Fracture Behaviour of Aluminium Alloys under Coastal Environmental Conditions: A Review
Abstract
1. Introduction
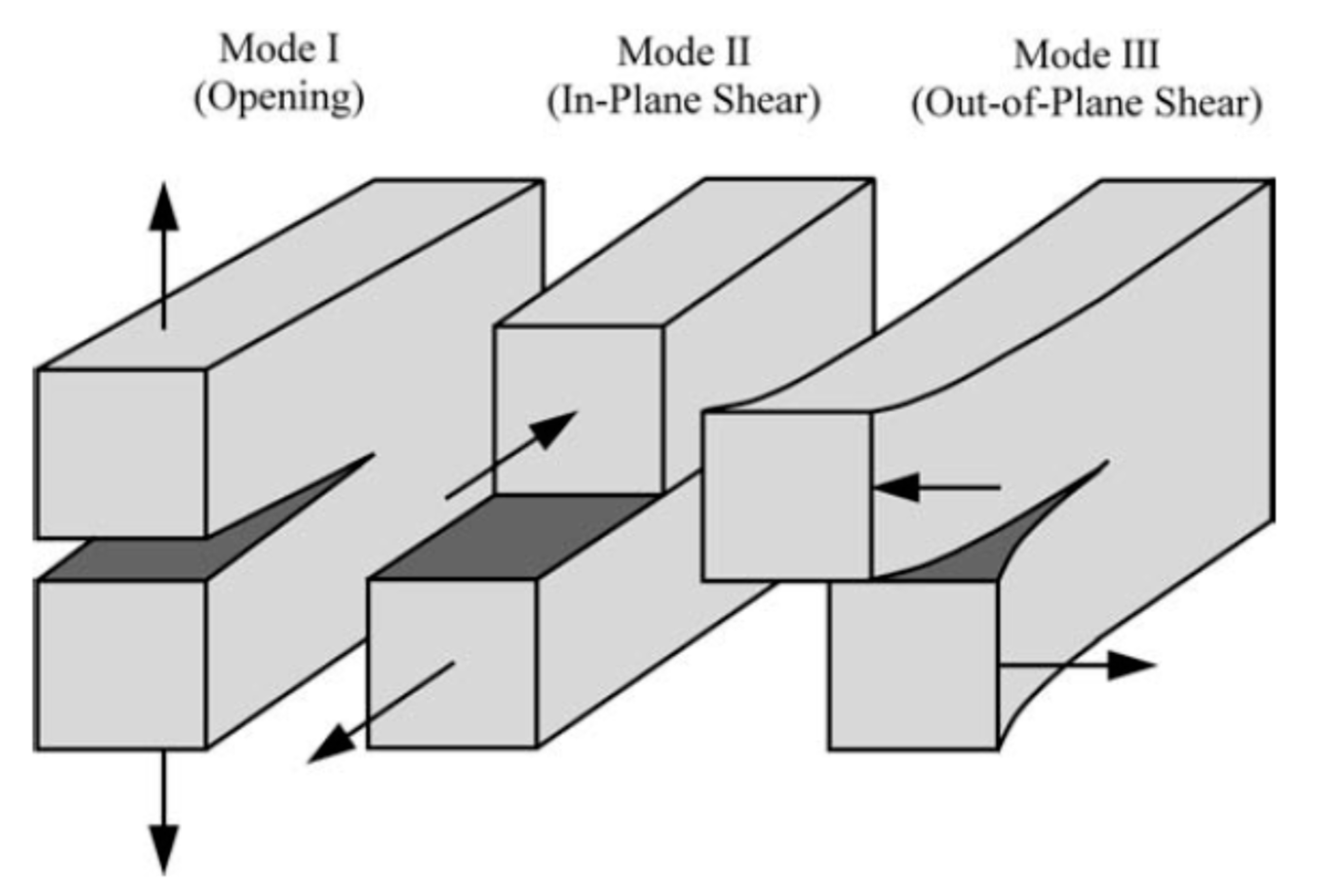
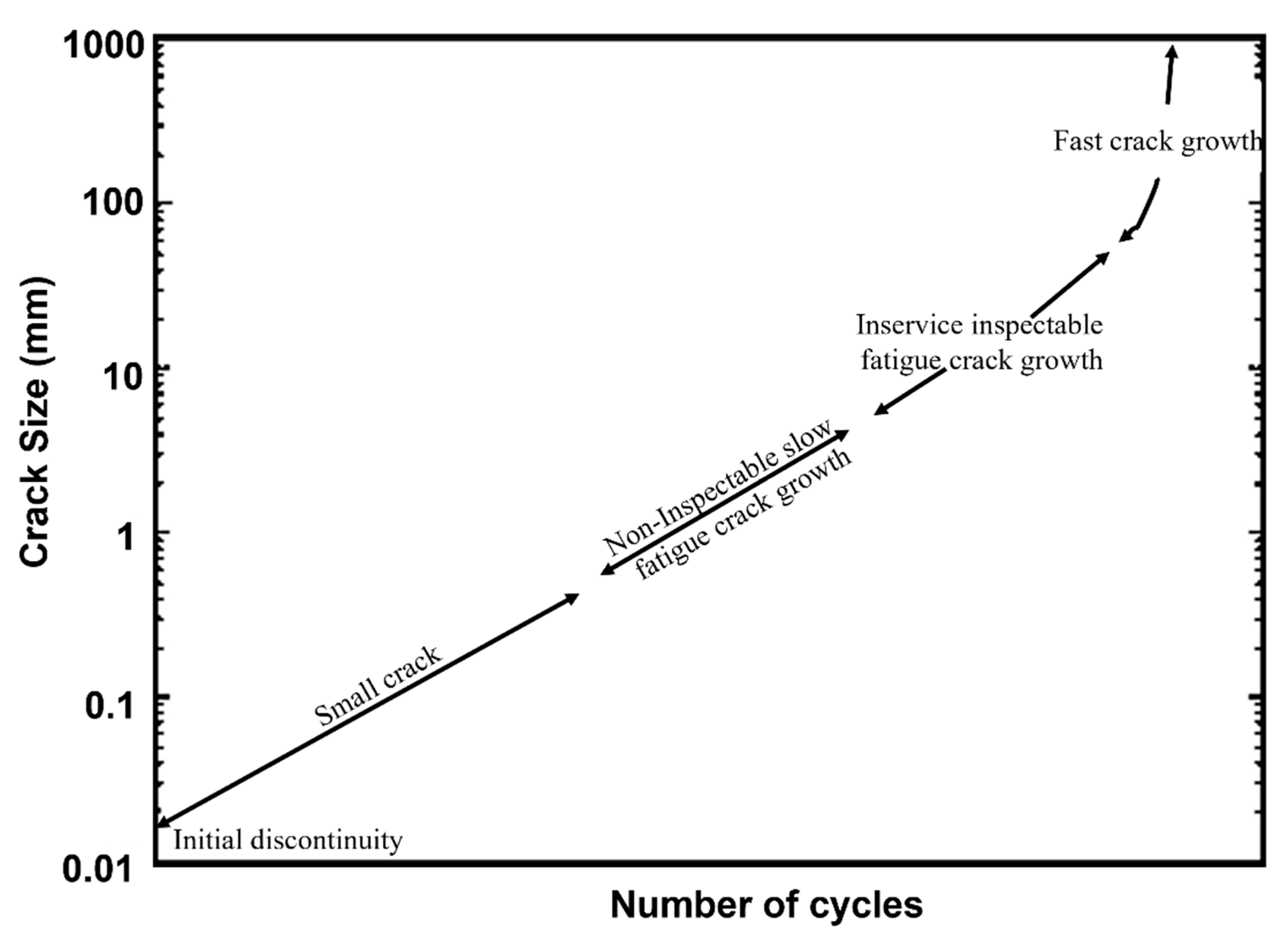
2. Fundamentals of Fracture and Fatigue Crack Growth Behaviour
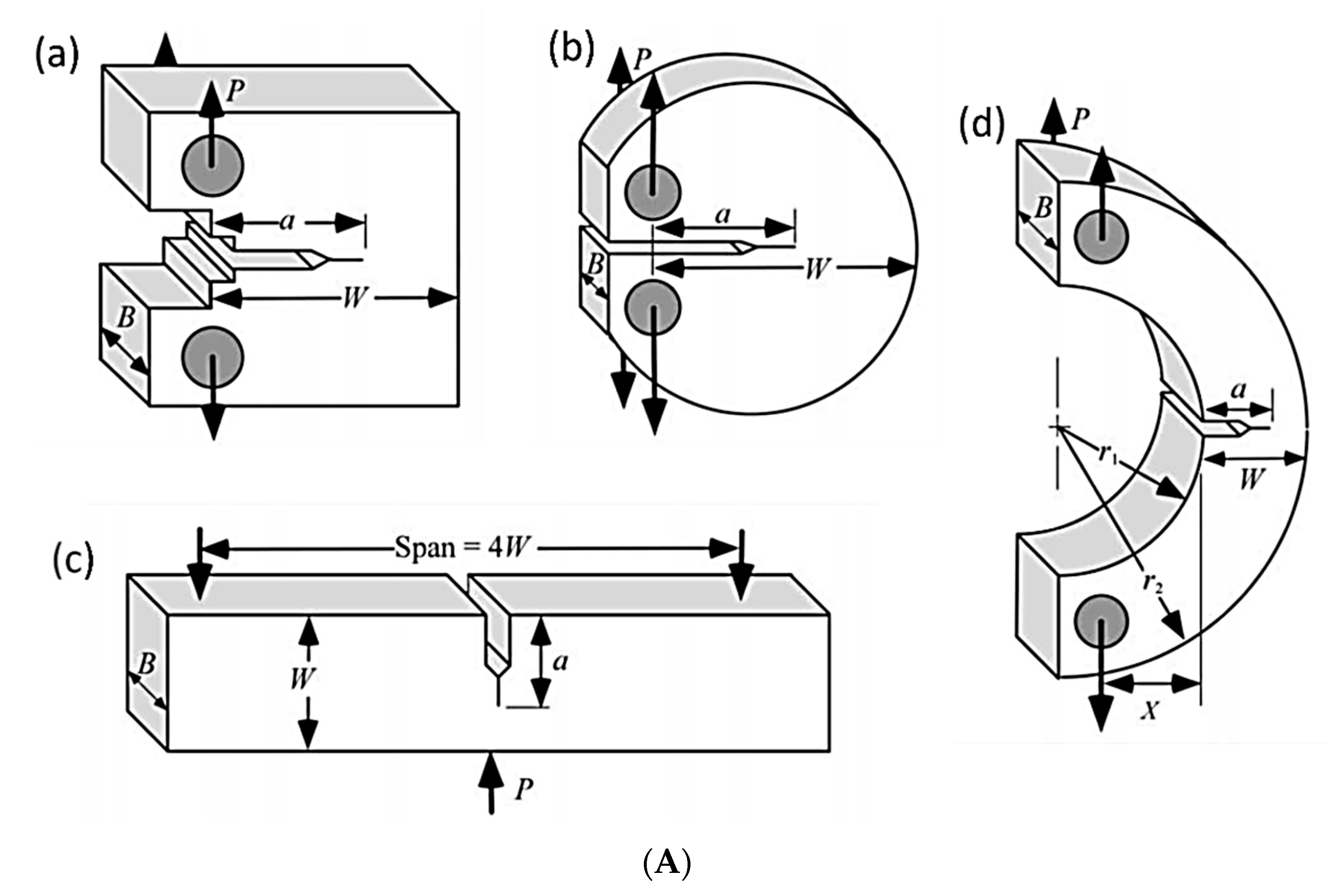
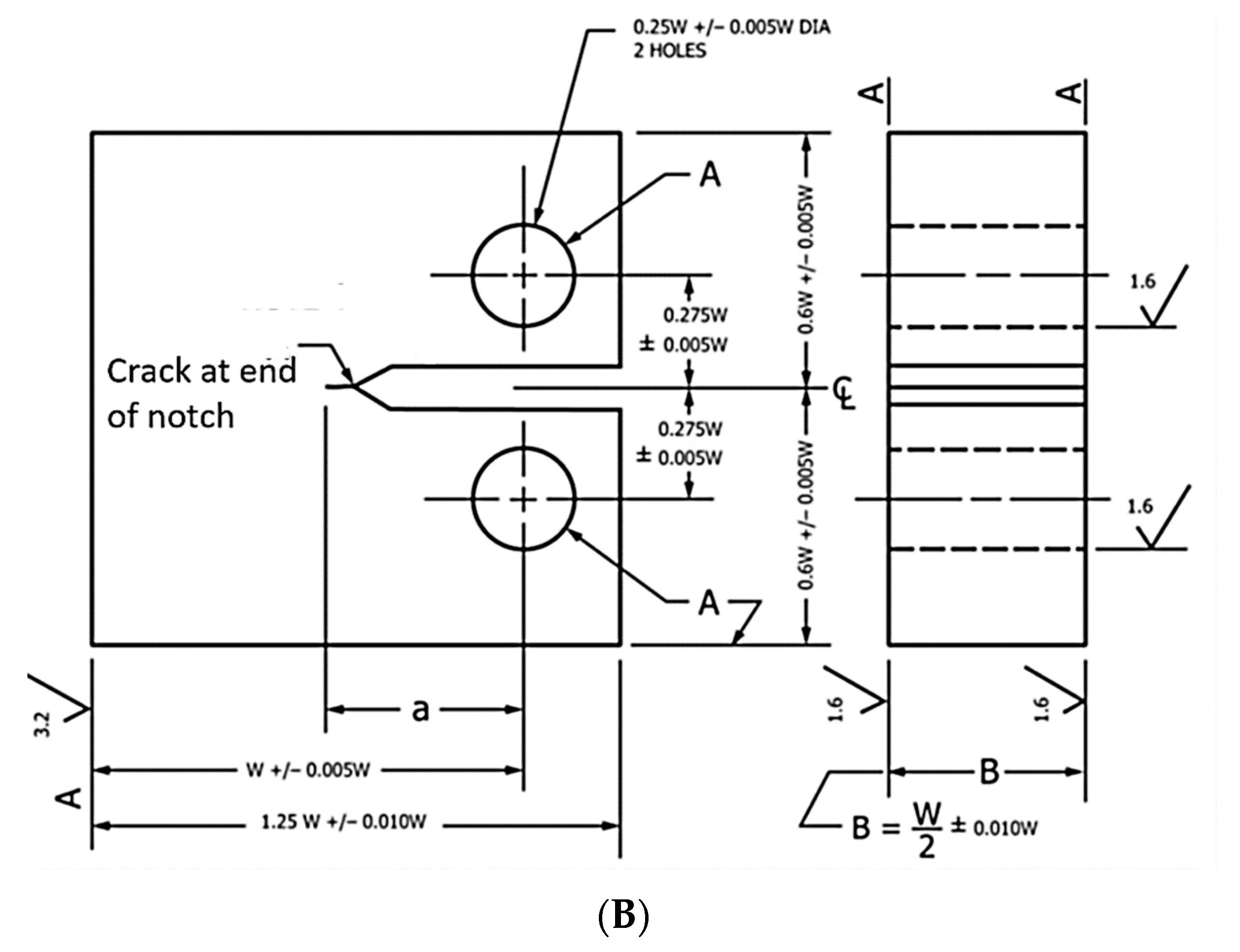
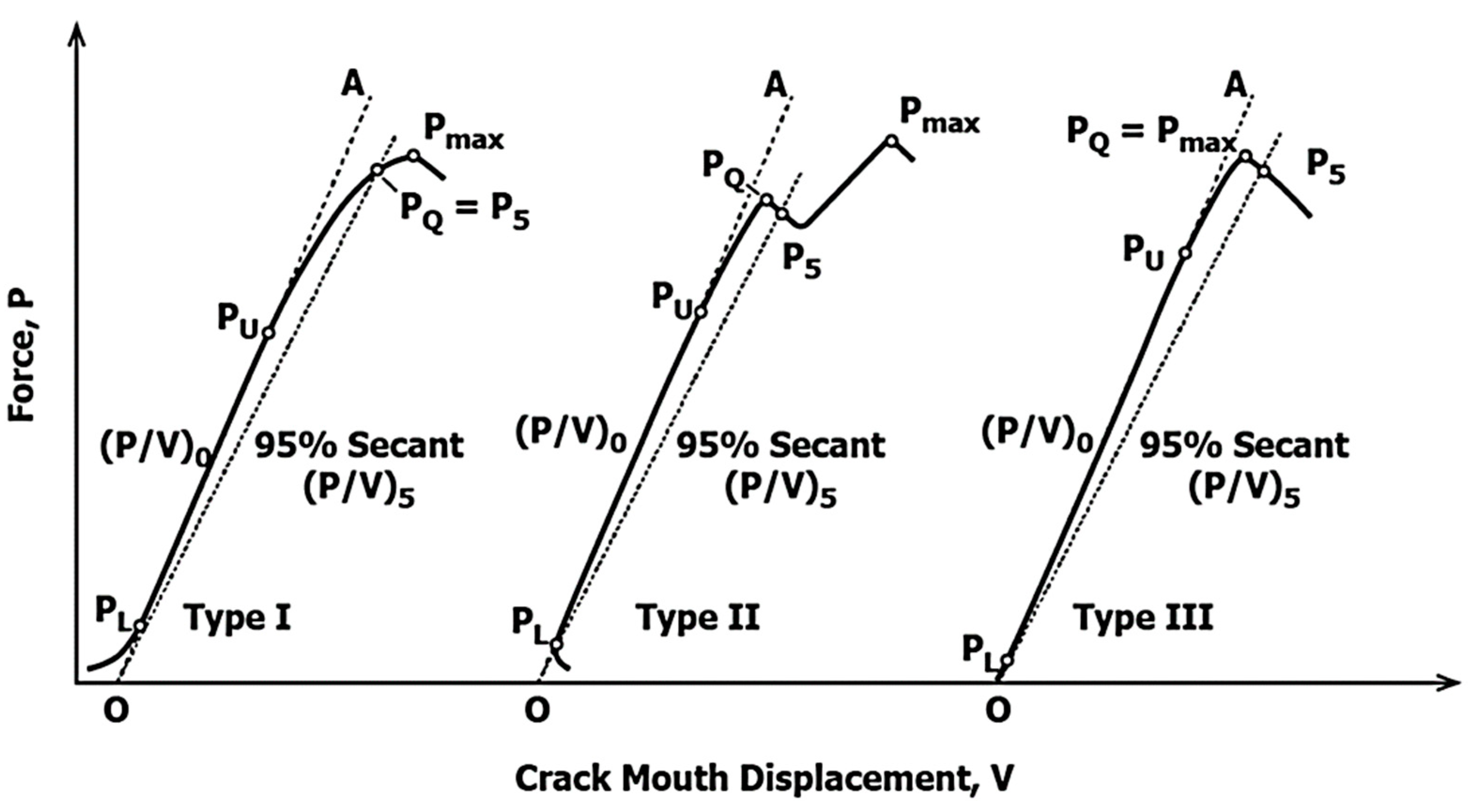
2.1. Fracture Toughness
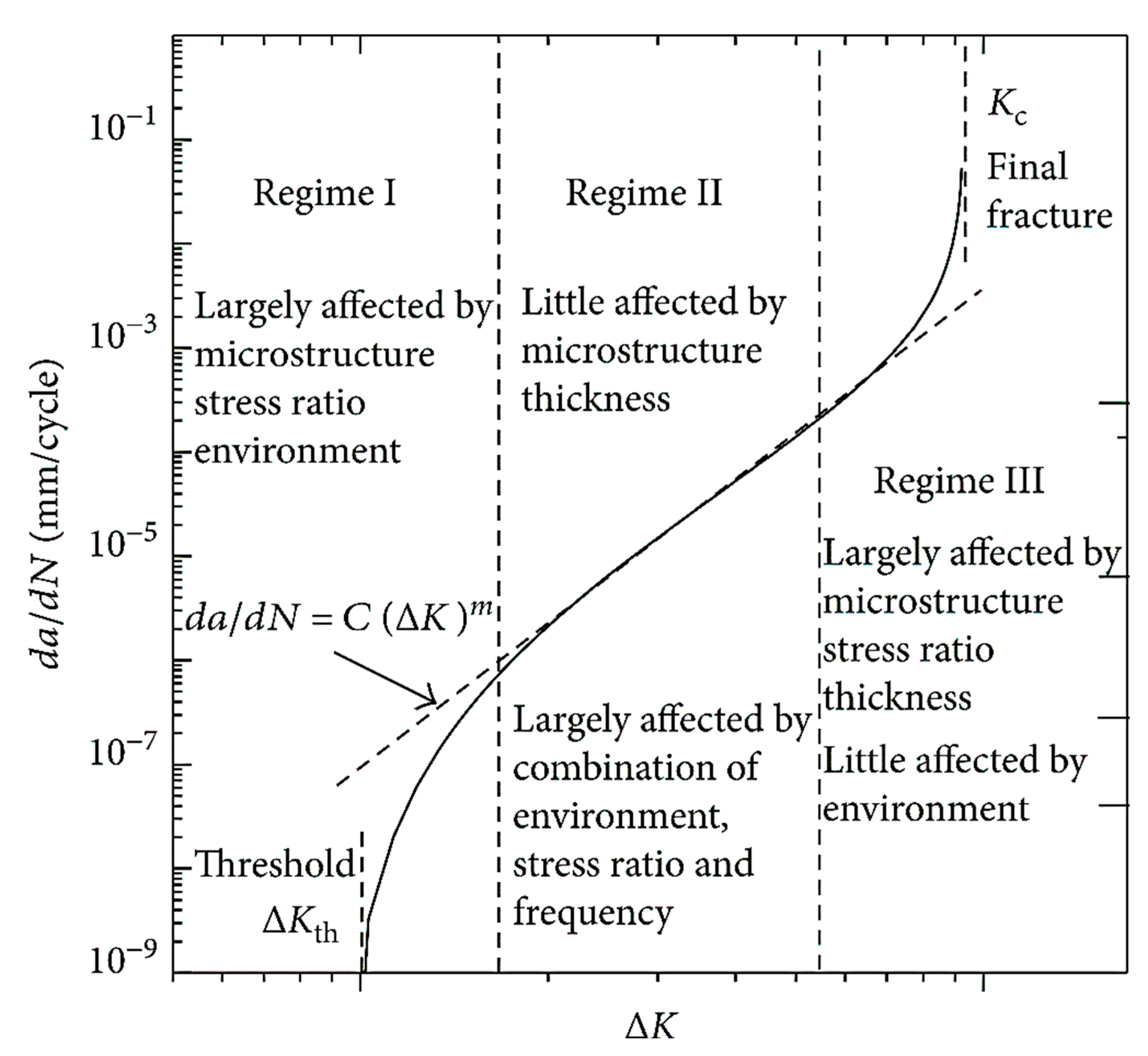
2.2. Fatigue Crack Growth Rate (FCGR)
2.3. Environmental Fracture
2.4. Threshold Stress Intensity Factor
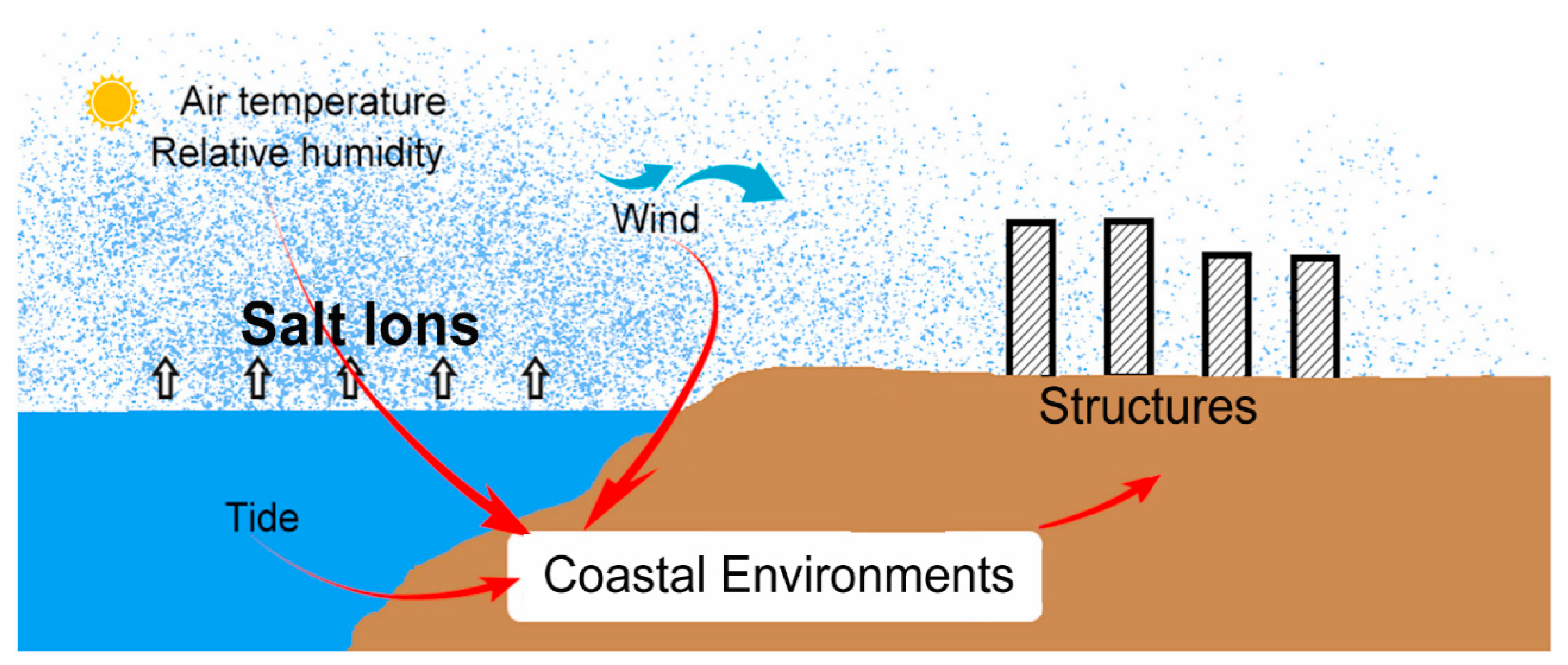
3. Coastal Environmental Conditions and Their Effects
3.1. Simulation of Coastal Conditions
