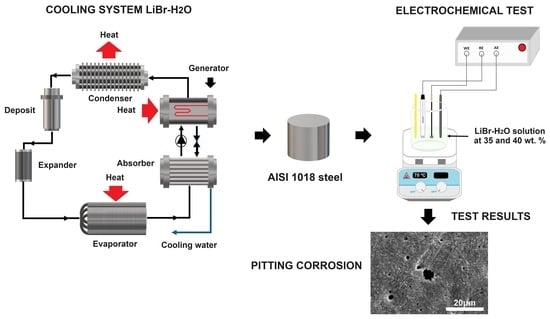
Corrosion Behavior of Carbon Steel in LiCl/H2O Mixtures
Abstract
1. Introduction
2. Experimental Procedure
2.1. Testing Material
2.2. Working Fluid
2.3. Electrochemical Techniques
3. Results and Discussion
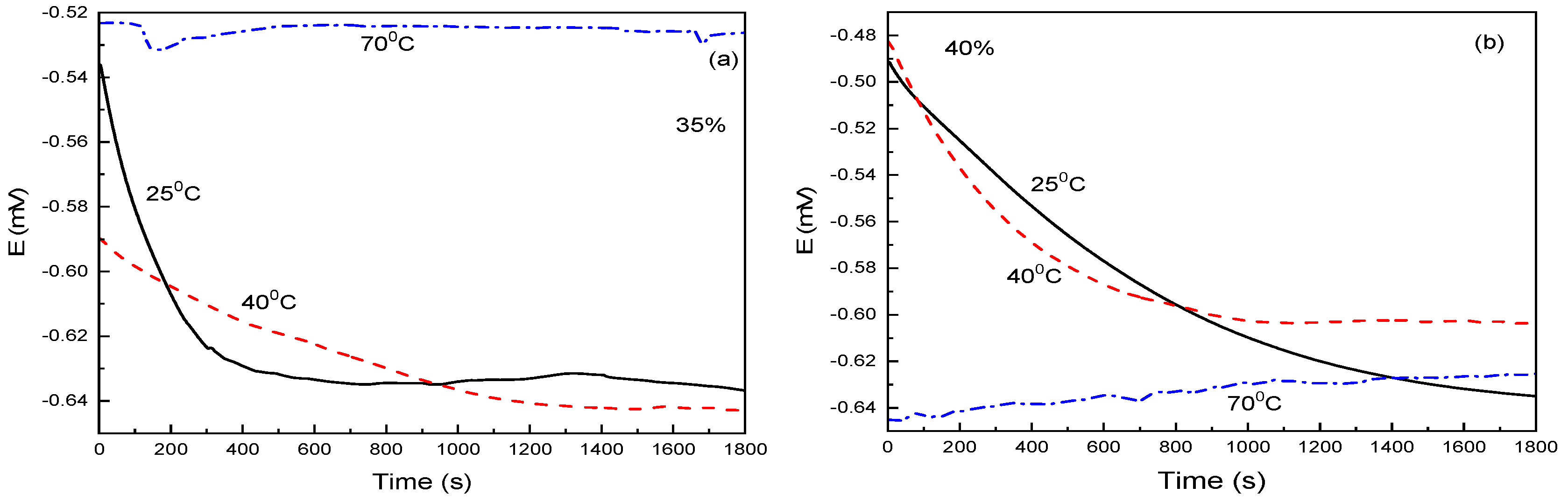
3.1. Open Circuit Potential
3.2. Potentiodynamic Polarization Curves (PPC)
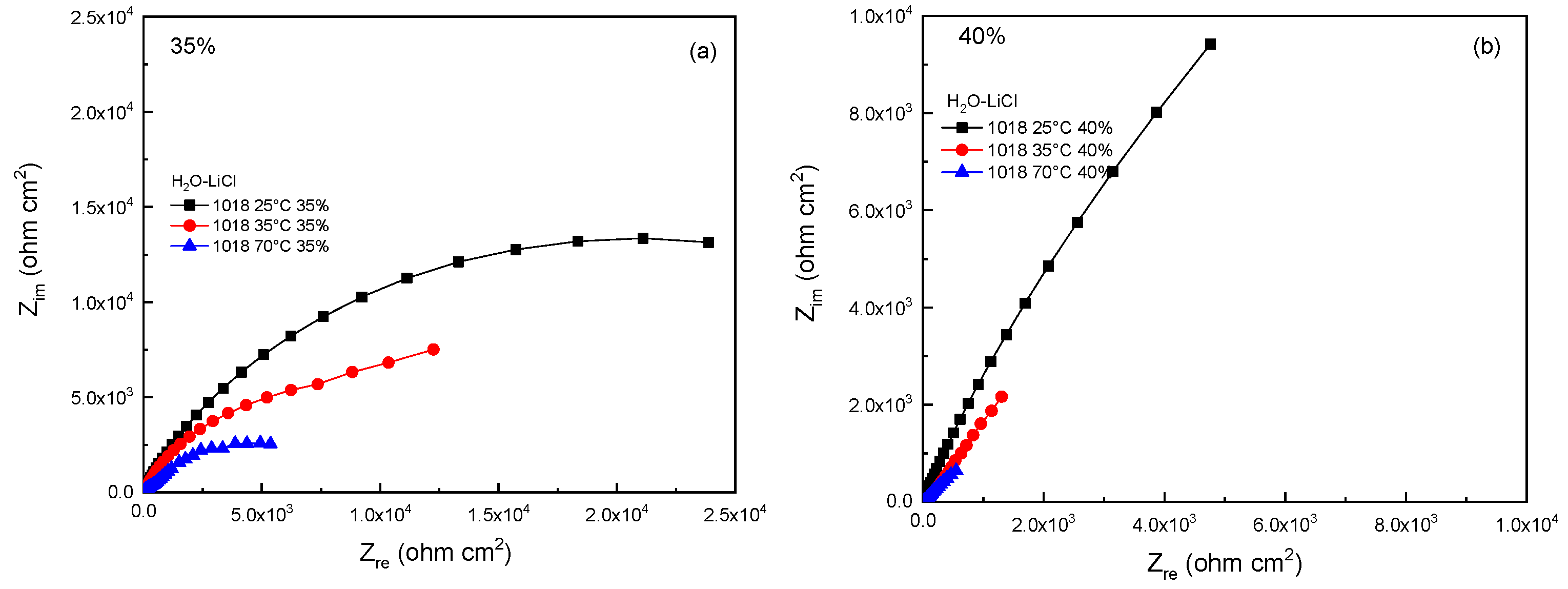
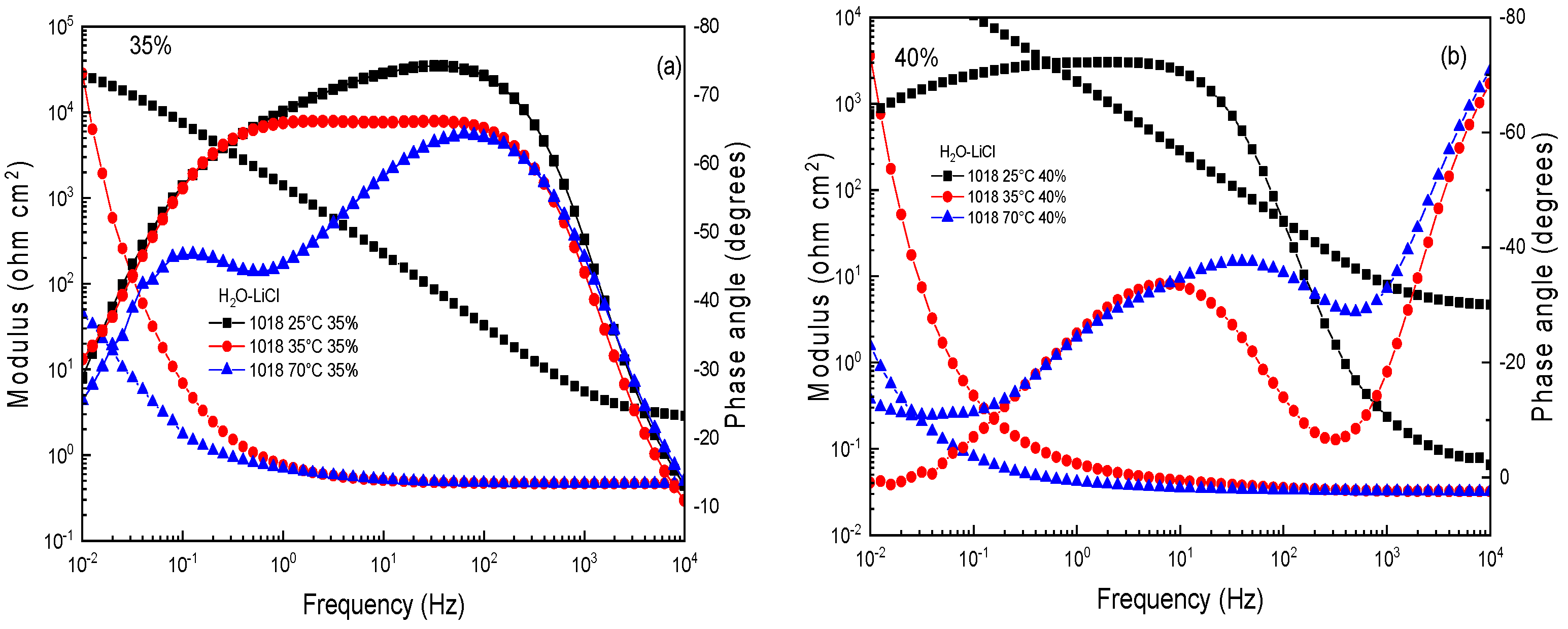
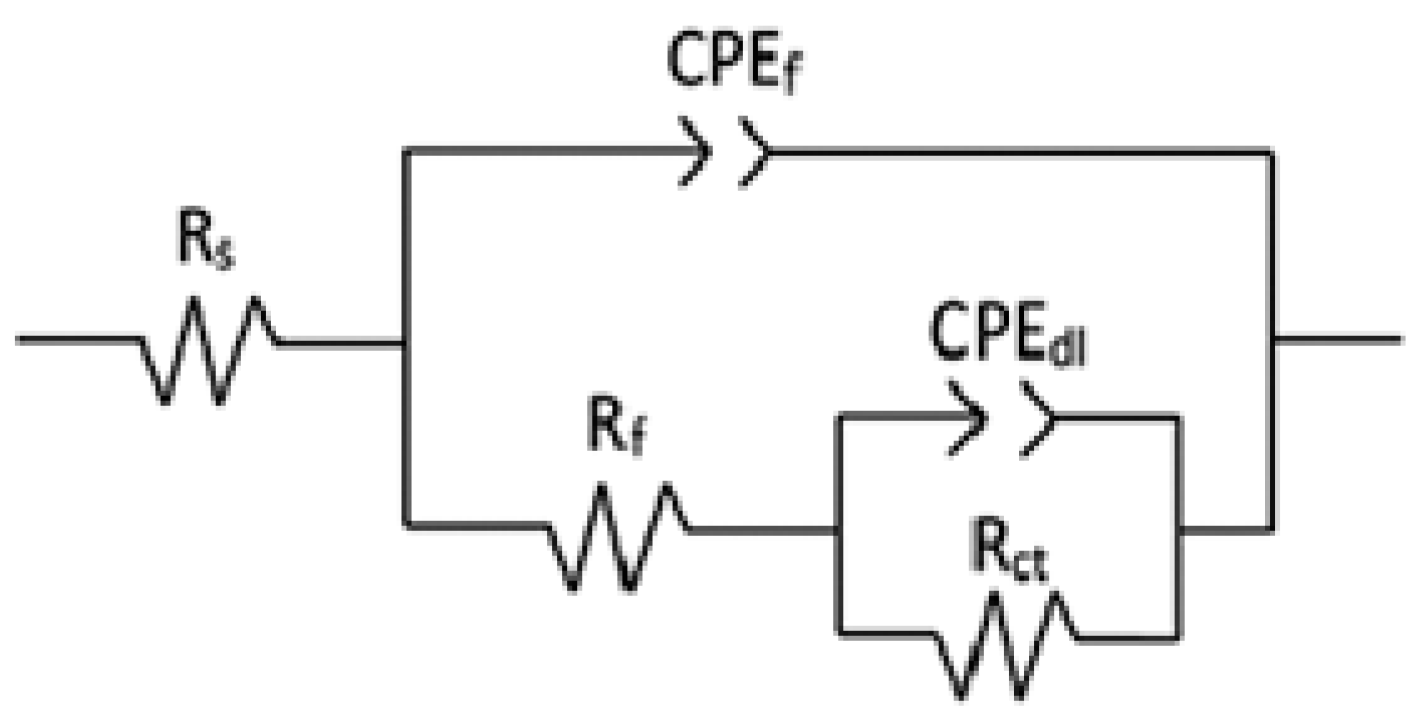
3.3. Electrochemical Impedance Spectroscopy (EIS)
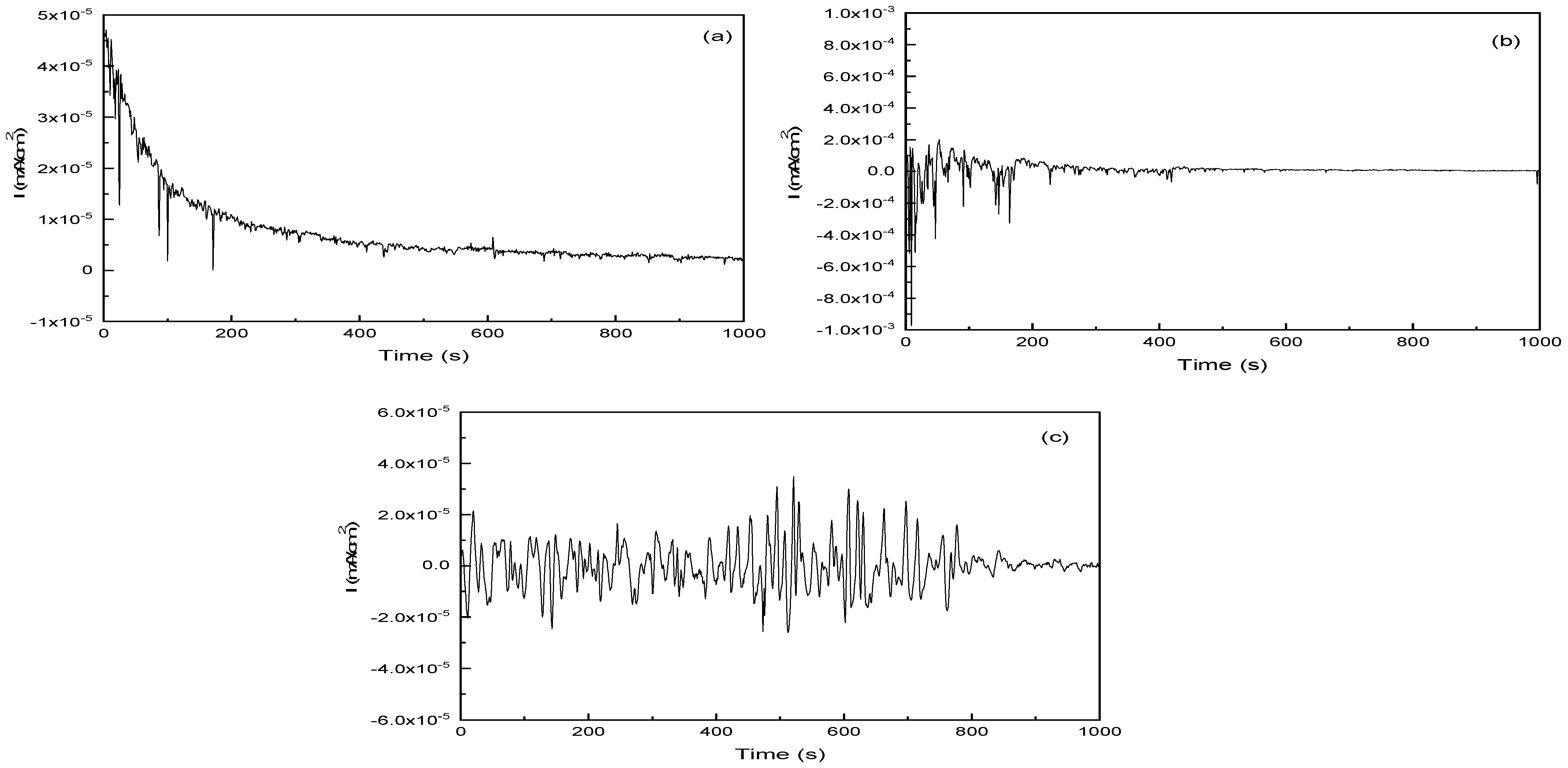
3.4. Electrochemical Noise Measurements (EN)
3.5. Calculation of Icorr by Using Different Techniques
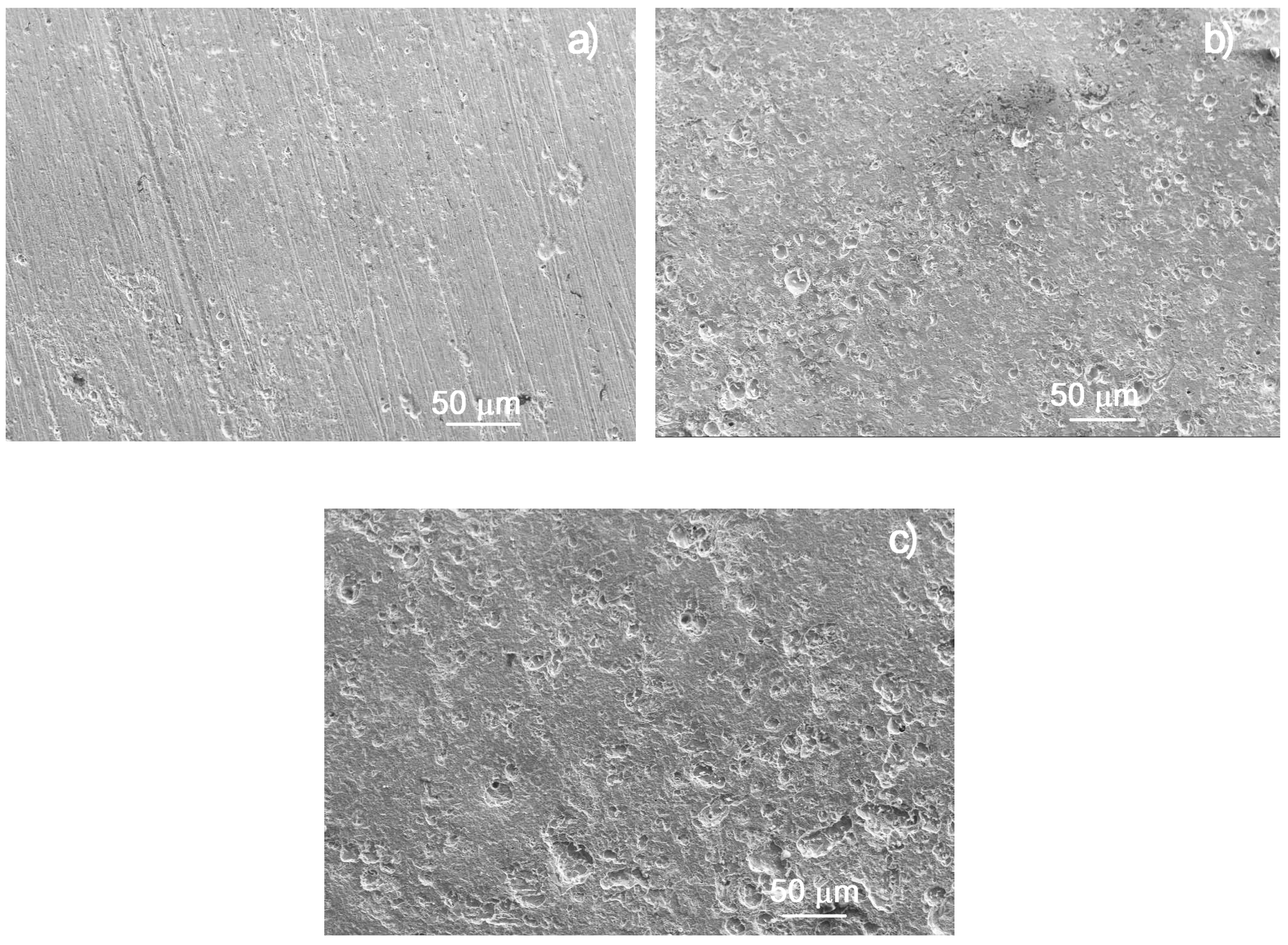
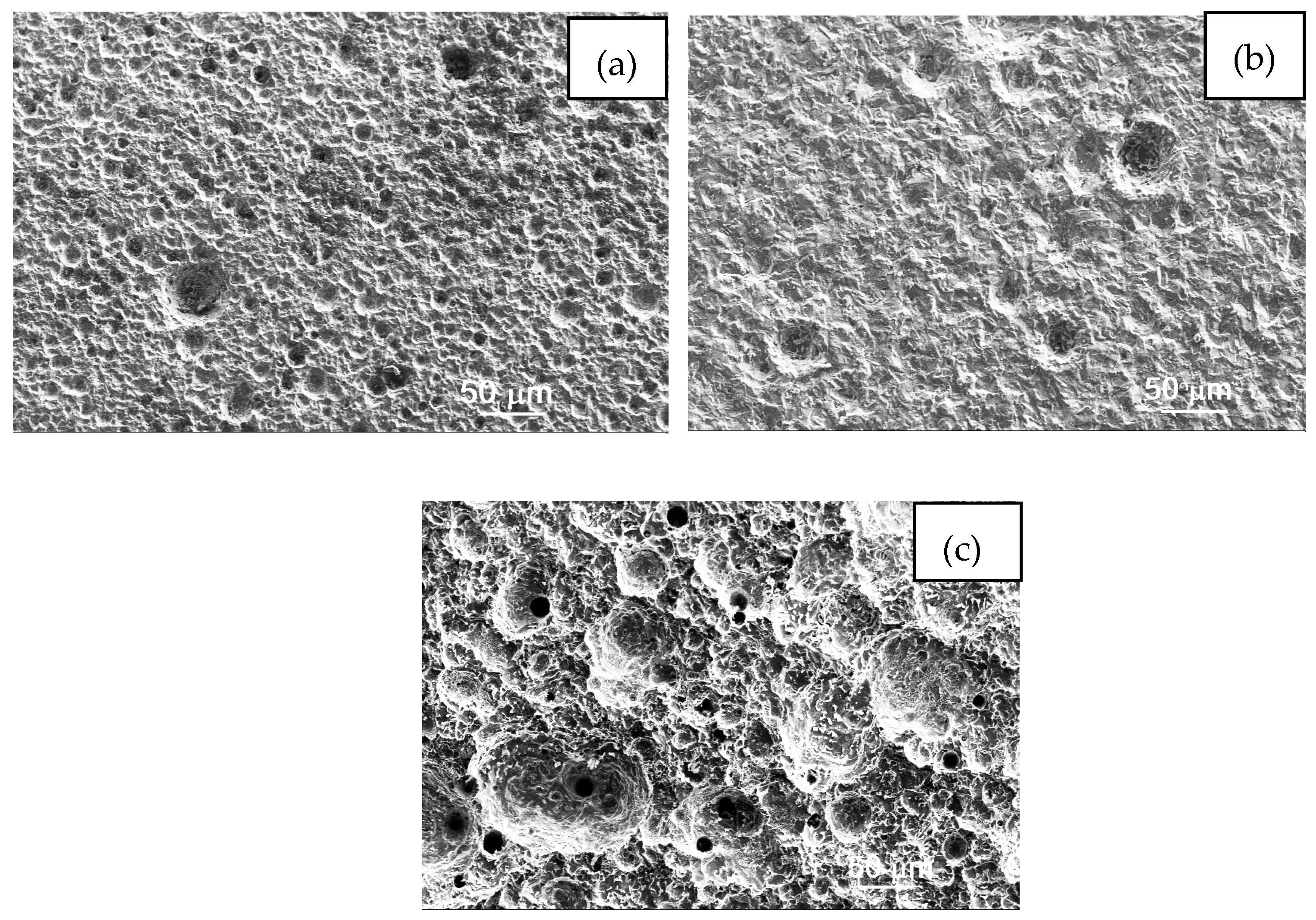
3.6. Analysis of Corroded Surfaces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mclinden, M.O.; Seeton, C.J.; Pearson, A. New refrigerants and system configurations for vapor-compression refrigeration. Science 2020, 370, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.T.; Kyriakides, A.S.; Khan, M.S.; Shehabi, M.; Papadopoulosb, A.I.; Hassan, I.; Rahman, M.A.; Seferlis, P. Thermo-economic and environmental assessment of hybrid vapor compression-absorption refrigeration systems for district cooling. Energy 2022, 243, 122991. [Google Scholar] [CrossRef]
