The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Mechanical Alloying
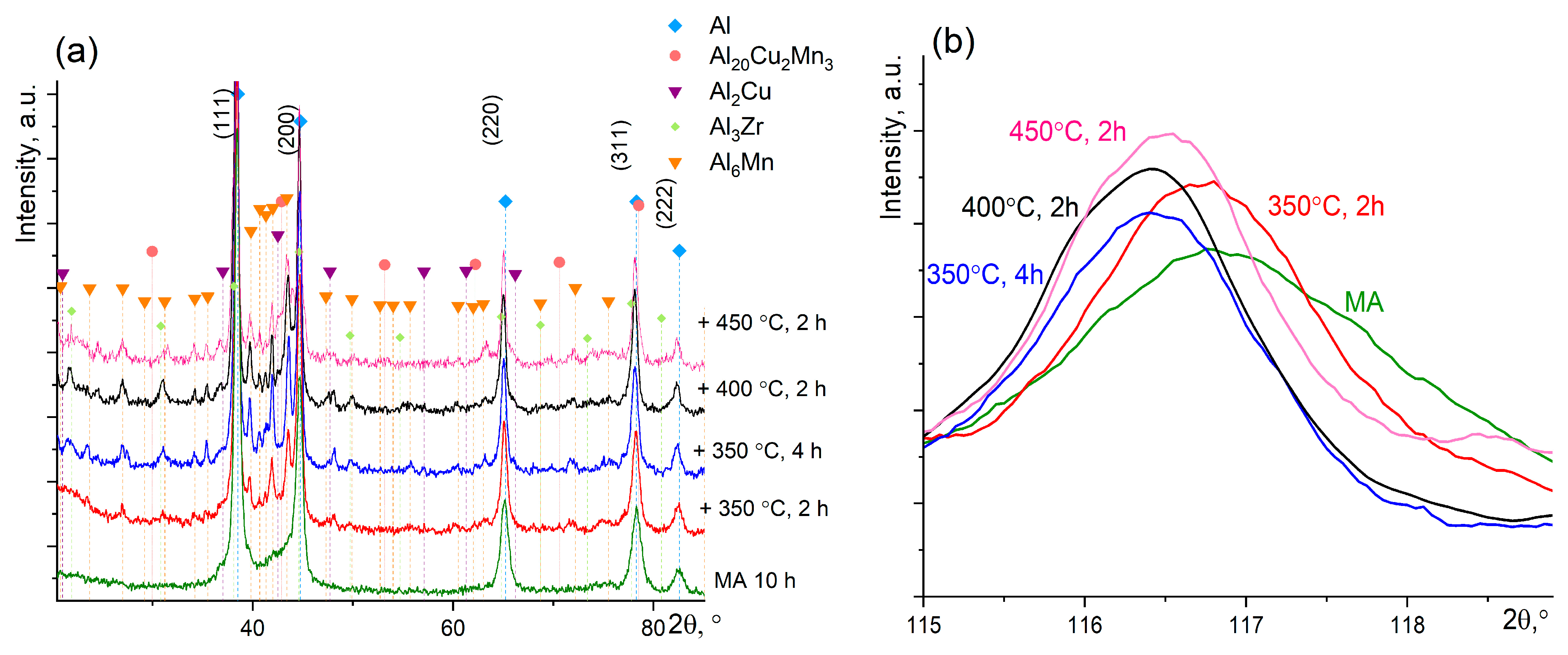
3.2. Annealing of the MA Granules
3.3. Hot-Press Sintering
4. Discussion
4.1. Effect of Mechanical Alloying
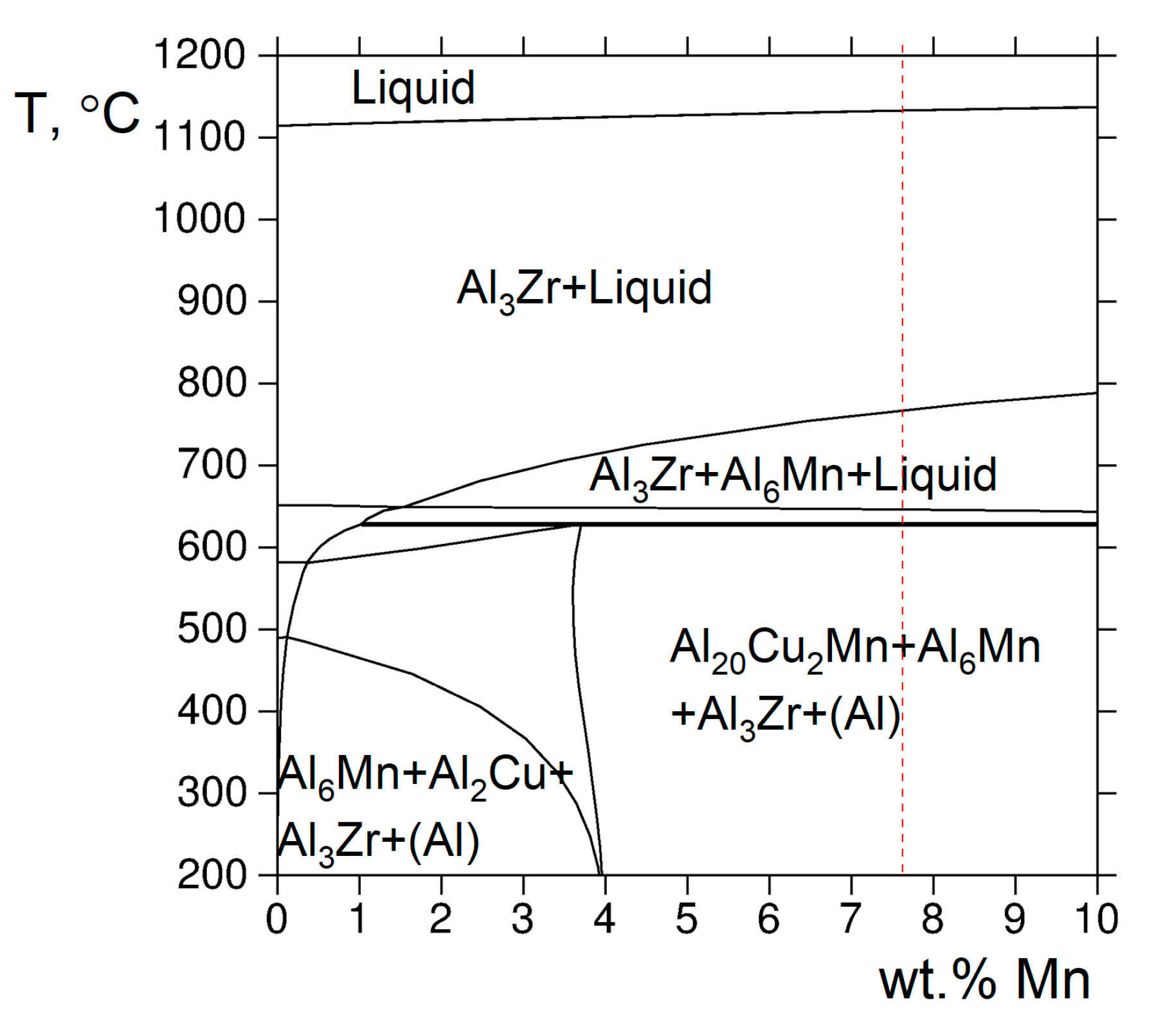
4.2. Phase Composition of the Alloy after Annealing and Hot-Press Sintering
4.3. Mechanical Properties of the Alloy after Annealing and Hot-Press Sintering
5. Summary
- The dissolution of Mn, Zr, and Cu with further precipitation of the Al6Mn phase occurred during mechanical alloying. For the PCA-free alloy, the dissolution of Zr, Cu, and the Mn solute content of about 5 wt% were observed after milling for 10 h. The grain size measured by TEM was 16 nm, which was the same as the crystallite size estimated by XRD. Due to the high solute content, fine precipitates and nano-grained structure, the MA granules exhibit a hardness of ~500–650 HV.
- The addition of stearic acid helped to refine the granules and facilitated Mn dissolution and precipitation of the secondary Al6Mn phase during milling but resulted in the formation of zirconium hydrate ZrH2 at early milling stages. Stearic acid decreased Zr solubility, increased the crystallite size of the MA granules to ~22 nm and decreased hardness to ~300–500 HV.