3.1.1. Corrosion Simulation
3.1.2. Environmental Chamber
3.2. Effect of Corrosive Solution
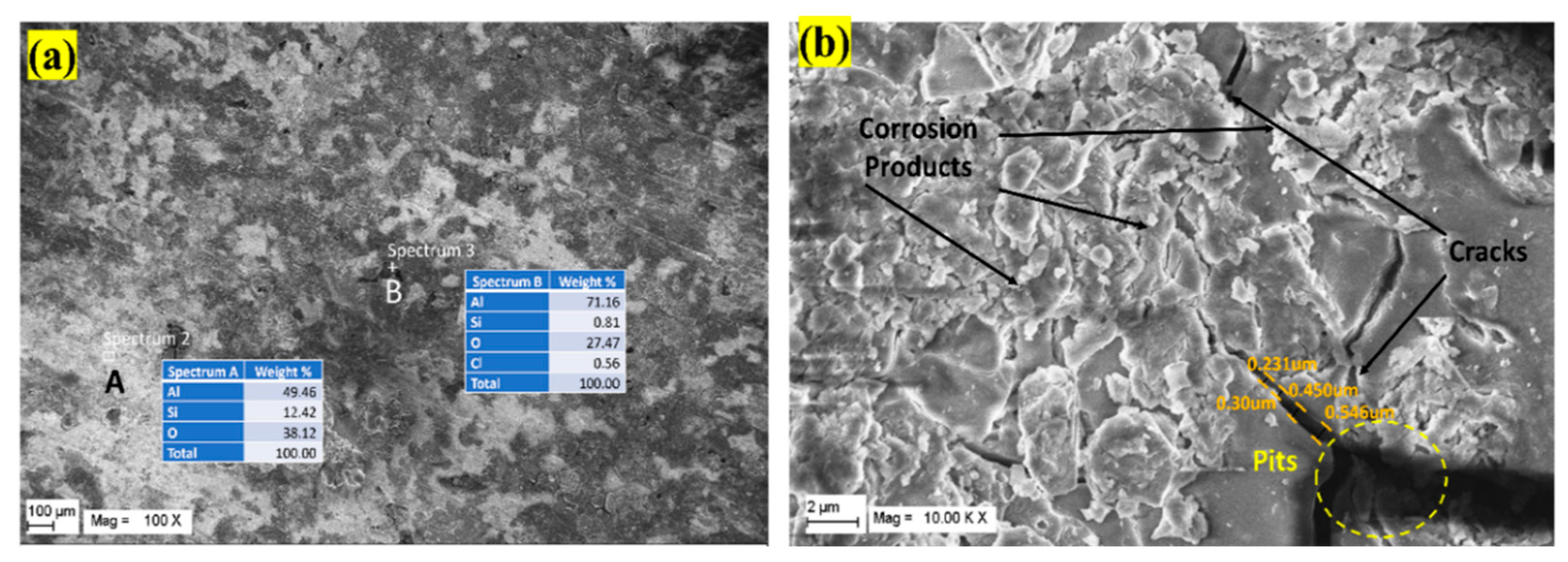
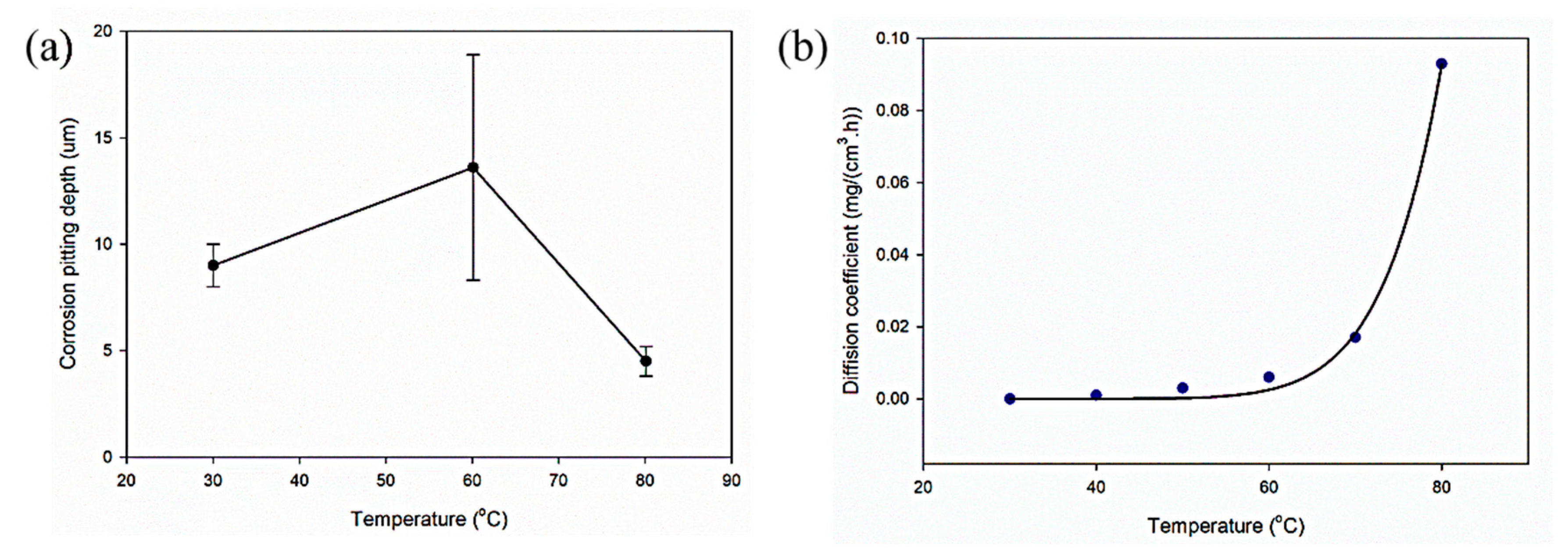
3.3. Effect of Temperature
3.4. Effect of Humidity
4. Fracture Mechanisms in Coastal Environments
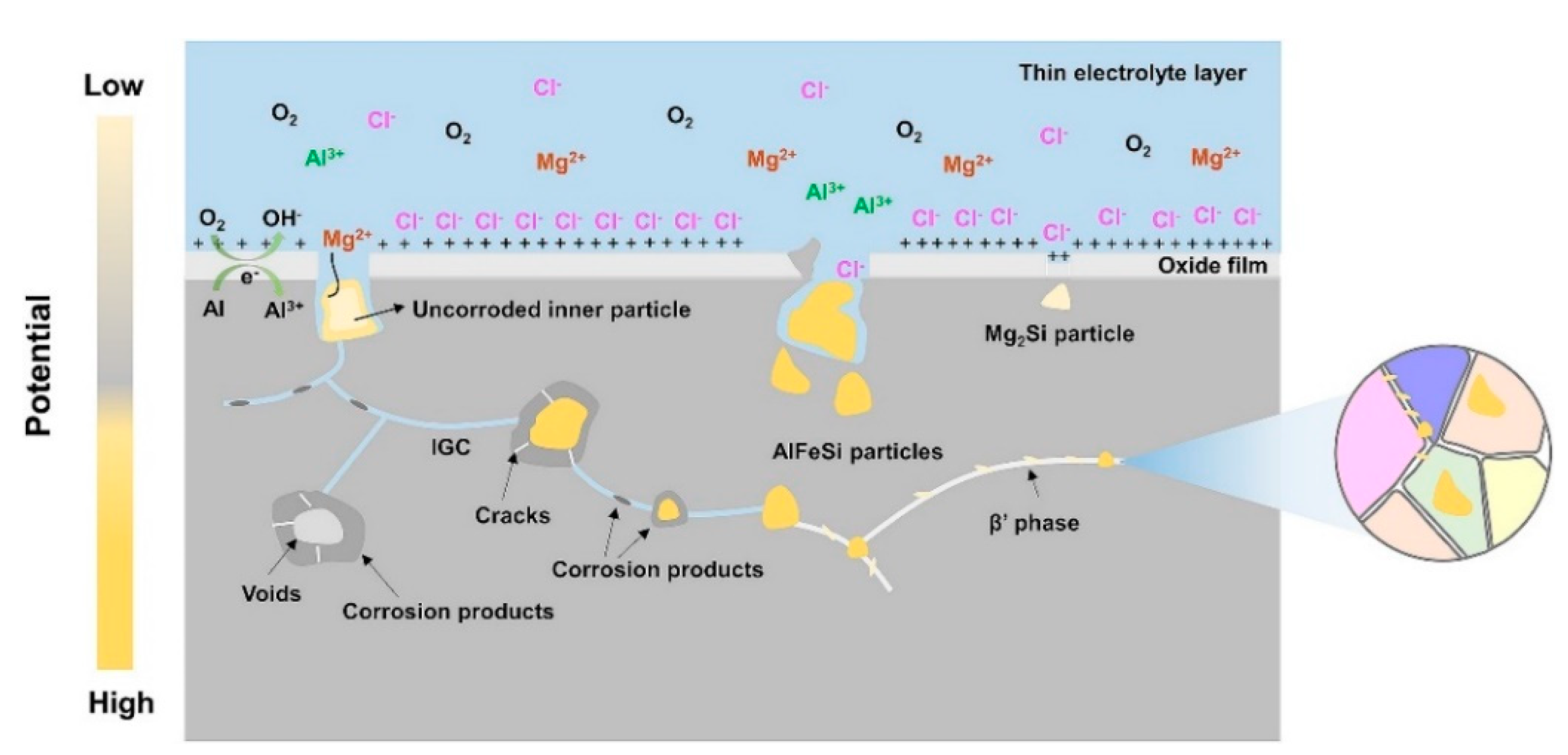
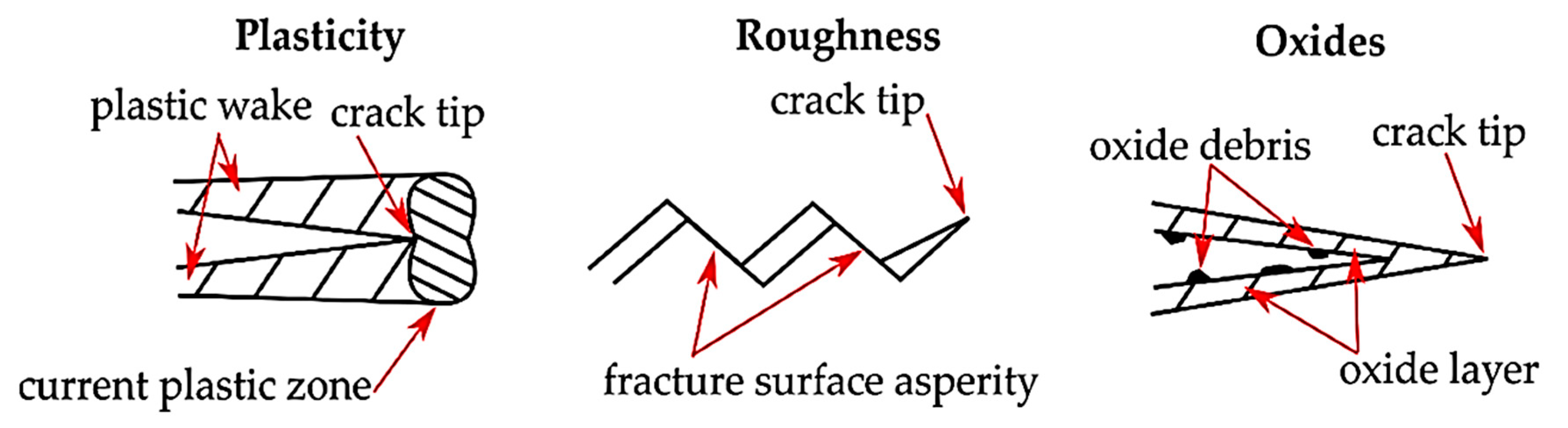
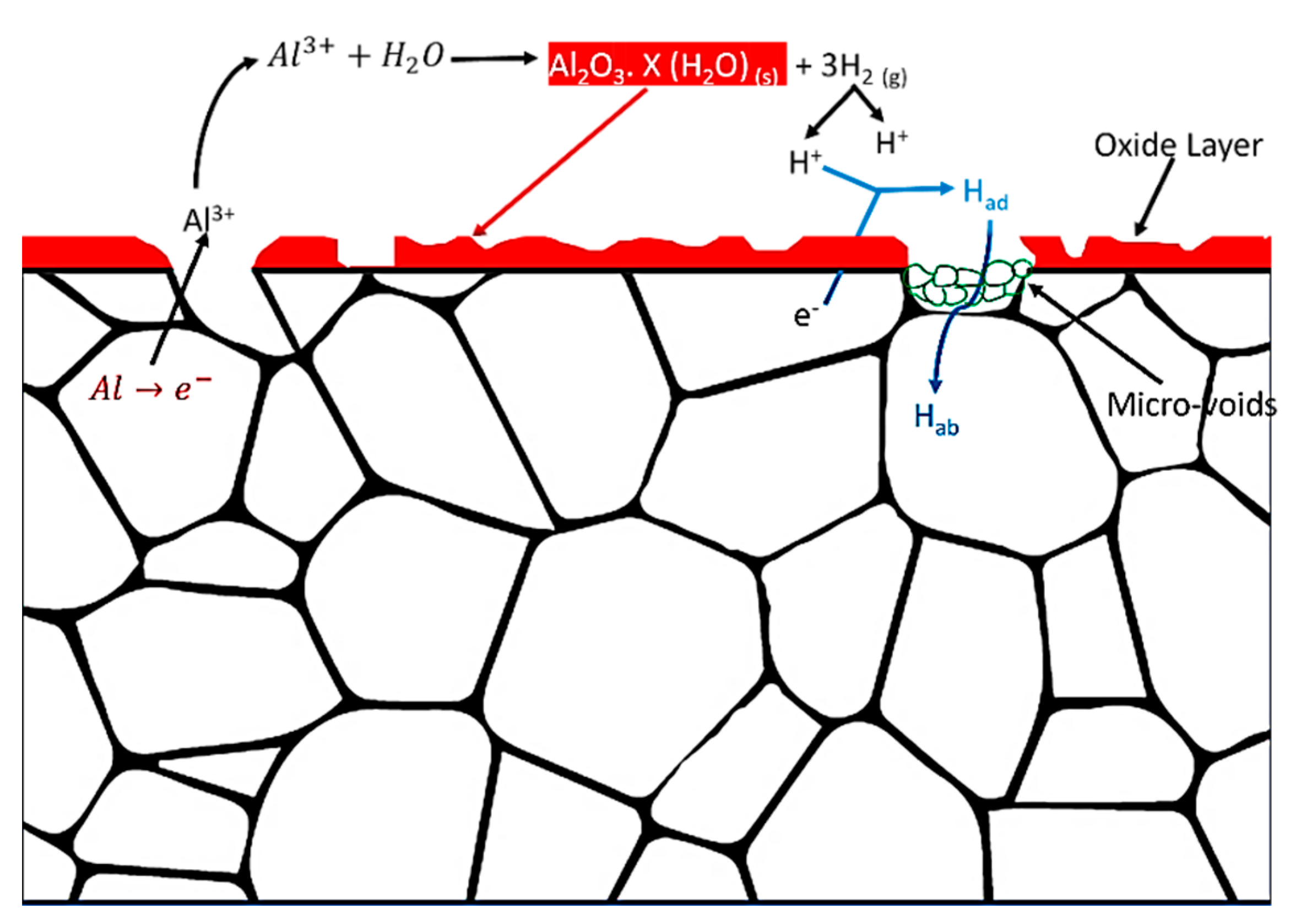
4.1. Oxide Layer
4.2. Crack Closure
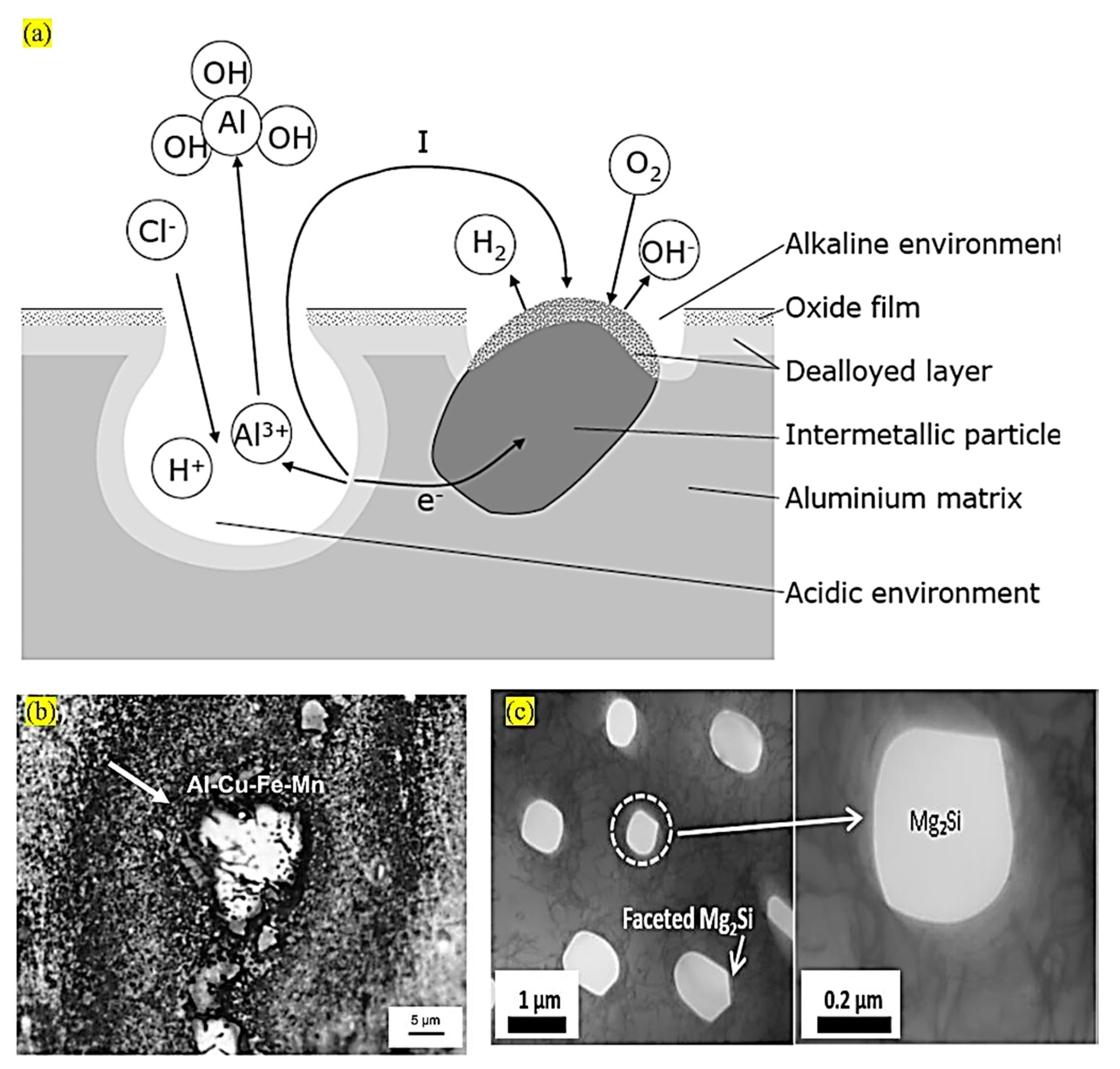
4.3. Phase Particles
4.4. Striations Spaces
4.5. Crack Propagation Path
4.6. Corrosion–Fatigue Interaction
4.7. Moisture-Assisted Crack Propagation
4.8. Hydrogen Embrittlement
5. Modelling and Predictive Approaches
6. Conclusions
- Aluminium alloys, especially the Al6000 series, are prone to corrosion in NaCl solutions, leading to reduced fracture toughness and corrosion fatigue under cyclic loading. Elevated temperatures exceeding 70 °C impact aircraft component performance, causing microscopic cracks and corrosion pits. Pitting corrosion is observed between 20 to 80 °C, decreasing above 70 °C with the formation of an aluminium oxide layer. Research on the aluminium alloy shows a reduction in corrosion pits with rising temperatures, emphasizing the correlation between temperature and the diffusion coefficient for protective oxide layer growth.