- Sharif, M.Z.; Azmi, W.H.; Ghazali, M.F.; Samykano, M.; Ali, H.M. Performance improvement strategies of R1234yf in vapor compression refrigeration system as a R134a replacement: A review. J. Taiwan Inst. Chem. Eng. 2023, 148, 105032. [Google Scholar] [CrossRef]
- Kong, D.; Yin, X.; Ding, X.; Fang, N.; Duan, P. Global optimization of a vapor compression refrigeration system with a self-adaptive differential evolution algorithm. Appl. Therm. Eng. 2021, 197, 117427. [Google Scholar] [CrossRef]
- Christopher, S.S.; Santosh, R.; Vikram, M.P.; Prabakaran, R.; Thakur, A.K.; Xu, H. Optimization of a solar water heating system for vapor absorption refrigeration system. Environ. Prog. Sustain. Energy 2021, 40, e13489. [Google Scholar] [CrossRef]
- Nikbakhti, R.; Wang, X.; Hussein, A.K.; Iranmanesh, A. Absorption cooling systems—Review of various techniques for energy performance enhancement. Alex. Eng. J. 2020, 59, 707–738. [Google Scholar] [CrossRef]
- Selvaraj, D.A.; Victor, K. Design and Performance of Solar PV Integrated Domestic Vapor Absorption Refrigeration System. Int. J. Photoenergy 2021, 2021, 6655113. [Google Scholar] [CrossRef]
- Abdullah, M.O.; Hieng, T.C. Comparative analysis of performance and techno-economics for an H2O-NH3-H2 absorption refrigerator driven by different energy sources. Appl. Energy 2010, 87, 1535–1545. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.W.; Lee, H.; Chung, J.T.; Kang, Y.T. Optimal design of generators for H2O-LiBr absorption chiller whit multi-heat sources. Appl. Energy 2019, 167, 47–59. [Google Scholar] [CrossRef]