- According to XRD data, annealing of the MA granules at the temperatures of 350–450 °C for 2–4 h resulted in decomposition of Al solid solution with precipitation of the Al6Mn, L12–Al3Zr, Al20Cu2Mn3, and Al2Cu secondary phases. The crystallite size increased to 26–33 nm and hardness decreased from 530 HV for as-milled condition to 380–500 HV after annealing.
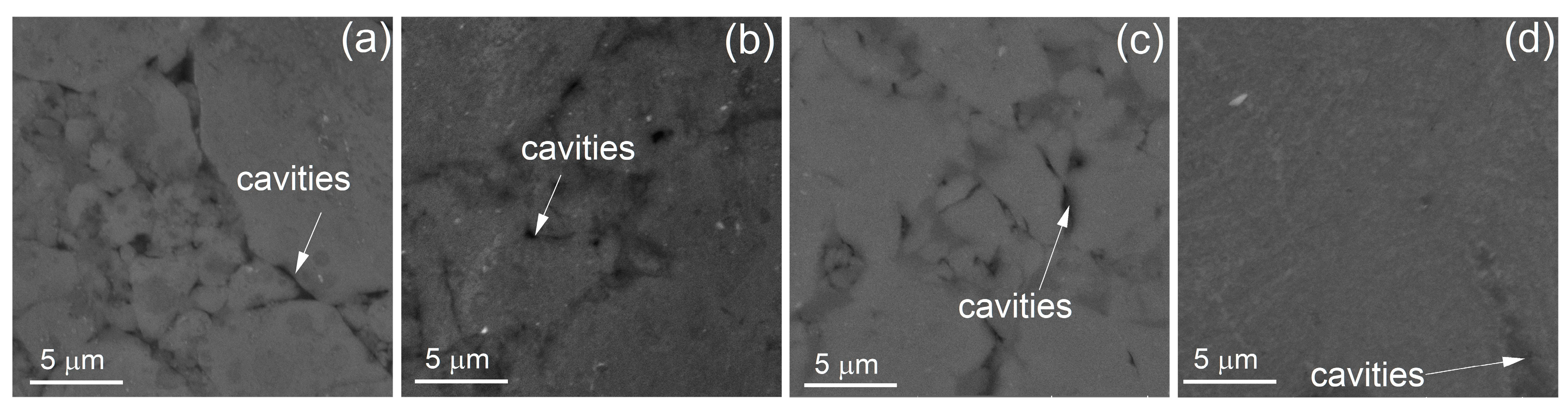
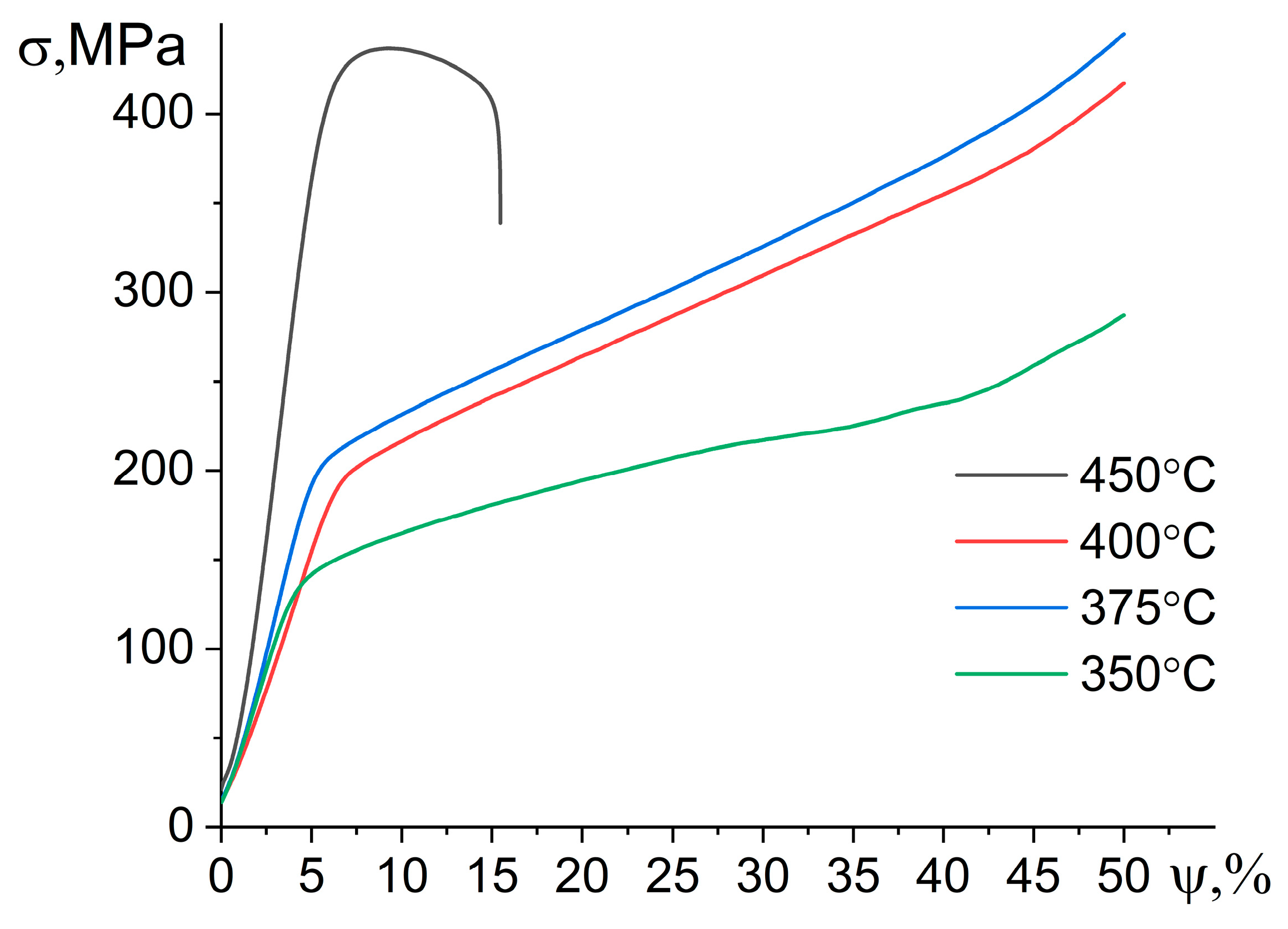
- Sintering and further annealing at temperatures of 350–400 °C provided a high hardness of 500–520 HV but a low density of ~2.7–2.9 g/sm3 and a large fraction of cavities of 6–12%. As a result, the studied alloy has a brittle fracture and low strength values at compression tests. In the elevated temperature test at 350 °C, the alloy exhibited an apparently stable flow with a low yield stress of 100–190 MPa and without the failure associated with the intragranular deformation mechanism accommodated by cavitation.