- Humidity significantly accelerates corrosion and affects mechanical properties in coastal areas, causing surface degradation and fatigue failure. Common corrosion types include intergranular and pitting corrosion. Cyclic loading in humid air increases fracture growth rates, and hydrogen embrittlement mechanisms involve water vapor reactions leading to hydrogen absorption.
Author Contributions
Funding
Conflicts of Interest
References
- Mohammed, Y. Abdellah Ductile Fracture and S–N Curve Simulation of a 7075-T6 Aluminum Alloy under Static and Constant Low-Cycle Fatigue. J. Fail. Anal. Prev. 2021, 21, 1476–1488. [Google Scholar] [CrossRef]
- Salam, I.; Muhammad, W.; Ejaz, N. Fatigue Crack Growth in an Aluminum Alloy-Fractographic Study. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Volume 146, 14th International Symposium on Advanced Materials, Islamabad, Pakistan, 12–16 October 2015; National Centre for Physics: Islamabad, Pakistan, 2016. [Google Scholar]
- Menan, F.; Henaff, G. Influence of Frequency and Exposure to a Saline Solution on the Corrosion Fatigue Crack Growth Behavior of the Aluminum Alloy 2024. Int. J. Fatigue 2009, 31, 1684–1695. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Alharthi, H. Fracture Toughness and Fatigue Crack Growth Analyses on a Biomedical Ti-27Nb Alloy under Constant Amplitude Loading Using Extended Finite Element Modelling. Materials 2023, 16, 4467. [Google Scholar] [CrossRef]
- Kamal, A.-S.H.; Ghazaly, N.M.; Abdellah, M.Y.; Seleem, A.-E.H.A.; Abdel-Jaber, G. Influence Parameters on the Essential Work of Fracture of 5754-H111 Aluminum Alloy Plate: Comparative Study. SVU-Int. J. Eng. Sci. Appl. 2023, 4, 243–259. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Ghazaly, N.M.; Kamal, A.-S.H.; Hagag, A.-E.; Seleem, A.; Abdel-Jaber, G. Ductile Fracture Toughness of Al 5754-H111 Alloy Using Essential Work of Fracture Method. AIMS Mater. Sci. 2023, 10, 370–389. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Fadhl, B.M.; Abu El-Ainin, H.M.; Hassan, M.K.; Backar, A.H.; Mohamed, A.F. Experimental Evaluation of Mechanical and Tribological Properties of Segregated Al-Mg-Si Alloy Filled with Alumina and Silicon Carbide through Different Types of Casting Molds. Metals 2023, 13, 316. [Google Scholar] [CrossRef]
- Doddamani, S.; Kaleemulla, M. Effect of Graphite on Fracture Toughness of 6061Al-Graphite. Strength Fract. Complex. 2018, 11, 295–308. [Google Scholar] [CrossRef]
- Igwemezie, V.; Mehmanparast, A. Waveform and Frequency Effects on Corrosion-Fatigue Crack Growth Behaviour in Modern Marine Steels. Int. J. Fatigue 2020, 134, 105484. [Google Scholar] [CrossRef]
- Alqahtani, I.; Starr, A.; Khan, M. Coupled Effects of Temperature and Humidity on Fracture Toughness of Al–Mg–Si–Mn Alloy. Materials 2023, 16, 4066. [Google Scholar] [CrossRef]
- Murphy, F.A.A.; Murphy, T.P. Influence of Material Toughness on Fracture Reliability in Steel Bridges. Natl. Acad. Sci. Transp. Res. Board 2021, 1–13. [Google Scholar] [CrossRef]
- Ramesh, R.S.; Santhosh Kumar, M.V.; Yasmin, B.; Doddamani, S.; Mohamed Kaleemulla, K. Fracture Toughness Investigations of AA6061-SiC Composites: Effect of Corrosion Parameters. Mater. Chem. Phys. 2023, 308, 128224. [Google Scholar] [CrossRef]
- Tenkamp, J.; Awd, M.; Siddique, S.; Starke, P.; Walther, F. Fracture–Mechanical Assessment of the Effect of Defects on the Fatigue Lifetime and Limit in Cast and Additively Manufactured Aluminum–Silicon Alloys from HCF to VHCF Regime. Metals 2020, 10, 943. [Google Scholar] [CrossRef]
- Abdellah, M.Y. Essential Work of Fracture Assessment for Thin Aluminium Strips Using Finite Element Analysis. Eng. Fract. Mech. 2017, 179, 190–202. [Google Scholar] [CrossRef]
- Saleemsab, D.; Kiran, J.O.; Kaleemulla, M.; Bakkappa, B. Fracture Toughness Testing of 6061Al-Graphite Composites Using SENB Specimens. J. Inst. Eng. (India)-Ser. D Springer 2019, 100, 195–201. [Google Scholar] [CrossRef]
- Anderson, T.L. Fracture Mechanics—Fundamentals and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Dowling, N.E. Mechanical Behavior of Materials—Engineering Methods for Deformation, Fracture and Fatigue, 4th ed.; Pearson Education Limited: Essex, UK, 2013. [Google Scholar]
- Golewski, G.L. The Phenomenon of Cracking in Cement Concretes and Reinforced Concrete Structures: The Mechanism of Cracks Formation, Causes of Their Initiation, Types and Places of Occurrence, and Methods of Detection—A Review. Buildings 2023, 13, 765. [Google Scholar] [CrossRef]
- Golewski, G.L. Fracture Performance of Cementitious Composites Based on Quaternary Blended Cements. Materials 2022, 15, 6023. [Google Scholar] [CrossRef]
- ASTME1823-10a; Standard Terminology Relating to Fatigue and Fracture Testing. ASTM: West Conshohocken, PA, USA, 2011.
- ASTME399-22; Standard Test Method for Linear-Elastic Plane Strain Fracture Toughness KIc of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2022.
- Bharath, K.N.; Saleemsab Doddamani, A.M.; Rajesh, K.M.K. Simulation of Various Fracture Models by Varying Geometrical Parameters Using Taguchi’s DOE. Struct. Integr. Life 2023, 23, 191–195. [Google Scholar]
- Dhummansure, V.; Kalyanrao, A.A.; Doddamani, S. Optimization of Process Parameters for Fracture Toughness of Al6061-Graphite Composites. Struct. Integr. Life 2020, 20, 51–55. [Google Scholar]
- Doddamani, S.; Kaleemulla, M. Comparisons of Experimental Fracture Toughness Testing Methods of Al6061–Graphite Particulate Composites. J. Fail. Anal. Prev. 2019, 19, 730–737. [Google Scholar] [CrossRef]
- Hegde, R.; Sivaram, N.M.; Ajaykumar, B.S.; Kirthan, L.J. Evaluation of Heat Treatment Effect on Fracture Behavior of Aluminum Silicon Carbide Graphite Hybrid Composite. Int. J. Appl. Eng. Res. 2017, 12, 605–610. [Google Scholar]
- Bergant, Z.; Trdan, U.; Grum, J. Effects of Laser Shock Processing on High Cycle Fatigue Crack Growth Rate and Fracture Toughness of Aluminium Alloy 6082-T651. Int. J. Fatigue 2016, 87, 444–455. [Google Scholar] [CrossRef]
- Raviraj, M.S.; Sharanaprabhu, C.M.; Mohankumar, G.C. Experimental Investigation of Effect of Specimen Thickness on Fracture Toughness of Al-TiC Composites. Frattura ed Integrita Strutturale 2016, 10, 360–368. [Google Scholar] [CrossRef]
- Chen, C.J.; Su, M.N.; Wang, Y.H.; Deng, X.W. Experimental Research on the Fatigue Crack Growth Behaviour of Q420C. J. Constr. Steel Res. 2022, 192, 107241. [Google Scholar] [CrossRef]
- Doddamani, S. Fracture Toughness Investigations of Al6061-Graphite Particulate Composite Using Compact Specimens. Frattura ed Integrita Strutturale 2017, 41, 484–490. [Google Scholar] [CrossRef]
- Zhu, X.-K.; Joyce, J.A. Review of Fracture Toughness (G, K, J, CTOD, CTOA) Testing and Standardization. Eng. Fract. Mech. 2012, 85, 1–46. [Google Scholar] [CrossRef]
- ASTM E647−13; Standard Test Method for Measurement of Fatigue Crack Growth Rates. ASTM: West Conshohocken, PA, USA, 2014; Volume 3, pp. 1–50.
- Wanhill, R.J.H.; Bray, G.H. Fatigue Crack Growth Behavior of Aluminum–Lithium Alloys. In Aluminum-Lithium Alloys; Prasad, N.E., Gokhale, A.A., Wanhill, R.J.H., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 381–413. [Google Scholar]
- Lee, E.E. Fatigue Behavior of Silicon Carbide Whisker/Aluminum Composite; Naval Air Development Center: Warminster, PA, USA, 1988. [Google Scholar]
- Yuan, R.; Kruzic, J.; Zhang, X.; De Jonghe, L.; Ritchie, R. Ambient to High-Temperature Fracture Toughness and Cyclic Fatigue Behavior in Al-Containing Silicon Carbide Ceramics. Acta Mater. 2003, 51, 6477–6491. [Google Scholar] [CrossRef]
- Achutha, M.V.; Sridhara, B.K.; Budan, A. Fatigue Life Estimation of Hybrid Aluminium Matrix Composites. Int. J. Des. Manuf. Technol. 2008, 2, 14–21. [Google Scholar]
- Uematsu, Y.; Tokaji, K.; Kawamura, M. Fatigue Behavior of SiC-Particulate-Reinforced Aluminium Alloy Composites with Different Particle Sizes at Elevated Temperatures. Compos. Sci. Technol. 2008, 68, 2785–2791. [Google Scholar] [CrossRef]
- Myriounis, D.P.; Hasan, S.T. Fatigue and Fracture Behaviour of Al-SiCp MMC NDE by IR Detection. In Proceedings of the 18TH International Conference on Composite Materials, Jeju Island, Republic of Korea, 21–26 August 2011. [Google Scholar]
- Huang, J.; Spowart, J.; Jones, J. The Role of Microstructural Variability on the Very High-Cycle Fatigue Behavior of Discontinuously-Reinforced Aluminum Metal Matrix Composites Using Ultrasonic Fatigue. Int. J. Fatigue 2010, 32, 1243–1254. [Google Scholar] [CrossRef]
- Sharma, M.; Ziemian, C.; Eden, T. Fatigue Behavior of SiC Particulate Reinforced Spray-Formed 7XXX Series Al-Alloys. Mater. Des. 2011, 32, 4304–4309. [Google Scholar] [CrossRef]
- Joadder, B.; Shit, J.; Acharyya, S.; Dhar, S. Fatigue Failure of Notched Specimen—A Strain-Life Approach. Mater. Sci. Appl. 2011, 2, 1730–1740. [Google Scholar] [CrossRef]
- Shahani, A.; Kashani, H.M. Assessment of Equivalent Initial Flaw Size Estimation Methods in Fatigue Life Prediction Using Compact Tension Specimen Tests. Eng. Fract. Mech. 2013, 99, 48–61. [Google Scholar] [CrossRef]
- Ciavarella, M.; D’antuono, P.; Demelio, G. Generalized Definition of “Crack-like” Notches to Finite Life and SN Curve Transition from “Crack-like” to “Blunt Notch” Behavior. Eng. Fract. Mech. 2017, 179, 154–164. [Google Scholar] [CrossRef]
- Raposo, P.; Correia, J.A.F.O.; De Jesus, A.M.P.; Calçada, R.A.B.; Lesiuk, G.; Hebdon, M.; Fernández-Canteli, A. Probabilistic Fatigue S-N Curves Derivation for Notched Components. Frattura ed Integrita Strutturale 2017, 42, 105–118. [Google Scholar] [CrossRef]
- Saleemsab Doddamani, M.K.K. Effect of Thickness on Fracture Toughness of Al6061-Graphite. J. Solid Mech. 2019, 11, 635–643. [Google Scholar]
- Farheen Kulsum, A.S.D. Review on Corrosion Behavior, Fatigue Behavior and Fracture Toughness of Al Alloy MMCS. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Antunes, F.V.; Serrano, S.; Branco, R.; Prates, P. Fatigue Crack Growth in the 2050-T8 Aluminium Alloy. Int. J. Fatigue 2018, 115, 79–88. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yuan, H. Application of a Cohesive Zone Model for Simulating Fatigue Crack Growth from Moderate to High ΔK Levels of Inconel 718. Int. J. Aerosp. Eng. 2018, 2018, 4048386. [Google Scholar] [CrossRef]