- Lee, R.J.; DiGuilio, R.M.; Jeteter, S.M.; Teja, A.S. Properties of LiBr-H2O solutions at high temperatures and concentrations. Part II. Density and viscosity, ASHRAE. Transactions 1990, 96, 709–714. [Google Scholar]
- Wen, T.; Luo, Y.; Sheng, L. Experimental study on the corrosion behavior and regeneration performance of KCOOH aqueous solution. Sol. Energy 2020, 201, 638–648. [Google Scholar] [CrossRef]
- Sun, D.W. Comparison of the performance of NH3-H2O, NH3-LiNO3, and NH3-NaSCN absorption refrigeration system. Appl. Energy Conv. Manag. 1998, 39, 357–368. [Google Scholar] [CrossRef]
- Best, R.; Eisa, M.A.; Holland, F.A. Thermodynamic design data for absorption heat pump system operating on NH3-H2O. Part 2. Heating. Heat Recovery Syst. 1987, 7, 177–185. [Google Scholar] [CrossRef]
- Srikhirin, P.; Aphornratana, S.; Chungpaibulpatana, S. A Review of Absorption Refrigeration Technologies. Appl. Renew. Sust. Energy Rev. 2001, 5, 343–372. [Google Scholar] [CrossRef]
- Ihamdane, R.; Tiskar, M.; Outemsaa, B.; Zelmat, L.; Dagdag, O.; Berisha, A.; Berdimurodov, E.; Ebenso, E.E.; Chaouch, A. Essential oil of Origanum vulgare as a green corrosion inhibitor for carbon steel in acidic medium. Arab. J. Sci. Eng. 2023, 48, 7685–7701. [Google Scholar] [CrossRef]