- Hot-press sintering at 450 °C, followed by annealing at 400 °C for 2 h resulted in a high density of ~3.1 g/sm3, which was similar to theoretical values, and a low fraction of cavities of ~1.5%. This sintering regime provided a lower hardness of 400 HV but high strength properties of the alloy in compression tests; a yield strength of 1100 MPa, an ultimate strength of 1200 MPa, and a strain at fracture of 0.5% were observed at room temperature, and a yield strength of 380 MPa, an ultimate strength of 440 MPa, and a strain at fracture of 3.5% were observed at 350 °C. The SEM studies of the surface structure after small strains at 350 °C showed that the deformation for the high-temperature sintered alloy was localized in the granular body.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, D.; Kumar, K. Application and Use of Different Aluminium Alloys with Respect to Workability, Strength and Welding Parameter Optimization. Ain Shams Eng. J. 2021, 12, 1143–1152. [Google Scholar] [CrossRef]
- Tzoumakis, G.; Fotopoulos, K.; Lampeas, G. Multi-Physics Digital Model of an Aluminum 2219 Liquid Hydrogen Aircraft Tank. Aerospace 2024, 11, 161. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making Sustainable Aluminum by Recycling Scrap: The Science of “Dirty” Alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Chernyshov, E.A.; Romanov, I.D.; Romanova, E.A. Problems and Perspectives of Implementation of Metalmatrix Composition Materials in Automotive Industry. IOP Conf. Ser. Earth Environ. Sci. 2020, 459, 062038. [Google Scholar] [CrossRef]
- Milojević, S.; Glišović, J.; Savić, S.; Bošković, G.; Bukvić, M.; Stojanović, B. Particulate Matter Emission and Air Pollution Reduction by Applying Variable Systems in Tribologically Optimized Diesel Engines for Vehicles in Road Traffic. Atmosphere 2024, 15, 184. [Google Scholar] [CrossRef]
- Bi, J.; Liu, L.; Zhang, D.S.; Wang, H.X.; Zheng, Q.L.; Dong, G.J. Research Progress of Casting, Rapid Solidified and Additive Manufactured Heat Resistant Aluminum Alloy. Zhongguo Youse Jinshu Xuebao/Chin. J. Nonferrous Met. 2023, 33, 969–996. [Google Scholar] [CrossRef]
- Eid, M.; Kaytbay, S.; El-Assal, A.; Elkady, O. Electrical, Thermal, and Mechanical Characterization of Hot Coined Carbon Fiber Reinforced Pure Aluminium Composites. Met. Mater. Int. 2022, 28, 2747–2765. [Google Scholar] [CrossRef]
- Lee, K.-B.; Nayak, K.C.; Shim, C.-H.; Lee, H.-I.; Kim, S.-H.; Choi, H.-J.; Ahn, J.-P. Tensile Properties of Aluminum Matrix Composites Produced via a Nitrogen-Induced Self-Forming Process. J. Compos. Sci. 2023, 7, 457. [Google Scholar] [CrossRef]
- Mondal, S.; Barik, S.; Mishra, D.P. Nanocarbon Reinforced Aluminium Matrix (NRAM) Composites: Fabrication, Structure and Properties. Mater. Sci. Technol. 2023, 39, 637–651. [Google Scholar] [CrossRef]
- De Paes Santos, C.V.; Oliveira, D.R.; Da Silva, F.P.; Lobato, M.Q.; Fernades, E.A.; Do Vale Quaresma, J.M. Application of Aluminum Alloy—Zirconium in Automotive Parts Manufacturing. In Proceedings of the 26th SAE BRASIL Inernational Congress and Display, São Paulo, Brasil, 7–9 November 2017; SAE Technical Papers. SAE International: Warrendale, PA, USA, 2017. Vol. 2017-Novem. [Google Scholar]
- Parveez, B.; Kittur, M.I.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; Umarfarooq, M.A. Scientific Advancements in Composite Materials for Aircraft Applications: A Review. Polymers 2022, 14, 5007. [Google Scholar] [CrossRef]
- Estrada-Ruiz, R.H.; Flores-Campos, R.; Herrera-Ramírez, J.M.; Martínez-Sánchez, R. Mechanical Properties of Aluminum 7075—Silver Nanoparticles Powder Composite and Its Relationship with the Powder Particle Size. Adv. Powder Technol. 2016, 27, 1694–1699. [Google Scholar] [CrossRef]
- Sadhu, K.K.; Mandal, N.; Sahoo, R.R. SiC/Graphene Reinforced Aluminum Metal Matrix Composites Prepared by Powder Metallurgy: A Review. J. Manuf. Process. 2023, 91, 10–43. [Google Scholar] [CrossRef]
- Menachery, N.; Thomas, S.; Deepanraj, B.; Senthilkumar, N. Processing of Nanoreinforced Aluminium Hybrid Metal Matrix Composites and the Effect of Post-Heat Treatment: A Review. Appl. Nanosci. 2023, 13, 4075–4099. [Google Scholar] [CrossRef]
- Okokpujie, I.P.; Tartibu, L.K. Aluminum Alloy Reinforced with Agro-Waste, and Eggshell as Viable Material for Wind Turbine Blade to Annex Potential Wind Energy: A Review. J. Compos. Sci. 2023, 7, 161. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Qian, M.; Jia, Z.; Imran, M.; Geng, L. Recent Progress in Particulate Reinforced Aluminum Composites Fabricated via Spark Plasma Sintering: Microstructure and Properties. Crit. Rev. Solid State Mater. Sci. 2023, 1–56. [Google Scholar] [CrossRef]
- Ujah, C.O.; Kallon, D.V.V. Trends in Aluminium Matrix Composite Development. Crystals 2022, 12, 1357. [Google Scholar] [CrossRef]
- Lakshmikanthan, A.; Angadi, S.; Malik, V.; Saxena, K.K.; Prakash, C.; Dixit, S.; Mohammed, K.A. Mechanical and Tribological Properties of Aluminum-Based Metal-Matrix Composites. Materials 2022, 15, 6111. [Google Scholar] [CrossRef] [PubMed]