- Alqahtani, I.M.; Starr, A.; Khan, M. Experimental and Theoretical Aspects of Crack Assisted Failures of Metallic Alloys in Corrosive Environments—A Review. Mater. Today Proc. 2022, 66, 2530–2535. [Google Scholar] [CrossRef]
- Niazi, H.; Eadie, R.; Chen, W.; Zhang, H. High PH Stress Corrosion Cracking Initiation and Crack Evolution in Buried Steel Pipelines: A Review. Eng. Fail. Anal. 2021, 120, 105013. [Google Scholar] [CrossRef]
- Blanc, C.; Creus, J.; Touzet-Cortina, M. Stress Corrosion Cracking. Between the Corrosion Defect and the Long Crack: The Phase of the Initiation of the Cracks. In Mechanics—Microstructure—Corrosion Coupling; Christine Blanc, I.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–312. [Google Scholar]
- Alqahtani, I.; Starr, A.; Khan, M. Crack Propagation Behaviour under Corrosion and Thermomechanical Loads. In Proceedings of the 7th International Conference on Sustainable Materials and Recent Trends in Mechanical Engineering (SMARTME-2023), Bengaluru, India, 11–12 August 2023; pp. 37–45. [Google Scholar]
- Pedeferri, P. Stress Corrosion Cracking and Corrosion-Fatigue. In Corrosion Science and Engineering; Engineering Materials; Springer: Cham, Switzerland, 2018; pp. 243–273. [Google Scholar]
- Wang, C.Q.; Xiong, J.J.; Shenoi, R.A.; Liu, M.D.; Liu, J.Z. A Modified Model to Depict Corrosion Fatigue Crack Growth Behavior for Evaluating Residual Lives of Aluminum Alloys. Int. J. Fatigue 2016, 83, 280–287. [Google Scholar] [CrossRef]
- Adeboye, S.A.; Adebowale, A.D.; Siyanbola, T.O.; Ajanaku, K.O. Coatings and the Environment: A Review of Problems, Progress and Prospects. In Proceedings of the IOP Conference Series: 6th International Conference on Science and Sustainable Development (ICSSD 2022), Virtual, 9–10 December 2022; Volume 1197. [Google Scholar]
- Panda, R.; Fatma, K.; Tripathy, J. Anti-Corrosion and Anti-Wear Ceramic Coatings. In Advanced Ceramic Coatings; Gupta, R.K., Motallebzadeh, A., Kakooei, S., Nguyen, T.A., Behera, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–217. [Google Scholar]
- Shaikh, S. Nanomaterials Applied in Coatings: Synthesis, Structures, Properties, and Applications. Coatings 2022, 12, 1773. [Google Scholar] [CrossRef]
- Putatunda, S.K.; Bajajand, S.; Boileau, J. A Novel Method for Determining the Fatigue Threshold and Fracture Toughness from a Single Test. Int. J. Metall. Met. Phys. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Robertson, S.W.; Ritchie, R.O. A Fracture-Mechanics-Based Approach to Fracture Control in Biomedical Devices Manufactured from Superelastic Nitinol Tube. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 84, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Perez, N. Fracture Mechanics, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24997-1. [Google Scholar]
- Saleemsab Doddamani, M.K.K. Review of Experimental Fracture Toughness (K IC) of Aluminium Alloy and Aluminium MMCs Metal Matrix Composites. Int. J. Fract. Damage Mech. 2016, 2, 38–51. [Google Scholar]
- Lai, M.O.; Ferguson, W.G. Fracture Toughness of Aluminium Alloy 7075-T6 in the as-Cast Condition. Mater. Sci. Eng. 1985, 74, 133–138. [Google Scholar] [CrossRef]
- Hill, M.R.; Panontin, T.L. Micromechanical Modeling of Fracture Initiation in 7050 Aluminum. Eng. Fract. Mech. 2002, 69, 2163–2186. [Google Scholar] [CrossRef]
- Urrego, L.F.; García-Beltrán, O.; Arzola, N.; Araque, O. Mechanical Fracture of Aluminium Alloy (AA 2024-T4), Used in the Manufacture of a Bioproducts Plant. Metals 2023, 13, 1134. [Google Scholar] [CrossRef]
- Tsangarakis, N. All Modes Fracture Toughness of Two Aluminium Alloys. Eng. Fract. Mech. 1987, 26, 313–321. [Google Scholar] [CrossRef]
- Prasad, N.E.; Kamat, S.V.; Prasad, K.S.; Malakondaiah, G.; Kutumbarao, V. In-Plane Anisotropy in the Fracture Toughness of an Al-Li 8090 Alloy Plate. Eng. Fract. Mech. 1993, 46, 209–223. [Google Scholar] [CrossRef]
- Alrashed, F.; Asif, M. Climatic Classifications of Saudi Arabia for Building Energy Modelling. Energy Procedia 2015, 75, 1425–1430. [Google Scholar] [CrossRef]
- Alqahtani, I.M.; Starr, A.; Khana, M. Fracture Toughness Investigation of AL6082-T651 Alloy under Corrosive Environmental Conditions. In Proceedings of the 11th International Conference on Fracture Fatigue and Wear (FFW-2023), Ghent, Belgium, 29–31 August 2023. [Google Scholar]
- Tang, Y.; Meng, Q.; Ren, P. Spatial Distribution and Concentrations of Salt Fogs in a Coastal Urban Environment: A Case Study in Zhuhai City. Build. Environ. 2023, 234, 110156. [Google Scholar] [CrossRef]
- Loader, P.B.C. Effect of High Temperature Exposure on the Mechanical Properties of Cold Expanded Open Holes in 7050-T7451 Aluminium Alloy; Defence Science and Technology Organisation: Edinburgh, Australia, 2008.
- Cavalcante, T.R.F.; Pereira, G.S.; Koga, G.Y.; Bolfarini, C.; Bose Filho, W.W.; Avila, J.A. Fatigue Crack Propagation of Aeronautic AA7050-T7451 and AA2050-T84 Aluminum Alloys in Air and Saline Environments. Int. J. Fatigue 2022, 154, 106519. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Luo, B.H.; He, C.; Gao, Y.; Bai, Z.H. Corrosion Evolution and Behaviour of Al–2.1Mg–1.6Si Alloy in Chloride Media. Rare Met. 2021, 40, 908–919. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and Corrosion Behavior of Al/SiC Metal Matrix Composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Y.; Zhao, Y.; Zhang, W.; Yao, J.; Wei, M.; Zhou, K.; Ma, X. Quantitative Understanding of the Environmental Effect on B10 Copper Alloy Corrosion in Seawater. Metals 2021, 11, 1080. [Google Scholar] [CrossRef]
- Little, B.J.; Ray, R.I.; Lee, J. Understanding Marine Biocorrosion: Experiments with Artificial and Natural Seawater. In Understanding Biocorrosion; Liengen, T., Féron, D., Basséguy, R., Beech, I.B., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 329–340. [Google Scholar]
- Zai, B.A.; Khan, M.A.; Khan, K.A.; Mansoor, A.; Shah, A.; Shahzad, M. The Role of Dynamic Response Parameters in Damage Prediction. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 233, 4620–4636. [Google Scholar] [CrossRef]
- Sarah Galyon Dorman, S.A.F. Examination of the Effects of Specimen Geometry on Single Edge-Cracked Tension Specimens. Eng. Fract. Mech. 2019, 209, 221–227. [Google Scholar] [CrossRef]
- Ramsden, E. Development Tools. In Hall-Effect Sensors; Ramsden, E., Ed.; Newnes: Oxford, UK, 2006; pp. 187–202. ISBN 9780750679343. [Google Scholar]
- Darehshouri, S.; Michelsen, N.; Schüth, C.; Schulz, S. A Low-Cost Environmental Chamber to Simulate Warm Climatic Conditions. Vadose Zone J. 2020, 19, e20023. [Google Scholar] [CrossRef]
- Kurniawan, I.; Faridah; Utami, S.S. Characterizing of Climate Chamber Thermal Environment Using the CFD Simulation Method Using IES VE. AIP Conf. Proc. 2020, 2223, 050010. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef] [PubMed]
- Aboura, Y.; Garner, A.; Euesden, R.; Barrett, Z.; Engel, C.; Holroyd, N.; Prangnell, P.; Burnett, T. Understanding the Environmentally Assisted Cracking (EAC) Initiation and Propagation of New Generation 7xxx Alloys Using Slow Strain Rate Testing. Corros. Sci. 2022, 199, 110161. [Google Scholar] [CrossRef]
- Brown, M.D. Moisture Absorption and Desorption Effects on Mechanical Behavior in Specialty Polyamide Products; Lehigh University: Bethlehem, PA, USA, 2019. [Google Scholar]
- Yadav, V.; Gaur, V.; Singh, I. Corrosion-Fatigue Behavior of Welded Aluminum Alloy 2024-T3. Int. J. Fatigue 2023, 173, 107675. [Google Scholar] [CrossRef]
- Gyarmati, G.; Bubonyi, T.; Fegyverneki, G.; Tokár, M.; Mende, T. Interactions of Primary Intermetallic Compound Particles and Double Oxide Films in Liquid Aluminum Alloys. Intermetallics 2022, 149, 107681. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, S.; Kim, H. The Complex Microstructures in an As-Cast Al–Mg–Si Alloy. Mater. Lett. 1999, 41, 267–272. [Google Scholar] [CrossRef]
- Eckermann, F.; Suter, T.; Uggowitzer, P.J.; Afseth, A.; Schmutz, P. The Influence of MgSi Particle Reactivity and Dissolution Processes on Corrosion in Al-Mg-Si Alloys. Electrochim. Acta 2008, 54, 844–855. [Google Scholar] [CrossRef]
- Wei, Z.; Goehring, T.; Mioduszewski, M.; Luo, L.; Kotrba, A.; Rybarz, M.; Ellinghaus, K.; Pieszkalla, M. Failure Mechanisms and Modes Analysis of Vehicle Exhaust Components and Systems. In Handbook of Materials Failure Analysis with Case Studies from the Aerospace and Automotive Industries; Abdel Salam Hamdy Makhlouf, M.A., Ed.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 393–432. [Google Scholar]
- Peng, C.; Cao, G.; Gu, T.; Wang, C.; Wang, Z.; Sun, C. The Corrosion Behavior of the 6061 Al Alloy in Simulated Nansha Marine Atmosphere. J. Mater. Res. Technol. 2022, 19, 709–721. [Google Scholar] [CrossRef]
- Noell, P.J.; Karasz, E.; Schindelholz, E.J.; Polonsky, A.T.; Campbell, I.; Katona, R.M.; Melia, M.A. The Evolution of Pit Morphology and Growth Kinetics in Aluminum during Atmospheric Corrosion. npj Mater. Degrad. 2023, 7, 12. [Google Scholar] [CrossRef]
- Cao, M.; Liu, L.; Fan, L.; Yu, Z.; Li, Y.; Oguzie, E.E.; Wang, F. Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials 2018, 11, 235. [Google Scholar] [CrossRef]
- Amura, M.; Aiello, L.; Colavita, M.; De Paolis, F.; Bernabei, M. Failure of a Helicopter Main Rotor Blade. Procedia Mater. Sci. 2014, 3, 726–731. [Google Scholar] [CrossRef][Green Version]
- Romeyn, A. Main Rotor Blade Failure Analysis Report; IOP Publishing: Philadelphia, PA, USA, 2005. [Google Scholar]
- Sunrise Helicopters Inc., Canada. In-Flight Separation of Main Rotor Blade and Collision with Terrain. 2011. Available online: https://www.bst-tsb.gc.ca/eng/rapports-reports/aviation/2011/a11o0205/a11o0205.pdf (accessed on 1 March 2024).
