2018, 49, 272–281. [Google Scholar] [CrossRef]
- Dubinin, O.N.; Bondareva, J.V.; Kuzminova, Y.O.; Simonov, A.P.; Varfolomeev, I.A.; Yakimchuk, I.V.; Evlashin, S.A. A promising approach to 3D printing of metal foam with defined porosity. J. Porous Mat. 2023, 30, 1565–1573. [Google Scholar] [CrossRef]
- Almonti, D.; Baiocco, G.; Tagliaferri, V.; Ucciardello, N. Design and Mechanical Characterization of Voronoi Structures Manufactured by Indirect Additive Manufacturing. Materials 2020, 13, 1085. [Google Scholar] [CrossRef]
- Lu, T.J.; Stone, H.A.; Ashby, M.F. Heat transfer in open-cell metal foams. Acta Mater. 1998, 46, 3619–3635. [Google Scholar] [CrossRef]
- Baloyo, J.M. Open-cell porous metals for thermal management applications: Fluid flow and heat transfer. Mater. Sci. Technol. 2017, 33, 265–276. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, T.J.; Hodson, H.P.; Jackson, J.D. The temperature dependence of effective thermal conductivity of open-celled steel alloy foams. Mater. Sci. Eng. A 2004, 367, 123–131. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Maestri, M.; Groppi, G.; Tronconi, E. A fundamental analysis of the influence of the geometrical properties on the effective thermal conductivity of open-cell foams. Chem. Eng. Process. 2018, 129, 181–189. [Google Scholar] [CrossRef]
- Nie, Z.; Lin, Y.; Tong, Q. Numerical investigation of pressure drop and heat transfer through open cell foams with 3D Laguerre-Voronoi model. Int. J. Heat Mass Transf. 2017, 113, 819–839. [Google Scholar] [CrossRef]
- Poureslami, P.; Siavashi, M.; Moghimi, H.; Hosseini, M. Pore-scale convection-conduction heat transfer and fluid flow in open-cell metal foams: A three-dimensional multiple-relaxation time lattice Boltzmann (MRT-LBM) solution. Int. Commun. Heat Mass 2021, 126, 105465. [Google Scholar] [CrossRef]
- Calati, M.; de Monte, E.; Mancin, S. Numerical analysis of the effects of the structure shape and orientation of kelvin cell porous structures during air forced convection. Appl. Sci. 2021, 11, 6189. [Google Scholar] [CrossRef]
- Zhu, W.; Kan, A.; Chen, Z.; Zhang, Q.; Zhang, J.; Yu, J.; He, Z. A modified Lattice Boltzmann method for predicting the effective thermal conductivity of open-cell foam materials. Int. Commun. Heat Mass 2022, 133, 105957. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, X.; Wang, X.; Du, Y.; Xiao, G. A Predicting Model for the Effective Thermal Conductivity of Anisotropic Open-Cell Foam. Energies 2022, 15, 6091. [Google Scholar] [CrossRef]
- Kim, J.M.; Ha, T.K.; Han, B.S.; Kim, Y.J. Manufacturing Method and Thermal Properties of Open-Cell Type Aluminum Foam by Replication Casting Process. Sol. State Phenom. 2023, 353, 31–36. [Google Scholar] [CrossRef]
- Lara-Rodriguez, G.A.; Figueroa, I.A.; Suarez, M.A.; Novelo-Peralta, O.; Alfonso, I.; Goodall, R. A replication-casting device for manufacturing open-cell Mg Foams. J. Mater. Process. Tech. 2017, 243, 16–22. [Google Scholar] [CrossRef]