- Belov, N.; Akopyan, T.; Tsydenov, K.; Cherkasov, S.; Avxentieva, N. Effect of Fe-Bearing Phases on the Mechanical Properties and Fracture Mechanism of Al–2wt.%Cu–1.5wt.%Mn (Mg,Zn) Non-Heat Treatable Sheet Alloy. Metals 2023, 13, 1911. [Google Scholar] [CrossRef]
- Belov, N.A.; Korotkova, N.O.; Akopyan, T.K.; Pesin, A.M. Phase Composition and Mechanical Properties of Al–1.5%Cu–1.5%Mn–0.35%Zr(Fe,Si) Wire Alloy. J. Alloys Compd. 2019, 782, 735–746. [Google Scholar] [CrossRef]
- Chankitmunkong, S.; Wang, F.; Pandee, P.; Limmaneevichitr, C.; Eskin, D.G. Precipitation Hardening and Structure Evolution in Hypereutectic Al-6 % Fe-Zr Alloys Subjected to Ultrasonic Melt Processing. J. Alloys Compd. 2024, 970, 172613. [Google Scholar] [CrossRef]
- Cui, L.; Liu, K.; Zhang, Z.; Chen, X.-G. Enhanced Elevated-Temperature Mechanical Properties of Hot-Rolled Al–Cu Alloys: Effect of Zirconium Addition and Homogenization. J. Mater. Sci. 2023, 58, 11424–11439. [Google Scholar] [CrossRef]
- Shao, S.; Liang, Z.; Yin, P.; Li, X.; Zhang, Y. Microstructure and Mechanical Properties of Al–Li Alloys with Different Li Contents Prepared by Selective Laser Melting. Materials 2024, 17, 657. [Google Scholar] [CrossRef]
- Ye, Y.-C.; He, L.-J.; Li, P.-J. Differences of Grain-Refining Effect of Sc and Ti Additions in Aluminum by Empirical Electron Theory Analysis. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2010, 20, 465–470. [Google Scholar] [CrossRef]
- Li, P.; Wei, S.; Lei, X.; Yang, L.; Sun, B. Study on Flight Dynamics and Heat Transfer Solidification of Metal Droplets during Centrifugal Spray Deposition Forming Process. Metals 2023, 13, 1446. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, W.; Shi, W.; Zhou, X.; Wen, S.; Wu, X.; Gao, K.; Zhang, D.; Qi, P.; Huang, H.; et al. Microstructure and Mechanical Properties of Al-Mg-Mn-Er-Zr Alloys Fabricated by Laser Powder Bed Fusion. Mater. Des. 2022, 222, 111064. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Yang, X.; Zhu, H. Influence of Zr Content on Printability, Microstructure and Mechanical Properties of Al-4.6Zn-1.4Mg–Zr Alloys Fabricated by Laser Powder Bed Fusion. Mater. Sci. Eng. A 2022, 854, 143790. [Google Scholar] [CrossRef]
- Zhu, A.W.; Starke, E.A. Strengthening Effect of Unshearable Particles of Finite Size: A Computer Experimental Study. Acta Mater. 1999, 47, 3263–3269. [Google Scholar] [CrossRef]
- Souza, P.H.L.; de Oliveira, C.A.S.; do Vale Quaresma, J.M. Precipitation Hardening in Dilute Al–Zr Alloys. J. Mater. Res. Technol. 2018, 7, 66–72. [Google Scholar] [CrossRef]
- Bansal, U.; Singh, M.P.; Sinha, S.K.; Sahu, D.K.; Mondol, S.; Makineni, S.K.; Paul, A.; Chattopadhyay, K. Strength and Stability through Variable Micro Segregation Behaviour of Ta and Zr Solutes at Intermetallic Interfaces in Al-Cu Alloys. Acta Mater. 2023, 259, 119254. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Sýkorová, M.; Brůna, M.; Matejka, M.; Širanec, L. Effect of Zr Addition on Selected Properties and Microstructure of Aluminum Alloy AlSi5Cu2Mg. Int. J. Met. 2023, 17, 2598–2611. [Google Scholar] [CrossRef]
- Nokhrin, A.V.; Nagicheva, G.S.; Chuvil’deev, V.N.; Kopylov, V.I.; Bobrov, A.A.; Tabachkova, N.Y. Effect of Er, Si, Hf and Nb Additives on the Thermal Stability of Microstructure, Electrical Resistivity and Microhardness of Fine-Grained Aluminum Alloys of Al-0.25%Zr. Materials 2023, 16, 2114. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, W.; Shi, W.; Zhou, X.; Wen, S.; Wu, X.; Gao, K.; Rong, L.; Qi, P.; Huang, H.; et al. Synergistic Effect of Al3(Er, Zr) Precipitation and Hot Extrusion on the Microstructural Evolution of a Novel Al–Mg–Si–Er–Zr Alloy. J. Mater. Res. Technol. 2023, 22, 947–957. [Google Scholar] [CrossRef]
- Vafaeenezhad, H.; Shahverdi, H.R. Synergic Effects of Si and V Micro-Additions on Microstructural and Mechanical Properties of a Dilute Al–Sc–Zr Alloy Containing L12 Al3(Sc, Zr, V) Nanoprecipitates. J. Alloys Compd. 2023, 967, 171747. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation Evolution in Al–Zr and Al–Zr–Ti Alloys during Isothermal Aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Lefebvre, W.; Masquelier, N.; Houard, J.; Patte, R.; Zapolsky, H. Tracking the Path of Dislocations across Ordered Al3Zr Nano-Precipitates in Three Dimensions. Scr. Mater. 2014, 70, 43–46. [Google Scholar] [CrossRef]
- Knipling, K. Precipitation Evolution in Al–Zr and Al–Zr–Ti Alloys during Aging at 450–600 °C. Acta Mater. 2008, 56, 1182–1195. [Google Scholar] [CrossRef]
- Xun, C.; Li, X.; Wen, K.; Geng, L.; Chen, P.; You, W.; Li, Y.; Li, Z.; Zhang, Y.; Xiong, B. Precipitation of Al 3 Zr Dispersoids under Various Homogenization and Its Effect on Recrystallization in 2198 Al–Cu–Li Alloy. Adv. Eng. Mater. 2023, 25. [Google Scholar] [CrossRef]
- Elasheri, A.; Elgallad, E.M.; Parson, N.; Chen, X.-G. Nucleation and Transformation of Zr-Bearing Dispersoids in Al–Mg–Si 6xxx Alloys. J. Mater. Res. 2023, 38, 696–707. [Google Scholar] [CrossRef]
- Hansen, V.; Gjønnes, J. Quasicrystals in an Aluminium Alloy Matrix and the Transformation to A–AlMnSi via Intermediate Stages. Philos. Mag. A 1996, 73, 1147–1158. [Google Scholar] [CrossRef]