- Cicolin, D.; Trueba, M.; Trasatti, S. Effect of Chloride Concentration, PH and Dissolved Oxygen, on the Repassivation of 6082-T6 Al Alloy. Electrochim. Acta 2014, 124, 27–35. [Google Scholar] [CrossRef]
- Otieno, M.; Thomas, M. Marine Exposure Environments and Marine Exposure Sites. In Marine Concrete Structures; Alexander, M.G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 171–196. [Google Scholar]
- Vargel, C. Chapter D.1—Freshwater. In Corrosion of Aluminium; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 299–327. ISBN 9780080444956. [Google Scholar]
- Tao, J.; Xiang, L.; Zhang, Y.; Zhao, Z.; Su, Y.; Chen, Q.; Sun, J.; Huang, B.; Peng, F. Corrosion Behavior and Mechanical Performance of 7085 Aluminum Alloy in a Humid and Hot Marine Atmosphere. Materials 2022, 15, 7503. [Google Scholar] [CrossRef]
- Luo, D.; Li, F.; Xing, G. Corrosion Resistance of 6061-T6 Aluminium Alloy and Its Feasibility of near-Surface Reinforcements in Concrete Structure. Rev. Adv. Mater. Sci. 2022, 61, 638–653. [Google Scholar] [CrossRef]
- Free, B.; Marino, G.; Schindelholz, E.; Galyon Dorman, S.; (Warner) Locke, J.S. Measurement of Atmospheric Corrosion Fatigue Crack Growth Rates on AA7085-T7451. Int. J. Fatigue 2023, 167, 107368. [Google Scholar] [CrossRef]
- Mao, Y.; Zhu, Y.; Deng, C.-M.; Sun, S.; Xia, D.-H. Analysis of Localized Corrosion Mechanism of 2024 Aluminum Alloy at a Simulated Marine Splash Zone. Eng. Fail. Anal. 2022, 142, 106759. [Google Scholar] [CrossRef]
- Bray, G.H.; Bucci, R.J.; Brazill, R.L. Lessons Neglected: Effects of Moist Air on Fatigue and Fatigue Crack Growth in Aluminum Alloys. Mater. Sci. Forum 2000, 331–337, 1413–1426. [Google Scholar]
- Ahmad, Z. Chapter 10—Atmospheric Corrosion. In Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2006; pp. 550–575. ISBN 9780750659246. [Google Scholar]
- Bradshaw, F.J.; Wheeler, C. The Influence of Gaseous Environment and Fatigue Frequency on the Growth of Fatigue Cracks in Some Aluminium Alloys. Int. J. Fract. Mech. 1969, 5, 255–268. [Google Scholar] [CrossRef]
- Davidson, D.L.; Lankford, J. The Effect of Water Vapor on Fatigue Crack Tip Mechanics in 7075-T651 Aluminium Alloy. Fatigue Fract. Eng. Mater. Struct. 1983, 6, 241–256. [Google Scholar] [CrossRef]
- Holper, B.; Mayer, H.; Vasudevan, A.; Stanzl-Tschegg, S. Near Threshold Fatigue Crack Growth in Aluminium Alloys at Low and Ultrasonic Frequency: Influences of Specimen Thickness, Strain Rate, Slip Behaviour and Air Humidity. Int. J. Fatigue 2003, 25, 397–411. [Google Scholar] [CrossRef]
- Young, G.A.; Scully, J.R. The Effects of Test Temperature, Temper, and Alloyed Copper on the Hydrogen-Controlled Crack Growth Rate of an Al-Zn-Mg-(Cu) Alloy. Met. Mater. Trans. A 2002, 33, 1167–1181. [Google Scholar] [CrossRef]
- Safyari, M.; Hojo, T.; Moshtaghi, M. Effect of Environmental Relative Humidity on Hydrogen-Induced Mechanical Degradation in an Al–Zn–Mg–Cu Alloy. Vacuum 2021, 192, 110489. [Google Scholar] [CrossRef]
- L’haridon-Quaireau, S.; Laot, M.; Colas, K.; Kapusta, B.; Delpech, S.; Gosset, D. Effects of Temperature and PH on Uniform and Pitting Corrosion of Aluminium Alloy 6061-T6 and Characterisation of the Hydroxide Layers. J. Alloys Compd. 2020, 833, 155146. [Google Scholar] [CrossRef]
- Khodor, J.; Özenç, K.; Lin, G.; Kaliske, M. Characterization of Fatigue Crack Growth by Cyclic Material Forces. Eng. Fract. Mech. 2021, 243, 107514. [Google Scholar] [CrossRef]
- González-Velázquez, J. Environmentally-Assisted Fracture. In Fractography and Failure Analysis. Structural Integrity; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Navidirad, M.; Stepniowski, W.J.; Cartier, E.; Christ, T.; Watanabe, M.; Misiolek, W.Z. Investigation on the Strain Induced Oxide Layer Fracture and Bonding During Cold Rolling of Aluminum Alloys. In The Minerals, Metals & Materials Series; Daehn, G., Cao, J., Kinsey, B., Tekkaya, E.V., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Song, H.; Liu, C.; Zhang, H.; Leen, S.B. A DIC-Based Study on Fatigue Damage Evolution in Pre-Corroded Aluminum Alloy 2024-T4. Materials 2018, 11, 2243. [Google Scholar] [CrossRef]
- Pu, J.; Zhang, Y.; Zhang, X.; Yuan, X.; Ren, P.; Jin, Z. Mapping the Fretting Corrosion Behaviors of 6082 Aluminum Alloy in 3.5% NaCl Solution. Wear 2021, 482–483, 203975. [Google Scholar] [CrossRef]
- Chugh, B.; Taraphdar, P.K.; Biswal, H.J.; Devi, N.R.; Dorothy, R.; Manimaran, N.; Rajendran, S. Corrosion Inhibition by Aluminum Oxide. In Inorganic Anticorrosive Materials; Verma, C., Aslam, J., Hussain, C.M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–249. [Google Scholar]
- Wang, D.; Yang, D.; Zhang, D.; Li, K.; Gao, L.; Lin, T. Electrochemical and DFT Studies of Quinoline Derivatives on Corrosion Inhibition of AA5052 Aluminium Alloy in NaCl Solution. Appl. Surf. Sci. 2015, 357, 2176–2183. [Google Scholar] [CrossRef]
- Vargel, C. Oxides and Peroxides. In Corrosion of Aluminium; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 357–365. [Google Scholar]
- Dias, C.; Ventura, J. Understanding the Influence of Metal Oxide Layer Thickness and Defects on Resistive Switching Behavior Through Numerical Modeling. Phys. Status Solidi A-Appl. Mater. Sci. 2022, 220, 202200730. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, H.; Zhan, J.; Zhang, H.; Cui, S.; Tang, W. Microstructure, Growth Kinetics and Formation Mechanism of Oxide Layers on AlN Ceramic Substrates. J. Ceram. Sci. Technol. 2018, 9, 263–270. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Oxide Inclusion Defects in Al-Si-Mg Cast Alloys. Can. Metall. Q. 2005, 44, 435–448. [Google Scholar] [CrossRef]
- Pokorný, P.; Vojtek, T.; Jambor, M.; Náhlík, L.; Hutař, P. Effect of Underload Cycles on Oxide-Induced Crack Closure Development in Cr-Mo Low-Alloy Steel. Materials 2021, 14, 2530. [Google Scholar] [CrossRef]
- Yamada, Y.; Newman, J.C. Crack-Closure Behavior of 2324-T39 Aluminum Alloy near-Threshold Conditions for High Load Ratio and Constant Kmax Tests. Int. J. Fatigue 2009, 31, 1780–1787. [Google Scholar] [CrossRef]
- Casperson, M.C.; Carroll, J.D.; Lambros, J.; Sehitoglu, H.; Dodds, R.H. Investigation of Thermal Effects on Fatigue Crack Closure Using Multiscale Digital Image Correlation Experiments. Int. J. Fatigue 2014, 61, 10–20. [Google Scholar] [CrossRef]
- Pippan, R.; Hohenwarter, A. Fatigue Crack Closure: A Review of the Physical Phenomena. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 471–495. [Google Scholar] [CrossRef]
- Culliton, D.; Betts, A.J.; Kennedy, D. Impact of Intermetallic Precipitates on the Tribological and/or Corrosion Performance of Cast Aluminium Alloys: A Short Review. Int. J. Cast Met. Res. 2013, 26, 65–71. [Google Scholar] [CrossRef]
- Koleva, D.A.; Hu, J.; Stroeven, P. Microstructure Alterations Underlying Electrochemical Process of Chloride-Induced Corrosion. In Brittle Matrix Composites 8; Brandt, A.M., Li, V.C., Marshall, I.H., Eds.; Woodhead Publishing: Sawston, UK, 2006; pp. 571–580. [Google Scholar]
- Shrivastava, V.; Dubey, S.; Gupta, G.K.; Singh, I.B. Influence of Alpha Nanoalumina Reinforcement Content on the Microstructure, Mechanical and Corrosion Properties of Al6061-Al2O3 Composite. J. Mater. Eng. Perform. 2017, 26, 4424–4433. [Google Scholar] [CrossRef]
- Kciuk, M.; Kurc, A.; Szewczenko, J. Structure and Corrosion Resistance of Aluminium AlMg2.5; AlMg5Mn and AlZn5Mg1 Alloys. J. Achiev. Mater. Manuf. Eng. 2010, 41, 74–81. [Google Scholar]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic Phases in Aluminum Alloys and Their Roles in Localized Corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Zarif, M.; Spacil, I.; Pabel, T.; Schumacher, P.; Li, J. Effect of Ca and P on the Size and Morphology of Eutectic Mg2Si in High-Purity Al-Mg-Si Alloys. Metals 2023, 13, 784. [Google Scholar] [CrossRef]
- Blau, P.J. Elevated-Temperature Tribology of Metallic Materials. Tribol. Int. 2010, 43, 1203–1208. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Temperature and Humidity Effects on Microstructure and Mechanical Properties of an Environmentally Friendly Sn–Ag–Cu Material. J. Mater. Sci. 2019, 54, 12863–12874. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Merino, S.; Mohedano, M.; Casajús, P. Corrosion Behaviour of Mg/Al Alloys in High Humidity Atmospheres. Mater. Corros. Und. Korros. 2011, 62, 326–334. [Google Scholar] [CrossRef]
- Shyam, A.; Lara-Curzio, E. A Model for the Formation of Fatigue Striations and Its Relationship with Small Fatigue Crack Growth in an Aluminum Alloy. Int. J. Fatigue 2010, 32, 1843–1852. [Google Scholar] [CrossRef]
- Laird, C. The Influence of Metallurgical Structure on the Mechanisms of Fatigue Crack Propagation. ASTM STP 415 Am. Soc. Test. Mats 1967, 131, 1–10. [Google Scholar]
- Data, F.M. Linear Elastic Fracture Mechanics (LEFM): Part Two. Available online: https://www.totalmateria.com/page.aspx?ID=CheckArticle&site=kts&LN=IT&NM=299 (accessed on 3 September 2023).
- Liu, Y.; Pan, Q.; Liu, B.; Yu, Q.; Li, G.; Pan, D. Effect of Aging Treatments on Fatigue Properties of 6005A Aluminum Alloy Containing Sc. Int. J. Fatigue 2022, 163, 107103. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, S.; Bai, Y.; Fan, C. Microstructure and Fatigue Damage Mechanism of 6082-T6 Aluminium Alloy Welded Joint. Mater. Res. Express 2021, 8, 056505. [Google Scholar] [CrossRef]
- Anis, S.F.; Koyama, M.; Hamada, S.; Noguchi, H. Mode I Fatigue Crack Growth Induced by Strain-Aging in Precipitation-Hardened Aluminum Alloys. Theor. Appl. Fract. Mech. 2019, 104, 102340. [Google Scholar] [CrossRef]