- Fan, G.; Liu, H.; Fana, B.; Ma, Y.; Hao, H.; Yang, B. Trazodone as an efficient corrosion inhibitor for carbon steel in acidic and neutral chloride-containing media: Facile synthesis, experimental and theoretical evaluations. J. Mol. Liq. 2020, 311, 113302. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhao, T.; Xiong, C.; Xu5, P.; Zhou, J.; Fan, Z. Effectiveness Protection Performance of an Internal Blending Organic Corrosion Inhibitor for Carbon Steel in Chloride Contaminated Simulated Concrete Pore Solution. J. Adv. Concrete Technol. 2020, 18, 116–128. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of Carbon Steel in Marine Environments: Role of the Corrosion Product Layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Bourouis, M.; Vallès, M.; Medrano, M.; Coronas, A. Performance of air-cooled absorption air-conditioning systems working with water-(LiBr+ Lil+ LiNO3+ LiCl). J. Proc. Mech. Eng. 2005, 219, 205–213. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Luo, C.; Su, Q. Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle. Entropy 2019, 21, 546. [Google Scholar] [CrossRef]
- Li, N.; Luo, C.; Su, Q. A working pair of CaCl2-LiBr-LiNO3/H2O and its application in a single-stage solar-driven absorption refrigeration cycle. Appl. Int. J. Refrig. 2017, 86, 1–13. [Google Scholar] [CrossRef]
- Torres-Díaz, T.; Siqueiros, J.; Coronas, A.; Salavera, D.; Huicochea, A.; Juárez-Romero, D. Performance of Li salts (LiBr + LiI + LiNO3+ LiCl) for an absorption cycle of an experimental absorption heat transformer for water purification. Desalination Water Treat. 