- Ruan, S.; Schuh, C.A. Electrodeposited Al-Mn Alloys with Microcrystalline, Nanocrystalline, Amorphous and Nano-Quasicrystalline Structures. Acta Mater. 2009, 57, 3810–3822. [Google Scholar] [CrossRef]
- Rozman, N.; Medved, J.; Zupanič, F. Microstructural Evolution in Al-Mn-Cu-(Be) Alloys. Philos. Mag. 2011, 91, 4230–4246. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. Nanodispersoids of the Quasicrystalline I-Phase in Mn- and Mg-Bearing Aluminum-Based Alloys. Mater. Lett. 2021, 284, 128945. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.-G. Effects of Al(MnFe)Si Dispersoids with Different Sizes and Number Densities on Microstructure and Ambient/Elevated-Temperature Mechanical Properties of Extruded Al–Mg–Si AA6082 Alloys with Varying Mn Content. J. Alloys Compd. 2021, 861, 157937. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Huang, S.; Yi, Y.; He, H. Effect of Three-Dimensional Deformation at Different Temperatures on Microstructure, Strength, Fracture Toughness and Corrosion Resistance of 7A85 Aluminum Alloy. J. Alloys Compd. 2022, 928, 167200. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Prangnell, P.B.; Sigli, C.; Bès, B. Effects of Combined Zr and Mn Additions on Dispersoid Formation and Recrystallisation Behaviour of AA2198 Sheet. Adv. Mater. Res. 2010, 89–91, 568–573. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Tabachkova, N.Y.; Ghayoumabadi, M.E.; Cheverikin, V.V.; Mikhaylovskaya, A.V. Joint Effect of Quasicrystalline Icosahedral and L1<inf>2</Inf>-Strucutred Phases Precipitation on the Grain Structure and Mechanical Properties of Aluminum-Based Alloys. J. Mater. Sci. Technol. 2021, 87, 196–206. [Google Scholar] [CrossRef]
- Jia, Z.; Hu, G.; Forbord, B.; Solberg, J.K. Effect of Homogenization and Alloying Elements on Recrystallization Resistance of Al–Zr–Mn Alloys. Mater. Sci. Eng. A 2007, 444, 284–290. [Google Scholar] [CrossRef]
- Pan, S.; Wang, Z.; Li, C.; Wan, D.; Chen, X.; Chen, K.; Li, Y. Achieving Superior Dispersion-Strengthening Effect in an AA5xxx Al-Mg-Mn Alloy by Mico-Alloying. Mater. Des. 2023, 226, 111647. [Google Scholar] [CrossRef]
- Forbord, B.; Hallem, H.; Ryum, N.; Marthinsen, K. Precipitation and Recrystallisation in Al–Mn–Zr with and without Sc. Mater. Sci. Eng. A 2004, 387–389, 936–939. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically Alloyed Nanocomposites. Prog. Mater. Sci. 2013, 58, 383–502. [Google Scholar] [CrossRef]
- Batista, C.D.; Fernandes, A.A.M.d.N.d.P.; Vieira, M.T.F.; Emadinia, O. From Machining Chips to Raw Material for Powder Metallurgy—A Review. Materials 2021, 14, 5432. [Google Scholar] [CrossRef]
- Christudasjustus, J.; Larimian, T.; Esquivel, J.; Gupta, S.; Darwish, A.A.; Borkar, T.; Gupta, R.K. Aluminum Alloys with High Elastic Modulus. Mater. Lett. 2022, 320, 132292. [Google Scholar] [CrossRef]
- Kundan, N.; Parida, B.; Keshri, A.K.; Soni, P.R. Synthesis and Characterization of the Nanostructured Solid Solution with Extended Solubility of Graphite in Nickel by Mechanical Alloying. Int. J. Miner. Metall. Mater. 2019, 26, 1031–1037. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Khelge, S.; Kumar, V.; Shetty, V. Kumaraswamy J Effect of Reinforcement Particles on the Mechanical and Wear Properties of Aluminium Alloy Composites: Review. Mater. Today Proc. 2022, 52, 571–576. [Google Scholar] [CrossRef]
- Chen, Y.; Hwang, T.; Marsh, M.; Williams, J.S. Study on Mechanism of Mechanical Activation. Mater. Sci. Eng. A 1997, 226–228, 95–98. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Maria Jackson, A.; Baskar, N.; Ganesan, M.; Varatharajulu, M. Evaluation of Stir Cast AlSiC Metal Matrix Composite by Energy-Dispersive Spectroscopy and Study of Influences of Milling Parameters by Particle Swarm Optimization. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 125. [Google Scholar] [CrossRef]
- Nokhrin, A.; Malekhonova, N.; Chuvil’deev, V.; Melekhin, N.; Bragov, A.; Filippov, A.; Boldin, M.; Lantsev, E.; Sakharov, N. Effect of High-Energy Ball Milling Time on the Density and Mechanical Properties of W-7%Ni-3%Fe Alloy. Metals 2023, 13, 1432. [Google Scholar] [CrossRef]
- Calka, A.; Kaczmarek, W.; Williams, J.S. Extended Solid Solubility in Ball-Milled Al-Mg Alloys. J. Mater. Sci. 1993, 28, 15–18. [Google Scholar] [CrossRef]
- Maity, S.; Sinha, A.; Bera, S. A Novel Study on Mechanically Alloyed Al–Mg System by X-Ray Diffraction Technique. Nano-Struct. Nano-Objects 2018, 16, 63–68. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Influence of the V Content on Microstructure and Hardness of High-Energy Ball Milled Nanocrystalline Al-V Alloys. J. Alloys Compd. 2018, 760, 63–70. [Google Scholar] [CrossRef]