- Ma, L.; Liu, C.; Ma, M.; Wang, Z.; Wu, D.; Liu, L.; Song, M. Fatigue Fracture Analysis on 2524 Aluminum Alloy with the Influence of Creep-Aging Forming Processes. Materials 2022, 15, 3244. [Google Scholar] [CrossRef]
- Doré, M.J.; Maddox, S.J. Accelerated Fatigue Crack Growth in 6082 T651 Aluminium Alloy Subjected to Periodic Underloads. Procedia Eng. 2013, 66, 313–322. [Google Scholar] [CrossRef][Green Version]
- Sharma, V.M.J.; Sree Kumar, K.; Nageswara Rao, B.; Pathak, S.D. Effect of Aging Treatments on the Fatigue Crack Growth and Threshold Behavior of AA 2219 Aluminium Alloy. Trans. Indian Inst. Met. 2010, 63, 535–540. [Google Scholar] [CrossRef]
- Chapetti, M.D.; Gubeljak, N.; Kozak, D. Intrinsic Fatigue Limit and the Minimum Fatigue Crack Growth Threshold. Materials 2023, 16, 5874. [Google Scholar] [CrossRef]
- Mathieu, F.; Hild, F.; Roux, S. Identification of a Crack Propagation Law by Digital Image Correlation. Int. J. Fatigue 2012, 36, 146–154. [Google Scholar] [CrossRef]
- Kallien, Z.; Knothe-Horstmann, C.; Klusemann, B. Fatigue Crack Propagation in AA5083 Structures Additively Manufactured via Multi-Layer Friction Surfacing. Addit. Manuf. Lett. 2023, 6, 100154. [Google Scholar] [CrossRef]
- Belan, J.; Kuchariková, L.; Tillová, E.; Závodská, D.; Chalupová, M. Effect of Fatigue Loading Mode on 718 Alloy Fatigue Properties. Period. Polytech. Transp. Eng. 2019, 47, 335–341. [Google Scholar] [CrossRef]
- Ma, J.; Li, D.; Du, S.; Han, Z.; Luo, P.; Zhao, J. Comparison of Subcritical Crack Growth and Dynamic Fracture Propagation in Rocks under Double-Torsion Tests. Int. J. Rock Mech. Min. Sci. 2023, 170, 105481. [Google Scholar] [CrossRef]
- Hana, N.; Umeda, M.; Akiyoshi, M.; Mitamura, K.; Amaya, K. Crack Identification by Digital Image Correlation Method Using Crack Shape as Prior Information. ASME J. Press. Vessel Technol. 2023, 145, 041601. [Google Scholar] [CrossRef]
- Ermakova, A.; Ganguly, S.; Razavi, N.; Berto, F.; Mehmanparast, A. Experimental Investigation of the Fatigue Crack Growth Behavior in Wire Arc Additively Manufactured ER100S-1 Steel Specimens. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 371–385. [Google Scholar] [CrossRef]
- Milella, P.P. Fatigue and Corrosion in Metals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Arzaghi, E.; Abbassi, R.; Garaniya, V.; Binns, J.; Chin, C.; Khakzad, N.; Reniers, G. Developing a Dynamic Model for Pitting and Corrosion-Fatigue Damage of Subsea Pipelines. Ocean Eng. 2018, 150, 391–396. [Google Scholar] [CrossRef]
- Guo, Y.; Shao, Y.; Gao, X.; Li, T.; Zhong, Y.; Luo, X. Corrosion Fatigue Crack Growth of Serviced API 5L X56 Submarine Pipeline. Ocean Eng. 2022, 256, 111502. [Google Scholar] [CrossRef]
- Gangloff, R.P. Environmental Cracking Corrosion Fatigue. In ASTM International: West Conshohocken; ASTM International: West Conshohocken, PA, USA, 2005; pp. 302–321. [Google Scholar]
- Wang, Q.Y.; Pidaparti, R.M.; Palakal, M.J. Comparative Study of Corrosion-Fatigue in Aircraft Materials. AIAA J. 2001, 39, 325–330. [Google Scholar] [CrossRef]
- Luer, K.; DuPont, J.; Marder, A.; Skelonis, C. Corrosion Fatigue of Alloy 625 Weld Claddings in Combustion Environments. Mater. High Temp. 2001, 18, 11–19. [Google Scholar] [CrossRef]
- Muñoz, A.F.; Buenhombre, J.L.M.; García-Diez, A.I.; Fabal, C.C.; Díaz, J.J.G. Fatigue Study of the Pre-Corroded 6082-T6 Aluminum Alloy in Saline Atmosphere. Metals 2020, 10, 1260. [Google Scholar] [CrossRef]
- Correia, J.A.F.O.; De Jesus, A.M.P.; Alves, A.S.F.; Lesiuk, G.; Tavares, P.J.S.; Moreira, P.M.G.P. Fatigue Crack Growth Behaviour of the 6082-T6 Aluminium Using CT Specimens with Distinct Notches. Procedia Struct. Integr. 2016, 2, 3272–3279. [Google Scholar] [CrossRef]
- Alireza Behvar, M.H. A Critical Review on Very High Cycle Corrosion Fatigue: Mechanisms, Methods, Materials, and Models. J. Space Saf. Eng. 2023, 10, 284–323. [Google Scholar] [CrossRef]
- Saintier, N.; El May, M.; Odemer, G.; Hénaff, G.; Bosch, C.; Feaugas, X.; Couvant, T. Corrosion and Hydrogen Fatigue at Different Scales. In Mechanics—Microstructure—Corrosion Coupling; Christine Blanc, I.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 385–411. [Google Scholar]
- Mudali, U.K. Materials for Hostile Corrosive Environments. In Materials Under Extreme Conditions; Tyagi, A.K., Banerjee, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–128. [Google Scholar]
- Gong, K.; Wu, M.; Liu, X.; Liu, G. Nucleation and Propagation of Stress Corrosion Cracks: Modeling by Cellular Automata and Finite Element Analysis. Mater. Today Commun. 2022, 33, 104886. [Google Scholar] [CrossRef]
- Chen, W.; Lu, W.; Gou, G.; Dian, L.; Zhu, Z.; Jin, J. The Effect of Fatigue Damage on the Corrosion Fatigue Crack Growth Mechanism in A7N01P-T4 Aluminum Alloy. Metals 2023, 13, 104. [Google Scholar] [CrossRef]
- Ge, F.; Fan, L.; Liang, J.; Pang, K.; Li, H.; Wang, X.; Cui, Z. Corrosion Evolution of High-Strength Aluminum Alloys in the Simulated Service Environment of Amphibious Aircraft in the Presence of Chloride and Bisulfite. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1679–1694. [Google Scholar] [CrossRef]
- Mahmood, S.; Gallagher, C.; Engelberg, D.L. Atmospheric Corrosion of Aluminum Alloy 6063 Beneath Ferric Chloride Corrosion Product Droplets. Corrosion 2020, 76, 985–994. [Google Scholar] [CrossRef]
- Kumar, P.; Verma, B.B. Propagation of Corrosion Induced Fatigue Crack in Aluminum Alloy. AIMS Mater. Sci. 2022, 9, 512–521. [Google Scholar] [CrossRef]
- Chen, H.; Chow, C.L.; Lau, D. Deterioration Mechanisms and Advanced Inspection Technologies of Aluminum Windows. Materials 2022, 15, 354. [Google Scholar] [CrossRef]
- Shan, J.; Hou, D.; Zhang, J.; Xin, X.; Cao, G.; Genzhe, H. Effects of the Extrusion Ratio on the Intergranular Corrosion Behaviour of 6082 Aluminium Alloy. Mater. Corros. Und. Korros. 2018, 69, 365–375. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement, 1st ed.; Springer: Singapore, 2018. [Google Scholar]
- Sergeev, N.N.; Sergeev, A.N.; Kutepov, S.N.; Kolmakov, A.G.; Gvozdev, A.E. Mechanism of the Hydrogen Cracking of Metals and Alloys, Part II (Review). Inorg. Mater. Appl. Res. 2019, 10, 32–41. [Google Scholar] [CrossRef]
- Albrecht, J.; Bernstein, I.M.; Thompson, A.W. Evidence for Dislocation Transport of Hydrogen in Aluminum. Met. Mater. Trans. A 1982, 13, 811–820. [Google Scholar] [CrossRef]
- Bochkaryova, A.V.; Li, Y.V.; Barannikova, S.A.; Zuev, L.B. The Effect of Hydrogen Embrittlement on the Mechanical Properties of Aluminum Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2015, 71, 012057. [Google Scholar] [CrossRef]
- Ambat, R.; Dwarakadasa, E.S. Effect of Hydrogen in Aluminium and Aluminium Alloys: A Review. Bull. Mater. Sci. 1990, 19, 103–114. [Google Scholar] [CrossRef]
- Xie, D.; Li, S.; Li, M.; Wang, Z.; Gumbsch, P.; Sun, J.; Ma, E.; Li, J.; Shan, Z. Hydrogenated Vacancies Lock Dislocations in Aluminium. Nat. Commun. 2016, 7, 13341. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ahn, D.; Sofronis, P.; Dodds, R.; Bammann, D. Effect of Hydrogen Trapping on Void Growth and Coalescence in Metals and Alloys. Mech. Mater. 2008, 40, 115–132. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, B.; Xu, Y.; Shimizu, K.; Fujihara, H.; Hirayama, K.; Takeuchi, A.; Uesugi, M.; Cheng, G.; Toda, H. Switching Nanoprecipitates to Resist Hydrogen Embrittlement in High-Strength Aluminum Alloys. Nat. Commun. 2022, 13, 6860. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Amirkhizi, A.V.; Nemat-Nasser, S. An Empirical Model for Estimating Fracture Toughness Using the DCDC Geometry. Int. J. Fract. 2014, 188, 113–118. [Google Scholar] [CrossRef][Green Version]
- Begum, Y.; Bharath, K.N.; Doddamani, S.; Rajesh, A.M.; Kaleemulla, K.M. Optimization of Process Parameters of Fracture Toughness Using Simulation Technique Considering Aluminum–Graphite Composites. Trans. Indian Inst. Met. 2020, 73, 3095–3103. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Hassan, M.K.; Hashem, A.M. Finite Element Computational Approach of Fracture Toughness in Composite Compact-Tension Specimen. Int. J. Mech. Mechatron. Eng. 2012, 12, 57–61. [Google Scholar]
- Achebo, J. Development of a Predictive Model for Determining Mechanical Properties of AA 6061 Using Regression Analysis. Prod. Manuf. Res. 2015, 3, 169–184. [Google Scholar] [CrossRef][Green Version]
- Gander, M.J.; Hennicker, J.; Masson, R. Modeling and Analysis of the Coupling in Discrete Fracture Matrix Models. SIAM J. Numer. Anal. 2021, 59, 195–218. [Google Scholar] [CrossRef]
- Zhong, Y.; Shao, Y.; Gao, X.; Luo, X.; Zhu, H. Fatigue Crack Growth of EH36 Steel in Air and Corrosive Marine Environments. J. Constr. Steel Res. 2023, 210, 108104. [Google Scholar] [CrossRef]
- Vishnuvardhan, S.; Saravanan, M.; Gandhi, P.; Raghava, G. Fatigue Crack Growth Studies on Power Plant Piping Materials under Corrosive Environment. Procedia Struct. Integr. 2019, 14, 482–490. [Google Scholar] [CrossRef]
- Brückner, A.; Munz, D. The Effect of Curve Fitting on the Prediction of Failure Probabilities from the Scatter in Crack Geometry and Fracture Toughness. Reliab. Eng. 1983, 5, 139–156. [Google Scholar] [CrossRef]
- Guddhur, H.; Naganna, C.; Doddamani, S. Taguchi’s Method of Optimization of Fracture Toughness Parameters of Al-SiCp Composite Using Compact Tension Specimens. Int. J. Optim. Control. Theor. Appl. 2021, 11, 152–157. [Google Scholar] [CrossRef]