2017, 82, 292–299. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Pavlovic, S.; Stefanovic, V. Thermodynamic investigation of LiCl-H2O working pair in a double effect absorption chiller driven by parabolic trough collectors. Therm. Sci. Eng. Prog. 2017, 3, 75–87. [Google Scholar] [CrossRef]
- Luo, C.; Su, Q.; Li, N.; Li, Y. Corrosion of Copper in a Concentrated LiNO3 Solution at a High Temperature. Int. J. Electrochem. Sci. 2017, 12, 1896–1914. [Google Scholar] [CrossRef]
- Larios-Galvez, A.K.; Vazquez-Velez, E.; Martinez-Valencia, H.; Gonzalez-Rodriguez, J.G. Effect of Plasma Nitriding and Oxidation on the Corrosion Resistance of 304 Stainless Steel in LiBr/H2O and CaCl2-LiBr-LiNO3-H2O Mixtures. Metals 2023, 13, 920. [Google Scholar] [CrossRef]
- Flores-Guadarrama, I.; Gónzalez-Rodríguez, J.G.; García-Rosas, D.; Guardían-Tapia, R.; León-Hernández, V.; López-Sesenes, R. Corrosion behavior of brass immersed in CaCl2-LiBr-LiNO3/H2O blends. J. Solid State Electrochem. 2023, 27, 3017–3027. [Google Scholar] [CrossRef]
- Flores-Guadarrama, I.; Gonzalez-Rodriguez, J.G.; Uruchurtu-Chavarín, J.; Ramírez-Arteaga, A.M.; Bustos Rivera-Bahena, G.; Guardián-Tapia, R.; López-Sesenes, R. Effect of refrigerant absorbent combinations in the corrosion resistance of copper as structural material in absorption refrigeration systems (ARS). Corros. Eng. Sci. Technol. 2023, 58, 787–798. [Google Scholar] [CrossRef]