- Witharamage, C.S.; Christudasjustus, J.; Gupta, R.K. The Effect of Milling Time and Speed on Solid Solubility, Grain Size, and Hardness of Al-V Alloys. J. Mater. Eng. Perform. 2021, 30, 3144–3158. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.K.; Suryanarayana, C.; Froes, F.H. Structural Evolution in Mechanically Alloyed Al-Fe Powders. Metall. Mater. Trans. A 1995, 26, 1939–1946. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Ohkubo, T.; Hono, K. Microstructure and Mechanical Properties of Bulk Nanocrystalline Al–Fe Alloy Processed by Mechanical Alloying and Spark Plasma Sintering. Acta Mater. 2009, 57, 3529–3538. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Armstrong, L.; Kapoor, D.; Tschopp, M.A.; Kecskes, L.J.; Mathaudhu, S.N. Influence of Mn Solute Content on Grain Size Reduction and Improved Strength in Mechanically Alloyed Al–Mn Alloys. Mater. Sci. Eng. A 2014, 589, 57–65. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Sundaresan, R. Metastable Phases in Mechanically Alloyed Al-Mn Powder Mixtures. Mater. Sci. Eng. A 1991, 131, 237–242. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Bazlov, A.I.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum. Metals 2023, 13, 1765. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Catalano, J.E.; Tschopp, M.A.; Kecskes, L.J. Effect of Processing Parameters on the Microstructure of Mechanically Alloyed Nanostructured Al-Mn Alloys. Adv. Compos. Aerosp. Mar. Land Appl. II 2016, 3–11. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D. Strengthening of Mechanically Alloyed Al-Based Alloy with High Zr Contents. Mater. Sci. Eng. A 2018, 713, 174–179. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Bazlov, A.I.; Churyumov, A.Y.; Mikhaylovskaya, A.V. A Study on the Influence of Zr on the Strengthening of the Al-10% Al2O3 Composite Obtained by Mechanical Alloying. Metals 2023, 13, 2008. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Investigation of Nanostructured Al-10 Wt.% Zr Material Prepared by Ball Milling for High Temperature Applications. Mater. Charact. 2017, 123, 173–177. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Influence of Pre-Milling on the Mn Solid Solubility in the Al-Mn-Cu Alloy during Mechanical Alloying. Metals 2023, 13, 756. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Microstructural Evolution and Grain Subdivision Mechanisms during Severe Plastic Deformation of Aluminum Particles by Ball Milling. Philos. Mag. 2015, 95, 1425–1447. [Google Scholar] [CrossRef]
- Senkov, O.; Froes, F.; Stolyarov, V.; Valiev, R.; Liu, J. Microstructure of Aluminum-Iron Alloys Subjected to Severe Plastic Deformation. Scr. Mater. 1998, 38, 1511–1516. [Google Scholar] [CrossRef]
- Sauvage, X.; Ganeev, A.; Ivanisenko, Y.; Enikeev, N.; Murashkin, M.; Valiev, R. Grain Boundary Segregation in UFG Alloys Processed by Severe Plastic Deformation. Adv. Eng. Mater. 2012, 14, 968–974. [Google Scholar] [CrossRef]
- Bachmaier, A.; Kerber, M.; Setman, D.; Pippan, R. The Formation of Supersaturated Solid Solutions in Fe–Cu Alloys Deformed by High-Pressure Torsion. Acta Mater. 2012, 60, 860–871. [Google Scholar] [CrossRef]
- Bachmaier, A.; Rathmayr, G.B.; Bartosik, M.; Apel, D.; Zhang, Z.; Pippan, R. New Insights on the Formation of Supersaturated Solid Solutions in the Cu–Cr System Deformed by High-Pressure Torsion. Acta Mater. 2014, 69, 301–313. [Google Scholar] [CrossRef]
- Song, Z.Z.; Niu, R.M.; Cui, X.Y.; Bobruk, E.V.; Murashkin, M.; Enikeev, N.A.; Valiev, R.Z.; Ringer, S.P.; Liao, X.Z. Room-Temperature-Deformation-Induced Chemical Short-Range Ordering in a Supersaturated Ultrafine-Grained Al-Zn Alloy. Scr. Mater. 2022, 210, 114423. [Google Scholar] [CrossRef]
- Zhou, E.; Suryanarayana, C.; Froes, F.H. Effect of Premilling Elemental Powders on Solid Solubility Extension of Magnesium in Titanium by Mechanical Alloying. Mater. Lett. 1995, 23, 27–31. [Google Scholar] [CrossRef]
- Wei, L.; Abd Rahim, S.; Al Bakri Abdullah, M.; Yin, A.; Ghazali, M.; Omar, M.; Nemeș, O.; Sandu, A.; Vizureanu, P.; Abdellah, A. Producing Metal Powder from Machining Chips Using Ball Milling Process: A Review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Nayak, K.C.; Han, J.Y.; Jung, C.H.; Joo, M.R.; Lee, K.B.; Bae, D.H.; Choi, H.J. Synergetic Effect of Milling Speed and Duration on Particle Morphology and Mechanical Properties of Nanocrystalline Al Matrix Containing SiC. Powder Metall. 2023, 66, 519–529. [Google Scholar] [CrossRef]
- Çinici, H.; Karakoç, H.; Şahin, Ö.; Ovalı, İ. Investigation of Microstructure, Mechanical, and Tribological Properties of SiC/h-BN/Gr Hybrid Reinforced Al6061 Matrix Composites Produced by Hot Extrusion Method. Diam. Relat. Mater. 2023, 136, 110028. [Google Scholar] [CrossRef]
- Tkachenko, S.; Slámečka, K.; Oliver-Urrutia, C.; Ksenzova, O.; Bednaříková, V.; Remešová, M.; Dvořák, K.; Baláž, M.; Deák, A.; Kachlík, M.; et al. Effect of Powder Milling on Sintering Behavior and Monotonic and Cyclic Mechanical Properties of Mo and Mo–Si Lattices Produced by Direct Ink Writing. J. Mater. Res. Technol. 2023, 27, 2475–2489. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M.F.; Abomostafa, H.M. Fabrication of Al/Al2O3/ SiC/Graphene Hybrid Nanocomposites from Al-Dross by Powder Metallurgy: Sinterability, Mechanical and Electrical Properties. Ceram. Int. 2022, 48, 20923–20932. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Qian, M.; Li, A.; Geng, L. Effect of SiC Nanoparticle on Microstructure and Mechanical Properties of Graphene Nanosheet (GNS) Reinforced Aluminum Composites. J. Alloys Compd. 2023, 968, 172177. [Google Scholar] [CrossRef]