- Younis, H.B.; Kamal, K.; Sheikh, M.F.; Hamza, A. Prediction of Fatigue Crack Growth Rate in Aircraft Aluminum Alloys Using Optimized Neural Networks. Theor. Appl. Fract. Mech. 2022, 117, 103196. [Google Scholar] [CrossRef]
- Kovalov, D.; Fekete, B.; Engelhardt, G.R.; Macdonald, D.D. Prediction of Corrosion Fatigue Crack Growth Rate in Alloys. Part II: Effect of Electrochemical Potential, NaCl Concentration, and Temperature on Crack Propagation in AA2024-T351. Corros. Sci. 2019, 152, 130–139. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Li, C.; Zhang, T.; Zhang, S. Effect of Alternate Corrosion and Fatigue on Fatigue Crack Growth Characterization of 2024-T4 Aluminum Alloy. Math. Probl. Eng. 2020, 2020, 7314241. [Google Scholar] [CrossRef]
- Bergner, F.; Zouhar, G. New Approach to the Correlation between the Coefficient and the Exponent in the Power Law Equation of Fatigue Crack Growth. Int. J. Fatigue 2000, 22, 229–239. [Google Scholar] [CrossRef]
- Alqahtani, I.; Starr, A.; Khan, M. Investigation of the Combined Influence of Temperature and Humidity on Fatigue Crack Growth Rate in Al6082 Al-Loy in a Coastal Environment. Materials 2023, 16, 6833. [Google Scholar] [CrossRef]
- He, F.; Khan, M.; Aldosari, S. Interdependencies between Dynamic Response and Crack Growth in a 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Cantilever Beam under Thermo-Mechanical Loads. Polymers 2022, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, H.; Feng, W.; Xu, S.; Li, Y.; Zhang, R. Fatigue Life Analysis for 6061-T6 Aluminum Alloy Based on Surface Roughness. PLoS ONE 2021, 16, e0252772. [Google Scholar] [CrossRef] [PubMed]
- Zai, B.A.; Khan, M.; Khan, S.Z.; Asif, M.; Khan, K.A.; Saquib, A.N.; Mansoor, A.; Shahzad, M.; Mujtaba, A. Prediction of Crack Depth and Fatigue Life of an Acrylonitrile Butadiene Styrene Cantilever Beam Using Dynamic Response. J. Test. Eval. 2020, 48, 1520–1536. [Google Scholar] [CrossRef]
- Guo, M.; Li, D.; Rao, S.X.; Guo, B.L. Effect of Environmental Factors on the Corrosion of 2024T3 Aluminium Alloy. In Proceedings of the 9th International Conference on Aluminium Alloys, Brisbane, Australia, 2–5 August 2004; Nie, J.F., Morton, A.J., Muddle, B.C., Eds.; Institute of Materials Engineering Australasia Ltd.: Melbourne, Australia, 2004; pp. 433–438. [Google Scholar]
- Ichitani, K. Effect of Experimental Humidity on Fatigue Fracture of 6XXX-Series Aluminum Alloys. Mater. Sci. Eng. 2012, 15–21. [Google Scholar]
- Bayoumi, M. Fatigue Behaviour of a Commercial Aluminium Ahoy in Sea Water at Different Temperatures. Eng. Fract. Mech. 1993, 45, 297–307. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.Z.; Sohail, W.; Khan, H.S.M.; Nisar, S. Mechanical Fatigue in Aluminum at Elevated Temperature and Remaining Life Prediction Based on Natural Frequency Evolution. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 897–903. [Google Scholar] [CrossRef]
- Omar, I.K.M.; Starr, A. Comparative Analysis of Machine Learning Models for Predicting Crack Propagation under Coupled Load and Temperature. Appl. Sci. 2023, 13, 7212. [Google Scholar] [CrossRef]
- He, F.; Khan, M. Effects of Printing Parameters on the Fatigue Behaviour of 3d-Printed Abs under Dynamic Thermo-Mechanical Loads. Polymers 2021, 13, 2362. [Google Scholar] [CrossRef]
- Kamei, K.; Khan, M.A. Current Challenges in Modelling Vibrational Fatigue and Fracture of Structures: A Review. J. Braz. Soc. Mech. Sci. Eng. 2020, 43, 77. [Google Scholar] [CrossRef]
- Agala, A.; Khan, M.; Starr, A. Degradation Mechanisms Associated with Metal Pipes and the Effective Impact of LDMs and LLMs in Water Transport and Distribution. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2023, 237, 1855–1876. [Google Scholar] [CrossRef]
- Khan, S.Z.; Khan, T.M.; Joya, Y.F.; Khan, M.A.; Ahmed, S.; Shah, A. Assessment of Material Properties of AISI 316L Stainless Steel Using Non-Destructive Testing. Nondestruct. Test. Eval. 2016, 31, 360–370. [Google Scholar] [CrossRef]
- Gairola, S.; Rengaswamy, J.; Verma, R. A Study on XFEM Simulation of Tensile, Fracture Toughness, and Fatigue Crack Growth Behavior of Al 2024 Alloy through Fatigue Crack Growth Rate Models Using Genetic Algorithm. Fatigue Fract. Eng. Mater. Struct. 2023, 46, 2121–2138. [Google Scholar] [CrossRef]
- Hareesha, G.; Chikkanna, N.; Doddamani, S. Finite Element Simulation of Fracture Toughness of Al6061 Reinforced with Silicon Carbide. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1065, 012036. [Google Scholar] [CrossRef]
- Saleemsab Doddamani, M.K. Indentation Fracture Toughness of Alumnum6061-Graphite Composites. Int. J. Fract. Damage Mech. 2016, 1, 40–46. [Google Scholar]
- Zhou, Z.; Bhamare, S.; Qian, D. Ductile Fracture in Thin Sheet Metals: A FEM Study of the Sandia Fracture Challenge Problem Based on the Gurson–Tvergaard–Needleman Fracture Model. Int. J. Fract. 2014, 186, 185–200. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, Y.; Liu, Y. Life Prediction of Corrosion-Fatigue Based on a New Crack Growth Rate Model with Damage and the Extended Finite Element Method. Eng. Fract. Mech. 2023, 289, 109445. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, X.; Hu, B.; Chen, L. Equivalent Crack Size Model for Pre-Corrosion Fatigue Life Prediction of Aluminum Alloy 7075-T6. Int. J. Fatigue 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Hu, P.; Meng, Q.; Hu, W.; Shen, F.; Zhan, Z.; Sun, L. A Continuum Damage Mechanics Approach Coupled with an Improved Pit Evolution Model for the Corrosion Fatigue of Aluminum Alloy. Corros. Sci. 2016, 113, 78–90. [Google Scholar] [CrossRef]
- Safyari, M.; Khossossi, N.; Meisel, T.; Dey, P.; Prohaska, T.; Moshtaghi, M. New Insights into Hydrogen Trapping and Embrittlement in High Strength Aluminum Alloys. Corros. Sci. 2023, 223, 111453. [Google Scholar] [CrossRef]
- Gholami, M.D.; Hashemi, R.; Sedighi, M. The Effect of Temperature on the Mechanical Properties and Forming Limit Diagram of Aluminum Strips Fabricated by Accumulative Roll Bonding Process. J. Mater. Res. Technol. 2020, 9, 1831–1846. [Google Scholar] [CrossRef]
- Mouritz, A.P. 19—Fracture Toughness Properties of Aerospace Materials. In Introduction to Aerospace Materials; Mouritz, A.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 454–468. ISBN 9781855739468. [Google Scholar]


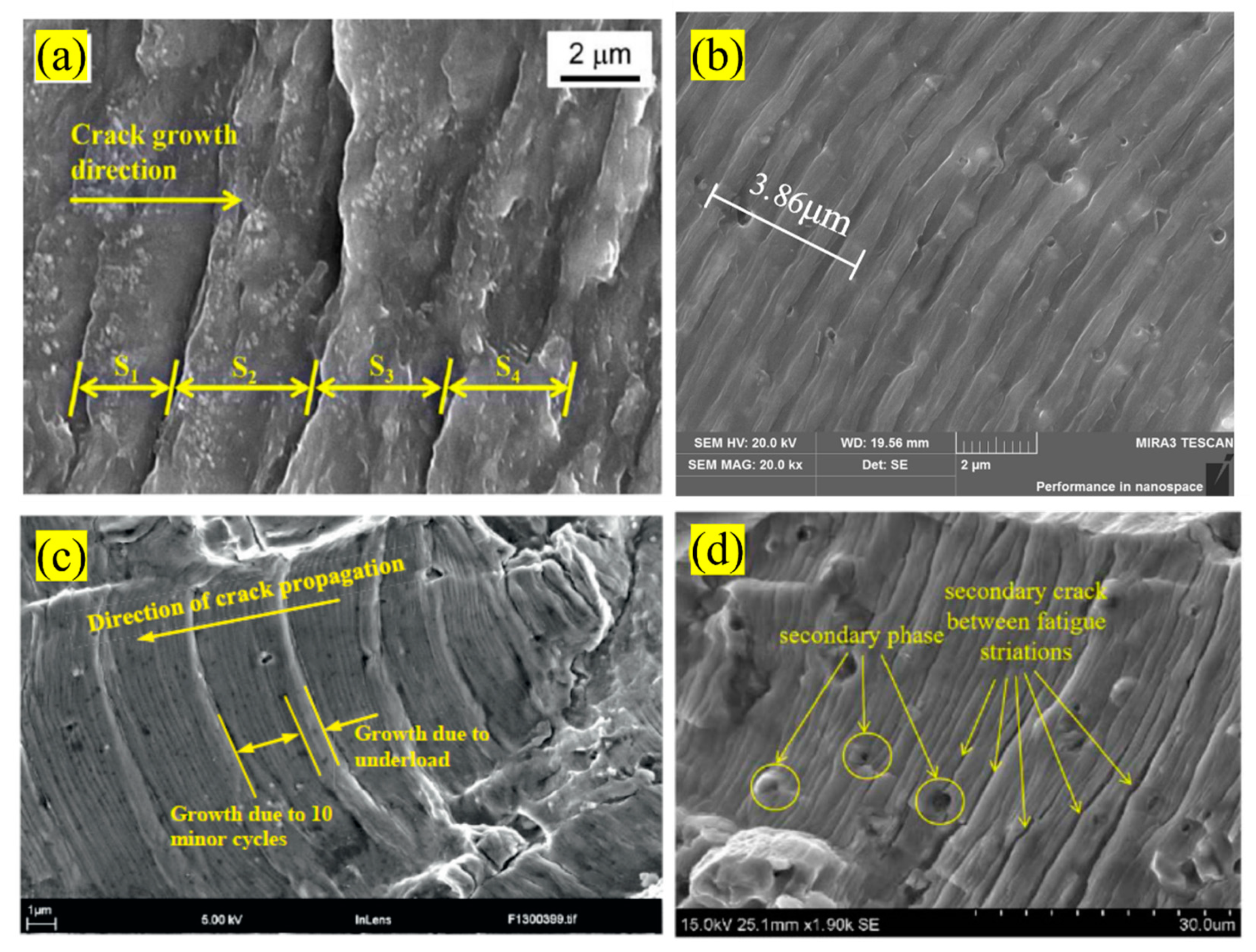
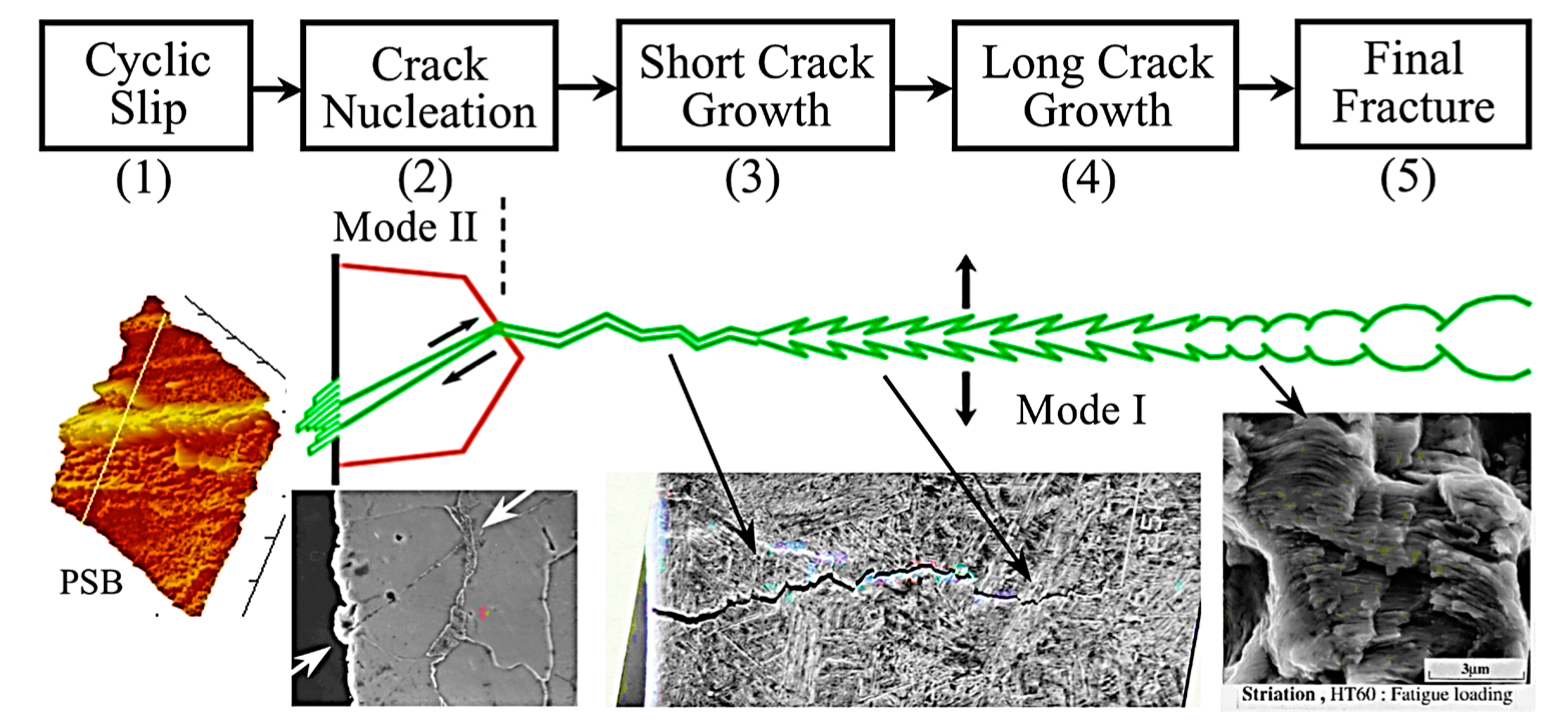
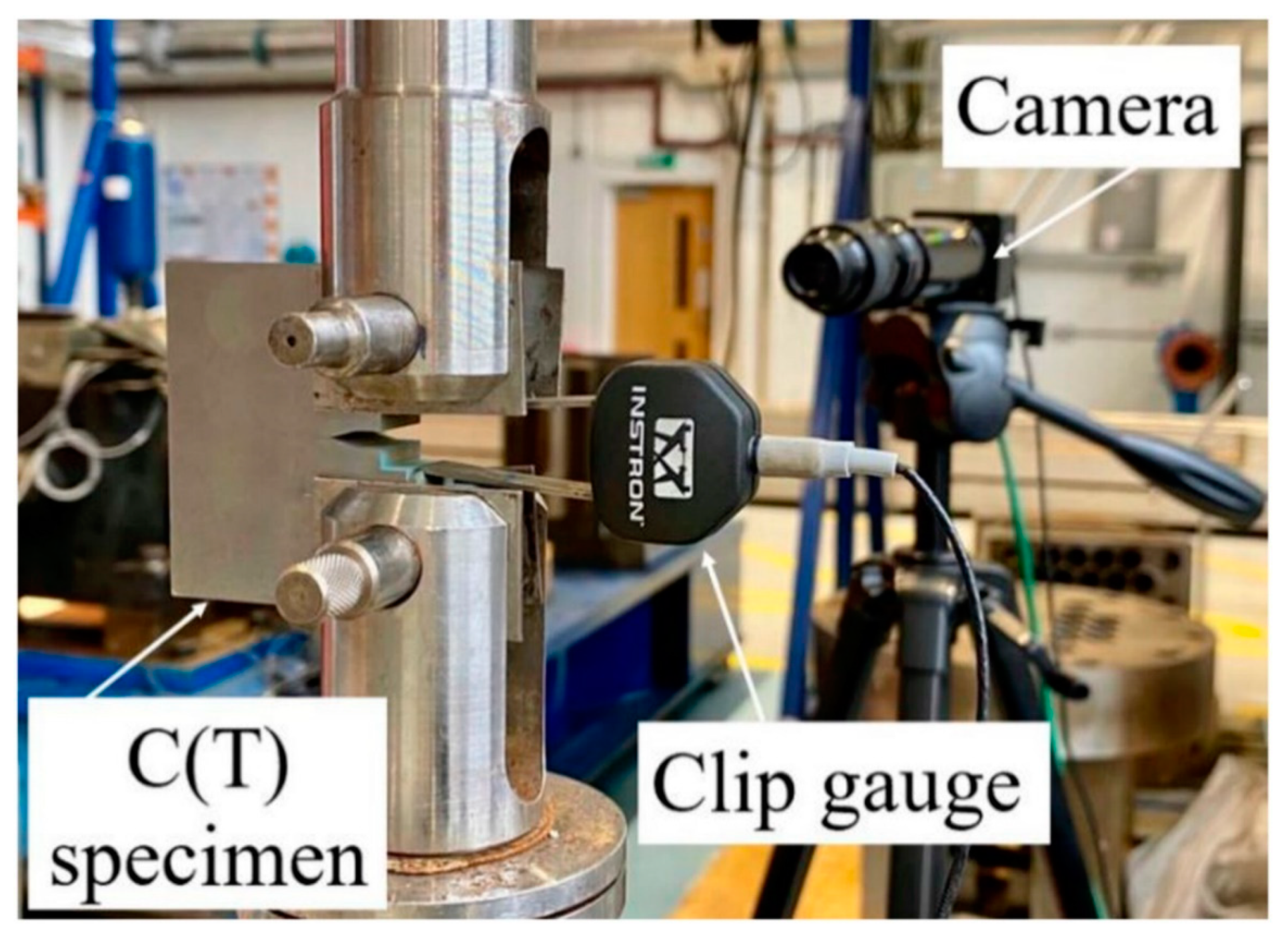
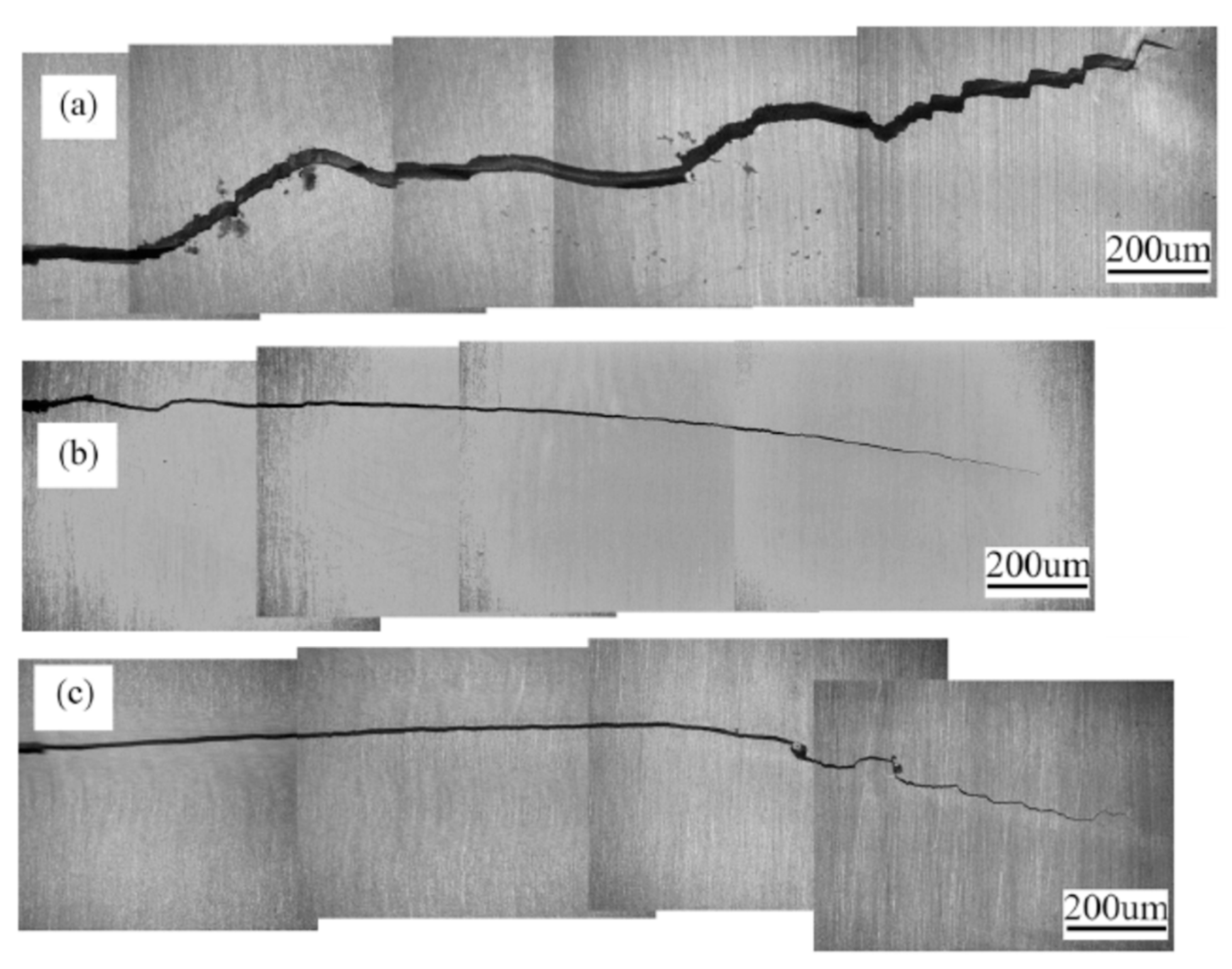


| Al Alloy | Elastic Modulus (GPa) | Yield Strength (MPa) | Fatigue Strength (MPa) | Fracture Toughness (MPa.√m) | Reference |
|---|---|---|---|---|---|
| AA6061 | 68–74 | 193–290 | 207 | 18.21 | [60] |
| AA6082 | 67.1 | 276 | - | 19–25 | [60] |
| AA7075 | 71 | 482 | - | 27.5 | [61] |
| AA7050 | 70–80 | 455 | 240 | 27.5 | [62] |
| AA2024 | 72–75.7 | 345–381 | 138 | 18.5 | [63] |
| AA5083 | 70–73.6 | 269–297 | - | 28.3 | [64] |
| AA8090 | 77 | 370 | 100 | 28 | [65] |
| Author | Model Used | Methodology | Application | Limitation |
|---|---|---|---|---|
| Zhiying et al. (2016) [205] | Corrosion–fatigue | Paris model and Trantina–Johnson model | Accurate results in corrosion conditions | Does not consider the temperature and humidity |
| C.Q. Wang et al. (2023) [53] | Corrosion–fatigue | Trantina–Johnson model | ||
| Huang et al. (2016) [206] | Pre-corrosion fatigue | Equivalent crack size (ECS) models and experiment | It focuses on single and multi-crack initiations | Pre-corrosion; does not consider the temperature and humidity |
| Ping et al. (2016) [207] | Theoretical model and numerical simulation | Pitting corrosion model | Fatigue damage was evaluated for the model of pit growth | Does not consider the temperature and humidity |
| Safyari et al. (2021) [107] and (2023) [208] | Humidity model | Hydrogen embrittlement mechanism | Hydrogen sensitivity index | Does not consider the temperature and corrosion |
| Delshad et al. (2020) [209] | Temperature model | Ductility and Yielding | Mechanical properties | Does not consider the humidity and corrosion |
| Mouritz et al. (2012) [210] | Temperature model | Fracture toughness | Aerospace materials | Does not consider the humidity and corrosion |
| Kimberly et al. (2020) [108] | Temperature and corrosion model | Pitting corrosion | Mg2Si intermetallic formation | Does not consider the humidity conditions |
| Sarah et al. (2023) [99] | Atmospheric corrosion | Frequency and salinity, atmospheric conditions | Atmospheric corrosion FCGR | Does not consider the Temperature and humidity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, I.; Starr, A.; Khan, M. Fracture Behaviour of Aluminium Alloys under Coastal Environmental Conditions: A Review. Metals 2024, 14, 336. https://doi.org/10.3390/met14030336
Alqahtani I, Starr A, Khan M. Fracture Behaviour of Aluminium Alloys under Coastal Environmental Conditions: A Review. Metals. 2024; 14(3):336. https://doi.org/10.3390/met14030336
Chicago/Turabian StyleAlqahtani, Ibrahim, Andrew Starr, and Muhammad Khan. 2024. "Fracture Behaviour of Aluminium Alloys under Coastal Environmental Conditions: A Review" Metals 14, no. 3: 336. https://doi.org/10.3390/met14030336
APA StyleAlqahtani, I., Starr, A., & Khan, M. (2024). Fracture Behaviour of Aluminium Alloys under Coastal Environmental Conditions: A Review. Metals, 14(3), 336. https://doi.org/10.3390/met14030336