- Parham, K.; Atikol, U.; Yari, M.; Agboola, O.P. Evaluation and optimization of single stage absorption chiller using (LiCl + H2O) as the working pair. Adv. Mech. Eng. 2013, 5, 453–462. [Google Scholar] [CrossRef]
- She, X.; Yin, Y.; Xu, M.; Zhang, X. A novel low-grade heat-driven absorption refrigeration system whit LiCl-H2O and LiBr-H2O working pair. Appl. Int. J. Refrig. 2015, 58, 219–234. [Google Scholar] [CrossRef]
- Cerezo, J.; Romero, R.J.; Ibarra, J.; Rodríguez, A.; Montero, G.; Acuña, A. Dynamic Simulation of an Absorption Cooling System with Different Working Mixtures. Energies 2018, 11, 259. [Google Scholar] [CrossRef]
- Conde, R.M. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- Liang, C.H.; Hu, X.Q.; Ma, L. Effects of Na3PW12O40 on the corrosion behavior of carbon steel in 55% LiBr solution. Mater. Corros. 2007, 58, 39–43. [Google Scholar] [CrossRef]
- Guo, J.; Liang, C. Corrosion of carbon steel in concentrated LiNO3 solution at high temperature. Corros. Eng. Sci. Technol. 2002, 14, 197–204. [Google Scholar]
- Tanno, K.; Itoh, M.; Sekiya, H.; Yashiro, H.; Kumagai, N. The corrosion inhibition of carbon steel in lithium bromide solution by hydroxide and molybdate at moderate temperaturas. Corros. Sci. 1993, 34, 1453–1461. [Google Scholar] [CrossRef]
- Smulko, J.; Darowicki, K.; Zielinski, A. Pitting corrosion in steel and electrochemical noise intensity. Electrochem. Commun. 2002, 4, 388–391. [Google Scholar] [CrossRef]