- Prashanth, M.; Karunanithi, R.; Sivasankaran, S.; MilicaVlahovic; Bhowmik, A. Synergistic Strengthening Mechanism and Microstructural Evolution of Al-Zn-Mg-Cu/ Al2O3/Y2O3 Hybrid Nanocomposite via Mechanical Alloying and Hot Pressing. Powder Technol. 2024, 434, 119377. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Robert, M.H.; Torralba, J.M. Mechanically Alloyed AlN Particle-Reinforced Al-6061 Matrix Composites: Powder Processing, Consolidation and Mechanical Strength and Hardness of the as-Extruded Materials. Mater. Sci. Eng. A 2006, 426, 85–94. [Google Scholar] [CrossRef]
- Guoxian, L.; Zhimin, L.; Erde, W.; Zhongren, W. Hot Hydrostatic Extrusion and Microstructures of Mechanically Alloyed Al-4.9Fe-4.9Ni Alloy. J. Mater. Process. Technol. 1995, 55, 37–42. [Google Scholar] [CrossRef]
- Bathula, S.; Anandani, R.C.; Dhar, A.; Srivastava, A.K. Microstructural Features and Mechanical Properties of Al 5083/SiCp Metal Matrix Nanocomposites Produced by High Energy Ball Milling and Spark Plasma Sintering. Mater. Sci. Eng. A 2012, 545, 97–102. [Google Scholar] [CrossRef]
- Muthuchamy, A.; Srikanth, M.; Agrawal, D.K.; Annamalai, A.R. Effect of Microwave and Conventional Modes of Heating on Sintering Behavior, Microstructural Evolution and Mechanical Properties of Al-Cu-Mn Alloys. Molecules 2021, 26, 3675. [Google Scholar] [CrossRef]
- Annamalai, A.R.; Srikanth, M.; Muthuchamy, A.; Acharya, S.; Khisti, A.; Agrawal, D.K.; Jen, C.-P. Spark Plasma Sintering and Characterization of Al-TiB2 Composites. Metals 2020, 10, 1110. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Kawasaki, M.; Langdon, T.G. Seventy Years of Hall-Petch, Ninety Years of Superplasticity and a Generalized Approach to the Effect of Grain Size on Flow Stress. Prog. Mater. Sci. 2023, 137, 101131. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Knight, B.E.; Schuh, C.A. Six Decades of the Hall–Petch Effect—A Survey of Grain-Size Strengthening Studies on Pure Metals. Int. Mater. Rev. 2016, 61, 495–512. [Google Scholar] [CrossRef]
- Rofman, O.V.; Prosviryakov, A.S.; Mikhaylovskaya, A.V.; Kotov, A.D.; Bazlov, A.I.; Cheverikin, V.V. Processing and Microstructural Characterization of Metallic Powders Produced from Chips of AA2024 Alloy. JOM 2019, 71, 2986–2995. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction; Springer: Boston, MA, USA, 1998; ISBN 978-1-4899-0150-7. [Google Scholar]
- Yakovtseva, O.A.; Bazlov, A.I.; Prosviryakov, A.S.; Emelina, N.B.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The Influence of the Al<inf>2</Inf>O<inf>3</Inf> Particles on the Microstructure of the Mechanically Alloyed Al-Mn-Cu Alloy. J. Alloys Compd. 2023, 930, 167452. [Google Scholar] [CrossRef]
- Muthaiah, V.M.S.; Mula, S. Effect of Zirconium on Thermal Stability of Nanocrystalline Aluminium Alloy Prepared by Mechanical Alloying. J. Alloys Compd. 2016, 688, 571–580. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Bazlov, A.I.; Mikhaylovskaya, A.V. Development of Heat-Resistant Composites Based on Al-Mg-Si Alloy Mechanically Alloyed with Aluminide Particles. JOM 2024, 76, 1306–1318. [Google Scholar] [CrossRef]
- Zhou, F.; Lee, Z.; Nutt, S.R.; Lavernia, E.J. The Influence of Sc on Thermal Stability of a Nanocrystalline Al-Mg Alloy Processed by Cryogenic Ball Milling. Metall. Mater. Trans. A 2005, 36, 1587–1594. [Google Scholar] [CrossRef]
- Zhou, F.; Lee, Z.; Lavernia, E.J.; Nutt, S.R. Bimodal Microstructures in Nanocrystalline Al and Al-Mg Alloy Powders Prepared by Cryogenic Ball Milling. MRS Proc. 2004, 821, 185–190. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mukhamejanova, A.; Kotov, A.D.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mochugovskiy, A.G. Precipitation Behavior of the Metastable Quasicrystalline I-Phase and Θ′-Phase in Al-Cu-Mn Alloy. Metals 2023, 13, 469. [Google Scholar] [CrossRef]
- Belov, N.A.; Alabin, A.N. Energy Efficient Technology for Al–Cu–Mn–Zr Sheet Alloys. Mater. Sci. Forum 2013, 765, 13–17. [Google Scholar] [CrossRef]
- Zupanič, F.; Gspan, C.; Burja, J.; Bončina, T. Quasicrystalline and L12 Precipitates in a Microalloyed Al-Mn-Cu Alloy. Mater. Today Commun. 2020, 22, 100809. [Google Scholar] [CrossRef]
- Bončina, T.; Albu, M.; Zupanič, F. Ageing of Al-Mn-Cu-Be Alloys for Stimulating Precipitation of Icosahedral Quasicrystals. Metals. 2020, 10, 937. [Google Scholar] [CrossRef]
- Mochugovskiy, A.; Tabachkova, N.; Mikhaylovskaya, A. Annealing Induced Precipitation of Nanoscale Icosahedral Quasicrystals in Aluminum Based Alloy. Mater. Lett. 2019, 247, 200–203. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Jang, J.; Lee, J.; Lee, C. Orowan Strengthening Effect on the Nanoindentation Hardness of the Ferrite Matrix in Microalloyed Steels. Mater. Sci. Eng. A 2008, 487, 552–557. [Google Scholar] [CrossRef]
- Yakovtseva, O.; Mochugovskiy, A.; Zanaeva, E.N.; Emelina, N.; Prosviryakov, A.; Mihaylovskaya, A. Strenghthening of the Mechanically Alloyed Al-Mn-Cu Alloy. Meatllirgist, 2024; in press. [Google Scholar]