- Dairon, J.; Gaillard, Y.; Tissier, J.C.; Balloy, D.; Degallaix, G. Parts containing open-celled metal foam manufactured by the foundry route: Processes, performances, and applications. Adv. Eng. Mater. 2011, 13, 1066–1071. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Q.; Bu, L.; Nie, Z.; Wang, Y.; Zhang, J.; Zhang, K.; Renchen, N.; He, T.; He, Z. Experimental study on improving lithium extraction efficiency of salinity-gradient solar pond through sodium carbonate addition and agitation. Sol. Energy 2022, 242, 364–377. [Google Scholar] [CrossRef]
- Richardson, J.T.; Peng, Y.; Remue, D. Properties of ceramic foam catalyst supports: Pressure drop. Appl. Catal. A-Gen. 2000, 204, 19–32. [Google Scholar] [CrossRef]
- Liu, J.F.; Wu, W.T.; Chiu, W.C.; Hsieh, W.H. Measurement and correlation of friction characteristic of flow through foam matrixes. Exp. Therm. Fluid Sci. 2006, 30, 329–336. [Google Scholar] [CrossRef]
- Guarino, S.; Rubino, G.; Tagliaferri, V.; Ucciardello, N. Thermal behavior of open cell aluminum foams in forced air: Experimental analysis. Measurement 2015, 242, 364–377. [Google Scholar] [CrossRef]
- Wu, D.; Huang, C. Thermal conductivity model of open-cell foam suitable for wide span of porosities. Int. J. Heat Mass Transf. 2019, 130, 1075–1086. [Google Scholar] [CrossRef]
- Babcsán, N.; Mészáros, I.; Hegman, N. Thermal and Electrical Conductivity Measurements on Aluminum Foams. Mater. Werkst. 2003, 34, 391–394. [Google Scholar] [CrossRef]
- Pandey, A.; Dubey, R.; Jain, H.; Abhas, A.; Kumar, R.; Gupta, G.K.; Siram, S.; Chilla, V.; Mondal, D.P. Effect of cell size on the microarchitectural and physicomechanical response in open-cell Al foam made through template method. Mater. Chem. Phys. 2023, 296, 127341. [Google Scholar] [CrossRef]
- Hernandez, J.N.C.; Link, G.; Schubert, M.; Hampel, U. Novel Mixing Relations for Determining the Effective Thermal Conductivity of Open-Cell Foams. Materials 2022, 15, 2168. [Google Scholar] [CrossRef] [PubMed]


| Elements | Si | Fe | Cu | Mn | Mg | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| wt.% | 7.08 | 0.20 | 0.08 | 0.05 | 0.29 | 0.02 | 0.12 | bal. |
| Ramming Count | Space Holder Size (mm) | Relative Density | (%) | Average Cell Wall Thickness (mm) | , 100 LPM (mbar) | , 50 LPM (mbar) |
|---|---|---|---|---|---|---|
| 5 | 2.8~3.4 | 0.373 | 62.7 | 1.76 | 61 | 15 |
| 5 | 1.0~2.0 | 0.381 | 61.9 | 0.88 | 80 | 25 |
| 5 | 0.7~1.0 | 0.388 | 61.2 | 0.22 | 108 | 34 |
| 2 | 2.8~3.4 | 0.441 | 55.9 | 2.03 | 70 | 18 |
| 2 | 1.0~2.0 | 0.447 | 55.3 | 1.01 | 89 | 28 |
| 2 | 0.7~1.0 | 0.452 | 54.8 | 0.26 | 119 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ha, T.; Lee, Y.; Kang, B.; Kim, Y. Heat Dissipation of Open-Cell-Type Aluminum Foams Manufactured by Replication-Casting Process. Metals 2024, 14, 206. https://doi.org/10.3390/met14020206
Kim J, Ha T, Lee Y, Kang B, Kim Y. Heat Dissipation of Open-Cell-Type Aluminum Foams Manufactured by Replication-Casting Process. Metals. 2024; 14(2):206. https://doi.org/10.3390/met14020206
Chicago/Turabian StyleKim, Jongmin, Taekyu Ha, Youngki Lee, Byungil Kang, and Youngjig Kim. 2024. "Heat Dissipation of Open-Cell-Type Aluminum Foams Manufactured by Replication-Casting Process" Metals 14, no. 2: 206. https://doi.org/10.3390/met14020206