- Sasaki, K.; Isaacs, H.S. Origins of Electrochemical Noise during Pitting Corrosion of Aluminum. J. Electrochem. Soc. 2004, 151, B124–B129. [Google Scholar] [CrossRef]
- Barnatt, S. Electrochemical Nature of Corrosion. In Electrochemical Techniques for Corrosion Engineering; Baboian, R., Ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1985; pp. 1–8. [Google Scholar]
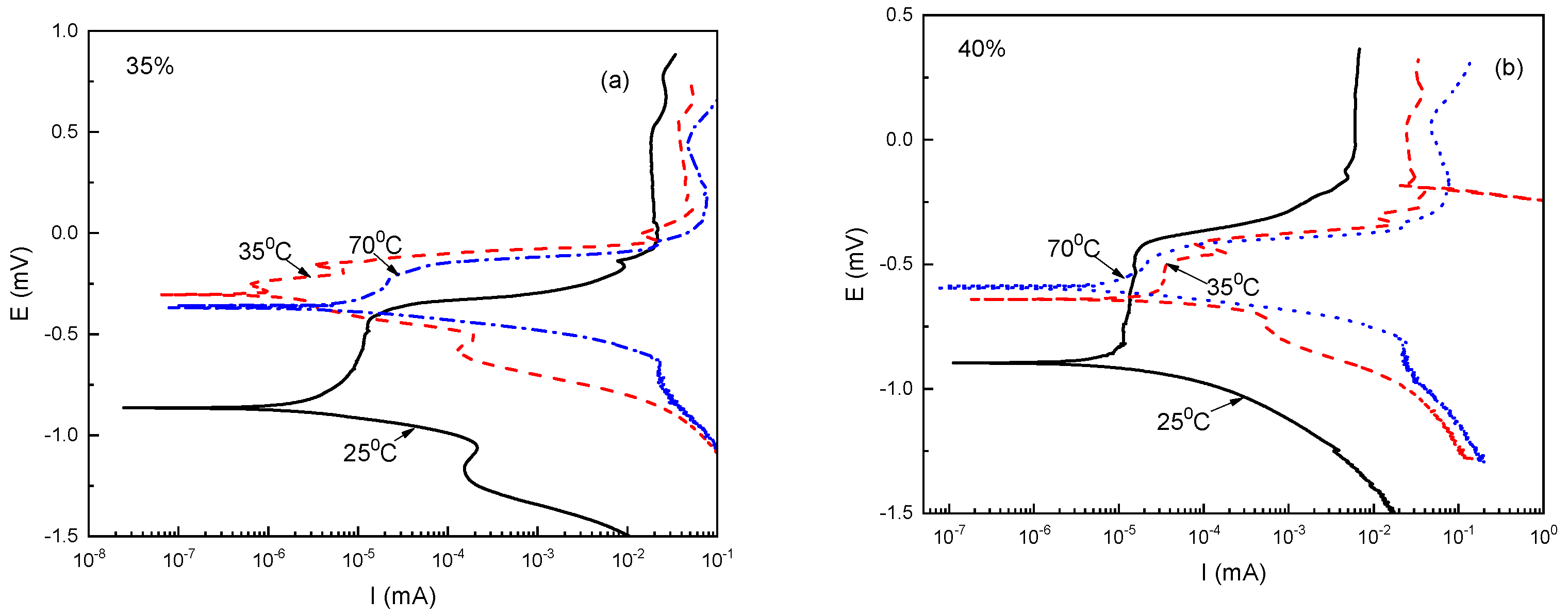

| T (°C) | Ecorr (mV) | Icorr × 10−6 (mA/cm2) | Epit (mV) | Epit-Ecorr (mV) | ||||
|---|---|---|---|---|---|---|---|---|
| 35% | 40% | 35% | 40% | 35% | 40% | 35% | 40% | |
| 25 | −840 | −890 | 5.7 | 11 | −425 | −460 | 415 | 435 |
| 35 | −300 | −590 | 15 | 120 | −240 | −450 | 60 | 140 |
| 70 | −360 | −630 | 290 | 510 | −220 | −455 | 140 | 135 |
| % | T (°C) | Rs (ohm cm2) | Rct (ohm cm2) | CPEdl (F cm−2) | ndl | Rf (ohm cm2) | CPEf (Fcm−2) | nf |
|---|---|---|---|---|---|---|---|---|
| 35 | 25 | 3 | 2950 | 1.20 × 10−4 | 0.9 | 35,109 | 7.23 × 10−5 | 0.9 |
| 35 | 2 | 770 | 5.23 × 10−4 | 0.8 | 15,702 | 1.50 × 10−4 | 0.8 | |
| 70 | 2 | 260 | 8.44 × 10−4 | 0.7 | 6652 | 6.17 × 10−4 | 0.7 | |
| 40 | 25 | 4 | 1177 | 3.38 × 10−4 | 0.8 | 30,825 | 3.24 × 10−5 | 0.8 |
| 35 | 2 | 677 | 6.16 × 10−4 | 0.7 | 9305 | 2.17 × 10−4 | 0.7 | |
| 70 | 2 | 145 | 8.92 × 10−4 | 0.6 | 2539 | 5.20 × 10−4 | 0.6 |
| T (°C) | PPC | EIS | EN | |||
|---|---|---|---|---|---|---|
| 35% | 40% | 35% | 40% | 35% | 40% | |