- Schurack, F.; Eckert, J.; Schultz, L. Synthesis and Mechanical Properties of Cast Quasicrystal-Reinforced Al-Alloys. Acta Mater. 2001, 49, 1351–1361. [Google Scholar] [CrossRef]
- Lava Kumar, P.; Lombardi, A.; Byczynski, G.; Narayana Murty, S.V.S.; Murty, B.S.; Bichler, L. Recent Advances in Aluminium Matrix Composites Reinforced with Graphene-Based Nanomaterial: A Critical Review. Prog. Mater. Sci. 2022, 128, 100948. [Google Scholar] [CrossRef]
- Srivatsan, T.S. Microstructure, Tensile Properties and Fracture Behaviour of Al2O3 Particulate-Reinforced Aluminium Alloy Metal Matrix Composites. J. Mater. Sci. 1996, 31, 1375–1388. [Google Scholar] [CrossRef]
- Mohammed, S.M.A.K.; Chen, D.L.; Liu, Z.Y.; Wang, Q.Z.; Ni, D.R.; Xiao, B.L.; Ma, Z.Y. Cyclic Deformation Behavior and Fatigue Life Modeling of CNT-Reinforced Heterogeneous Aluminum-Based Nanocomposite. Mater. Sci. Eng. A 2022, 840, 142881. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Minak, G.; Morri, A.; Tarterini, F. Effect of Friction Stir Welding on Microstructure, Tensile and Fatigue Properties of the AA7005/10 Vol.%Al2O3 Composite. Compos. Sci. Technol. 2007, 67, 605–615. [Google Scholar] [CrossRef]
- Ahmed, A.; Neely, A.J.; Shankar, K.; Nolan, P.; Moricca, S.; Eddowes, T. Synthesis, Tensile Testing, and Microstructural Characterization of Nanometric SiC Particulate-Reinforced Al 7075 Matrix Composites. Metall. Mater. Trans. A 2010, 41, 1582–1591. [Google Scholar] [CrossRef]
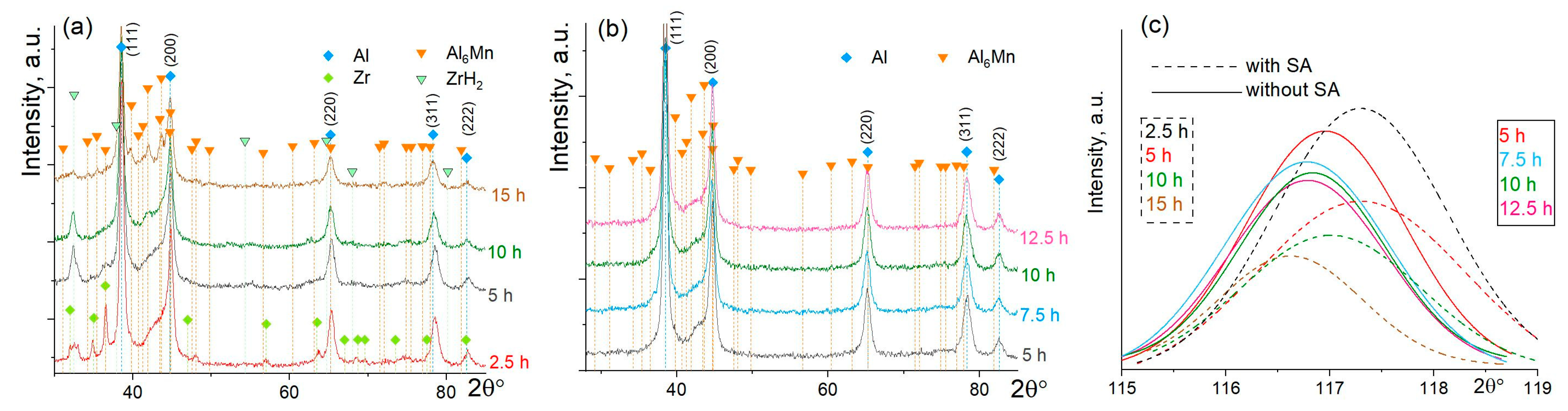
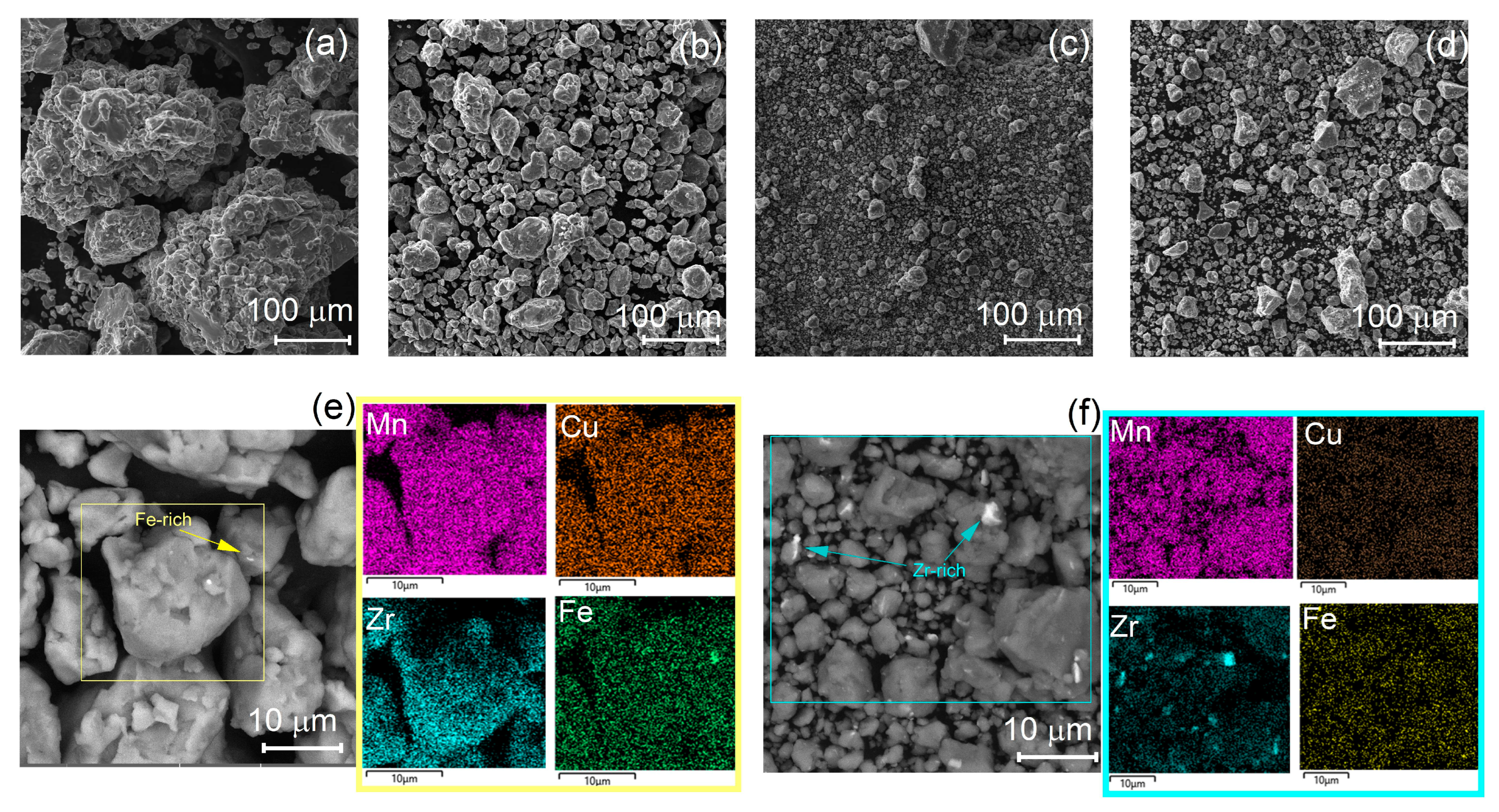


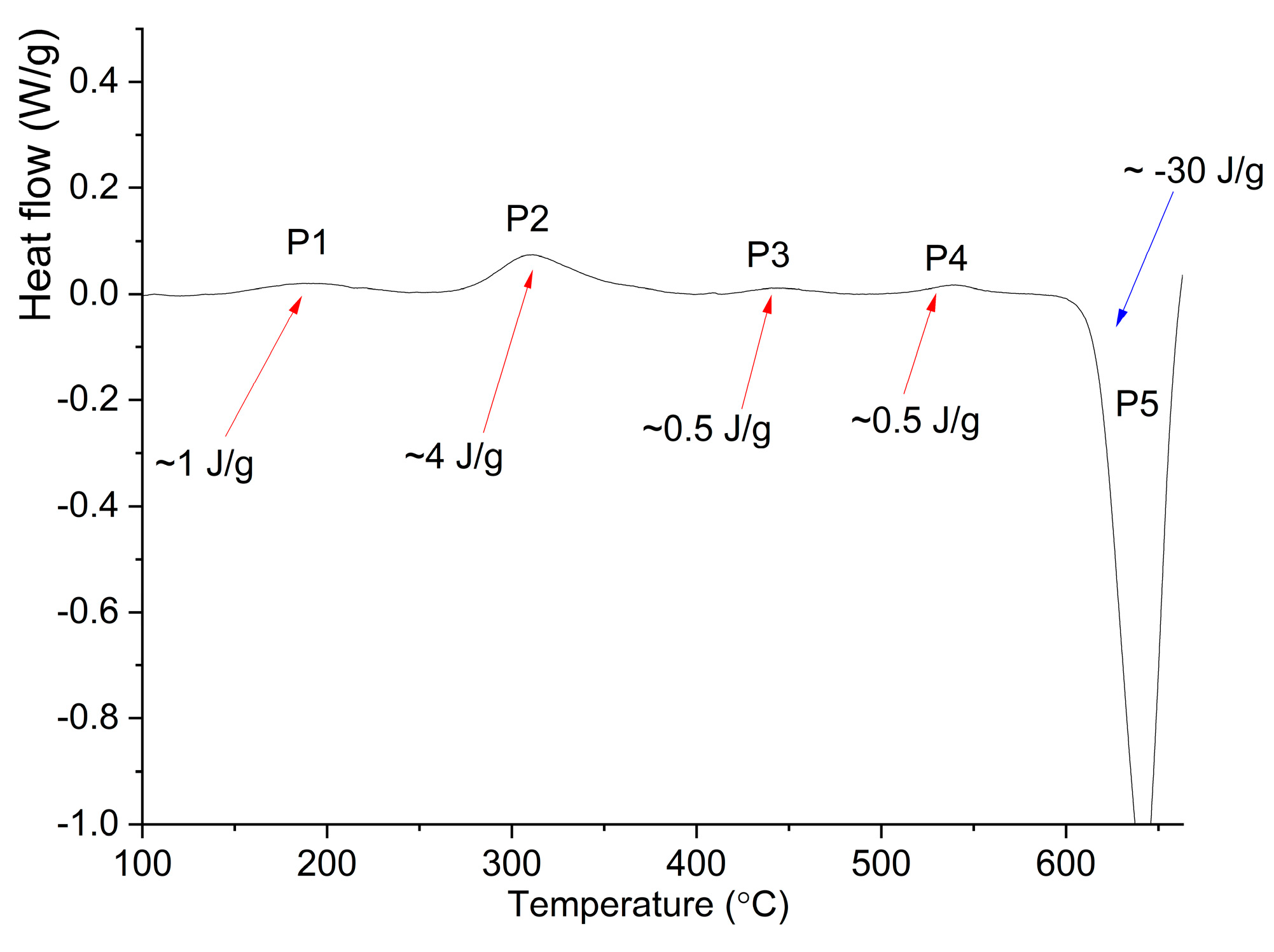
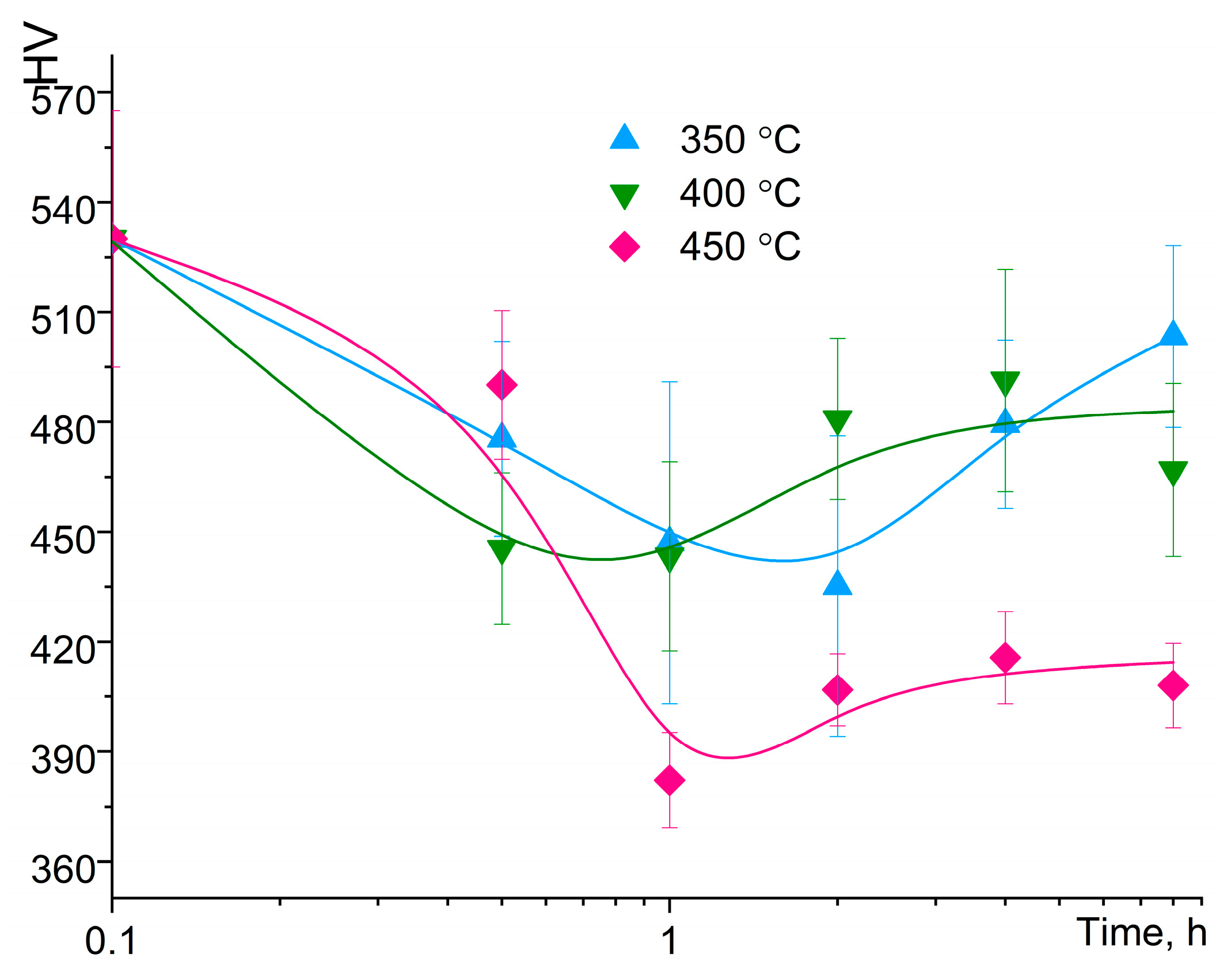


| Treatment | Lattice Parameter a, nm | Coherent Scattering Region, CSR, nm |
|---|---|---|
| Mechanical alloying | 0.4039 | 17 ± 3 |
| Annealing at 350 °C for 2 h | 0.4048 | 23 ± 5 |
| Annealing at 350 °C for 4 h | 0.4055 | 31 ± 6 |
| Annealing at 400 °C for 2 h | 0.4055 | 26 ± 5 |
| Annealing at 450 °C for 2 h | 0.4053 | 29 ± 8 |
| Hot pressing at 350 °C | 0.4042 | 34 ± 2 |
| Hot pressing at 375 °C | 0.4043 | 25 ± 5 |
| Hot pressing at 400 °C | 0.4047 | 33 ± 7 |
| Hot pressing at 450 °C | 0.4053 | 33 ± 8 |
| Consolidation Temperature and Heat Treatment Regime | HV, kgs/mm2 | Density, g/sm3 | Ultimate Compressive Strength (σmax) at 25 °C, MPa | Yield Strength (σ0.2) at 350 °C, MPa |
|---|---|---|---|---|
| 350 °C | 500 ± 30 | 2.70 ± 0.03 | 180 ± 10 | 125 ± 20 |
| 350 °C and annealing at 350 °C for 2 h | 475 ± 30 | 2.69 ± 0.02 | 160 ± 20 | 100 ± 15 |
| 375 °C | 520 ± 20 | 2.77 ± 0.02 | 415 ± 15 | 160 ± 15 |
| 375 °C and annealing at 375 °C for 2 h | 515 ± 20 | 2.89 ± 0.02 | 475 ± 15 | 190 ± 10 |
| 400 °C | 470 ± 20 | 2.78 ± 0.02 | 350 ± 30 | 165 ± 10 |
| 400 °C and annealing at 400 °C for 2 h | 510 ± 15 | 2.85 ± 0.01 | 610 ± 25 | 185 ± 15 |
| 450 °C | 380 ± 20 | 3.05 ± 0.02 | - | - |
| 450 °C and annealing at 400 °C for 2 h | 400 ± 15 | 3.15 ± 0.05 | 1100 ± 20 */1200 ± 30 ** | 380 ± 12 */440 ± 13 ** |
| Alloy/Composite | Hardness, HV, kgs/mm2 | Yield Strength (RT/Elevated Temperature), MPa | Ultimate Compressive or Tensile Strength, (RT/Elevated Temperature) MPa | Strain–to–Fracture, (RT/Elevated Temperature)% | Ref. |
|---|---|---|---|---|---|
| Al–7.7 wt%Mn–3.2 wt%Cu–4.9 wt%Zr | 530–630 | 1100RT/380350 | 1200RT/440350 | 0.5RT/3.5350 | This study |
| Al–7.7 wt%Mn–3.1 wt%Cu | 500 | 870RT/220350 | 900RT/350350 | 0.27RT/40350 | [109] |
| Al–3.17%Mn–3.08 %Cu–0.35%Be–0.21%Sc–0.14%Zr | ~110 | 304RT/150350 | 437RT/155350 | 180350 | [105] |
| Al–Mn–Cu–Zr | - | 450RT | 590RT | 0.6RT | [20] |
| Al–3%Mn–1%Cr–2%Cu | - | ≈300RT | 600RT | ≥20 RT | [110] |
| Al–10 wt%Zr | 270 | 310300 | 822RT/344300 | 0.1RT/0.2300 | [73] |
| Al–10 wt%Fe–PCA | - | 1000RT/400350 | 1200RT/500350 | 0.1–0.2RT/≥15350 | [66] |
| Al alloy + graphene NP | - | - | 1200RT/110350 | - | [111] |
| 7075 + Ag–C NPs | 300 | - | - | - | [12] |
| 2024 + 10%Al2O3 | - | 460RT/325180 | 495RT/340180 | 2.0RT/6.6180 | [112] |
| Al–5 wt%Zr–10%Al203 | 520 | 220350 | 340350 | 25350 | [72] |
| AA6063 + 1 wt%Cu + Al3Ti | 265 | 872RT/273300 | ~970RT/290300 | ~8300 | [100] |
| AA6063 + 1 wt%Cu + Al3Zr | 265 | 841RT/242300 | ~900RT/254300 | ~5300 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovtseva, O.A.; Mochugovskiy, A.G.; Prosviryakov, A.S.; Bazlov, A.I.; Emelina, N.B.; Mikhaylovskaya, A.V. The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering. Metals 2024, 14, 310. https://doi.org/10.3390/met14030310
Yakovtseva OA, Mochugovskiy AG, Prosviryakov AS, Bazlov AI, Emelina NB, Mikhaylovskaya AV. The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering. Metals. 2024; 14(3):310. https://doi.org/10.3390/met14030310
Chicago/Turabian StyleYakovtseva, Olga A., Andrey G. Mochugovskiy, Alexey S. Prosviryakov, Andrey I. Bazlov, Nadezhda B. Emelina, and Anastasia V. Mikhaylovskaya. 2024. "The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering" Metals 14, no. 3: 310. https://doi.org/10.3390/met14030310
APA StyleYakovtseva, O. A., Mochugovskiy, A. G., Prosviryakov, A. S., Bazlov, A. I., Emelina, N. B., & Mikhaylovskaya, A. V. (2024). The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering. Metals, 14(3), 310. https://doi.org/10.3390/met14030310