| 25 | 5.7 | 11 | 7.21 | 12.8 | 36 | 44 |
| 35 | 15 | 120 | 17.3 | 131 | 51 | 139 |
| 70 | 290 | 510 | 302.1 | 522 | 81 | 211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés-Flores, M.; Larios-Gálvez, A.K.; Martínez-González, J.; Lopez-Sesenes, R.; Brito-Franco, A.; Cerezo, J.; Tejeda, F.C.M.; Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G. Corrosion Behavior of Carbon Steel in LiCl/H2O Mixtures. Metals 2024, 14, 376. https://doi.org/10.3390/met14040376
Avilés-Flores M, Larios-Gálvez AK, Martínez-González J, Lopez-Sesenes R, Brito-Franco A, Cerezo J, Tejeda FCM, Ramirez-Arteaga AM, Gonzalez-Rodriguez JG. Corrosion Behavior of Carbon Steel in LiCl/H2O Mixtures. Metals. 2024; 14(4):376. https://doi.org/10.3390/met14040376
Chicago/Turabian StyleAvilés-Flores, Modesto, Ana Karen Larios-Gálvez, José Martínez-González, Roy Lopez-Sesenes, Alfredo Brito-Franco, Jesús Cerezo, Francisco Christian Martinez Tejeda, America Maria Ramirez-Arteaga, and Jose Gonzalo Gonzalez-Rodriguez. 2024. "Corrosion Behavior of Carbon Steel in LiCl/H2O Mixtures" Metals 14, no. 4: 376. https://doi.org/10.3390/met14040376
APA StyleAvilés-Flores, M., Larios-Gálvez, A. K., Martínez-González, J., Lopez-Sesenes, R., Brito-Franco, A., Cerezo, J., Tejeda, F. C. M., Ramirez-Arteaga, A. M., & Gonzalez-Rodriguez, J. G. (2024). Corrosion Behavior of Carbon Steel in LiCl/H2O Mixtures. Metals, 14(4), 376. https://doi.org/10.3390/met14040376